Meta-Analysis of Methamphetamine Modulation on Amyloid Precursor Protein through HMGB1 in Alzheimer’s Disease
Abstract
1. Introduction
2. Results
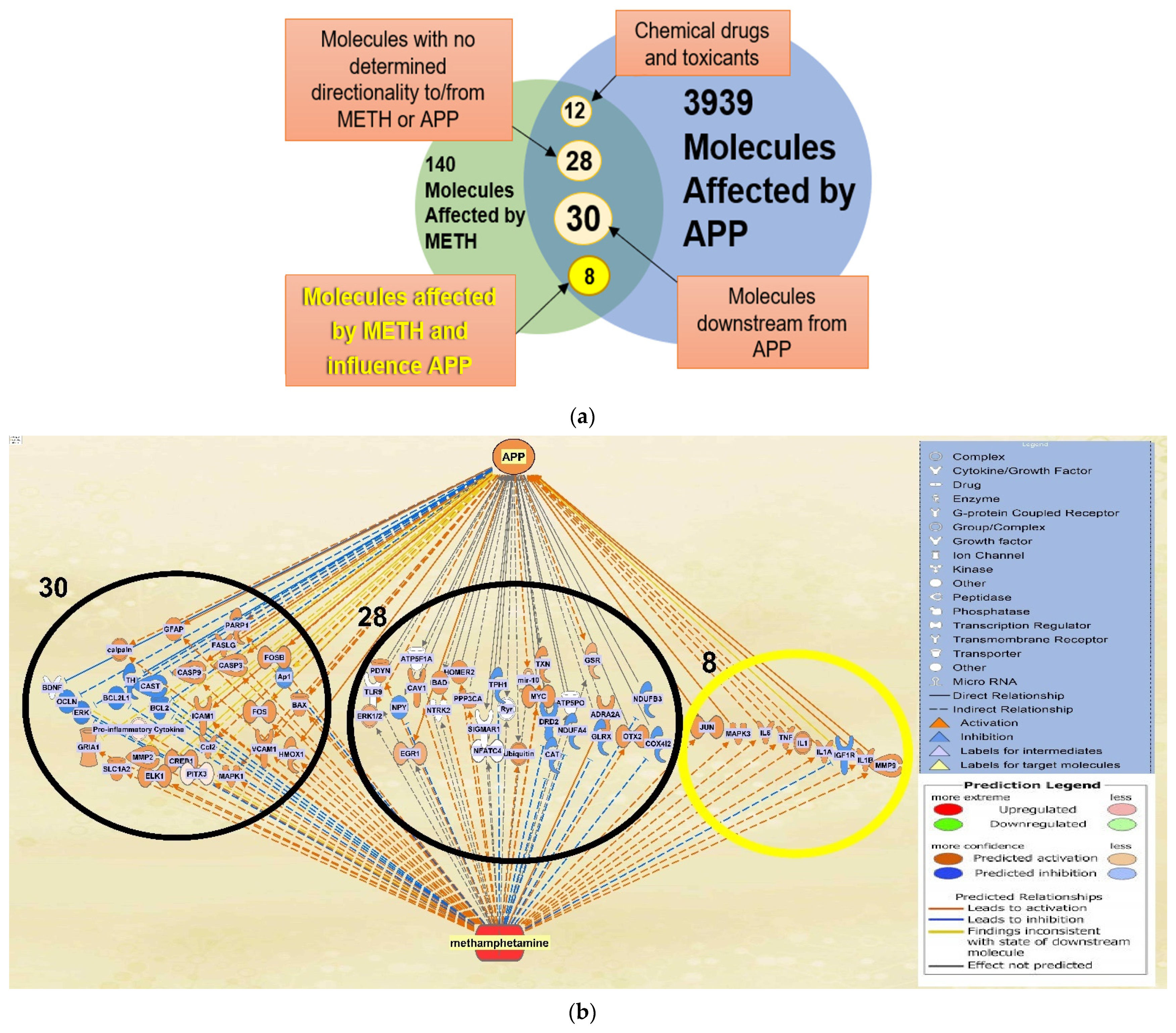
2.1. Molecules Affected by METH and APP
2.2. Quantitative Characterization of the influence of METH Exposure on APP Expression
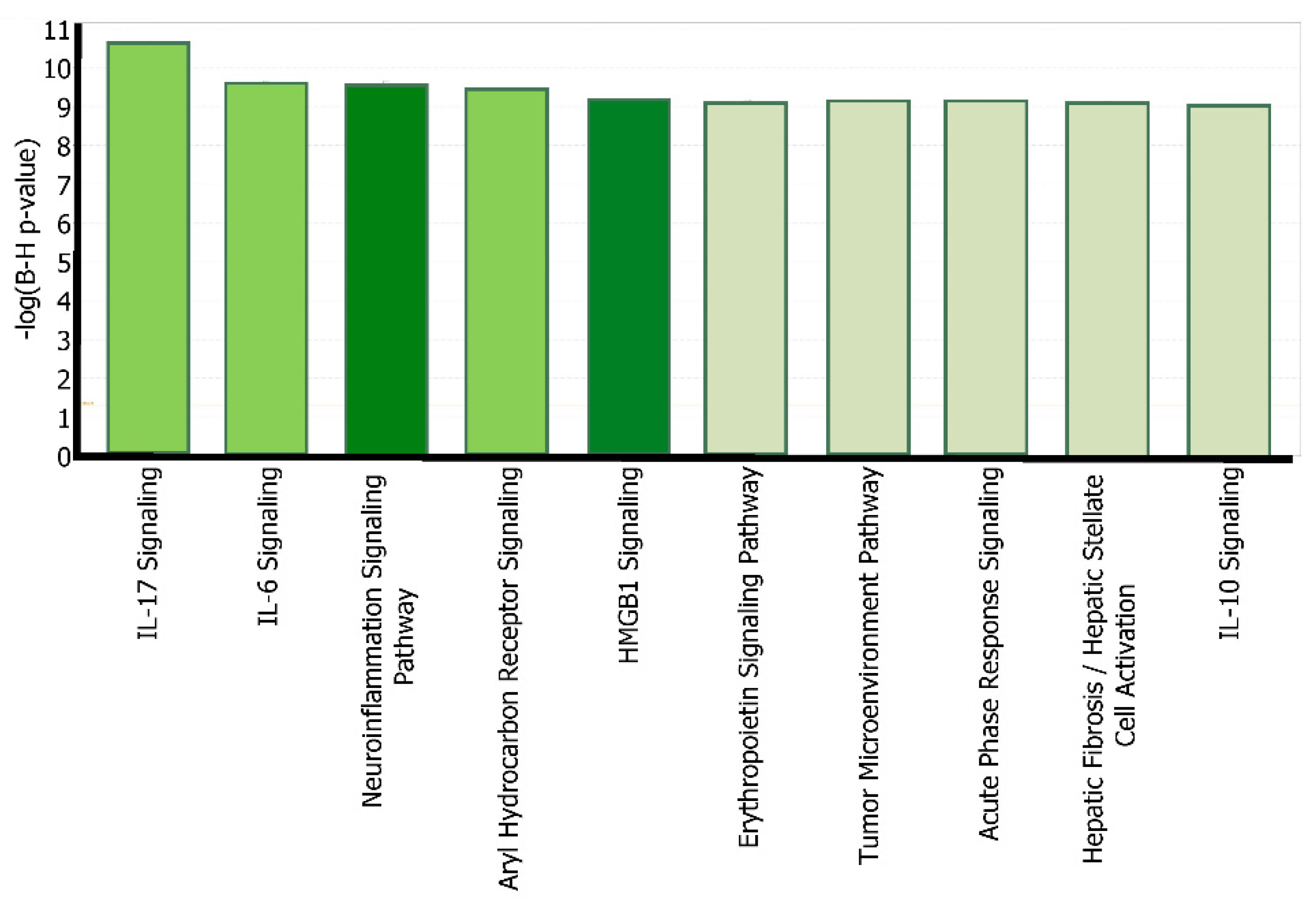
2.3. Canonical Pathway Analysis of the Intermediate Molecules Dataset
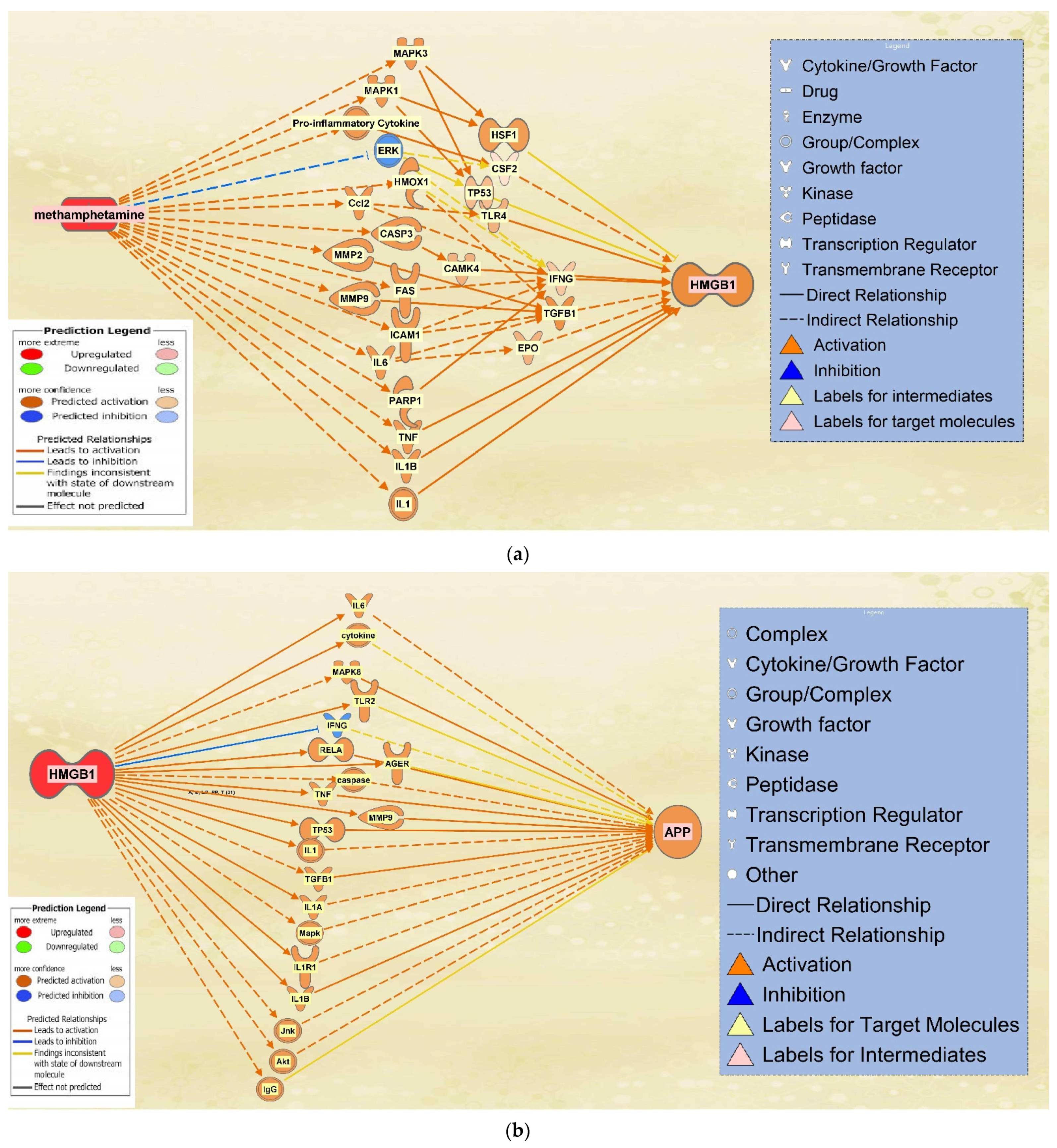
2.4. The Integrated Network of Upregulated APP Expression through HMGB1 Following METH Exposure
2.5. The Effect of HMGB1 Inhibition on the Network
3. Discussion
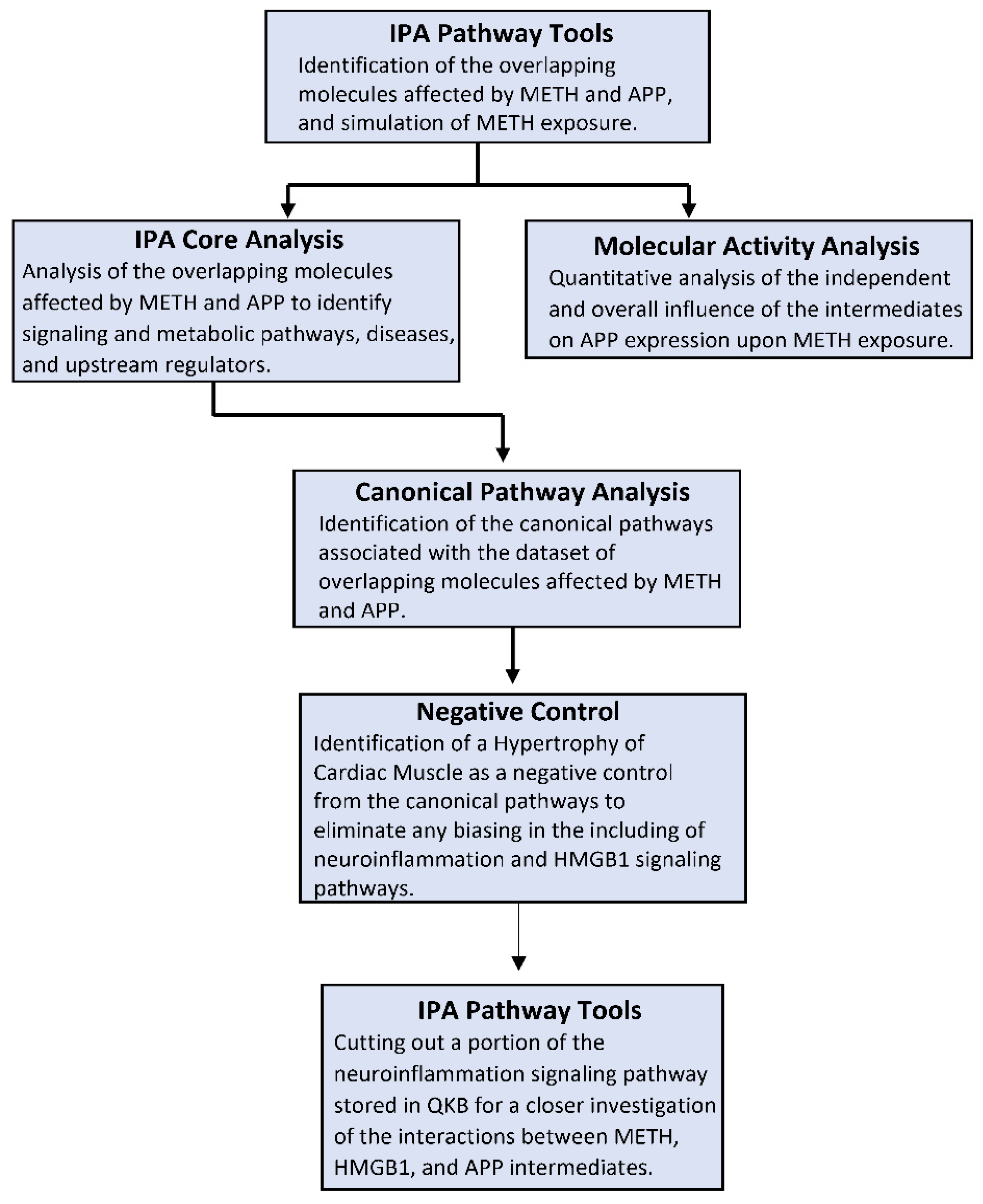
4. Materials and Methods
4.1. Ingenuity Pathway Analysis Software
4.2. Identification of Overlapping Molecules Affected by METH, HMGB1, and APP
4.3. Connectivity and Molecule Activity Predictor (MAP)
4.4. Canonical Pathway Analysis through the Core Analysis
4.5. Negative Control of the Canonical Pathways
4.6. Quantitative Analysis of the Influence of METH Exposure on APP Expression
4.7. Generation of the Neuroinflammation Signaling Pathway Featuring METH, HMGB1, APP, and Relevant Molecules and Diseases/Functions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Paula, V.J.R.; Guimaraes, F.M.; Diniz, B.S.; Forlenza, O.V. Neurobiological pathways to Alzheimer’s disease: Amyloid-beta, TAU protein or both? Dement. Neuropsychol. 2009, 3, 188–194. [Google Scholar] [CrossRef]
- Roberts, G.W.; Gentleman, S.M.; Lynch, A.; Murray, L.; Landon, M.; Graham, D.I. Beta amyloid protein deposition in the brain after severe head injury: Implications for the pathogenesis of Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 1994, 57, 419–425. [Google Scholar] [CrossRef]
- Verdile, G.; Fuller, S.; Atwood, C.S.; Laws, S.M.; Gandy, S.E.; Martins, R.N. The role of beta amyloid in Alzheimer’s disease: Still a cause of everything or the only one who got caught? Pharmacol. Res. 2004, 50, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Andersson, U.; Erlandsson-Harris, H.; Yang, H.; Tracey, K.J. HMGB1 as a DNA-binding cytokine. J. Leukoc. Biol. 2002, 72, 1084–1091. [Google Scholar] [PubMed]
- Nan, K.; Han, Y.; Fang, Q.; Huang, C.; Yu, L.; Ge, W.; Xiang, F.; Tao, Y.X.; Cao, H.; Li, J. HMGB1 gene silencing inhibits neuroinflammation via down-regulation of NF-kappaB signaling in primary hippocampal neurons induced by Abeta25-35. Int. Immunopharmacol. 2019, 67, 294–301. [Google Scholar] [CrossRef]
