Preferential Expression of Programmed Death Ligand 1 Protein in Tumor-Associated Macrophages and Its Potential Role in Immunotherapy for Hepatocellular Carcinoma
Abstract
:1. Introduction
2. Results
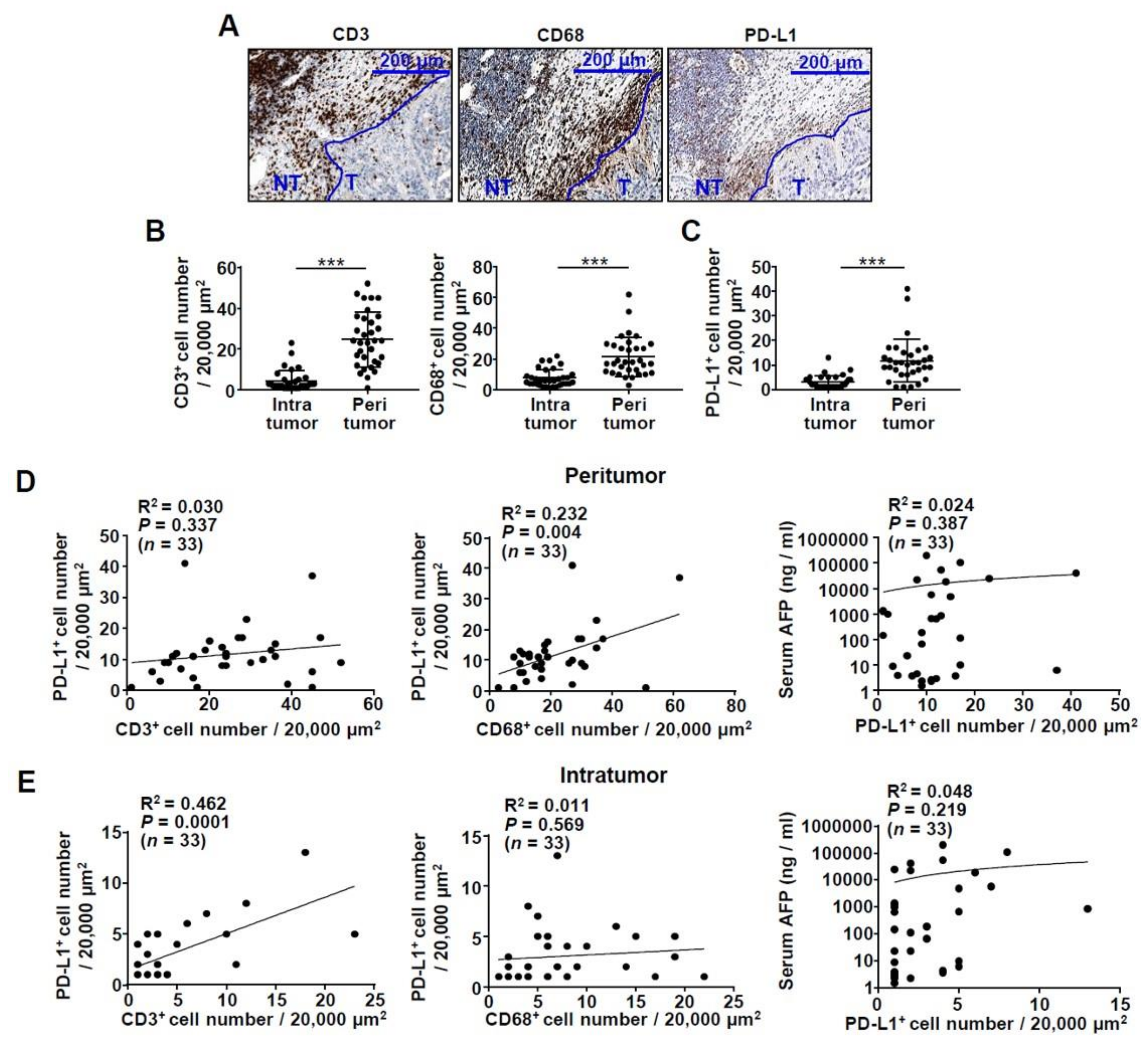
2.1. Infiltration of CD3+ T Cells and CD68+ Macrophages in the HCC Tissue of Patients
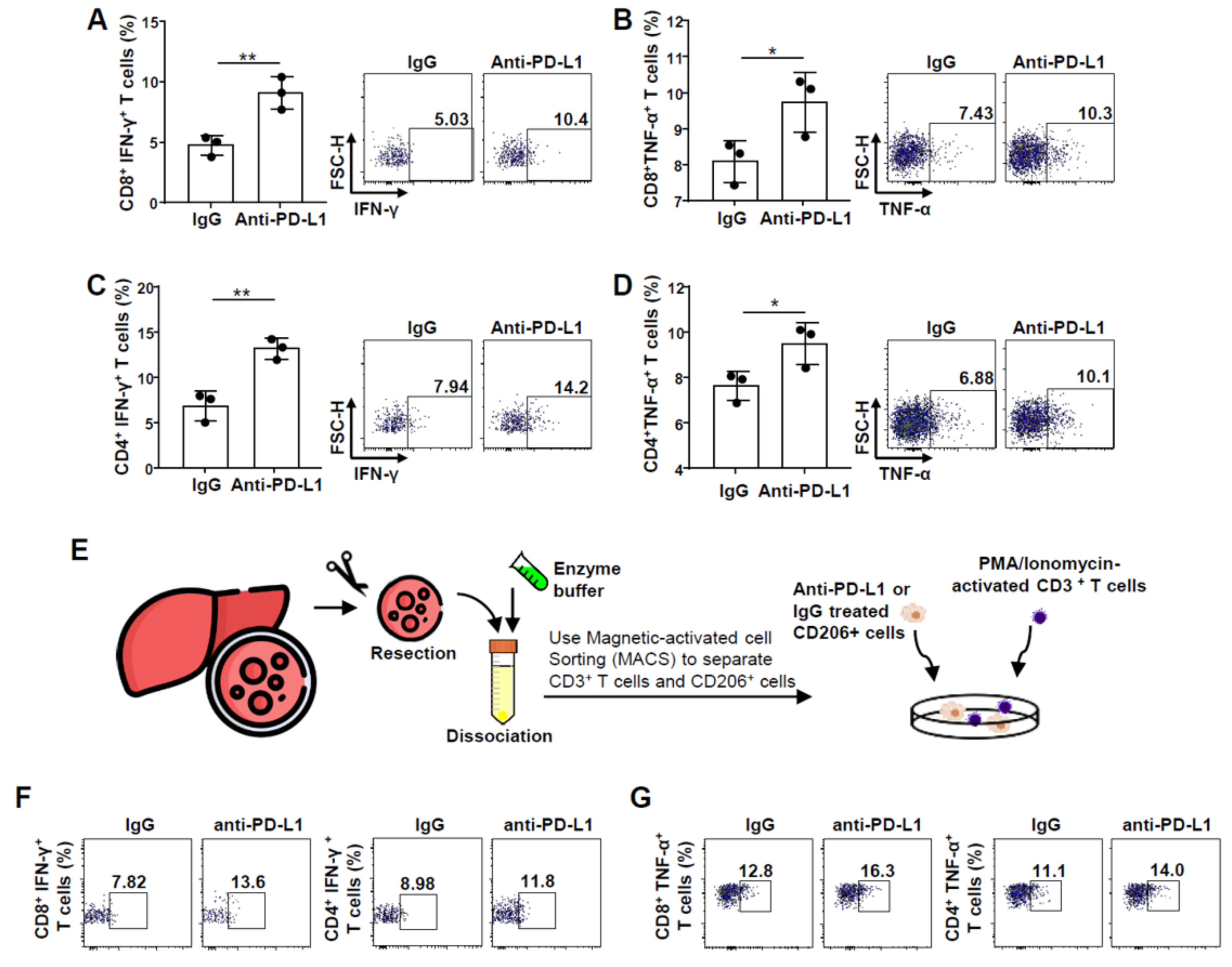
2.2. Improvement in CD8+ and CD4+ T Cell Functions after PD-L1 Expression Blockade on M2 Macrophages
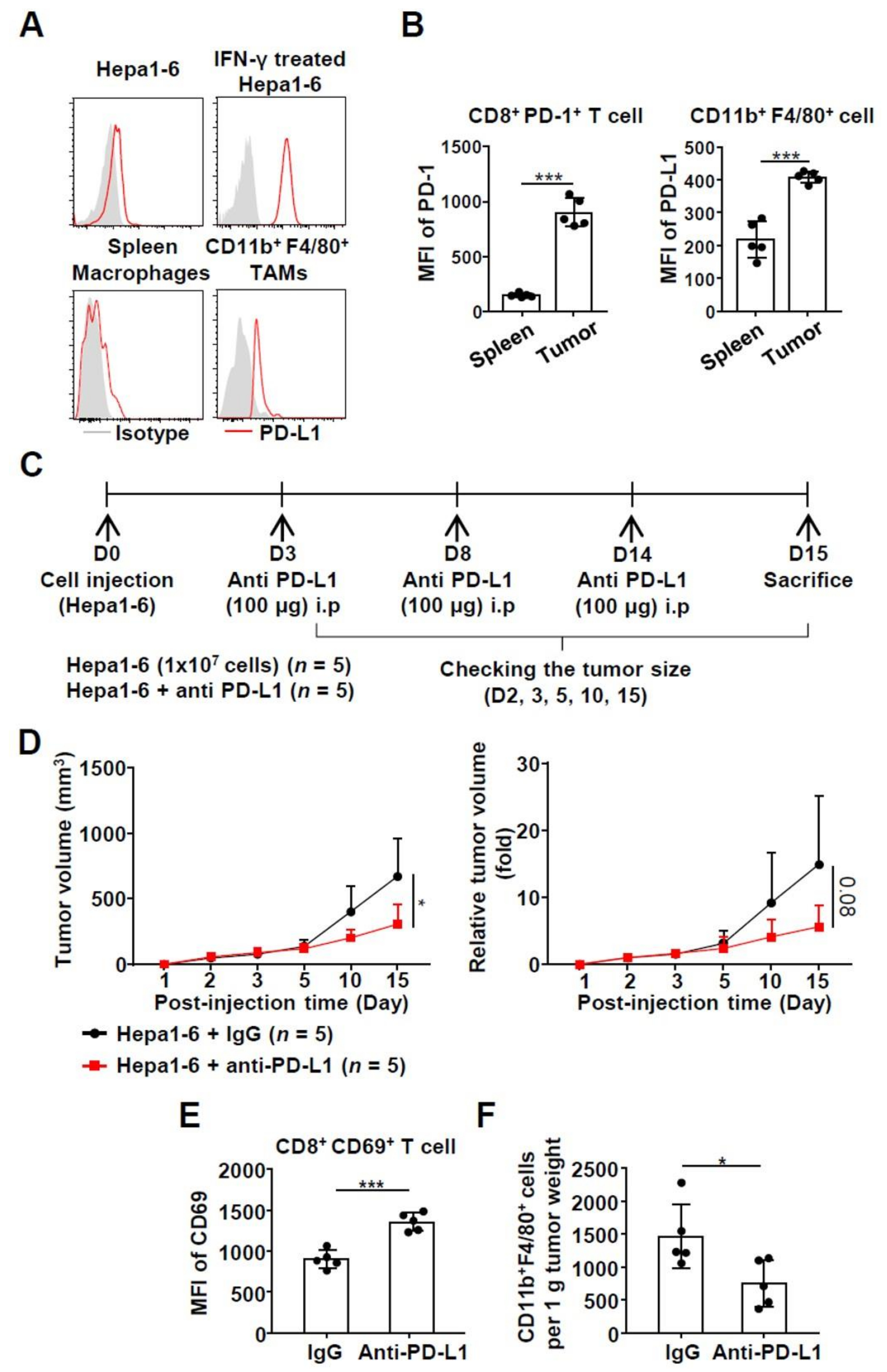
2.3. Effects of Anti-PD-L1 Treatment in a Syngeneic HCC Mouse Model
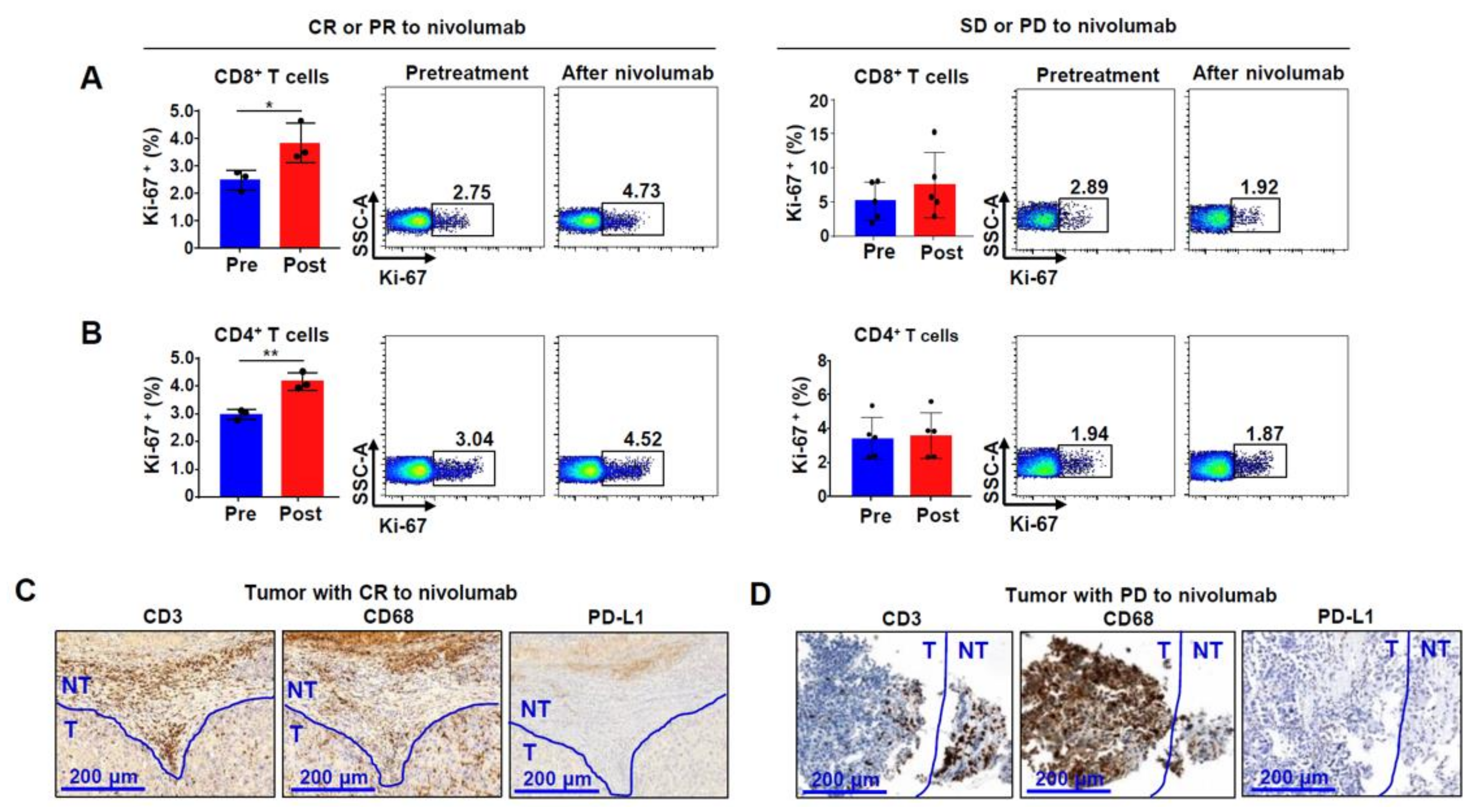
2.4. Ki-67 Expression in Bulk CD8+ and CD4+ T Cells from Patients Reflects Nivolumab Response in HCC
3. Discussion
4. Materials and Methods
4.1. Patient Samples and Clinical Information
4.2. Flow Cytometry
4.3. Intracellular Cytokine Staining
4.4. Purification of CD3+ T Cells and CD14+ Monocytes
4.5. Separation of CD206+ Macrophages in Human HCC Tissue
4.6. Monocyte Polarization to M2 Macrophages
4.7. Co-Culture of CD3+ T Cells and M2 Macrophages
4.8. Immunohistochemistry
4.9. In Vivo Mouse Model
4.10. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [Green Version]
- Park, D.J.; Sung, P.S.; Kim, J.H.; Lee, G.W.; Jang, J.W.; Jung, E.S.; Yoon, S.K. EpCAM-high liver cancer stem cells resist natural killer cell-mediated cytotoxicity by upregulating CEACAM1. J. Immunother. Cancer 2020, 8, e000301. [Google Scholar] [CrossRef] [Green Version]
