The Potential of Calcium/Phosphate Containing MAO Implanted in Bone Tissue Regeneration and Biological Characteristics
Abstract
:1. Introduction
2. Experimental
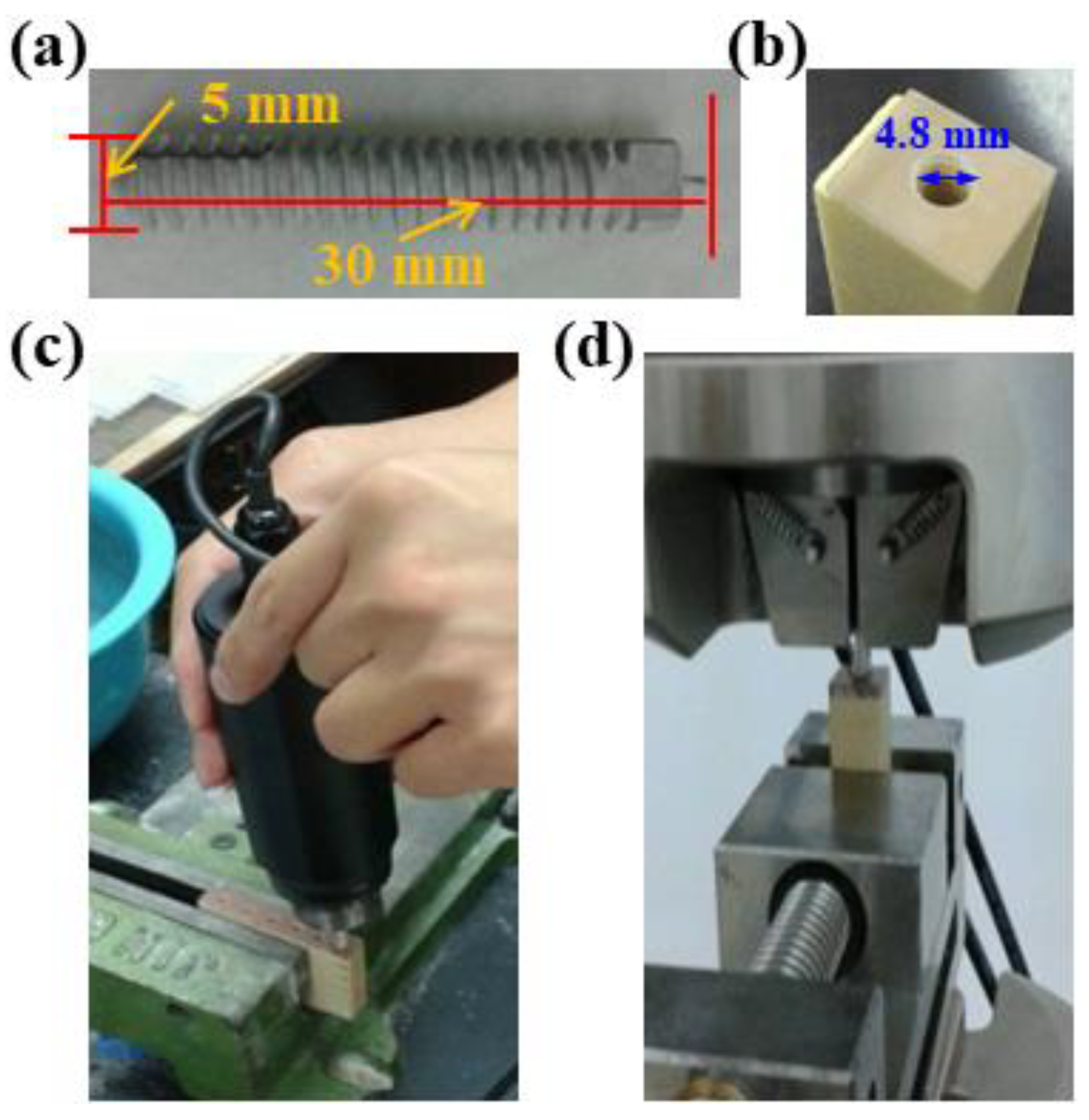
2.1. Preparation of Specimens
2.2. Characterization
2.3. Electrochemical Measurements and Corrosion Test
2.4. Mechanical Testing
2.5. Animal Surgery and Implant Harvest
3. Results and Discussion
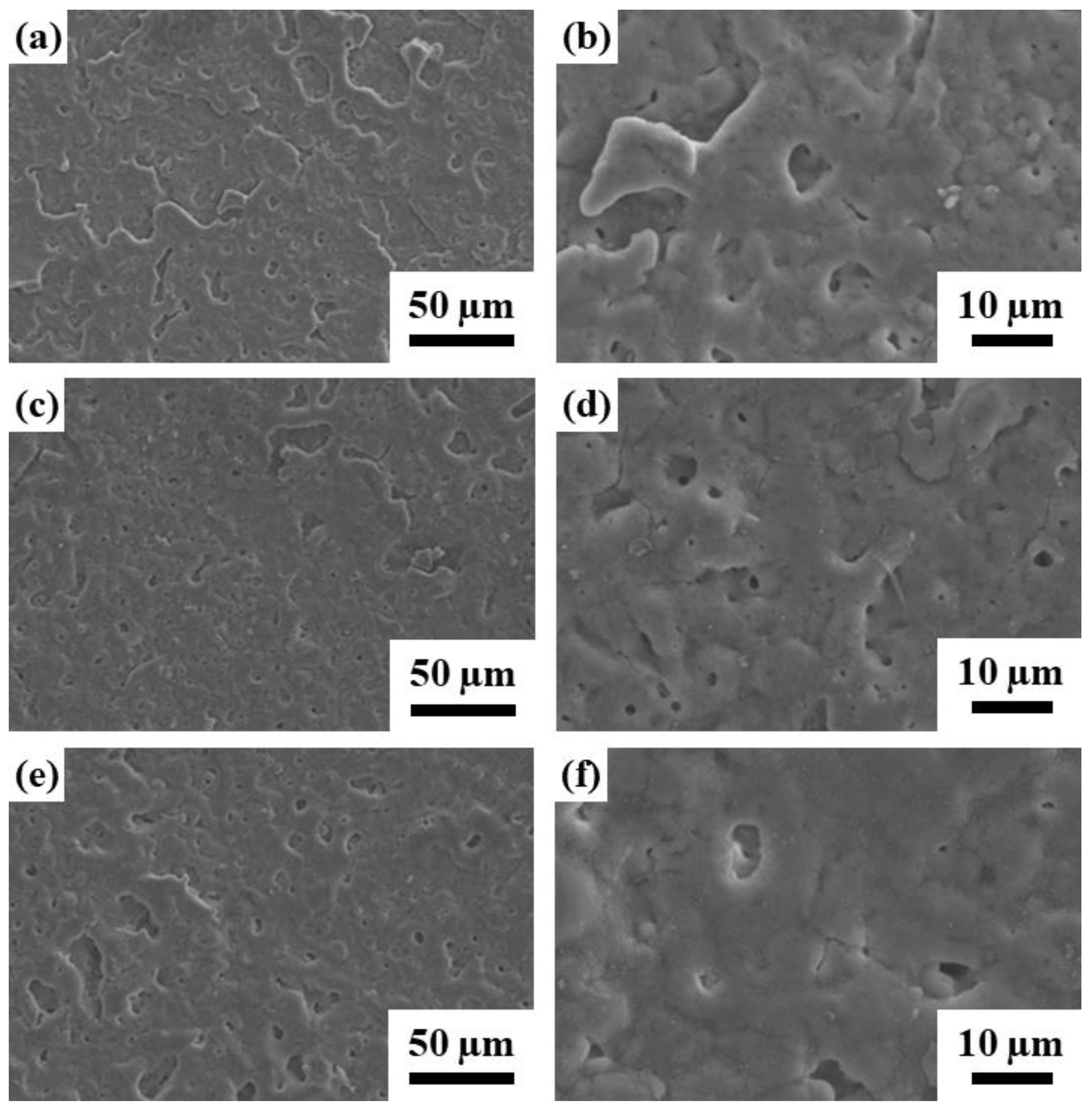
3.1. Specimen Surface Characteristic Analysis
3.1.1. Surface Morphology and Chemical Composition
3.1.2. Cross-Section SEM Characterization
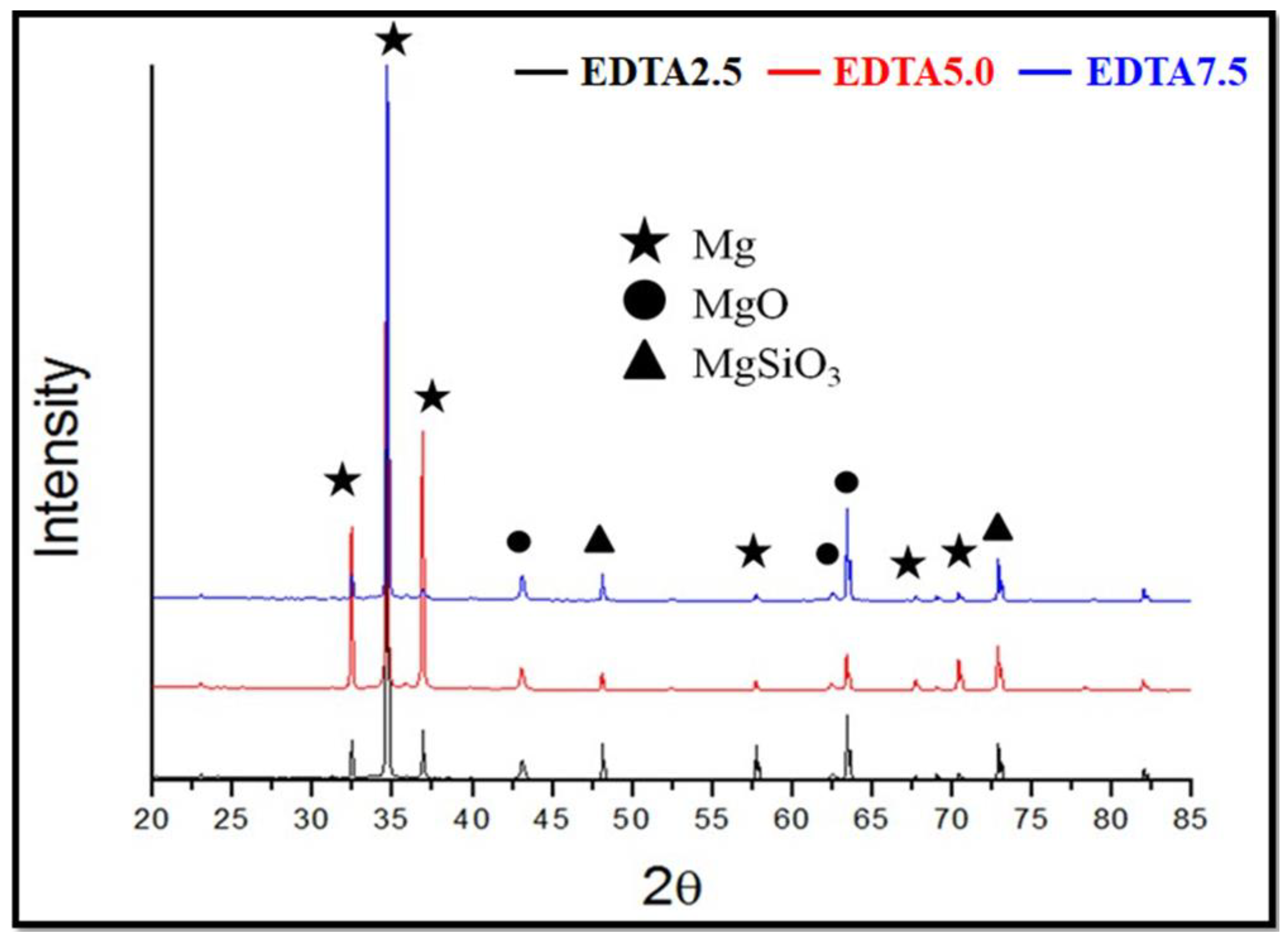
3.1.3. Surface Phase Composition Analysis
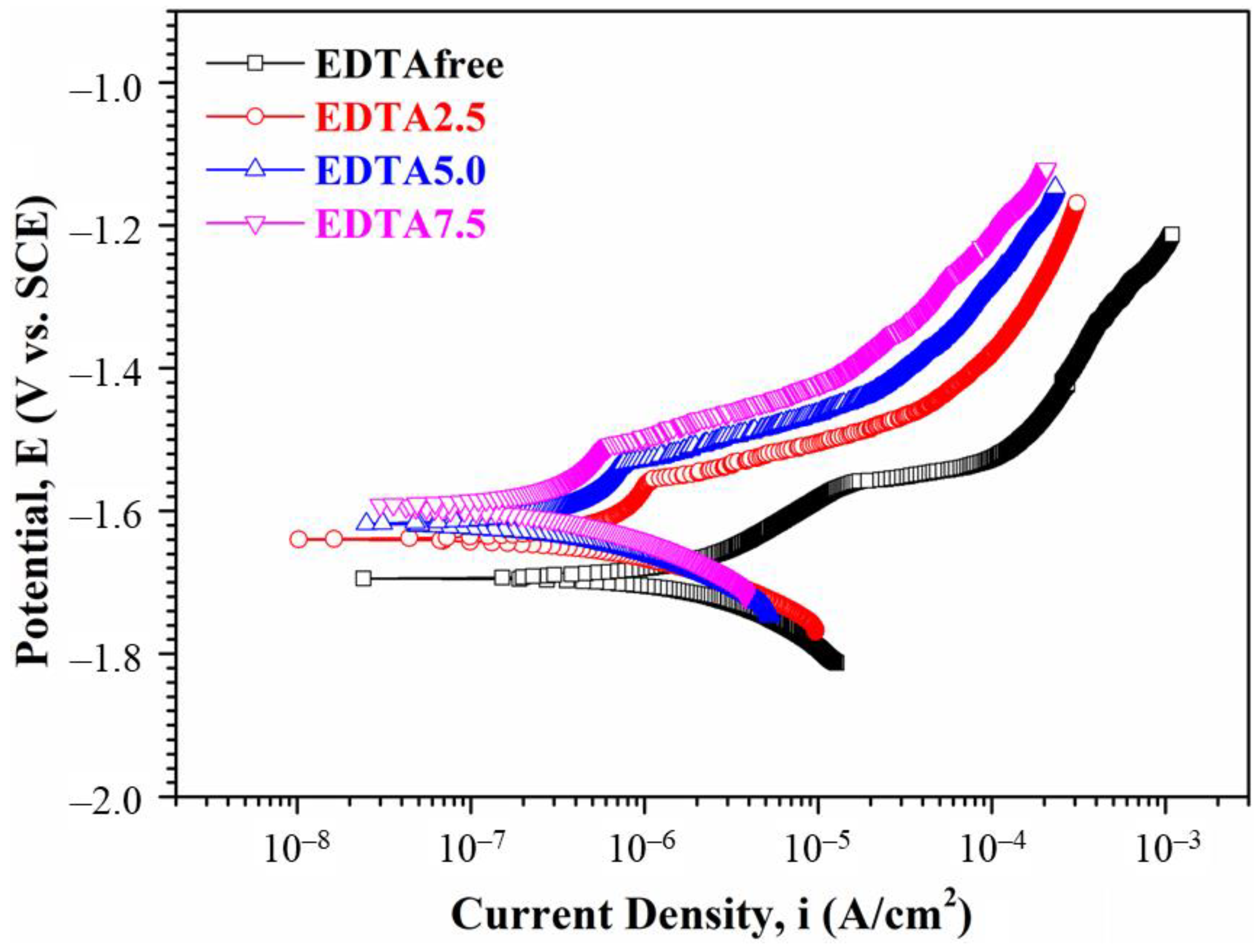
3.1.4. Electrochemical Measurements and Corrosion Test
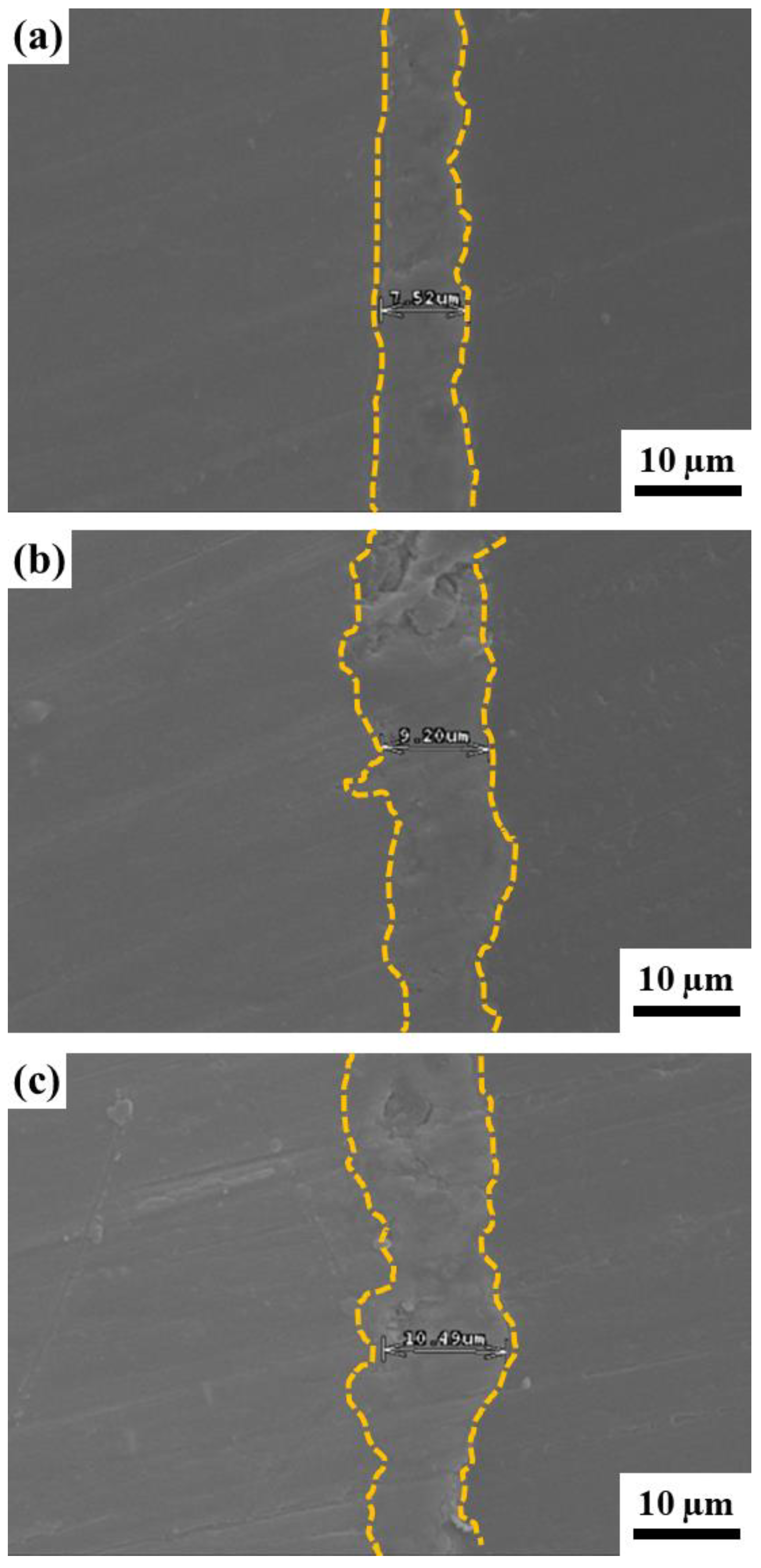
3.1.5. Surface Roughness and Porosity
3.1.6. Mechanical Properties
3.2. Biocompatibility Test
3.2.1. In Vitro Cytotoxicity Test-MTT Assay
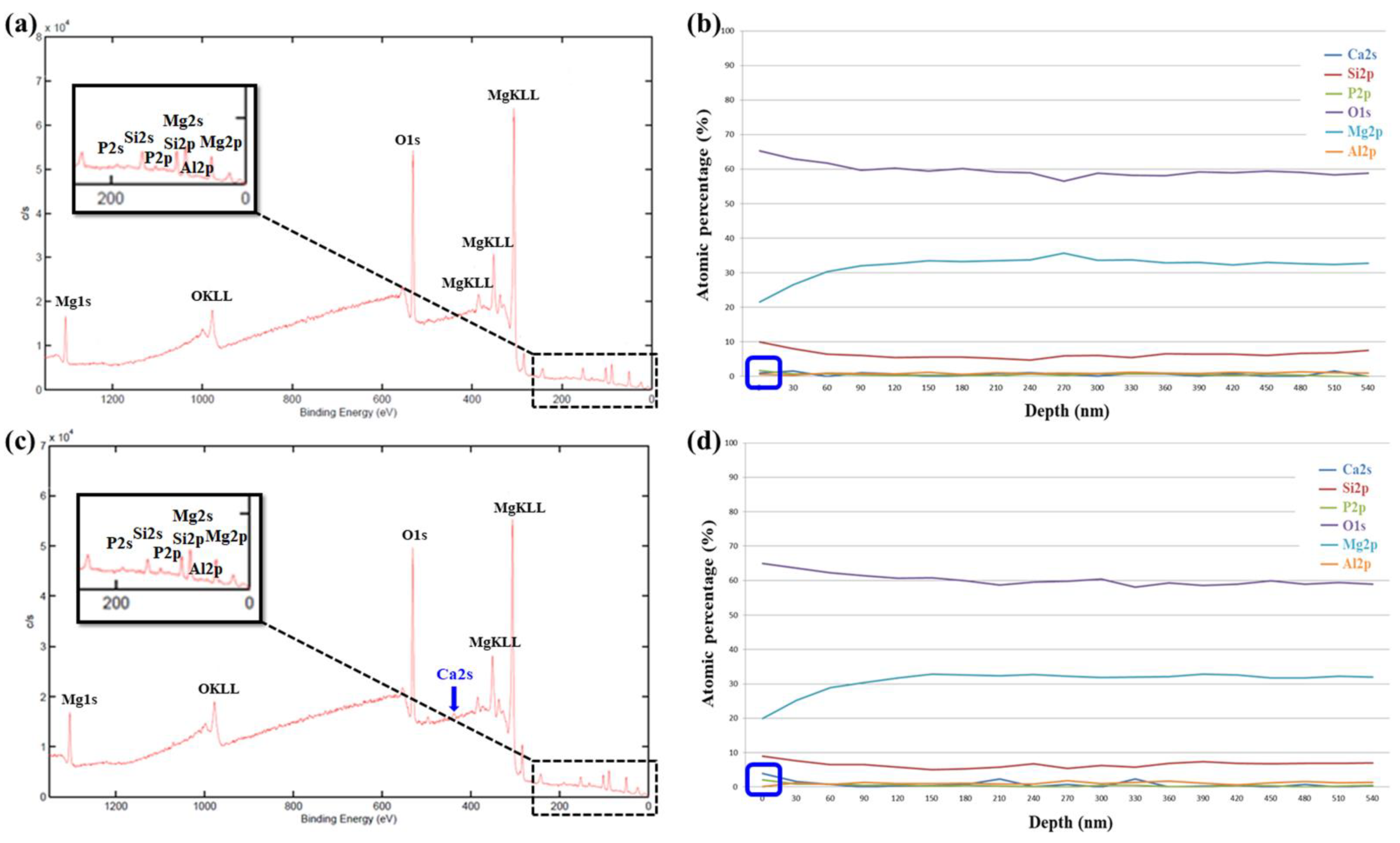
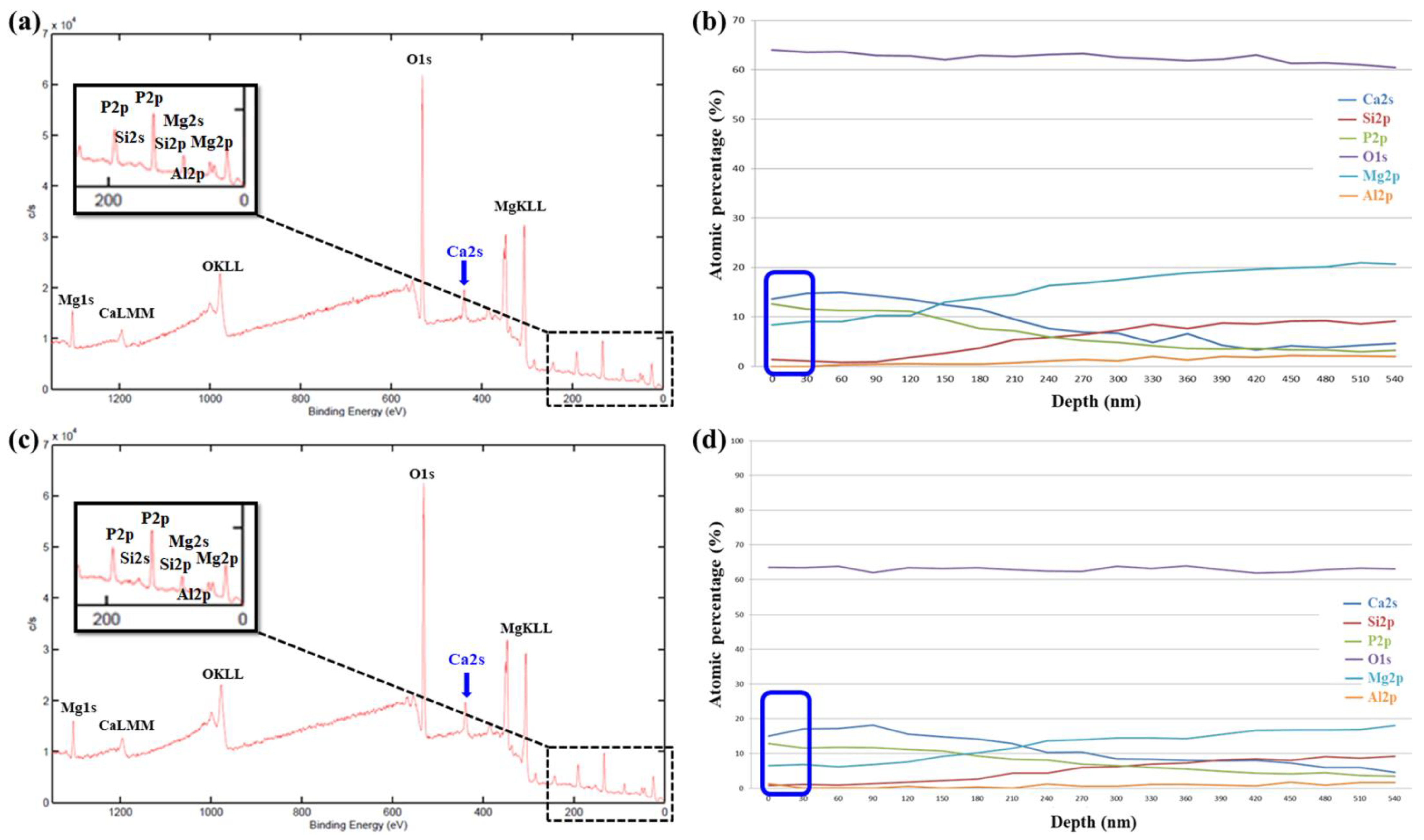
3.2.2. XPS Analysis
3.3. Animal Experiments
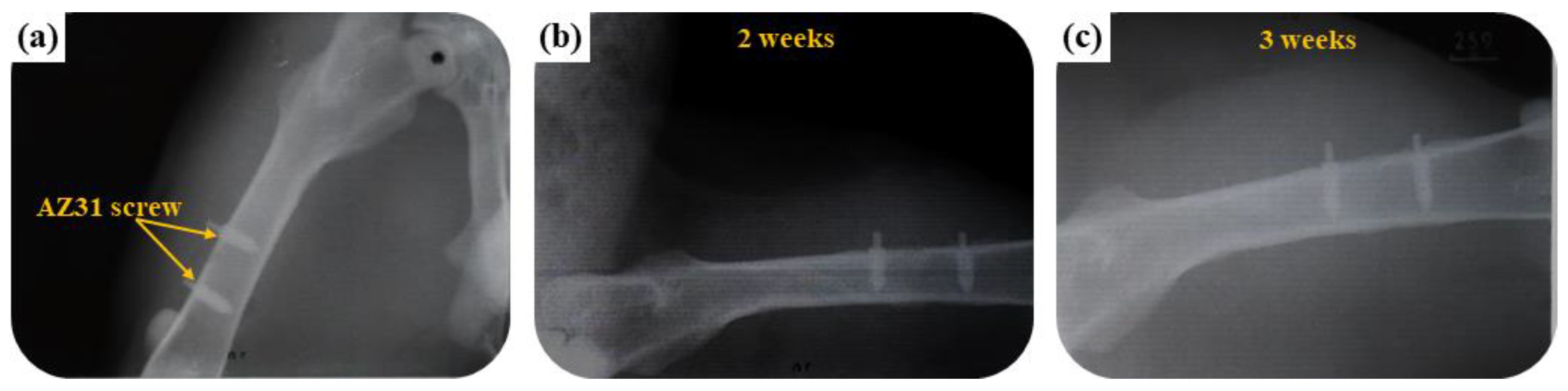
3.3.1. Radiological Examination
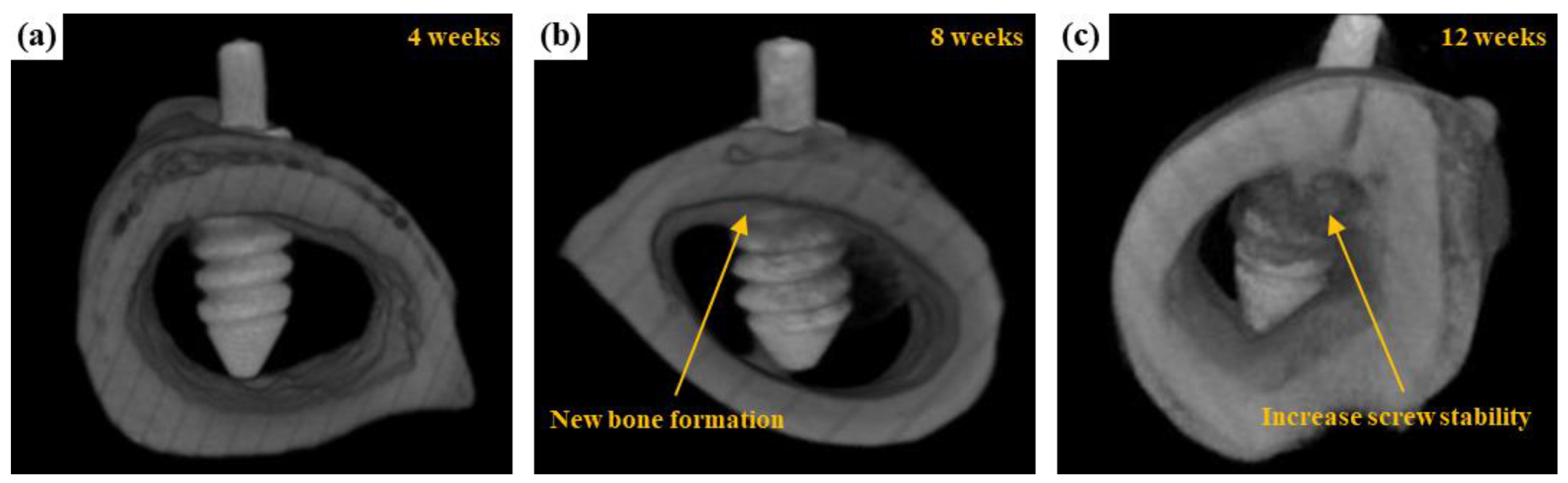
3.3.2. μCT Scanning
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Li, Y.; Jahr, H.; Zhou, J.; Zadpoor, A.A. Additively manufactured biodegradable porous metals. Acta Biomater. 2020, 115, 29–50. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Witte, F.; Xi, T.; Zheng, Y.; Yang, K.; Yang, Y.; Zhao, D.; Meng, J.; Li, Y.; Li, W.; et al. Recommendation for modifying current cytotoxicity testing standards for biodegradable magnesium-based materials. Acta Biomater. 