- Falcão, A.S.; Carvalho, L.A.R.; Lidónio, G.; Vaz, A.R.; Lucas, S.D.; Moreira, R.; Brites, D. Dipeptidyl Vinyl Sulfone as a Novel Chemical Tool to Inhibit HMGB1/NLRP3-Inflammasome and Inflamma-miRs in Aβ-Mediated Microglial Inflammation. ACS Chem. Neurosci. 2017, 8, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Antoine, D.J.; Andersson, U.; Tracey, K.J. The many faces of HMGB1: Molecular structure-functional activity in inflammation, apoptosis, and chemotaxis. J. Leukoc. Biol. 2013, 93, 865–873. [Google Scholar] [CrossRef]
- Fujita, K.; Motoki, K.; Tagawa, K.; Chen, X.; Hama, H.; Nakajima, K.; Homma, H.; Tamura, T.; Watanabe, H.; Katsuno, M.; et al. HMGB1, a pathogenic molecule that induces neurite degeneration via TLR4-MARCKS, is a potential therapeutic target for Alzheimer’s disease. Sci. Rep. 2016, 6, 31895. [Google Scholar] [CrossRef]
- Kong, Z.H.; Chen, X.; Hua, H.P.; Liang, L.; Liu, L.J. The Oral Pretreatment of Glycyrrhizin Prevents Surgery-Induced Cognitive Impairment in Aged Mice by Reducing Neuroinflammation and Alzheimer’s-Related Pathology via HMGB1 Inhibition. J. Mol. Neurosci. 2017, 63, 385–395. [Google Scholar] [CrossRef]
- Takata, K.; Takada, T.; Ito, A.; Asai, M.; Tawa, M.; Saito, Y.; Ashihara, E.; Tomimoto, H.; Kitamura, Y.; Shimohama, S. Microglial Amyloid-beta1-40 Phagocytosis Dysfunction Is Caused by High-Mobility Group Box Protein-1: Implications for the Pathological Progression of Alzheimer’s Disease. Int. J. Alzheimer’s Dis. 2012, 2012, 685739. [Google Scholar] [CrossRef]
- Takata, K.; Kitamura, Y.; Tsuchiya, D.; Kawasaki, T.; Taniguchi, T.; Shimohama, S. High mobility group box protein-1 inhibits microglial Abeta clearance and enhances Abeta neurotoxicity. J. Neurosci. Res. 2004, 78, 880–891. [Google Scholar] [CrossRef]
- Festoff, B.W.; Sajja, R.K.; van Dreden, P.; Cucullo, L. HMGB1 and thrombin mediate the blood-brain barrier dysfunction acting as biomarkers of neuroinflammation and progression to neurodegeneration in Alzheimer’s disease. J. Neuroinflammation 2016, 13, 194. [Google Scholar] [CrossRef]
- Nishibori, M.; Wang, D.; Ousaka, D.; Wake, H. High Mobility Group Box-1 and Blood-Brain Barrier Disruption. Cells 2020, 9, 2650. [Google Scholar] [CrossRef]
- Wongprayoon, P.; Govitrapong, P. Melatonin attenuates methamphetamine-induced neuroinflammation through the melatonin receptor in the SH-SY5Y cell line. Neurotoxicology 2015, 50, 122–130. [Google Scholar] [CrossRef]
- Rippeth, J.D.; Heaton, R.K.; Carey, C.L.; Marcotte, T.D.; Moore, D.J.; Gonzalez, R.; Wolfson, T.; Grant, I. Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons. J. Int. Neuropsychol. Soc. 2004, 10, 1–14. [Google Scholar] [CrossRef]
- Liu, X.; Chang, L.; Vigorito, M.; Kass, M.; Li, H.; Chang, S.L. Methamphetamine-Induced Behavioral Sensitization is Enhanced in the HIV-1 Transgenic Rat. J. Neuroimmune Pharmacol. 2009, 4, 309–316. [Google Scholar] [CrossRef]
- Kass, M.D.; Liu, X.; Vigorito, M.; Chang, L.; Chang, S.L. Methamphetamine-induced behavioral and physiological effects in adolescent and adult HIV-1 transgenic rats. J. Neuroimmune Pharmacol. 2010, 5, 566–573. [Google Scholar] [CrossRef]
- Kiyatkin, E.A.; Sharma, H.S. Breakdown of Blood-Brain and Blood-Spinal Cord Barriers During Acute Methamphetamine Intoxication: Role of Brain Temperature. CNS Neurol. Disord. Drug Targets 2016, 15, 1129–1138. [Google Scholar] [CrossRef]
- Shukla, M.; Vincent, B. The multi-faceted impact of methamphetamine on Alzheimer’s disease: From a triggering role to a possible therapeutic use. Ageing Res. Rev. 2020, 60, 101062. [Google Scholar] [CrossRef]
- Cadet, J.L.; Krasnova, I.N. Molecular bases of methamphetamine-induced neurodegeneration. Int. Rev. Neurobiol. 2009, 88, 101–119. [Google Scholar] [CrossRef]
- McFadden, L.M.; Vieira-Brock, P.L. The Persistent Neurotoxic Effects of Methamphetamine on Dopaminergic and Serotonergic Markers in Male and Female Rats. Toxicol. Open Access 2016, 2. [Google Scholar] [CrossRef]
- Tzeng, N.S.; Chien, W.C.; Chung, C.H.; Chang, H.A.; Kao, Y.C.; Liu, Y.P. Association between amphetamine-related disorders and dementia-a nationwide cohort study in Taiwan. Ann. Clin. Transl. Neurol. 2020, 7, 1284–1295. [Google Scholar] [CrossRef]
- Gonzales, R.; Mooney, L.; Rawson, R.A. The Methamphetamine Problem in the United States. Annu. Rev. Public Health 2010, 31, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Rau, T.F.; Kothiwal, A.S.; Rova, A.R.; Brooks, D.M.; Rhoderick, J.F.; Poulsen, A.J.; Hutchinson, J.; Poulsen, D.J. Administration of low dose methamphetamine 12 h after a severe traumatic brain injury prevents neurological dysfunction and cognitive impairment in rats. Exp. Neurol. 2014, 253, 31–40. [Google Scholar] [CrossRef]
- Rau, T.; Ziemniak, J.; Poulsen, D. The neuroprotective potential of low-dose methamphetamine in preclinical models of stroke and traumatic brain injury. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2016, 64, 231–236. [Google Scholar] [CrossRef]
- Shukla, M.; Maitra, S.; Hernandez, J.-F.; Govitrapong, P.; Vincent, B. Methamphetamine regulates βAPP processing in human neuroblastoma cells. Neurosci. Lett. 2019, 701, 20–25. [Google Scholar] [CrossRef]
- Mockett, B.G.; Richter, M.; Abraham, W.C.; Müller, U.C. Therapeutic Potential of Secreted Amyloid Precursor Protein APPsα. Front. Mol. Neurosci. 2017, 10. [Google Scholar] [CrossRef]
- Frank, M.G.; Adhikary, S.; Sobesky, J.L.; Weber, M.D.; Watkins, L.R.; Maier, S.F. The danger-associated molecular pattern HMGB1 mediates the neuroinflammatory effects of methamphetamine. Brain Behav. Immun. 2016, 51, 99–108. [Google Scholar] [CrossRef]
- Kramer, A.; Green, J.; Pollard, J.; Tugendreich, S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 2014, 30, 523–530. [Google Scholar] [CrossRef]
- Stouffer, S.A.; Suchman, E.A.; Devinney, L.C.; Star, S.A.; Williams, R.M. The American Soldier: Adjustment during Army Life (Studies in Social Psychology in World War II), Vol. 1; Princeton University Press: Oxford, UK, 1949; pp. xii, 599. [Google Scholar]
- Ramos-Garcia, N.A.; Orozco-Ibarra, M.; Estudillo, E.; Elizondo, G.; Apo, E.G.; Macias, L.G.C.; Sosa-Ortiz, A.L.; Torres-Ramos, M.A. Aryl Hydrocarbon Receptor in Post-Mortem Hippocampus and in Serum from Young, Elder, and Alzheimer’s Patients. Int. J. Mol. Sci. 2020, 21, 1983. [Google Scholar] [CrossRef] [PubMed]
- Andersson, U.; Wang, H.; Palmblad, K.; Aveberger, A.C.; Bloom, O.; Erlandsson-Harris, H.; Janson, A.; Kokkola, R.; Zhang, M.; Yang, H.; et al. High Mobility Group 1 Protein (Hmg-1) Stimulates Proinflammatory Cytokine Synthesis in Human Monocytes. J. Exp. Med. 2000, 192, 565–570. [Google Scholar] [CrossRef]
- Cheng, X.; Yang, Y.L.; Yang, H.; Wang, Y.H.; Du, G.H. Kaempferol alleviates LPS-induced neuroinflammation and BBB dysfunction in mice via inhibiting HMGB1 release and down-regulating TLR4/MyD88 pathway. Int. Immunopharmacol. 2018, 56, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.M.; Walker, P.D.; Benjamins, J.A.; Geddes, T.J.; Kuhn, D.M. Methamphetamine Neurotoxicity in Dopamine Nerve Endings of the Striatum Is Associated with Microglial Activation. J. Pharmacol. Exp. Ther. 2004, 311, 1–7. [Google Scholar] [CrossRef]
- Medeiros, R.; Prediger, R.D.S.; Passos, G.F.; Pandolfo, P.; Duarte, F.S.; Franco, J.L.; Dafre, A.L.; Di Giunta, G.; Figueiredo, C.P.; Takahashi, R.N.; et al. Connecting TNF- Signaling Pathways to iNOS Expression in a Mouse Model of Alzheimer’s Disease: Relevance for the Behavioral and Synaptic Deficits Induced by Amyloid Protein. J. Neurosci. 2007, 27, 5394–5404. [Google Scholar] [CrossRef] [PubMed]
- Shahrara, S.; Pickens, S.R.; Mandelin, A.M.; Karpus, W.J.; Huang, Q.; Kolls, J.K.; Pope, R.M. IL-17–Mediated Monocyte Migration Occurs Partially through CC Chemokine Ligand 2/Monocyte Chemoattractant Protein-1 Induction. J. Immunol. 2010, 184, 4479–4487. [Google Scholar] [CrossRef] [PubMed]
- Li, J.K.; Nie, L.; Zhao, Y.P.; Zhang, Y.Q.; Wang, X.; Wang, S.S.; Liu, Y.; Zhao, H.; Cheng, L. IL-17 mediates inflammatory reactions via p38/c-Fos and JNK/c-Jun activation in an AP-1-dependent manner in human nucleus pulposus cells. J. Transl. Med. 2016, 14, 77. [Google Scholar] [CrossRef] [PubMed]
- Faour, W.H.; Mancini, A.; He, Q.W.; Di Battista, J.A. T-cell-derived interleukin-17 regulates the level and stability of cyclooxygenase-2 (COX-2) mRNA through restricted activation of the p38 mitogen-activated protein kinase cascade: Role of distal sequences in the 3’-untranslated region of COX-2 mRNA. J. Biol. Chem. 2003, 278, 26897–26907. [Google Scholar] [CrossRef] [PubMed]
- Martel-Pelletier, J.; Mineau, F.; Jovanovic, D.; Di Battista, J.A.; Pelletier, J.P. Mitogen-activated protein kinase and nuclear factor kappaB together regulate interleukin-17-induced nitric oxide production in human osteoarthritic chondrocytes: Possible role of transactivating factor mitogen-activated protein kinase-activated proten kinase (MAPKAPK). Arthritis Rheum. 1999, 42, 2399–2409. [Google Scholar] [CrossRef]
- Tocharus, J.; Chongthammakun, S.; Govitrapong, P. Melatonin inhibits amphetamine-induced nitric oxide synthase mRNA overexpression in microglial cell lines. Neurosci. Lett. 2008, 439, 134–137. [Google Scholar] [CrossRef]
- Tocharus, J.; Khonthun, C.; Chongthammakun, S.; Govitrapong, P. Melatonin attenuates methamphetamine-induced overexpression of pro-inflammatory cytokines in microglial cell lines. J. Pineal Res. 2010, 48, 347–352. [Google Scholar] [CrossRef]
- Sriram, K.; Miller, D.B.; O’Callaghan, J.P. Minocycline attenuates microglial activation but fails to mitigate striatal dopaminergic neurotoxicity: Role of tumor necrosis factor-alpha. J. Neurochem. 2006, 96, 706–718. [Google Scholar] [CrossRef] [PubMed]
- Dickson, D.W.; Lee, S.C.; Mattiace, L.A.; Yen, S.H.; Brosnan, C. Microglia and cytokines in neurological disease, with special reference to AIDS and Alzheimer’s disease. Glia 1993, 7, 75–83. [Google Scholar] [CrossRef]
- Liao, Y.F.; Wang, B.J.; Cheng, H.T.; Kuo, L.H.; Wolfe, M.S. Tumor Necrosis Factor-α, Interleukin-1β, and Interferon-γ Stimulate γ-Secretase-mediated Cleavage of Amyloid Precursor Protein through a JNK-dependent MAPK Pathway. J. Biol. Chem. 2004, 279, 49523–49532. [Google Scholar] [CrossRef] [PubMed]
- Permpoonputtana, K.; Govitrapong, P. The Anti-inflammatory Effect of Melatonin on Methamphetamine-Induced Proinflammatory Mediators in Human Neuroblastoma Dopamine SH-SY5Y Cell Lines. Neurotox. Res. 2013, 23, 189–199. [Google Scholar] [CrossRef]