- Kang, F.-B.; Wang, L.; Li, N.; Zhang, Y.-G.; Sun, D.-X. Hepatocellular carcinomas promote tumor-associated macrophage M2-polarization via increased B7-H3 expression. Oncol. Rep. 2014, 33, 274–282. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Sung, P.S.; Yang, H.; Lee, S.K.; Nam, H.C.; Yoo, S.H.; Lee, H.L.; Kim, H.Y.; Lee, S.W.; Kwon, J.H.; et al. A Real-World Comparative Analysis of Lenvatinib and Sorafenib as a Salvage Therapy for Transarterial Treatments in Unresectable HCC. J. Clin. Med. 2020, 9, 4121. [Google Scholar] [CrossRef] [PubMed]
- Hilmi, M.; Neuzillet, C.; Calderaro, J.; Lafdil, F.; Pawlotsky, J.-M.; Rousseau, B. Angiogenesis and immune checkpoint inhibitors as therapies for hepatocellular carcinoma: Current knowledge and future research directions. J. Immunother. Cancer 2019, 7, 1–13. [Google Scholar] [CrossRef]
- El-Khoueiry, A.B.; Sangro, B.; Yau, T.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Kim, T.-Y.; Choo, S.-P.; Trojan, J.; Welling, T.H.; et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017, 389, 2492–2502. [Google Scholar] [CrossRef]
- Sung, P.S.; Jang, J.W.; Lee, J.; Lee, S.K.; Lee, H.L.; Yang, H.; Nam, H.C.; Lee, S.W.; Bae, S.H.; Choi, J.Y.; et al. Real-World Outcomes of Nivolumab in Patients with Unresectable Hepatocellular Carcinoma in an Endemic Area of Hepatitis B Virus Infection. Front. Oncol. 2020, 10, 1043. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Sung, P.S.; Lee, J.; Yoon, S.K.; Jung, E.S.; Park, C.-S.; Bae, S.H. Heterogenous responses to nivolumab in a single metastatic nodule in hepatocellular carcinoma: Role of salvage surgery. HepatoBiliary Surg. Nutr. 2019, 8, 569–571. [Google Scholar] [CrossRef]
- Cheng, A.-L.; Hsu, C.; Chan, S.L.; Choo, S.-P.; Kudo, M. Challenges of combination therapy with immune checkpoint inhibitors for hepatocellular carcinoma. J. Hepatol. 2020, 72, 307–319. [Google Scholar] [CrossRef] [Green Version]
- Vitale, A.; Trevisani, F.; Farinati, F.; Cillo, U. Treatment of Hepatocellular Carcinoma in the Precision Medicine Era: From Treatment Stage Migration to Therapeutic Hierarchy. Hepatology 2020, 72, 2206–2218. [Google Scholar] [CrossRef]
- Herbst, R.S.; Soria, J.-C.; Kowanetz, M.; Fine, G.D.; Hamid, O.; Gordon, M.S.; Sosman, J.A.; McDermott, D.F.; Powderly, J.D.; Gettinger, S.N.; et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nat. Cell Biol. 2014, 515, 563–567. [Google Scholar] [CrossRef] [Green Version]
- Peranzoni, E.; Lemoine, J.; Vimeux, L.; Feuillet, V.; Barrin, S.; Kantari-Mimoun, C.; Bercovici, N.; Guérin, M.; Biton, J.; Ouakrim, H.; et al. Macrophages impede CD8 T cells from reaching tumor cells and limit the efficacy of anti–PD-1 treatment. Proc. Natl. Acad. Sci. USA 2018, 115, E4041–E4050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeung, O.W.; Lo, C.-M.; Ling, C.-C.; Qi, X.; Geng, W.; Li, C.-X.; Ng, K.T.; Forbes, S.J.; Guan, X.-Y.; Poon, R.T.; et al. Alternatively activated (M2) macrophages promote tumour growth and invasiveness in hepatocellular carcinoma. J. Hepatol. 2015, 62, 607–616. [Google Scholar] [CrossRef]
- Ding, W.; Tan, Y.; Qian, Y.; Xue, W.; Wang, Y.; Jiang, P.; Xu, X. Clinicopathologic and prognostic significance of tumor-associated macrophages in patients with hepatocellular carcinoma: A meta-analysis. PLoS ONE 2019, 14, e0223971. [Google Scholar] [CrossRef] [PubMed]
- Keenan, B.P.; Fong, L.; Kelley, R.K. Immunotherapy in hepatocellular carcinoma: The complex interface between inflammation, fibrosis, and the immune response. J. Immunother. Cancer 2019, 7, 267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeGroote, H.; Van Dierendonck, A.; Geerts, A.; Van Vlierberghe, H.; Devisscher, L. Preclinical and Clinical Therapeutic Strategies Affecting Tumor-Associated Macrophages in Hepatocellular Carcinoma. J. Immunol. Res. 2018, 2018, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singhal, S.; Stadanlick, J.; Annunziata, M.J.; Rao, A.S.; Bhojnagarwala, P.S.; O’Brien, S.; Moon, E.K.; Cantu, E.; Danet-Desnoyers, G.; Ra, H.-J.; et al. Human tumor-associated monocytes/macrophages and their regulation of T cell responses in early-stage lung cancer. Sci. Transl. Med. 2019, 11, eaat1500. [Google Scholar] [CrossRef]
- Marin, J.J.G.; Herraez, E.; Lozano, E.; Macias, R.I.R.; Briz, O. Models for Understanding Resistance to Chemotherapy in Liver Cancer. Cancers 2019, 11, 1677. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; Zhao, Q.; Gao, Z.; Lao, X.-M.; Lin, W.-M.; Chen, D.-P.; Mu, M.; Huang, C.-X.; Liu, Z.-Y.; Li, B.; et al. The local immune landscape determines tumor PD-L1 heterogeneity and sensitivity to therapy. J. Clin. Investig. 2019, 129, 3347–3360. [Google Scholar] [CrossRef] [Green Version]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [Green Version]
- Sangro, B.; Melero, I.; Wadhawan, S.; Finn, R.S.; Abou-Alfa, G.K.; Cheng, A.-L.; Yau, T.; Furuse, J.; Park, J.-W.; Boyd, Z.; et al. Association of inflammatory biomarkers with clinical outcomes in nivolumab-treated patients with advanced hepatocellular carcinoma. J. Hepatol. 2020, 73, 1460–1469. [Google Scholar] [CrossRef]
- Spahn, S.; Roessler, D.; Pompilia, R.; Gabernet, G.; Gladstone, B.P.; Horger, M.; Biskup, S.; Feldhahn, M.; Nahnsen, S.; Hilke, F.J.; et al. Clinical and Genetic Tumor Characteristics of Responding and Non-Responding Patients to PD-1 Inhibition in Hepatocellular Carcinoma. Cancers 2020, 12, 3830. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jie, H.B.; Lei, Y.; Gildener-Leapman, N.; Trivedi, S.; Green, T.; Kane, L.P.; Ferris, R.L. PD-1/SHP-2 inhibits Tc1/Th1 phenotypic responses and the activation of T cells in the tumor microenvironment. Cancer Res. 2015, 75, 508–518. [Google Scholar] [CrossRef] [Green Version]
- Kamada, T.; Togashi, Y.; Tay, C.; Ha, D.; Sasaki, A.; Nakamura, Y.; Sato, E.; Fukuoka, S.; Tada, Y.; Tanaka, A.; et al. PD-1+ regulatory T cells amplified by PD-1 blockade promote hyperprogression of cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 9999–10008. [Google Scholar] [CrossRef] [Green Version]
- Zong, Z.; Zou, J.; Mao, R.; Ma, C.; Li, N.; Wang, J.; Wang, X.; Zhou, H.; Zhang, L.; Shi, Y. M1 Macrophages Induce PD-L1 Expression in Hepatocellular Carcinoma Cells Through IL-1β Signaling. Front. Immunol. 2019, 10, 1643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benner, B.; Scarberry, L.; Suarez-Kelly, L.P.; Duggan, M.C.; Campbell, A.R.; Smith, E.; Lapurga, G.; Jiang, K.; Butchar, J.P.; Tridandapani, S.; et al. Generation of monocyte-derived tumor-associated macrophages using tumor-conditioned media provides a novel method to study tumor-associated macrophages in vitro. J. Immunother. Cancer 2019, 7, 140. [Google Scholar] [CrossRef] [Green Version]
- Zhou, D.; Luan, J.; Huang, C.; Li, J. Tumor-Associated Macrophages in Hepatocellular Carcinoma: Friend or Foe? Gut Liver 2020. [Google Scholar] [CrossRef]
- Pathria, P.; Louis, T.L.; Varner, J.A. Targeting Tumor-Associated Macrophages in Cancer. Trends Immunol. 2019, 40, 310–327. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, R.; Su, X.; Pan, Y.; Han, X.; Shao, C.; Shi, Y. Harnessing tumor-associated macrophages as aids for cancer immunotherapy. Mol. Cancer 2019, 18, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakanishi, Y.; Nakatsuji, M.; Seno, H.; Ishizu, S.; Akitake-Kawano, R.; Kanda, K.; Ueo, T.; Komekado, H.; Kawada, M.; Minami, M.; et al. COX-2 inhibition alters the phenotype of tumor-associated macrophages from M2 to M1 in ApcMin/+ mouse polyps. Carcinogenesis 2011, 32, 1333–1339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, A.; Hsiao, Y.-J.; Chen, H.-Y.; Chen, H.-W.; Ho, C.-C.; Chen, Y.-Y.; Liu, Y.-C.; Hong, T.-H.; Yu, S.-L.; Chen, J.J.; et al. Opposite Effects of M1 and M2 Macrophage Subtypes on Lung Cancer Progression. Sci. Rep. 2015, 5, 14273. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Gao, A.; Zhang, F.; Yang, Z.; Wang, S.; Fang, Y.; Li, J.; Wang, J.; Shi, W.; Wang, L.; et al. ILT4 inhibition prevents TAM- and dysfunctional T cell-mediated immunosuppression and enhances the efficacy of anti-PD-L1 therapy in NSCLC with EGFR activation. Theranostics 2021, 11, 3392–3416. [Google Scholar] [CrossRef] [PubMed]
- Kubota, K.; Moriyama, M.; Furukawa, S.; Rafiul, H.A.S.M.; Maruse, Y.; Jinno, T.; Tanaka, A.; Ohta, M.; Ishiguro, N.; Yamauchi, M.; et al. CD163+CD204+ tumor-associated macrophages contribute to T cell regulation via interleukin-10 and PD-L1 production in oral squamous cell carcinoma. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Lu, L.-C.; Lee, Y.-H.; Chang, C.-J.; Shun, C.-T.; Fang, C.-Y.; Shao, Y.-Y.; Liu, T.-H.; Cheng, A.-L.; Hsu, C.-H. Increased Expression of Programmed Death-Ligand 1 in Infiltrating Immune Cells in Hepatocellular Carcinoma Tissues after Sorafenib Treatment. Liver Cancer 2019, 8, 110–120. [Google Scholar] [CrossRef]
- Sung, P.S.; Cho, S.W.; Lee, J.; Yang, H.; Jang, J.W.; Bae, S.H.; Choi, J.Y.; Yoon, S.K. Infiltration of T Cells and Programmed Cell Death Ligand 1-expressing Macrophages as a Potential Predictor of Lenvatinib Response in Hepatocellular Carcinoma. J. Liver Cancer 2020, 20, 128–134. [Google Scholar] [CrossRef]
- Sung, P.S.; Park, D.J.; Kim, J.-H.; Han, J.W.; Lee, E.B.; Lee, G.W.; Nam, H.C.; Jang, J.W.; Bae, S.H.; Choi, J.Y.; et al. Ex vivo Detection and Characterization of Hepatitis B Virus-Specific CD8+ T Cells in Patients Considered Immune Tolerant. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Rey-Giraud, F.; Hafner, M.; Ries, C.H. In Vitro Generation of Monocyte-Derived Macrophages under Serum-Free Conditions Improves Their Tumor Promoting Functions. PLoS ONE 2012, 7, e42656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, D.-J.; Sung, P.-S.; Lee, G.-W.; Cho, S.-W.; Kim, S.-M.; Kang, B.-Y.; Hur, W.-H.; Yang, H.; Lee, S.-K.; Lee, S.-H.; et al. Preferential Expression of Programmed Death Ligand 1 Protein in Tumor-Associated Macrophages and Its Potential Role in Immunotherapy for Hepatocellular Carcinoma. Int. J. Mol. Sci. 2021, 22, 4710. https://doi.org/10.3390/ijms22094710
Park D-J, Sung P-S, Lee G-W, Cho S-W, Kim S-M, Kang B-Y, Hur W-H, Yang H, Lee S-K, Lee S-H, et al. Preferential Expression of Programmed Death Ligand 1 Protein in Tumor-Associated Macrophages and Its Potential Role in Immunotherapy for Hepatocellular Carcinoma. International Journal of Molecular Sciences. 2021; 22(9):4710. https://doi.org/10.3390/ijms22094710
Chicago/Turabian StylePark, Dong-Jun, Pil-Soo Sung, Gil-Won Lee, Sung-Woo Cho, Sung-Min Kim, Byung-Yoon Kang, Won-Hee Hur, Hyun Yang, Soon-Kyu Lee, Sung-Hak Lee, and et al. 2021. "Preferential Expression of Programmed Death Ligand 1 Protein in Tumor-Associated Macrophages and Its Potential Role in Immunotherapy for Hepatocellular Carcinoma" International Journal of Molecular Sciences 22, no. 9: 4710. https://doi.org/10.3390/ijms22094710
APA StylePark, D.-J., Sung, P.-S., Lee, G.-W., Cho, S.-W., Kim, S.-M., Kang, B.-Y., Hur, W.-H., Yang, H., Lee, S.-K., Lee, S.-H., Jung, E.-S., Seo, C.-H., Ahn, J., Choi, H.-J., You, Y.-K., Jang, J.-W., Bae, S.-H., Choi, J.-Y., & Yoon, S.-K. (2021). Preferential Expression of Programmed Death Ligand 1 Protein in Tumor-Associated Macrophages and Its Potential Role in Immunotherapy for Hepatocellular Carcinoma. International Journal of Molecular Sciences, 22(9), 4710. https://doi.org/10.3390/ijms22094710