2015, 21, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Witte, F.; Lu, F.; Wang, J.; Li, J.; Qin, L. Current status on clinical applications of magnesium-based orthopaedic implants: A review from clinical translational perspective. Biomaterials 2017, 112, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Wan, P.; Tan, L.; Yang, K. Surface Modification on Biodegradable Magnesium Alloys as Orthopedic Implant Materials to Improve the Bio-adaptability: A Review. J. Mater. Sci. Technol. 2016, 32, 827–834. [Google Scholar] [CrossRef]
- Li, Z.; Gu, X.; Lou, S.; Zheng, Y. The development of binary Mg–Ca alloys for use as biodegradable materials within bone. Biomaterials 2008, 29, 1329–1344. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Z.; Smith, C.; Sankar, J. Recent advances on the development of magnesium alloys for biodegradable implants. Acta Biomater. 2014, 10, 4561–4573. [Google Scholar] [CrossRef] [PubMed]
- Song, G. Control of biodegradation of biocompatable magnesium alloys. Corros. Sci. 2007, 49, 1696–1701. [Google Scholar] [CrossRef]
- Liu, C.; Ren, Z.; Xu, Y.; Pang, S.; Zhao, X.; Zhao, Y. Biodegradable Magnesium Alloys Developed as Bone Repair Materials: A Review. Scanning 2018, 2018, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Maier, H.J.; Julmi, S.; Behrens, S.; Klose, C.; Gartzke, A.-K.; Wriggers, P.; Waselau, A.-C.; Meyer-Lindenberg, A. Magnesium Alloys for Open-Pored Bioresorbable Implants. JOM 2020, 72, 1859–1869. [Google Scholar] [CrossRef] [Green Version]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef]
- Zheng, Y.; Gu, X.; Witte, F. Biodegradable metals. Mater. Sci. Eng. R Rep. 2014, 77, 1–34. [Google Scholar] [CrossRef]
