Micro- and Nanosized Substances Cause Different Autophagy-Related Responses
Abstract
1. Introduction
2. Classification of Micro- and Nanosized Substances (MSs and NSs)
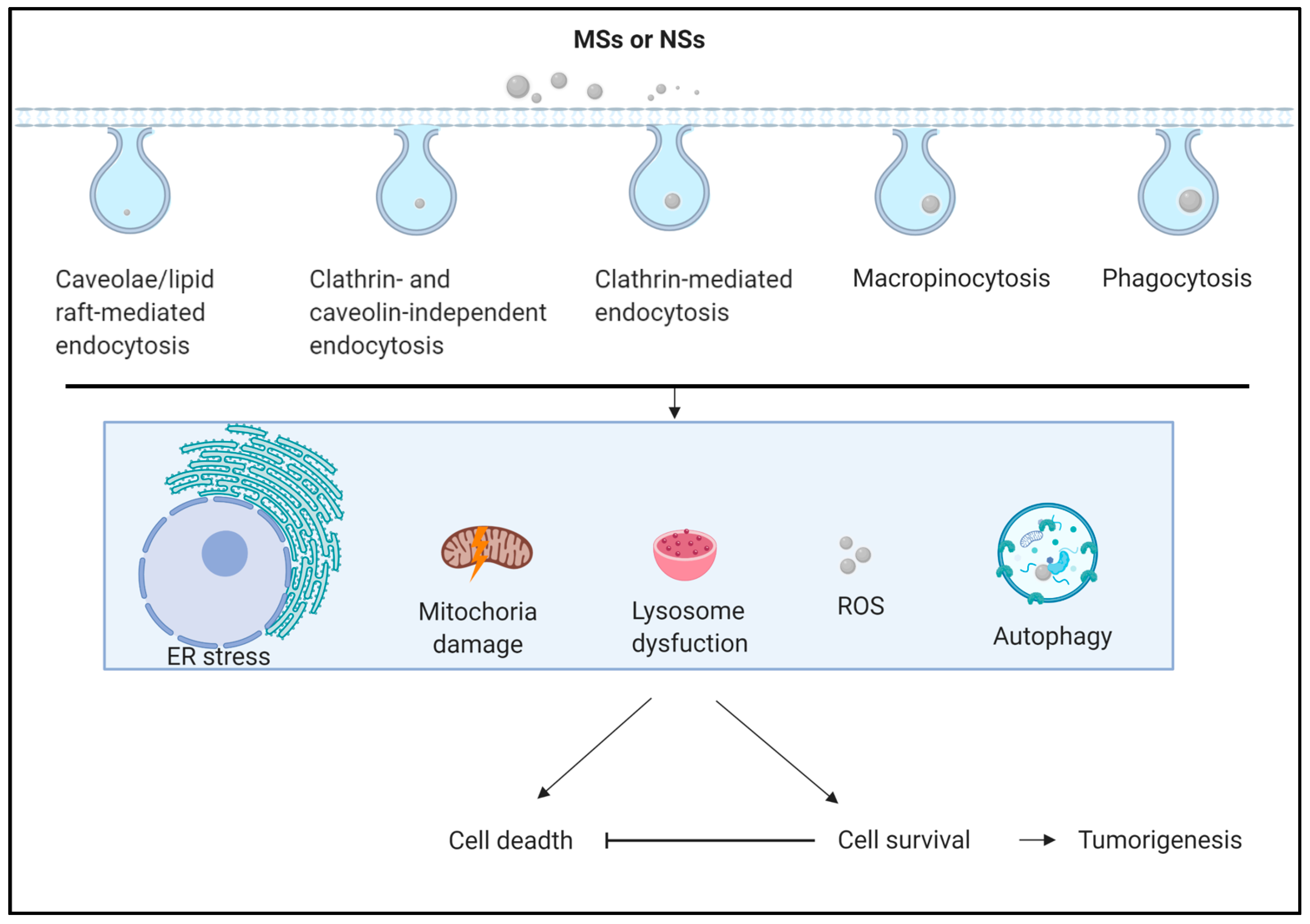
3. Autophagy-Related Responses to MSs and NSs in Animal and Cell Lines
4. Autophagy-Related Responses in Undecomposed MSs and NSs
5. Autophagy and Tumorigenesis
6. Solutions for MS- and NS-Caused Pollution
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reed, N.A.; Raliya, R.; Tang, R.; Xu, B.; Mixdorf, M.; Achilefu, S.; Biswas, P. Electrospray Functionalization of Titanium Dioxide Nanoparticles with Transferrin for Cerenkov Radiation Induced Cancer Therapy. ACS Appl. Bio. Mater. 2019, 2, 1141–1147. [Google Scholar] [CrossRef]
- Chu, C.; Lu, C.; Yuan, J.; Xing, C. Fate of Fe3O4@NH2 in soil and their fixation effect to reduce lead translocation in two rice cultivars. Food Sci. Nutr. 2020, 8, 3673–3681. [Google Scholar] [CrossRef] [PubMed]
- Proquin, H.; Rodriguez-Ibarra, C.; Moonen, C.G.; Urrutia Ortega, I.M.; Briede, J.J.; de Kok, T.M.; van Loveren, H.; Chirino, Y.I. Titanium dioxide food additive (E171) induces ROS formation and genotoxicity: Contribution of micro and nano-sized fractions. Mutagenesis 2017, 32, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Kazimirova, A.; El Yamani, N.; Rubio, L.; Garcia-Rodriguez, A.; Barancokova, M.; Marcos, R.; Dusinska, M. Effects of Titanium Dioxide Nanoparticles on the Hprt Gene Mutations in V79 Hamster Cells. Nanomaterials 2020, 10, 465. [Google Scholar] [CrossRef]
- Brandao, F.; Fernandez-Bertolez, N.; Rosario, F.; Bessa, M.J.; Fraga, S.; Pasaro, E.; Teixeira, J.P.; Laffon, B.; Valdiglesias, V.; Costa, C. Genotoxicity of TiO2 Nanoparticles in Four Different Human Cell Lines (A549, HEPG2, A172 and SH-SY5Y). Nanomaterials 2020, 10, 412. [Google Scholar] [CrossRef] [PubMed]
- Aryal, S.; Remant, B.K.; Narayan, B.; Kim, C.K.; Kim, H.Y. Study of electrolyte induced aggregation of gold nanoparticles capped by amino acids. J. Colloid. Interface Sci. 2006, 299, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Y.; Westerhoff, P.; Crittenden, J. Impact of natural organic matter and divalent cations on the stability of aqueous nanoparticles. Water Res. 2009, 43, 4249–4257. [Google Scholar] [CrossRef]
- Proquin, H.; Jonkhout, M.C.M.; Jetten, M.J.; van Loveren, H.; de Kok, T.M.; Briede, J.J. Transcriptome changes in undifferentiated Caco-2 cells exposed to food-grade titanium dioxide (E171): Contribution of the nano-and micro-sized particles. Sci. Rep. 2019, 9, 18287. [Google Scholar] [CrossRef]
- Judy, J.D.; Unrine, J.M.; Bertsch, P.M. Evidence for biomagnification of gold nanoparticles within a terrestrial food chain. Environ. Sci. Technol. 2011, 45, 776–781. [Google Scholar] [CrossRef]
- Judy, J.D.; Unrine, J.M.; Rao, W.; Bertsch, P.M. Bioaccumulation of gold nanomaterials by Manduca sexta through dietary uptake of surface contaminated plant tissue. Environ. Sci. Technol. 2012, 46, 12672–12678. [Google Scholar] [CrossRef]
- Cho, Y.; Shim, W.J.; Jang, M.; Han, G.M.; Hong, S.H. Abundance and characteristics of microplastics in market bivalves from South Korea. Environ. Pollut. 2019, 245, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.; Love, D.C.; Rochman, C.M.; Neff, R.A. Microplastics in Seafood and the Implications for Human Health. Curr. Environ. Health Rep. 2018, 5, 375–386. [Google Scholar] [CrossRef]
- Kniggendorf, A.K.; Wetzel, C.; Roth, B. Microplastics Detection in Streaming Tap Water with Raman Spectroscopy. Sensors 2019, 19, 1839. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Mohamed Nor, N.H.; Hermsen, E.; Kooi, M.; Mintenig, S.M.; De France, J. Microplastics in freshwaters and drinking water: Critical review and assessment of data quality. Water Res. 2019, 155, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Zhou, Y.; Wu, J.; Gong, Z. Influencing Factors of PM2.5 Pollution: Disaster Points of Meteorological Factors. Int. J. Environ. Res. Public Health 2019, 16, 3891. [Google Scholar] [CrossRef] [PubMed]
- Fei, Y.X.; Zhao, B.; Yin, Q.Y.; Qiu, Y.Y.; Ren, G.H.; Wang, B.W.; Wang, Y.F.; Fang, W.R.; Li, Y.M. Ma Xing Shi Gan Decoction Attenuates PM2.5 Induced Lung Injury via Inhibiting HMGB1/TLR4/NFkappaB Signal Pathway in Rat. Front. Pharmacol. 2019, 10, 1361. [Google Scholar] [CrossRef] [PubMed]
- Stueckle, T.A.; Davidson, D.C.; Derk, R.; Kornberg, T.G.; Schwegler-Berry, D.; Pirela, S.V.; Deloid, G.; Demokritou, P.; Luanpitpong, S.; Rojanasakul, Y.; et al. Evaluation of tumorigenic potential of CeO2 and Fe2O3 engineered nanoparticles by a human cell in vitro screening model. NanoImpact 2017, 6, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Lary, D.J.; Zewdie, G.K.; Liu, X. Using machine learning to understand the temporal morphology of the PM2.5 annual cycle in East Asia. Environ. Monit. Assess. 2019, 191, 272. [Google Scholar] [CrossRef]
- Sah, D.; Verma, P.K.; Kandikonda, M.K.; Lakhani, A. Chemical fractionation, bioavailability, and health risks of heavy metals in fine particulate matter at a site in the Indo-Gangetic Plain, India. Environ. Sci. Pollut Res. Int. 2019, 26, 19749–19762. [Google Scholar] [CrossRef]
- Jeon, Y.M.; Lee, M.Y. Airborne nanoparticles (PM0.1) induce autophagic cell death of human neuronal cells. J. Appl. Toxicol. 2016, 36, 1332–1342. [Google Scholar] [CrossRef]
- Corbin, J.C. PM0.1 particles from aircraft may increase risk of vascular disease. BMJ 2013, 347, f6783. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wu, S.D.; Shen, L.J.; Zhao, T.X.; Wei, Y.; Tang, X.L.; Long, C.L.; Zhou, Y.; He, D.W.; Lin, T.; et al. Spermatogenesis dysfunction induced by PM2.5 from automobile exhaust via the ROS-mediated MAPK signaling pathway. Ecotoxicol. Environ. Saf. 2019, 167, 161–168. [Google Scholar] [CrossRef]
- Wei, Y.; Cao, X.N.; Tang, X.L.; Shen, L.J.; Lin, T.; He, D.W.; Wu, S.D.; Wei, G.H. Urban fine particulate matter (PM2.5) exposure destroys blood-testis barrier (BTB) integrity through excessive ROS-mediated autophagy. Toxicol. Mech. Methods 2018, 28, 302–319. [Google Scholar] [CrossRef] [PubMed]
- Hime, N.J.; Marks, G.B.; Cowie, C.T. A Comparison of the Health Effects of Ambient Particulate Matter Air Pollution from Five Emission Sources. Int. J. Environ. Res. Public Health 2018, 15, 1206. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Ding, C.; Jiang, X.; Pan, G.; Wei, X.; Sun, Y. Chemical Compositions and Sources Contribution of Atmospheric Particles at a Typical Steel Industrial Urban Site. Sci. Rep. 2020, 10, 7654. [Google Scholar] [CrossRef]
- Fang, X.; Li, R.; Xu, Q.; Bottai, M.; Fang, F.; Cao, Y. A Two-Stage Method to Estimate the Contribution of Road Traffic to PM(2).(5) Concentrations in Beijing, China. Int. J. Environ. Res. Public Health 2016, 13, 124. [Google Scholar] [CrossRef] [PubMed]
- Khreis, H.; Nieuwenhuijsen, M.J. Traffic-Related Air Pollution and Childhood Asthma: Recent Advances and Remaining Gaps in the Exposure Assessment Methods. Int. J. Environ. Res. Public Health 2017, 14, 312. [Google Scholar] [CrossRef]
- Qi, Z.; Song, Y.; Ding, Q.; Liao, X.; Li, R.; Liu, G.; Tsang, S.; Cai, Z. Water soluble and insoluble components of PM2.5 and their functional cardiotoxicities on neonatal rat cardiomyocytes in vitro. Ecotoxicol. Environ. Saf. 2019, 168, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Feng, L.; Zhang, Y.; Hu, H.; Shi, Y.; Liang, S.; Zhao, T.; Fu, Y.; Duan, J.; Sun, Z. Cytotoxicity induced by fine particulate matter (PM2.5) via mitochondria-mediated apoptosis pathway in human cardiomyocytes. Ecotoxicol. Environ. Saf. 2018, 161, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Ji, X.; Shou, Y.; Huang, Y.; Hu, Y.; Wang, H. Recent advances in understanding the mechanisms of PM2.5-mediated neurodegenerative diseases. Toxicol. Lett. 2020, 329, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Xia, R.; Fang, N.; Yang, Y.; Xu, F.; Zhang, L.; Ji, S. PM2.5 promotes apoptosis of alveolar epithelial cells via targeting ROS/p38 signaling pathway and thus leads to emphysema in mice. Minerva Med. 2020. [Google Scholar] [CrossRef]
- Li, R.; Zhou, R.; Zhang, J. Function of PM2.5 in the pathogenesis of lung cancer and chronic airway inflammatory diseases. Oncol Lett. 2018, 15, 7506–7514. [Google Scholar] [CrossRef]
- Wang, Y.; Zhong, Y.; Hou, T.; Liao, J.; Zhang, C.; Sun, C.; Wang, G. PM2.5 induces EMT and promotes CSC properties by activating Notch pathway in vivo and vitro. Ecotoxicol. Environ. Saf. 2019, 178, 159–167. [Google Scholar] [CrossRef]
- Zhou, L.; Su, X.; Li, B.; Chu, C.; Sun, H.; Zhang, N.; Han, B.; Li, C.; Zou, B.; Niu, Y.; et al. PM2.5 exposure impairs sperm quality through testicular damage dependent on NALP3 inflammasome and miR-183/96/182 cluster targeting FOXO1 in mouse. Ecotoxicol. Environ. Saf. 2019, 169, 551–563. [Google Scholar] [CrossRef]
- Ogino, K.; Nagaoka, K.; Ito, T.; Takemoto, K.; Okuda, T.; Nakayama, S.F.; Ogino, N.; Seki, Y.; Hamada, H.; Takashiba, S.; et al. Involvement of PM2.5-bound protein and metals in PM2.5-induced allergic airway inflammation in mice. Inhal. Toxicol. 2018, 30, 498–508. [Google Scholar] [CrossRef]
- Shou, Y.; Huang, Y.; Zhu, X.; Liu, C.; Hu, Y.; Wang, H. A review of the possible associations between ambient PM2.5 exposures and the development of Alzheimer’s disease. Ecotoxicol. Environ. Saf. 2019, 174, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Zhang, F.; Rui, W.; Long, F.; Wang, L.; Feng, Z.; Chen, D.; Ding, W. PM2.5-induced oxidative stress triggers autophagy in human lung epithelial A549 cells. Toxicol. In Vitro 2013, 27, 1762–1770. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, M. PM2.5 induces autophagy and apoptosis through endoplasmic reticulum stress in human endothelial cells. Sci. Total Environ. 2020, 710, 136397. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Kim, J.; Herrera, M.; Mukherjee, A.; Kabanov, A.V.; Sahay, G. Brief update on endocytosis of nanomedicines. Adv. Drug Deliv Rev. 2019, 144, 90–111. [Google Scholar] [CrossRef] [PubMed]
- Gehr, P.; Clift, M.J.; Brandenberger, C.; Lehmann, A.; Herzog, F.; Rothen-Rutishauser, B. Endocytosis of environmental and engineered micro- and nanosized particles. Compr. Physiol. 2011, 1, 1159–1174. [Google Scholar] [CrossRef] [PubMed]
- Kaksonen, M.; Roux, A. Mechanisms of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 2018, 19, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.L.; Wang, S.W.; Sun, W.C.; Shu, C.W.; Kao, Y.C.; Shiao, M.S.; Chen, C.L. Sorafenib suppresses TGF-beta responses by inducing caveolae/lipid raft-mediated internalization/degradation of cell-surface type II TGF-beta receptors: Implications in development of effective adjunctive therapy for hepatocellular carcinoma. Biochem. Pharmacol. 2018, 154, 39–53. [Google Scholar] [CrossRef]
- Johannes, L.; Parton, R.G.; Bassereau, P.; Mayor, S. Building endocytic pits without clathrin. Nat. Rev. Mol. Cell Biol. 2015, 16, 311–321. [Google Scholar] [CrossRef] [PubMed]
- King, J.S.; Kay, R.R. The origins and evolution of macropinocytosis. Philos. Trans. R Soc. Lond. B Biol. Sci. 2019, 374, 20180158. [Google Scholar] [CrossRef]
- Gray, M.; Botelho, R.J. Phagocytosis: Hungry, Hungry Cells. Methods Mol. Biol. 2017, 1519, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Birgisdottir, A.B.; Johansen, T. Autophagy and endocytosis-interconnections and interdependencies. J. Cell Sci. 2020, 133. [Google Scholar] [CrossRef] [PubMed]
- Bejarano, E.; Cuervo, A.M. Chaperone-mediated autophagy. Proc. Am. Thorac. Soc. 2010, 7, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Parzych, K.R.; Klionsky, D.J. An overview of autophagy: Morphology, mechanism, and regulation. Antioxid. Redox Signal. 2014, 20, 460–473. [Google Scholar] [CrossRef] [PubMed]
- Yim, W.W.; Mizushima, N. Lysosome biology in autophagy. Cell Discov. 2020, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Mijaljica, D.; Prescott, M.; Devenish, R.J. Microautophagy in mammalian cells: Revisiting a 40-year-old conundrum. Autophagy 2011, 7, 673–682. [Google Scholar] [CrossRef]
- Li, W.W.; Li, J.; Bao, J.K. Microautophagy: Lesser-known self-eating. Cell Mol. Life Sci. 2012, 69, 1125–1136. [Google Scholar] [CrossRef]
- Massey, A.; Kiffin, R.; Cuervo, A.M. Pathophysiology of chaperone-mediated autophagy. Int. J. Biochem. Cell Biol. 2004, 36, 2420–2434. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, S.; Cuervo, A.M. The coming of age of chaperone-mediated autophagy. Nat. Rev. Mol. Cell Biol. 2018, 19, 365–381. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Baehrecke, E.H.; Ballabio, A.; Boya, P.; Bravo-San Pedro, J.M.; Cecconi, F.; Choi, A.M.; Chu, C.T.; Codogno, P.; Colombo, M.I.; et al. Molecular definitions of autophagy and related processes. EMBO J. 2017, 36, 1811–1836. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.; Kroemer, G. Biological Functions of Autophagy Genes: A Disease Perspective. Cell 2019, 176, 11–42. [Google Scholar] [CrossRef] [PubMed]
- Klionsky, D.J. Look people, “Atg” is an abbreviation for “autophagy-related.” That’s it. Autophagy 2012, 8, 1281–1282. [Google Scholar] [CrossRef]
- Li, Y.J.; Lei, Y.H.; Yao, N.; Wang, C.R.; Hu, N.; Ye, W.C.; Zhang, D.M.; Chen, Z.S. Autophagy and multidrug resistance in cancer. Chin. J. Cancer 2017, 36, 52. [Google Scholar] [CrossRef]
- He, C.; Bassik, M.C.; Moresi, V.; Sun, K.; Wei, Y.; Zou, Z.; An, Z.; Loh, J.; Fisher, J.; Sun, Q.; et al. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature 2012, 481, 511–515. [Google Scholar] [CrossRef]
- Sousa, C.M.; Biancur, D.E.; Wang, X.; Halbrook, C.J.; Sherman, M.H.; Zhang, L.; Kremer, D.; Hwang, R.F.; Witkiewicz, A.K.; Ying, H.; et al. Erratum: Pancreatic stellate cells support tumour metabolism through autophagic alanine secretion. Nature 2016, 540, 150. [Google Scholar] [CrossRef]
- Fernandez, A.F.; Sebti, S.; Wei, Y.; Zou, Z.; Shi, M.; McMillan, K.L.; He, C.; Ting, T.; Liu, Y.; Chiang, W.C.; et al. Author Correction: Disruption of the beclin 1-BCL2 autophagy regulatory complex promotes longevity in mice. Nature 2018, 561, E30. [Google Scholar] [CrossRef]
- Khaminets, A.; Heinrich, T.; Mari, M.; Grumati, P.; Huebner, A.K.; Akutsu, M.; Liebmann, L.; Stolz, A.; Nietzsche, S.; Koch, N.; et al. Regulation of endoplasmic reticulum turnover by selective autophagy. Nature 2015, 522, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Green, D.R.; Galluzzi, L.; Kroemer, G. Cell biology. Metabolic control of cell death. Science 2014, 345, 1250256. [Google Scholar] [CrossRef]
- Wei, Y.; Zou, Z.; Becker, N.; Anderson, M.; Sumpter, R.; Xiao, G.; Kinch, L.; Koduru, P.; Christudass, C.S.; Veltri, R.W.; et al. EGFR-mediated Beclin 1 phosphorylation in autophagy suppression, tumor progression, and tumor chemoresistance. Cell 2013, 154, 1269–1284. [Google Scholar] [CrossRef]
- Lőrincz, P.; Juhász, G. Autophagosome-lysosome fusion. J. Mol. Biol. 2020, 432, 2462–2482. [Google Scholar] [CrossRef]
- Schroder, B.A.; Wrocklage, C.; Hasilik, A.; Saftig, P. The proteome of lysosomes. Proteomics 2010, 10, 4053–4076. [Google Scholar] [CrossRef] [PubMed]
- Ishidoh, K.; Kominami, E. Processing and activation of lysosomal proteinases. Biol. Chem. 2002, 383, 1827–1831. [Google Scholar] [CrossRef]
- Suzuki, C.; Tanida, I.; Ohmuraya, M.; Oliva Trejo, J.A.; Kakuta, S.; Sunabori, T.; Uchiyama, Y. Lack of Cathepsin D in the Renal Proximal Tubular Cells Resulted in Increased Sensitivity against Renal Ischemia/Reperfusion Injury. Int. J. Mol. Sci. 2019, 20, 711. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.-W.; Chang, C.-L.; Lai, J.-S. Prevalence of and factors related to pneumoconiosis among foundry workers in central Taiwan. Occup. Health Ind. Med. 1999, 2, 79. [Google Scholar] [CrossRef]
- Chong, S.Y.; Lee, C.K.; Huang, C.; Ou, Y.H.; Charles, C.J.; Richards, A.M.; Neupane, Y.R.; Pavon, M.V.; Zharkova, O.; Pastorin, G.; et al. Extracellular Vesicles in Cardiovascular Diseases: Alternative Biomarker Sources, Therapeutic Agents, and Drug Delivery Carriers. Int. J. Mol. Sci. 2019, 20, 272. [Google Scholar] [CrossRef] [PubMed]
- Bei, Y.; Das, S.; Rodosthenous, R.S.; Holvoet, P.; Vanhaverbeke, M.; Monteiro, M.C.; Monteiro, V.V.S.; Radosinska, J.; Bartekova, M.; Jansen, F.; et al. Extracellular Vesicles in Cardiovascular Theranostics. Theranostics 2017, 7, 4168–4182. [Google Scholar] [CrossRef]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Thery, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.; Qiao, Y.; Concepcion, W.; Thakor, A.S. Stem cell-derived extracellular vesicles: Role in oncogenic processes, bioengineering potential, and technical challenges. Stem Cell Res. Ther. 2019, 10, 347. [Google Scholar] [CrossRef]
- Stahl, P.D.; Raposo, G. Extracellular Vesicles: Exosomes and Microvesicles, Integrators of Homeostasis. Physiology 2019, 34, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Vidal, M. Exosomes: Revisiting their role as “garbage bags”. Traffic 2019, 20, 815–828. [Google Scholar] [CrossRef]
- Trzepizur, W.; Martinez, M.C.; Priou, P.; Andriantsitohaina, R.; Gagnadoux, F. Microparticles and vascular dysfunction in obstructive sleep apnoea. Eur. Respir. J. 2014, 44, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Poon, I.K.; Lucas, C.D.; Rossi, A.G.; Ravichandran, K.S. Apoptotic cell clearance: Basic biology and therapeutic potential. Nat. Rev. Immunol. 2014, 14, 166–180. [Google Scholar] [CrossRef] [PubMed]
- Atkin-Smith, G.K.; Poon, I.K.H. Disassembly of the Dying: Mechanisms and Functions. Trends Cell Biol. 2017, 27, 151–162. [Google Scholar] [CrossRef]
- Meldolesi, J. Extracellular vesicles, news about their role in immune cells: Physiology, pathology and diseases. Clin. Exp. Immunol. 2019, 196, 318–327. [Google Scholar] [CrossRef]
- Slomka, A.; Urban, S.K.; Lukacs-Kornek, V.; Zekanowska, E.; Kornek, M. Large Extracellular Vesicles: Have We Found the Holy Grail of Inflammation? Front. Immunol. 2018, 9, 2723. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Tang, T.; Hou, Y.; Zeng, Q.; Wang, Y.; Fan, W.; Qu, S. Extracellular vesicles in atherosclerosis. Clin. Chim. Acta 2019, 495, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Liu, X.; Xu, G. Extracellular Vesicles as Messengers in Atherosclerosis. J. Cardiovasc. Transl. Res. 2020, 13, 121–130. [Google Scholar] [CrossRef]
- Szekeres-Bartho, J.; Sucurovic, S.; Mulac-Jericevic, B. The Role of Extracellular Vesicles and PIBF in Embryo-Maternal Immune-Interactions. Front. Immunol. 2018, 9, 2890. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Lam, E.W.; Sun, Y. Extracellular vesicles in the tumor microenvironment: Old stories, but new tales. Mol. Cancer 2019, 18, 59. [Google Scholar] [CrossRef]
- Bogatcheva, N.V.; Coleman, M.E. Conditioned Medium of Mesenchymal Stromal Cells: A New Class of Therapeutics. Biochemistry 2019, 84, 1375–1389. [Google Scholar] [CrossRef]
- Kichenbrand, C.; Velot, E.; Menu, P.; Moby, V. Dental Pulp Stem Cell-Derived Conditioned Medium: An Attractive Alternative for Regenerative Therapy. Tissue Eng. Part. B Rev. 2019, 25, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Fui, L.W.; Lok, M.P.W.; Govindasamy, V.; Yong, T.K.; Lek, T.K.; Das, A.K. Understanding the multifaceted mechanisms of diabetic wound healing and therapeutic application of stem cells conditioned medium in the healing process. J. Tissue Eng. Regen. Med. 2019, 13, 2218–2233. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; He, Y.; Feng, J.; Dong, Z.; Yao, Y.; Lu, F. Conditioned medium from 3D culture system of stromal vascular fraction cells accelerates wound healing in diabetic rats. Regen. Med. 2019, 14, 925–937. [Google Scholar] [CrossRef] [PubMed]
- Sato, C.; Yamamoto, Y.; Funayama, E.; Furukawa, H.; Oyama, A.; Murao, N.; Hosono, H.; Kawakubo, K.; Sakamoto, N.; Ohnishi, S. Conditioned Medium Obtained from Amnion-Derived Mesenchymal Stem Cell Culture Prevents Activation of Keloid Fibroblasts. Plast. Reconstr. Surg. 2018, 141, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, F.; Borsari, V.; Sartori, M.; Orciani, M.; Mattioli-Belmonte, M.; Fini, M. The use of cell conditioned medium for musculoskeletal tissue regeneration. J. Cell Physiol. 2018, 233, 4423–4442. [Google Scholar] [CrossRef]
- Fukuoka, H.; Narita, K.; Suga, H. Hair Regeneration Therapy: Application of Adipose-Derived Stem Cells. Curr. Stem Cell Res. Ther. 2017, 12, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Mathew, B.; Ravindran, S.; