Repurposing Bedaquiline for Effective Non-Small Cell Lung Cancer (NSCLC) Therapy as Inhalable Cyclodextrin-Based Molecular Inclusion Complexes
Abstract
1. Introduction
2. Results and Discussion
2.1. UV Method Development for Bedaquiline (BDQ)
2.2. Phase-Solubility Studies
2.3. Continuous Variation Method (Job’s Plot)
2.4. Solid State Characterization of BDQ-SBE-β-CD Complex
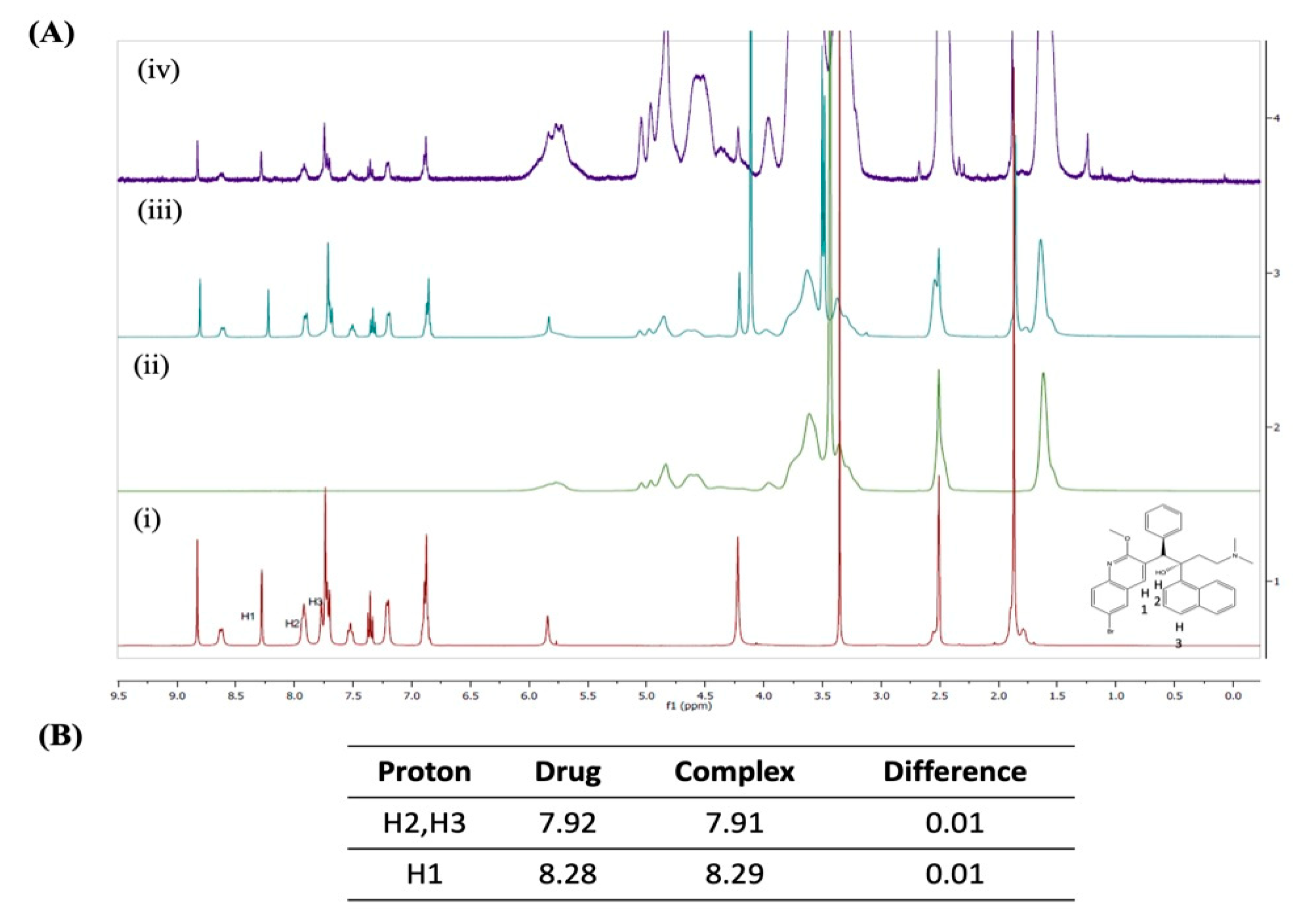
2.4.1. 1H-Nuclear Magnetic Resonance Spectroscopy (1H-NMR)
2.4.2. FT-IR Analysis
2.4.3. P-XRD Studies
2.4.4. DSC Studies
2.5. Accelerated Stability Study
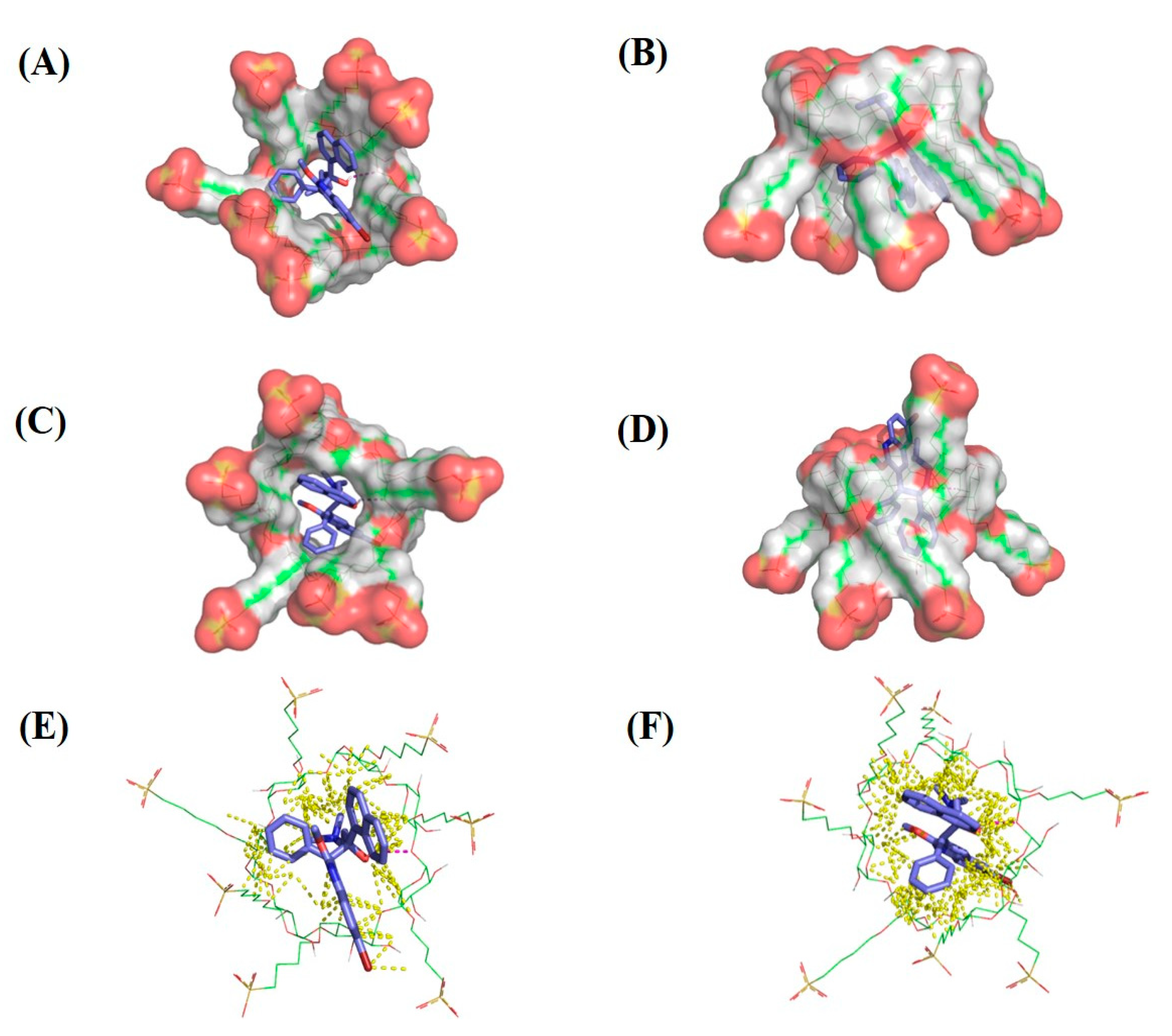
2.6. Molecular Modeling
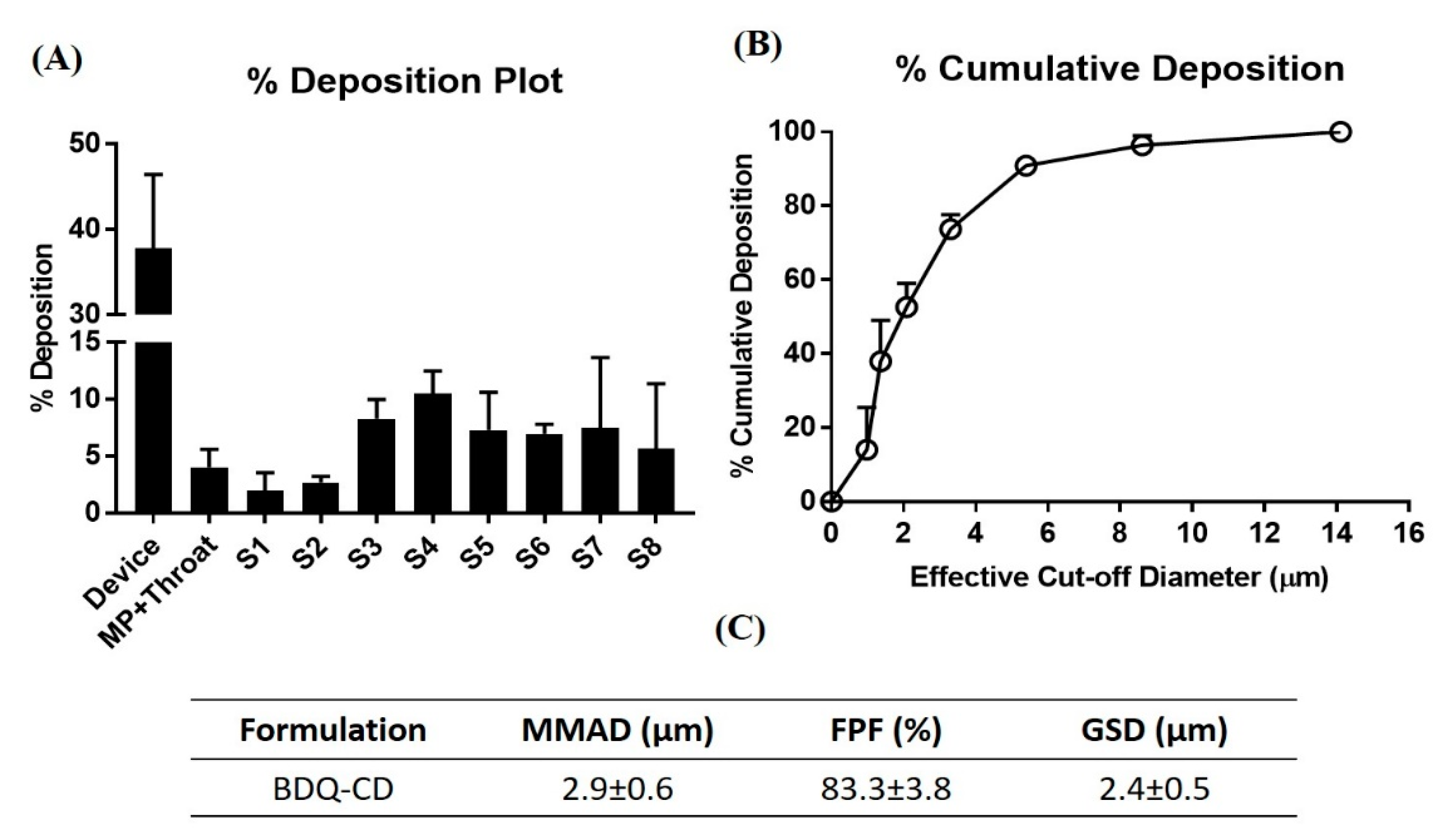
2.7. In Vitro Aerosol Performance
2.8. Cytotoxic Efficacy of BDQ and BDQ-CD Inclusion Complex in Different Cell Lines
3. Materials and Methods
3.1. Materials
3.2. Cell Lines
3.3. UV Spectrophotometric Method Development for Bedaquiline (BDQ)
3.4. Phase Solubility Studies
3.5. Continuous Variation Method (Job’s Plot Analysis)
3.6. Preparation of Solid BDQ-CD Complex
3.7. Solid State Characterization Studies
3.8. Accelerated Stability Study
3.9. Molecular Docking of Bedaquiline on Sulfobutyl Ether β-Cyclodextrins
3.10. In Vitro Aerosol Performance
3.11. Cytotoxicity Assay
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, G.; Pei, F.; Yang, F.; Li, L.; Amin, A.D.; Liu, S.; Buchan, J.R.; Cho, W.C. Role of Autophagy and Apoptosis in Non-Small-Cell Lung Cancer. Int. J. Mol. Sci. 2017, 18, 367. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, L.; Xie, N.; Nice, E.C.; Zhang, T.; Cui, Y.; Huang, C. Overcoming Cancer Therapeutic Bottleneck by Drug Repurposing. Signal Transduct. Target. Ther. 2020, 5, 1–25. [Google Scholar] [CrossRef]
- Shah, R.R.; Stonier, P.D. Repurposing Old Drugs in Oncology: Opportunities with Clinical and Regulatory Challenges Ahead. J. Clin. Pharm. Ther. 2019, 44, 6–22. [Google Scholar] [CrossRef]
- Parvathaneni, V.; Kulkarni, N.S.; Muth, A.; Gupta, V. Drug Repurposing: A Promising Tool to Accelerate the Drug Discovery Process. Drug Discov. Today 2019, 24, 2076–2085. [Google Scholar] [CrossRef]
- Senerovic, L.; Opsenica, D.; Moric, I.; Aleksic, I.; Spasić, M.; Vasiljevic, B. Quinolines and Quinolones as Antibacterial, Antifungal, Anti-virulence, Antiviral and Anti-parasitic Agents. In Advances in Microbiology, Infectious Diseases and Public Health: Volume 14; Donelli, G., Ed.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2020; pp. 37–69. ISBN 978-3-030-53647-3. [Google Scholar]
- da Gama, A.N.S.; de Nazaré Correia Soeiro, M. Quinoline-Based Compounds as Key Candidates to Tackle Drug Discovery Programs of Microbicidal Agents. Curr. Pharm. Des. 2020. [Google Scholar] [CrossRef]
- Ahsan, M.J.; Shastri, S.; Yadav, R.; Hassan, M.Z.; Bakht, M.A.; Jadav, S.S.; Yasmin, S. Synthesis and Antiproliferative Activity of Some Quinoline and Oxadiazole Derivatives. Org. Chem. Int. 2016, 2016, 9589517. [Google Scholar] [CrossRef]
- Tseng, C.-H.; Chen, Y.-L.; Chung, K.-Y.; Wang, C.-H.; Peng, S.-I.; Cheng, C.-M.; Tzeng, C.-C. Synthesis and Antiproliferative Evaluation of 2,3-Diarylquinoline Derivatives. Org. Biomol. Chem. 2011, 9, 3205–3216. [Google Scholar] [CrossRef]
- Wu, X.; Li, F.; Wang, X.; Li, C.; Meng, Q.; Wang, C.; Huang, J.; Chen, S.; Zhu, Z. Antibiotic Bedaquiline Effectively Targets Growth, Survival and Tumor Angiogenesis of Lung Cancer through Suppressing Energy Metabolism. Biochem. Biophys. Res. Commun. 2018, 495, 267–272. [Google Scholar] [CrossRef]
- Fiorillo, M.; Lamb, R.; Tanowitz, H.B.; Cappello, A.R.; Martinez-Outschoorn, U.E.; Sotgia, F.; Lisanti, M.P. Bedaquiline, an FDA-Approved Antibiotic, Inhibits Mitochondrial Function and Potently Blocks the Proliferative Expansion of Stem-like Cancer Cells (CSCs). Aging 2016, 8, 1593–1606. [Google Scholar] [CrossRef]
- Braga, S.S. Cyclodextrins: Emerging Medicines of the New Millennium. Biomolecules 2019, 9, 801. [Google Scholar] [CrossRef]
- Su, W.; Liang, Y.; Meng, Z.; Chen, X.; Lu, M.; Han, X.; Deng, X.; Zhang, Q.; Zhu, H.; Fu, T. Inhalation of Tetrandrine-Hydroxypropyl-β-Cyclodextrin Inclusion Complexes for Pulmonary Fibrosis Treatment. Mol. Pharm. 2020. [Google Scholar] [CrossRef]
- Szente, L.; Singhal, A.; Domokos, A.; Song, B. Cyclodextrins: Assessing the Impact of Cavity Size, Occupancy, and Substitutions on Cytotoxicity and Cholesterol Homeostasis. Molecules 2018, 23, 1228. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, B.; Parvathaneni, V.; Kulkarni, N.S.; Shukla, S.K.; Damon, J.K.; Sarode, A.; Kanabar, D.; Garcia, J.V.; Mitragotri, S.; Muth, A.; et al. Cyclodextrin Modified Erlotinib Loaded PLGA Nanoparticles for Improved Therapeutic Efficacy against Non-Small Cell Lung Cancer. Int. J. Biol. Macromol. 2019, 122, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Pillai, K.; Akhter, J.; Morris, D.L. Super Aqueous Solubility of Albendazole in β-Cyclodextrin for Parenteral Application in Cancer Therapy. J. Cancer 2017, 8, 913–923. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qian, Y.; Qian, C.; Shi, F.; Yao, J.; Bi, X.; Chen, Z. A Novel β-Cyclodextrin-Rhein Conjugate for Improving the Water Solubility and Bioavailability of Rhein. Carbohydr. Res. 2020, 490, 107958. [Google Scholar] [CrossRef]
- Vaidya, B.; Shukla, S.K.; Kolluru, S.; Huen, M.; Mulla, N.; Mehra, N.; Kanabar, D.; Palakurthi, S.; Ayehunie, S.; Muth, A.; et al. Nintedanib-Cyclodextrin Complex to Improve Bio-Activity and Intestinal Permeability. Carbohydr. Polym. 2019, 204, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Stella, V.J.; He, Q. Cyclodextrins. Toxicol. Pathol. 2008, 36, 30–42. [Google Scholar] [CrossRef]
- Haimhoffer, Á.; Rusznyák, Á.; Réti-Nagy, K.; Vasvári, G.; Váradi, J.; Vecsernyés, M.; Bácskay, I.; Fehér, P.; Ujhelyi, Z.; Fenyvesi, F. Cyclodextrins in Drug Delivery Systems and Their Effects on Biological Barriers. Sci. Pharm. 2019, 87, 33. [Google Scholar] [CrossRef]
- Goel, A.; Baboota, S.; Sahni, J.K.; Ali, J. Exploring Targeted Pulmonary Delivery for Treatment of Lung Cancer. Int. J. Pharm. Investig. 2013, 3, 8–14. [Google Scholar] [CrossRef]
- Dominique, N.P.; Nitesh, K.K.; Elliott, K.M. Inhaled Therapeutics against TB: The Promise of Pulmonary Treatment and Prevention Strategies in the Clinic; CRC Press: Boca Raton, FL, USA, 2019; pp. 361–375. ISBN 978-1-315-15976-8. [Google Scholar]
- Abdelaziz, H.M.; Gaber, M.; Abd-Elwakil, M.M.; Mabrouk, M.T.; Elgohary, M.M.; Kamel, N.M.; Kabary, D.M.; Freag, M.S.; Samaha, M.W.; Mortada, S.M.; et al. Inhalable Particulate Drug Delivery Systems for Lung Cancer Therapy: Nanoparticles, Microparticles, Nanocomposites and Nanoaggregates. J. Control. Release 2018, 269, 374–392. [Google Scholar] [CrossRef]
- Lee, W.-H.; Loo, C.-Y.; Traini, D.; Young, P.M. Inhalation of Nanoparticle-Based Drug for Lung Cancer Treatment: Advantages and Challenges. Asian J. Pharm. Sci. 2015, 10, 481–489. [Google Scholar] [CrossRef]
- Wauthoz, N.; Deleuze, P.; Saumet, A.; Duret, C.; Kiss, R.; Amighi, K. Temozolomide-Based Dry Powder Formulations for Lung Tumor-Related Inhalation Treatment. Pharm. Res. 2011, 28, 762–775. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Parvathaneni, V.; Shukla, S.K.; Kanabar, D.D.; Muth, A.; Gupta, V. Cyclodextrin Complexation for Enhanced Stability and Non-Invasive Pulmonary Delivery of Resveratrol—Applications in Non-Small Cell Lung Cancer Treatment. Aaps Pharmscitech 2020, 21, 183. [Google Scholar] [CrossRef] [PubMed]
- Binti Mohtar, N. Cyclodextrin-Based Formulations for Pulmonary Delivery of Chemotherapeutic Molecules. Ph.D. Thesis, University College London, London, UK, 2018. [Google Scholar]
- Vaidya, B.; Kulkarni, N.S.; Shukla, S.K.; Parvathaneni, V.; Chauhan, G.; Damon, J.K.; Sarode, A.; Garcia, J.V.; Kunda, N.; Mitragotri, S.; et al. Development of Inhalable Quinacrine Loaded Bovine Serum Albumin Modified Cationic Nanoparticles: Repurposing Quinacrine for Lung Cancer Therapeutics. Int. J. Pharm. 2020, 577, 118995. [Google Scholar] [CrossRef]
- Shukla, S.K.; Chan, A.; Parvathaneni, V.; Kanabar, D.D.; Patel, K.; Ayehunie, S.; Muth, A.; Gupta, V. Enhanced Solubility, Stability, Permeation and Anti-Cancer Efficacy of Celastrol-β-Cyclodextrin Inclusion Complex. J. Mol. Liq. 2020, 318, 113936. [Google Scholar] [CrossRef]
- Parvathaneni, V.; Elbatanony, R.S.; Shukla, S.K.; Kulkarni, N.S.; Kanabar, D.D.; Chauhan, G.; Ayehunie, S.; Chen, Z.-S.; Muth, A.; Gupta, V. Bypassing P-Glycoprotein Mediated Efflux of Afatinib by Cyclodextrin Complexation – Evaluation of Intestinal Absorption and Anti-Cancer Activity. J. Mol. Liq. 2020, 114866. [Google Scholar] [CrossRef]
- Higuchi T, C.K. Phase Solubility Techniques. Adv. Anal. Chem. Instrum. 1965, 4, 117–122. [Google Scholar]
- Angiolini, L.; Agnes, M.; Cohen, B.; Yannakopoulou, K.; Douhal, A. Formation, Characterization and PH Dependence of Rifampicin: Heptakis(2,6-Di-O-Methyl)-β-Cyclodextrin Complexes. Int. J. Pharm. 2017, 531, 668–675. [Google Scholar] [CrossRef]
- Salzano, G.; Wankar, J.; Ottani, S.; Villemagne, B.; Baulard, A.R.; Willand, N.; Brodin, P.; Manet, I.; Gref, R. Cyclodextrin-Based Nanocarriers Containing a Synergic Drug Combination: A Potential Formulation for Pulmonary Administration of Antitubercular Drugs. Int. J. Pharm. 2017, 531, 577–587. [Google Scholar] [CrossRef]
- Bosque-Sendra, J.M.; ALMANSA-LóPEZ, E.; García-Campaña, M.; Cuadros-Rodríguez, L. Data Analysis in the Determination of Stoichiometries and Stability Constants of Complexes. Anal. Sci. 2003, 19, 1431–1439. [Google Scholar] [CrossRef][Green Version]
- Kost, B.; Brzeziński, M.; Zimnicka, M.; Socka, M.; Wielgus, E.; Słowianek, M.; Biela, T. PLA Stereocomplexed Microspheres Modified with Methyl-β-Cyclodextrin as an Atropine Delivery System. Synthesis and Characterization. Mater. Today Commun. 2020, 25, 101605. [Google Scholar] [CrossRef]
- Momin, M.A.M.; Rangnekar, B.; Sinha, S.; Cheung, C.-Y.; Cook, G.M.; Das, S.C. Inhalable Dry Powder of Bedaquiline for Pulmonary Tuberculosis: In Vitro Physicochemical Characterization, Antimicrobial Activity and Safety Studies. Pharmaceutics 2019, 11, 502. [Google Scholar] [CrossRef]
- Sambasevam, K.P.; Mohamad, S.; Sarih, N.M.; Ismail, N.A. Synthesis and Characterization of the Inclusion Complex of β-Cyclodextrin and Azomethine. Int. J. Mol. Sci. 2013, 14, 3671–3682. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, F.; Wang, J.; He, H.; Duan, S.; Zhu, R.; Chen, C.; Yin, L.; Chen, Y. Biodegradable Nanoparticles Mediated Co-Delivery of Erlotinib (ELTN) and Fedratinib (FDTN) Toward the Treatment of ELTN-Resistant Non-Small Cell Lung Cancer (NSCLC) via Suppression of the JAK2/STAT3 Signaling Pathway. Front. Pharmacol. 2018, 9. [Google Scholar] [CrossRef]
- Vangara, K.K.; Ali, H.I.; Lu, D.; Liu, J.L.; Kolluru, S.; Palakurthi, S. SN-38-Cyclodextrin Complexation and Its Influence on the Solubility, Stability, and in Vitro Anticancer Activity against Ovarian Cancer. Aaps Pharmscitech 2014, 15, 472–482. [Google Scholar] [CrossRef]
- Sadaquat, H.; Akhtar, M. Comparative Effects of β-Cyclodextrin, HP-β-Cyclodextrin and SBE7-β-Cyclodextrin on the Solubility and Dissolution of Docetaxel via Inclusion Complexation. J. Incl. Phenom. Macrocycl. Chem. 2020, 96, 333–351. [Google Scholar] [CrossRef]
- Momin, M.A.M.; Rangnekar, B.; Larson, I.; Sinha, S.; Das, S.C. Dry Powder Formulation Combining Bedaquiline with Pyrazinamide for Latent and Drug-Resistant Tuberculosis. Adv. Powder Technol. 2019, 30, 2473–2482. [Google Scholar] [CrossRef]
- Du, F.; Pan, T.; Ji, X.; Hu, J.; Ren, T. Study on the Preparation of Geranyl Acetone and β-Cyclodextrin Inclusion Complex and Its Application in Cigarette Flavoring. Sci. Rep. 2020, 10, 12375. [Google Scholar] [CrossRef]
- Muthu, M.S.; Feng, S.-S. Pharmaceutical Stability Aspects of Nanomedicines. Nanomedicine 2009, 4, 857–860. [Google Scholar] [CrossRef]
- Carneiro, S.B.; Costa Duarte, F.Í.; Heimfarth, L.; de Souza Siqueira Quintans, J.; Quintans-Júnior, L.J.; da Veiga Júnior, V.F.; de Lima, Á.A.N. Cyclodextrin−Drug Inclusion Complexes: In Vivo and In Vitro Approaches. Int. J. Mol. Sci. 2019, 20, 642. [Google Scholar] [CrossRef]
- Maheriya, P. Cyclodextrin: A Promising Candidate in Enhancing Oral Bioavailability of Poorly Water Soluble Drugs. MOJ Bioequivalence Bioavailab. 2017, 3. [Google Scholar] [CrossRef]
- Sharma, N.; Baldi, A. Exploring Versatile Applications of Cyclodextrins: An Overview. Drug Deliv. 2016, 23, 729–747. [Google Scholar] [CrossRef]
- Drugs@FDA: FDA-Approved Drugs. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/ (accessed on 17 December 2020).
- Cabral Marques, H.M.; Hadgraft, J.; Kellaway, I.W.; Taylor, G. Studies of Cyclodextrin Inclusion Complexes. IV. The Pulmonary Absorption of Salbutamol from a Complex with 2-Hydroxypropyl-β-Cyclodextrin in Rabbits. Int. J. Pharm. 1991, 77, 303–307. [Google Scholar] [CrossRef]
- Srichana, T.; Suedee, R.; Reanmongkol, W. Cyclodextrin as a Potential Drug Carrier in Salbutamol Dry Powder Aerosols: The In vitro Deposition and Toxicity Studies of the Complexes. Respir. Med. 2001, 95, 513–519. [Google Scholar] [CrossRef]
- Rosati, J.A.; Leith, D.; Kim, C.S. Monodisperse and Polydisperse Aerosol Deposition in a Packed Bed. Aerosol Sci. Technol. 2003, 37, 528–535. [Google Scholar] [CrossRef]
- Evrard, B.; Bertholet, P.; Gueders, M.; Flament, M.-P.; Piel, G.; Delattre, L.; Gayot, A.; Leterme, P.; Foidart, J.-M.; Cataldo, D. Cyclodextrins as a Potential Carrier in Drug Nebulization. J. Control. Release 2004, 96, 403–410. [Google Scholar] [CrossRef]
- Wang, X.; Parvathaneni, V.; Shukla, S.K.; Kulkarni, N.S.; Muth, A.; Kunda, N.K.; Gupta, V. Inhalable Resveratrol-Cyclodextrin Complex Loaded Biodegradable Nanoparticles for Enhanced Efficacy against Non-Small Cell Lung Cancer. Int. J. Biol. Macromol. 2020, 164, 638–650. [Google Scholar] [CrossRef]
- Cyclolab. Available online: https://cyclolab.hu/products/pharma_grade_cyclodextrins-c8/sulfobutylated_betacyclodextrin_sodium_salt_ds65-p41/ (accessed on 23 March 2021).
- BIOVIA Discovery Studio—BIOVIA—Dassault Systèmes®. Available online: https://www.3ds.com/products-services/biovia/products/molecular-modeling-simulation/biovia-discovery-studio/ (accessed on 17 December 2020).
- PyMOL|Pymol.Org. Available online: https://pymol.org/2/ (accessed on 17 December 2020).
- Hidau, M.K.; Mehra, N.K.; Palakurthi, S.K. and S. Cyclodextrin Complexation of Meclizine Hydrochloride and Its Influence on the Solubility for Its Repurposing. Drug Deliv. Lett. 2017, 7, 54–61. [Google Scholar]
- Petit, S.; Coquerel, G.; Meyer, C.; Guillemont, J. Absolute Configuration and Structural Features of R207910, a Novel Anti-Tuberculosis Agent. J. Mol. Struct. 2007, 837, 252–256. [Google Scholar] [CrossRef]
- Case: What Is the Difference between the GoldScore, ChemScore, ASP and ChemPLP Scoring Functions Provided with GOLD?—The Cambridge Crystallographic Data Centre (CCDC). Available online: https://www.ccdc.cam.ac.uk/support-and-resources/support/case/?caseid=5d1a2fc0-c93a-49c3-a8e2-f95c472dcff0 (accessed on 17 December 2020).
- Abdou, E.M.; Kandil, S.M.; Morsi, A.; Sleem, M.W. In vitro and in-Vivo Respiratory Deposition of a Developed Metered Dose Inhaler Formulation of an Anti-Migraine Drug. Drug Deliv. 2019, 26, 689–699. [Google Scholar] [CrossRef]
- Carvalho, T.C.; Peters, J.I.; Williams, R.O. Influence of Particle Size on Regional Lung Deposition--What Evidence Is There? Int. J. Pharm. 2011, 406, 1–10. [Google Scholar] [CrossRef]
- Kunda, N.K.; Alfagih, I.M.; Dennison, S.R.; Tawfeek, H.M.; Somavarapu, S.; Hutcheon, G.A.; Saleem, I.Y. Bovine Serum Albumin Adsorbed PGA-Co-PDL Nanocarriers for Vaccine Delivery via Dry Powder Inhalation. Pharm. Res. 2015, 32, 1341–1353. [Google Scholar] [CrossRef]
- Parvathaneni, V.; Kulkarni, N.S.; Shukla, S.K.; Farrales, P.T.; Kunda, N.K.; Muth, A.; Gupta, V. Systematic Development and Optimization of Inhalable Pirfenidone Liposomes for Non-Small Cell Lung Cancer Treatment. Pharmaceutics 2020, 12, 206. [Google Scholar] [CrossRef]
- Shukla, S.K.; Chan, A.; Parvathaneni, V.; Gupta, V. Metformin-Loaded Chitosomes for Treatment of Malignant Pleural Mesothelioma—A Rare Thoracic Cancer. Int. J. Biol. Macromol. 2020, 160, 128–141. [Google Scholar] [CrossRef]
- Parvathaneni, V.; Kulkarni, N.S.; Chauhan, G.; Shukla, S.K.; Elbatanony, R.; Patel, B.; Kunda, N.K.; Muth, A.; Gupta, V. Development of Pharmaceutically Scalable Inhaled Anti-Cancer Nanotherapy—Repurposing Amodiaquine for Non-Small Cell Lung Cancer (NSCLC). Mater. Sci. Eng. C 2020, 115, 111139. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parvathaneni, V.; Elbatanony, R.S.; Goyal, M.; Chavan, T.; Vega, N.; Kolluru, S.; Muth, A.; Gupta, V.; Kunda, N.K. Repurposing Bedaquiline for Effective Non-Small Cell Lung Cancer (NSCLC) Therapy as Inhalable Cyclodextrin-Based Molecular Inclusion Complexes. Int. J. Mol. Sci. 2021, 22, 4783. https://doi.org/10.3390/ijms22094783
Parvathaneni V, Elbatanony RS, Goyal M, Chavan T, Vega N, Kolluru S, Muth A, Gupta V, Kunda NK. Repurposing Bedaquiline for Effective Non-Small Cell Lung Cancer (NSCLC) Therapy as Inhalable Cyclodextrin-Based Molecular Inclusion Complexes. International Journal of Molecular Sciences. 2021; 22(9):4783. https://doi.org/10.3390/ijms22094783
Chicago/Turabian StyleParvathaneni, Vineela, Rasha S. Elbatanony, Mimansa Goyal, Tejashri Chavan, Nathan Vega, Srikanth Kolluru, Aaron Muth, Vivek Gupta, and Nitesh K. Kunda. 2021. "Repurposing Bedaquiline for Effective Non-Small Cell Lung Cancer (NSCLC) Therapy as Inhalable Cyclodextrin-Based Molecular Inclusion Complexes" International Journal of Molecular Sciences 22, no. 9: 4783. https://doi.org/10.3390/ijms22094783
APA StyleParvathaneni, V., Elbatanony, R. S., Goyal, M., Chavan, T., Vega, N., Kolluru, S., Muth, A., Gupta, V., & Kunda, N. K. (2021). Repurposing Bedaquiline for Effective Non-Small Cell Lung Cancer (NSCLC) Therapy as Inhalable Cyclodextrin-Based Molecular Inclusion Complexes. International Journal of Molecular Sciences, 22(9), 4783. https://doi.org/10.3390/ijms22094783