- Witte, F. The history of biodegradable magnesium implants: A review. Acta Biomater. 2010, 6, 1680–1692. [Google Scholar] [CrossRef]
- Witte, F.; Hort, N.; Vogt, C.; Cohen, S.; Kainer, K.U.; Willumeit, R.; Feyerabend, F. Degradable biomaterials based on magnesium corrosion. Curr. Opin. Solid State Mater. Sci. 2008, 12, 63–72. [Google Scholar] [CrossRef] [Green Version]
- Jian, S.-Y.; Yang, C.-Y.; Chang, J.-K. Robust corrosion resistance and self-healing characteristics of a novel Ce / Mn conversion coatings on EV31 magnesium alloys. Appl. Surf. Sci. 2020, 510, 145385. [Google Scholar] [CrossRef]
- Jian, S.-Y.; Chang, K.-L. Effect of cerium ion on the microstructure and properties of permanganate conversion coating on LZ91 magnesium alloy. Appl. Surf. Sci. 2020, 509, 144767. [Google Scholar] [CrossRef]
- Jian, S.-Y.; Tzeng, Y.-C.; Ger, M.-D.; Chang, K.-L.; Shi, G.-N.; Huang, W.-H.; Chen, C.-Y.; Wu, C.-C. The study of corrosion behavior of manganese-based conversion coating on LZ91 magnesium alloy: Effect of addition of pyrophosphate and cerium. Mater. Des. 2020, 192, 108707. [Google Scholar] [CrossRef]
- Fisher, D.A.; Watts, M.; Davis, K.E. Implant position in knee surgery: A comparison of minimally invasive, open unicompart-mental, and total knee. J. Arthroplast. 2003, 18, 2–8. [Google Scholar] [CrossRef]
- Koeck, F.X.; Beckmann, J.; Lüring, C.; Rath, B.; Grifka, J.; Basad, E. Evaluation of implant position and knee alignment after patient-specific unicompartmental knee arthroplasty. Knee 2011, 18, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Jun, Y.; Choi, K. Design of patient-specific hip implants based on the 3D geometry of the human femur. Adv. Eng. Softw. 2010, 41, 537–547. [Google Scholar] [CrossRef]
- Aparicio, C.; Padrós, A.; Gil, F.-J. In vivo evaluation of micro-rough and bioactive titanium dental implants using histometry and pull-out tests. J. Mech. Behav. Biomed. Mater. 2011, 4, 1672–1682. [Google Scholar] [CrossRef]
- Brånemark, R.; Emanuelsson, L.; Palmquist, A.; Thomsen, P. Bone response to laser-induced micro- and nano-size titanium surface features. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Serhan, H.; Slivka, M.; Albert, T.; Kwak, S.D. Is galvanic corrosion between titanium alloy and stainless steel spinal implants a clinical concern? Spine J. 2004, 4, 379–387. [Google Scholar] [CrossRef]
- Vaccaro, A.R.; Singh, K.; Haid, R.; Kitchel, S.; Wuisman, P.; Taylor, W.; Branch, C.; Garfin, S. The use of bioabsorbable implants in the spine. Spine J. 2003, 3, 227–237. [Google Scholar] [CrossRef]
- Williams, D.F. On the mechanisms of biocompatibility. Biomaterials 2008, 29, 2941–2953. [Google Scholar] [CrossRef]
- De Groot, K. Bioceramics consisting of calcium phosphate salts. Biomaterials 1980, 1, 47–50. [Google Scholar] [CrossRef]
- Jarcho, M. Calcium Phosphate Ceramics as Hard Tissue Prosthetics. Clin. Orthop. Relat. Res. 1981, 157, 259–278. [Google Scholar] [CrossRef]
- Jahn, T.L. A Possible Mechanism for the Effect of Electrical Potentials on Apatite Formation in Bone. Clin. Orthop. Relat. Res. 1968, 56, 261–274. [Google Scholar] [CrossRef]
- Jarcho, M.; Salsbury, R.L.; Thomas, M.B.; Doremus, R.H. Synthesis and fabrication of β-tricalcium phosphate (whitlockite) ceramics for potential prosthetic applications. J. Mater. Sci. 1979, 14, 142–150. [Google Scholar] [CrossRef]
- Klein, C.P.A.T.; Driessen, A.A.; De Groot, K.; van den Hooff, A. Biodegradation behavior of various calcium phosphate materials in bone tissue. J. Biomed. Mater. Res. 1983, 17, 769–784. [Google Scholar] [CrossRef] [PubMed]
- Jian, S.-Y.; Ho, M.-L.; Shih, B.-C.; Wang, Y.-J.; Weng, L.-W.; Wang, M.-W.; Tseng, C.-C. Evaluation of the Corrosion Resistance and Cytocompatibility of a Bioactive Micro-Arc Oxidation Coating on AZ31 Mg Alloy. Coatings 2019, 9, 396. [Google Scholar] [CrossRef] [Green Version]
- Atrens, A.; Liu, M.; Zainal Abidin, N.I. Corrosion mechanism applicable to biodegradable magnesium implants. Mater. Sci. Eng. B 2011, 176, 1609–1636. [Google Scholar] [CrossRef]
- Neil, W.C.; Forsyth, M.; Howlett, P.C.; Hutchinson, C.R.; Hinton, B.R.W. Corrosion of heat treated magnesium alloy ZE41. Corros. Sci. 2011, 53, 3299–3308. [Google Scholar] [CrossRef]
- Wang, L.; Nancollas, G.H. Calcium Orthophosphates: Crystallization and Dissolution. Chem. Rev. 2008, 108, 4628–4669. [Google Scholar] [CrossRef] [Green Version]
- Hentrich, D.; Tauer, K.; Espanol, M.; Ginebra, M.-P.; Taubert, A. EDTA and NTA Effectively Tune the Mineralization of Calcium Phosphate from Bulk Aqueous Solution. Biomimetics 2017, 2, 24. [Google Scholar] [CrossRef] [Green Version]
- Fujishiro, Y.; Fujimoto, A.; Sato, T.; Okuwaki, A. Coating of Hydroxyapatite on Titanium Plates Using Thermal Dissociation of Calcium-EDTA Chelate Complex in Phosphate Solutions under Hydrothermal Conditions. J. Colloid Interface Sci. 1995, 173, 119–127. [Google Scholar] [CrossRef]
- Arce, H.; Montero, M.L.; Saenz, A.; Castaño, V.M. Effect of pH and temperature on the formation of hydroxyapatite at low temperatures by decomposition of a Ca–EDTA complex. Polyhedron 2004, 23, 1897–1901. [Google Scholar] [CrossRef]
- Mao, C.; Li, H.; Cui, F.; Feng, Q.; Ma, C. The functionalization of titanium with EDTA to induce biomimetic mineralization of hydroxyapatite. J. Mater. Chem. 1999, 9, 2573–2582. [Google Scholar] [CrossRef]
- Kamil, M.P.; Kaseem, M.; Ko, Y.G. Soft plasma electrolysis with complex ions for optimizing electrochemical performance. Sci. Rep. 2017, 7, srep44458. [Google Scholar] [CrossRef]
- Kyrylenko, S.; Warchoł, F.; Oleshko, O.; Husak, Y.; Kazek-Kęsik, A.; Korniienko, V.; Deineka, V.; Sowa, M.; Maciej, A.; Michalska, J.; et al. Effects of the sources of calcium and phosphorus on the structural and functional properties of ceramic coatings on titanium dental implants produced by plasma electrolytic oxidation. Mater. Sci. Eng. C 2021, 119, 111607. [Google Scholar] [CrossRef]
- Kumar, A.M.; Hassan, S.F.; Sorour, A.A.; Paramsothy, M.; Gupta, M. Electrochemical Corrosion and In vitro Biocompatibility Performance of AZ31Mg/Al2O3 Nanocomposite in Simulated Body Fluid. J. Mater. Eng. Perform. 2018, 27, 3419–3428. [Google Scholar] [CrossRef]
- Bornapour, M.; Muja, N.; Shum-Tim, D.; Cerruti, M.; Pekguleryuz, M. Biocompatibility and biodegradability of Mg–Sr alloys: The formation of Sr-substituted hydroxyapatite. Acta Biomater. 2013, 9, 5319–5330. [Google Scholar] [CrossRef] [PubMed]
- Zberg, B.; Uggowitzer, P.J.; Löffler, J.F. MgZnCa glasses without clinically observable hydrogen evolution for biodegradable implants. Nat. Mater. 2009, 8, 887–891. [Google Scholar] [CrossRef] [PubMed]
- Santos-Coquillat, A.; Mohedano, M.; Martinez-Campos, E.; Arrabal, R.; Pardo, A.; Matykina, E. Bioactive multi-elemental PEO-coatings on titanium for dental implant applications. Mater. Sci. Eng. C 2019, 97, 738–752. [Google Scholar] [CrossRef] [PubMed]


| Name | Na2SiO3 | NaOH | Na3PO4 | Ca3(PO4)2 | EDTA |
|---|---|---|---|---|---|
| EDTA2.5 | 60 g/L | 70 g/L | 20 g/L | 7.0 g/L | 2.5 g/L |
| EDTA5.0 | 60 g/L | 70 g/L | 20 g/L | 7.0 g/L | 5.0 g/L |
| EDTA7.5 | 60 g/L | 70 g/L | 20 g/L | 7.0 g/L | 7.5 g/L |
| Element-Atomic% | |||||||
|---|---|---|---|---|---|---|---|
| C | O | Ca | Mg | Al | Si | Total | |
| EDTA2.5 | 9.66 | 53.2 | - | 29.1 | 0.79 | 7.25 | 100 |
| EDTA5.0 | 10.3 | 53.8 | 0.75 | 27.9 | 0.7 | 6.55 | 100 |
| EDTA7.5 | 14.2 | 55.3 | 1.19 | 20.9 | 0.54 | 7.87 | 100 |
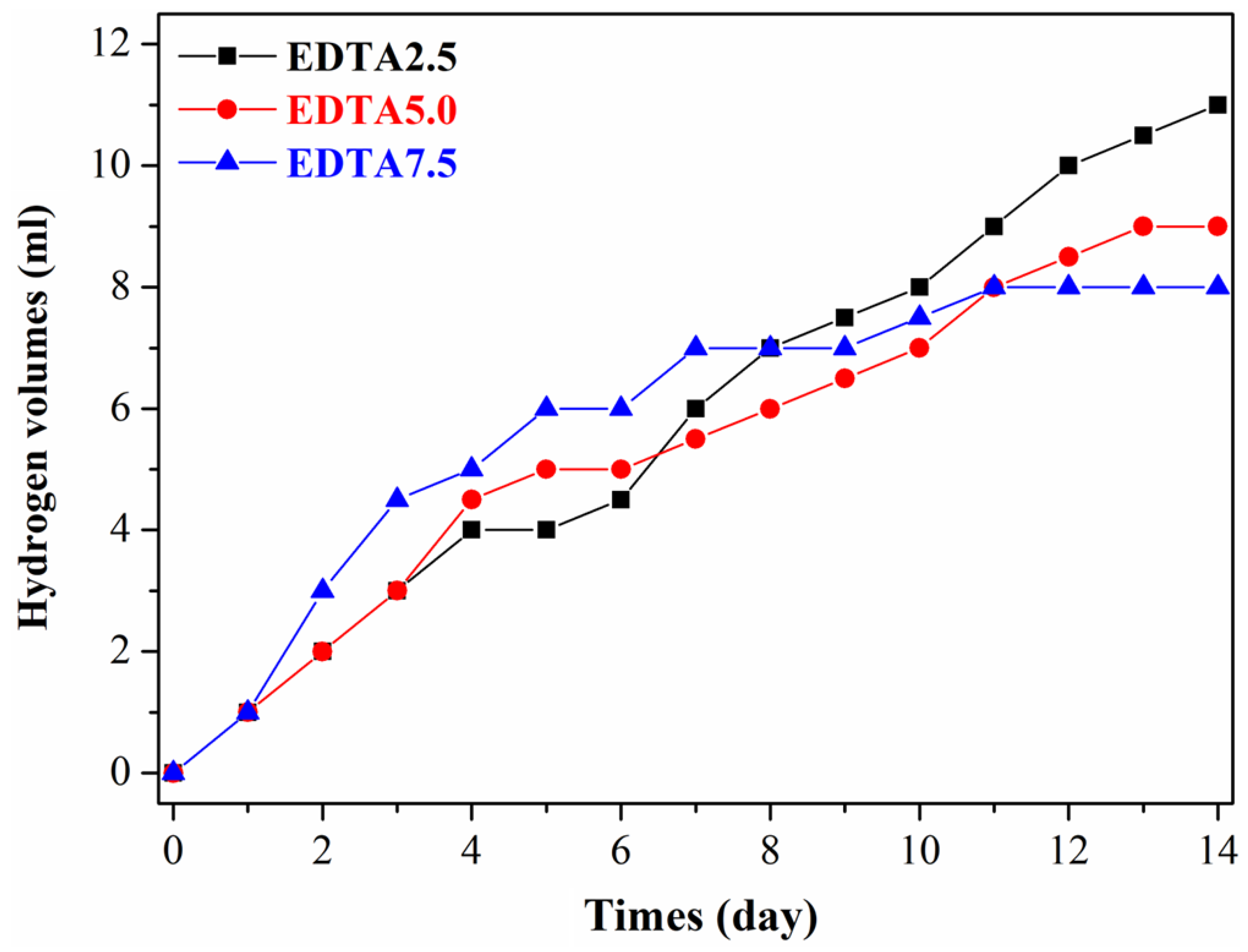
| Hydrogen Volumes (mL/14 day) | Hydrogen Evolution Corrosion Rate (mm/y) | Surface Roughness (µm) | Porosity (%) | |
|---|---|---|---|---|
| EDTA2.5 | 11 ± 1.3 | 1.79 ± 0.38 | 0.85 ± 0.19 | 2.59 ± 0.47 |
| EDTA5.0 | 9 ± 1.0 | 1.47 ± 0.29 | 0.79 ± 0.14 | 2.23 ± 0.45 |
| EDTA7.5 | 8 ± 1.0 | 1.30 ± 0.21 | 0.62 ± 0.10 | 2.08 ± 0.45 |
| AZ31 Mg Alloy Bone Screw | |
|---|---|
| Locking force with non-immersed SBF (A) | 308 ± 40 N |
| Locking force with immersed for 6 weeks (B) | 285 ± 40 N |
| Residual locking force with immersed for 6 weeks (B/A) | 92% |
| Locking force with immersed for 10 weeks (C) | 265 ± 42 N |
| Residual locking force with immersed for 10 weeks (C/A) | 86% |
| Extracts | Cell Morphology | Inhibition of Viability | Cytotoxicity |
|---|---|---|---|
| Ca-P containing MAO sample | 1 | <30% | None |
| Blank control | 0 | <30% | None |
| Positive control | 4 | 98.2% | Cytotoxicity |
| Negative control | 0 | <30% | None |
| Non-Immersed in SBF | After Immersed in SBF for 48 h | |||
|---|---|---|---|---|
| EDTA 0 g Bath | EDTA 7.5 g Bath | EDTA 0 G Bath | EDTA 7.5 g Bath | |
| Ca | 0% | 3.95% | 14.8% | 17.1% |
| P | 1.72% | 2.08% | 11.6% | 11.6% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jian, S.-Y.; Aktug, S.L.; Huang, H.-T.; Ho, C.-J.; Lin, S.-Y.; Chen, C.-H.; Wang, M.-W.; Tseng, C.-C. The Potential of Calcium/Phosphate Containing MAO Implanted in Bone Tissue Regeneration and Biological Characteristics. Int. J. Mol. Sci. 2021, 22, 4706. https://doi.org/10.3390/ijms22094706
Jian S-Y, Aktug SL, Huang H-T, Ho C-J, Lin S-Y, Chen C-H, Wang M-W, Tseng C-C. The Potential of Calcium/Phosphate Containing MAO Implanted in Bone Tissue Regeneration and Biological Characteristics. International Journal of Molecular Sciences. 2021; 22(9):4706. https://doi.org/10.3390/ijms22094706
Chicago/Turabian StyleJian, Shun-Yi, Salim Levent Aktug, Hsuan-Ti Huang, Cheng-Jung Ho, Sung-Yen Lin, Chung-Hwan Chen, Min-Wen Wang, and Chun-Chieh Tseng. 2021. "The Potential of Calcium/Phosphate Containing MAO Implanted in Bone Tissue Regeneration and Biological Characteristics" International Journal of Molecular Sciences 22, no. 9: 4706. https://doi.org/10.3390/ijms22094706
APA StyleJian, S.-Y., Aktug, S. L., Huang, H.-T., Ho, C.-J., Lin, S.-Y., Chen, C.-H., Wang, M.-W., & Tseng, C.-C. (2021). The Potential of Calcium/Phosphate Containing MAO Implanted in Bone Tissue Regeneration and Biological Characteristics. International Journal of Molecular Sciences, 22(9), 4706. https://doi.org/10.3390/ijms22094706