- McDaid, J.; Graham, M.P.; Napier, T.C. Methamphetamine-Induced Sensitization Differentially Alters pCREB and ΔFosB throughout the Limbic Circuit of the Mammalian Brain. Mol. Pharmacol. 2006, 70, 2064–2074. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Zhang, Y.; Zhang, J.; Wang, H.; Ren, B. ERK in Learning and Memory: A Review of Recent Research. Int. J. Mol. Sci. 2010, 11, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Mizoguchi, H.; Yamada, K.; Nabeshima, T. Neuropsychotoxicity of abused drugs: Involvement of matrix metalloproteinase-2 and -9 and tissue inhibitor of matrix metalloproteinase-2 in methamphetamine-induced behavioral sensitization and reward in rodents. J. Pharmacol. Sci. 2008, 106, 9–14. [Google Scholar] [CrossRef]
- Yong, V.W.; Power, C.; Forsyth, P.; Edwards, D.R. Metalloproteinases in biology and pathology of the nervous system. Nat. Rev. Neurosci. 2001, 2, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, A.; Lapinska, J.; Rylski, M.; McKay, R.D.G.; Kaczmarek, L. Matrix Metalloproteinase-9 Undergoes Expression and Activation during Dendritic Remodeling in Adult Hippocampus. J. Neurosci. 2002, 22, 920–930. [Google Scholar] [CrossRef]
- Fu, Y.; Lei, J.; Zhuang, Y.; Zhang, K.; Lu, D. Overexpression of HMGB1 A-box reduced IL-1β-induced MMP expression and the production of inflammatory mediators in human chondrocytes. Exp. Cell Res. 2016, 349, 184–190. [Google Scholar] [CrossRef]
- García-Arnandis, I.; Guillén, M.I.; Gomar, F.; Pelletier, J.P.; Martel-Pelletier, J.; Alcaraz, M.J. High mobility group box 1 potentiates the pro-inflammatory effects of interleukin-1β in osteoarthritic synoviocytes. Arthritis Res. Ther. 2010, 12, R165. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Xu, J.; Zheng, Y.; Wei, Y.; Zhu, X.; Lo, E.H.; Moskowitz, M.A.; Sims, J.R. High-Mobility Group Box 1 Promotes Metalloproteinase-9 Upregulation Through Toll-Like Receptor 4 After Cerebral Ischemia. Stroke 2010, 41, 2077–2082. [Google Scholar] [CrossRef]
- Hernandez-Guillamon, M.; Mawhirt, S.; Blais, S.; Montaner, J.; Neubert, T.A.; Rostagno, A.; Ghiso, J. Sequential Amyloid-beta Degradation by the Matrix Metalloproteases MMP-2 and MMP-9. J. Biol. Chem. 2015, 290, 15078–15091. [Google Scholar] [CrossRef]
- Hernandez-Guillamon, M.; Martinez-Saez, E.; Delgado, P.; Domingues-Montanari, S.; Boada, C.; Penalba, A.; Boada, M.; Pagola, J.; Maisterra, O.; Rodriguez-Luna, D.; et al. MMP-2/MMP-9 Plasma Level and Brain Expression in Cerebral Amyloid Angiopathy-Associated Hemorrhagic Stroke. Brain Pathol. 2012, 22, 133–141. [Google Scholar] [CrossRef]
- Cai, Z.; Liu, N.; Wang, C.; Qin, B.; Zhou, Y.; Xiao, M.; Chang, L.; Yan, L.J.; Zhao, B. Role of RAGE in Alzheimer’s Disease. Cell. Mol. Neurobiol. 2016, 36, 483–495. [Google Scholar] [CrossRef]
- Qin, Y.-H.; Dai, S.-M.; Tang, G.-S.; Zhang, J.; Ren, D.; Wang, Z.-W.; Shen, Q. HMGB1 Enhances the Proinflammatory Activity of Lipopolysaccharide by Promoting the Phosphorylation of MAPK p38 through Receptor for Advanced Glycation End Products. J. Immunol. 2009, 183, 6244–6250. [Google Scholar] [CrossRef]
- He, Z.-W.; Qin, Y.-H.; Wang, Z.-W.; Chen, Y.; Shen, Q.; Dai, S.-M. HMGB1 Acts in Synergy with Lipopolysaccharide in Activating Rheumatoid Synovial Fibroblasts via p38 MAPK and NF-κB Signaling Pathways. Mediat. Inflamm. 2013, 2013, 596716. [Google Scholar] [CrossRef]
- Tagawa, K.; Homma, H.; Saito, A.; Fujita, K.; Chen, X.; Imoto, S.; Oka, T.; Ito, H.; Motoki, K.; Yoshida, C.; et al. Comprehensive phosphoproteome analysis unravels the core signaling network that initiates the earliest synapse pathology in preclinical Alzheimer’s disease brain. Hum. Mol. Genet. 2015, 24, 540–558. [Google Scholar] [CrossRef]
- Deane, R.; Du Yan, S.; Submamaryan, R.K.; LaRue, B.; Jovanovic, S.; Hogg, E.; Welch, D.; Manness, L.; Lin, C.; Yu, J.; et al. RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nat. Med. 2003, 9, 907–913. [Google Scholar] [CrossRef]
- Garwood, C.J.; Pooler, A.M.; Atherton, J.; Hanger, D.P.; Noble, W. Astrocytes are important mediators of Abeta-induced neurotoxicity and tau phosphorylation in primary culture. Cell Death Dis. 2011, 2, e167. [Google Scholar] [CrossRef]
- Kitazawa, M.; Oddo, S.; Yamasaki, T.R.; Green, K.N.; LaFerla, F.M. Lipopolysaccharide-Induced Inflammation Exacerbates Tau Pathology by a Cyclin-Dependent Kinase 5-Mediated Pathway in a Transgenic Model of Alzheimer’s Disease. J. Neurosci. 2005, 25, 8843–8853. [Google Scholar] [CrossRef]
- Walters, A.; Phillips, E.; Zheng, R.; Biju, M.; Kuruvilla, T. Evidence for neuroinflammation in Alzheimer’s disease. Prog. Neurol. Psychiatry 2016, 20, 25–31. [Google Scholar] [CrossRef]
- Whitlock, M.C. Combining probability from independent tests: The weighted Z-method is superior to Fisher’s approach. J. Evol. Biol. 2005, 18, 1368–1373. [Google Scholar] [CrossRef] [PubMed]

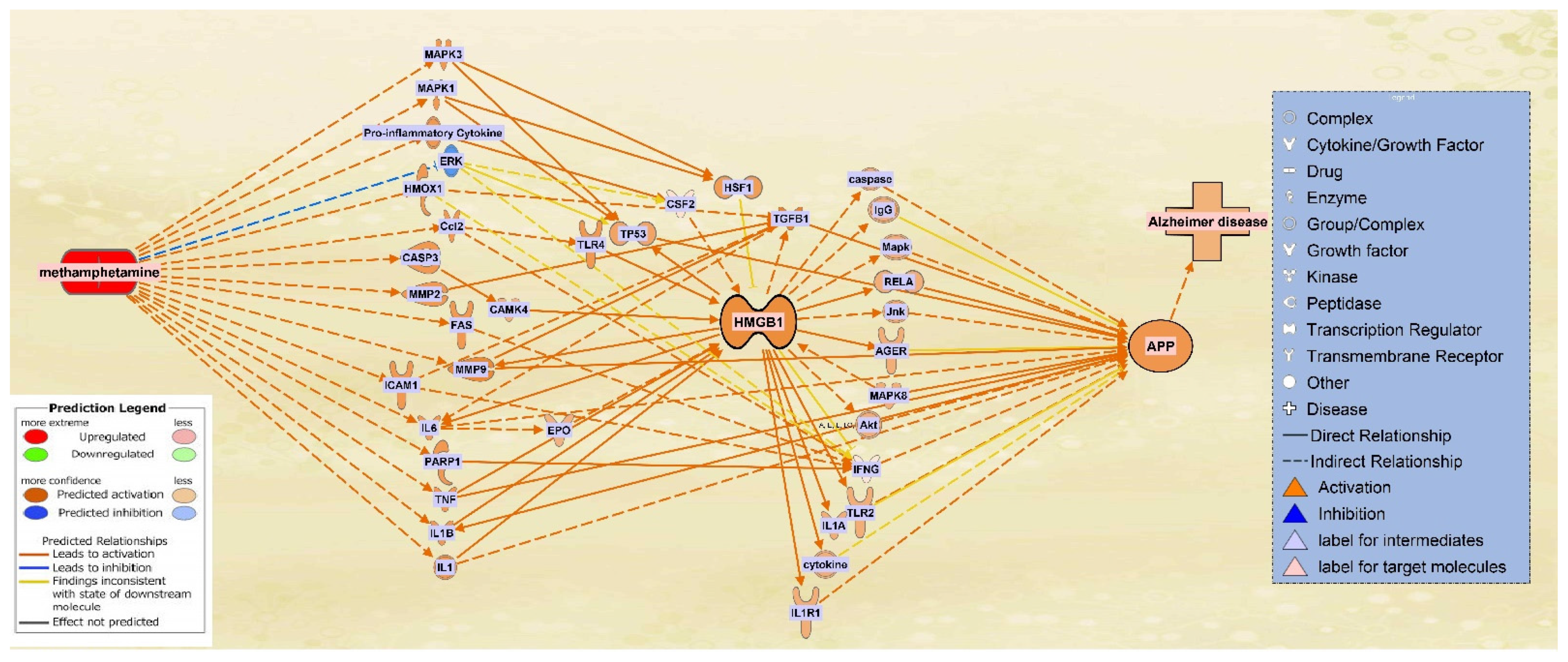

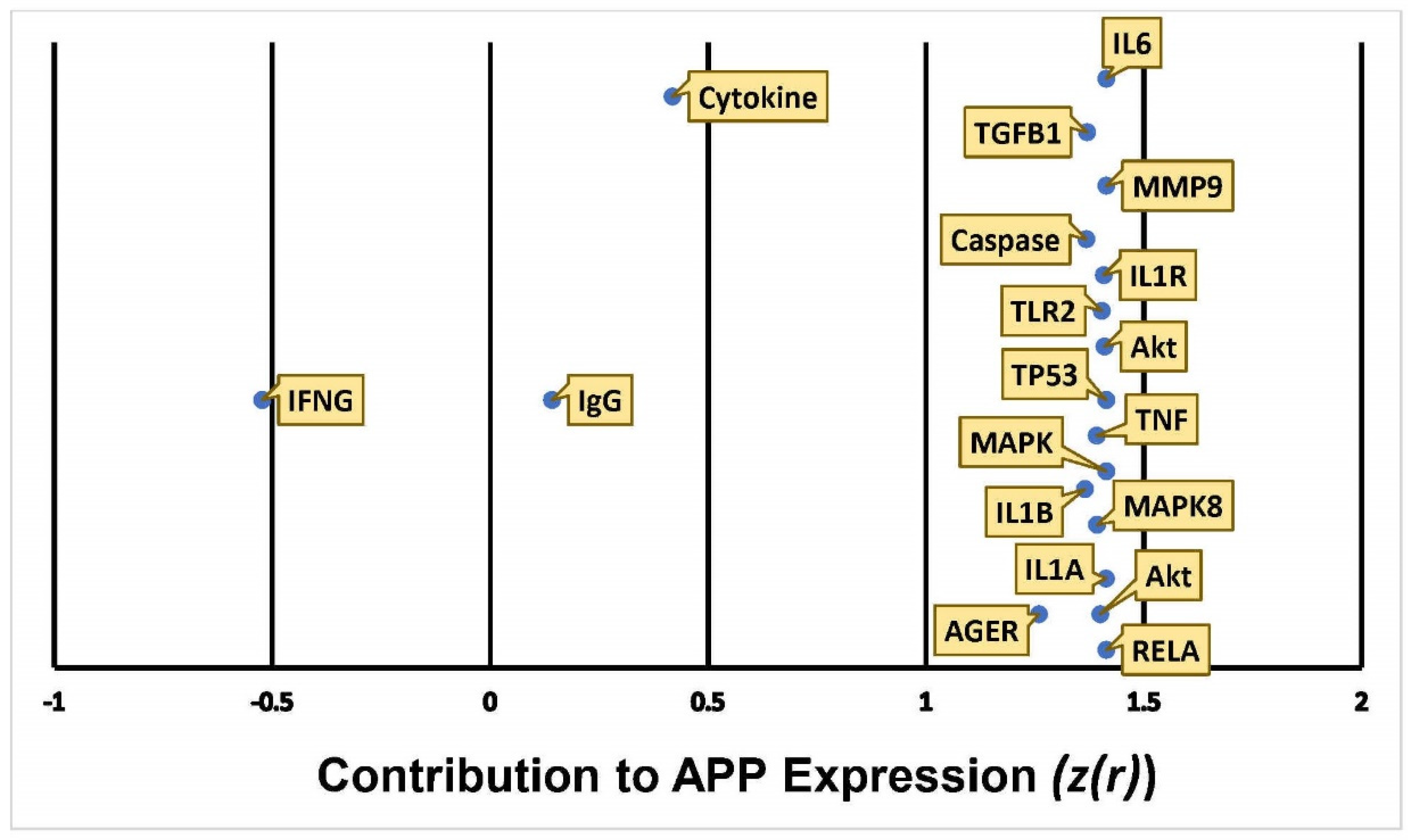
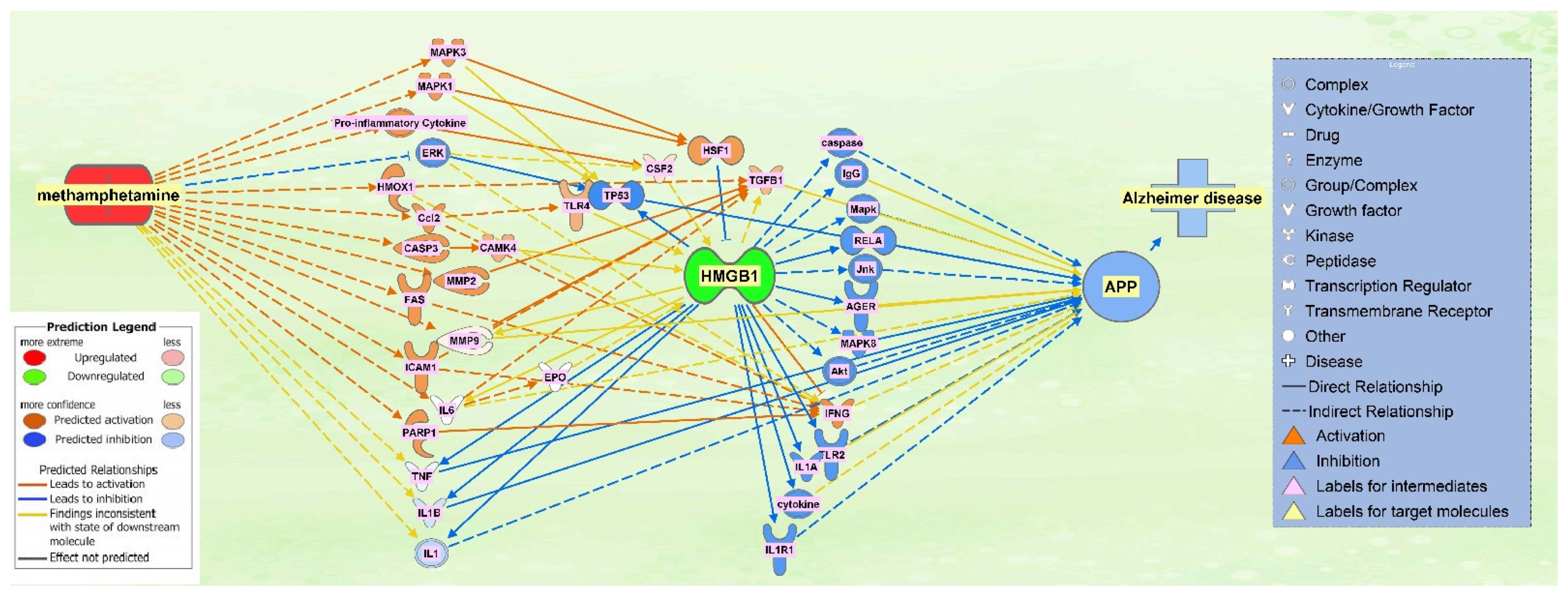
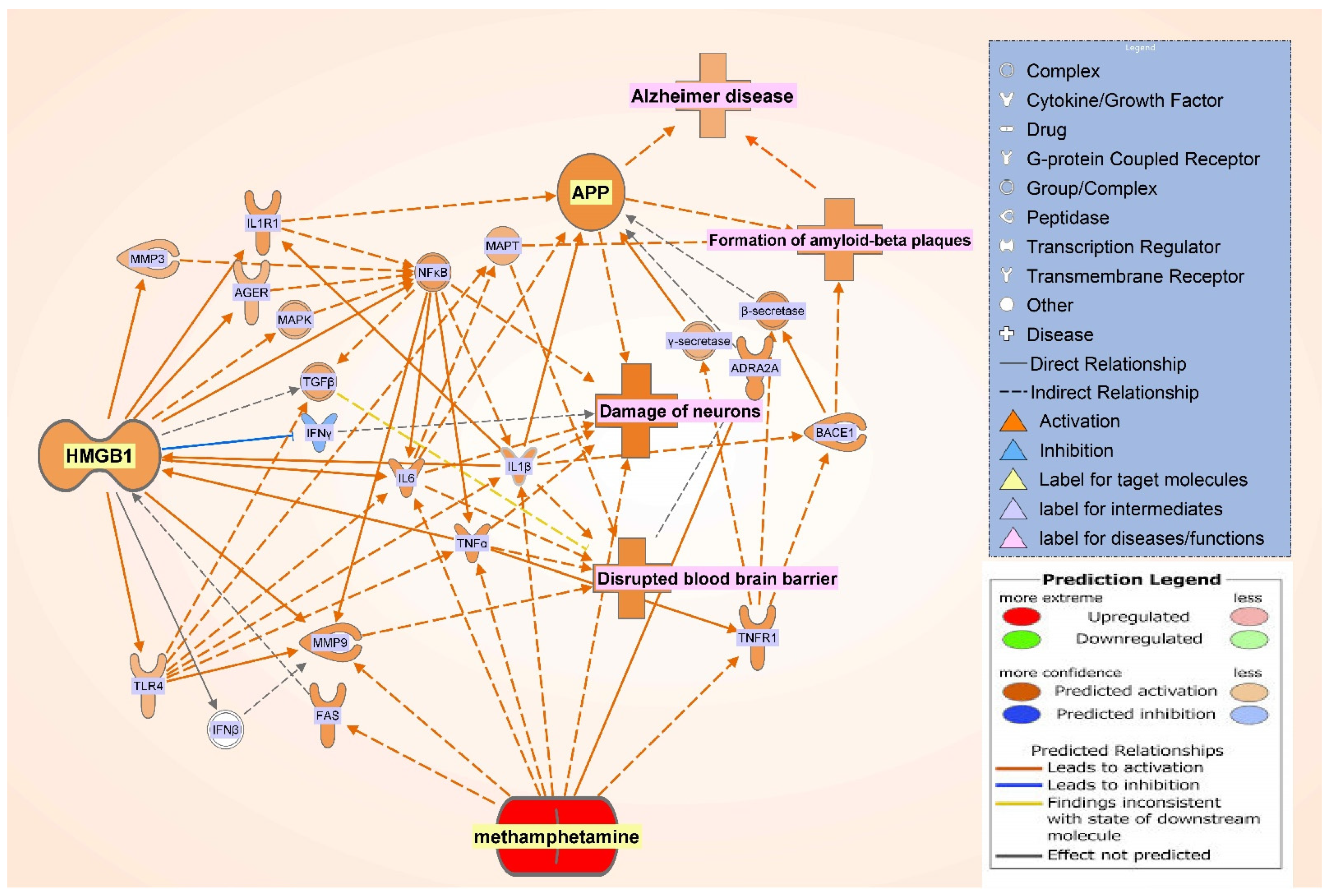
| Symbol | Entrez Gene Name | Location |
|---|---|---|
| AGER | advanced glycosylation end-product specific receptor | Plasma Membrane |
| Akt | Akt | Cytoplasm |
| CAMK4 | calcium/calmodulin dependent protein kinase IV | Nucleus |
| CASP3 | caspase 3 | Cytoplasm |
| caspase | caspase | Cytoplasm |
| Ccl2 | chemokine (C-C motif) ligand 2 | Extracellular Space |
| CSF2 | colony stimulating factor 2 | Extracellular Space |
| Cytokine * | cytokine | Extracellular Space |
| EPO | Erythropoietin | Extracellular Space |
| ERK | Extracellular Receptor Kinase | Other |
| FAS | Fas cell surface death receptor | Plasma Membrane |
| HMOX1 | heme oxygenase 1 | Cytoplasm |
| HSF1 | heat shock transcription factor 1 | Nucleus |
| ICAM1 | intercellular adhesion molecule 1 | Plasma Membrane |
| IFNG | interferon gamma | Extracellular Space |
| IgG | immunoglobulin G | Extracellular Space |
| IL1 | interleukin 1 | Extracellular Space |
| IL6 | interleukin 6 | Extracellular Space |
| IL1A | interleukin 1 alpha | Extracellular Space |
| IL1B | interleukin 1 beta | Extracellular Space |
| IL1R1 | interleukin 1 receptor type 1 | Plasma Membrane |
| Jnk | c-Jun N-terminal kinase | Cytoplasm |
| Mapk | Mitogen-activated protein kinase | Cytoplasm |
| MAPK1 | mitogen-activated protein kinase 1 | Cytoplasm |
| MAPK3 | mitogen-activated protein kinase 3 | Cytoplasm |
| MAPK8 | mitogen-activated protein kinase 8 | Cytoplasm |
| MMP2 | matrix metallopeptidase 2 | Extracellular Space |
| MMP9 | matrix metallopeptidase 9 | Extracellular Space |
| PARP1 | poly(ADP-ribose) polymerase 1 | Nucleus |
| Pro-inflammatory Cytokine * | Pro-inflammatory cytokine | Other |
| RELA | RELA proto-oncogene, NF-kB subunit | Nucleus |
| TGFB1 | transforming growth factor beta 1 | Extracellular Space |
| TLR2 | toll like receptor 2 | Plasma Membrane |
| TLR4 | toll like receptor 4 | Plasma Membrane |
| TNF | tumor necrosis factor | Extracellular Space |
| TP53 | tumor protein p53 | Nucleus |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alabed, S.; Zhou, H.; Sariyer, I.K.; Chang, S.L. Meta-Analysis of Methamphetamine Modulation on Amyloid Precursor Protein through HMGB1 in Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 4781. https://doi.org/10.3390/ijms22094781
Alabed S, Zhou H, Sariyer IK, Chang SL. Meta-Analysis of Methamphetamine Modulation on Amyloid Precursor Protein through HMGB1 in Alzheimer’s Disease. International Journal of Molecular Sciences. 2021; 22(9):4781. https://doi.org/10.3390/ijms22094781
Chicago/Turabian StyleAlabed, Sedra, Heping Zhou, Ilker K. Sariyer, and Sulie L. Chang. 2021. "Meta-Analysis of Methamphetamine Modulation on Amyloid Precursor Protein through HMGB1 in Alzheimer’s Disease" International Journal of Molecular Sciences 22, no. 9: 4781. https://doi.org/10.3390/ijms22094781
APA StyleAlabed, S., Zhou, H., Sariyer, I. K., & Chang, S. L. (2021). Meta-Analysis of Methamphetamine Modulation on Amyloid Precursor Protein through HMGB1 in Alzheimer’s Disease. International Journal of Molecular Sciences, 22(9), 4781. https://doi.org/10.3390/ijms22094781






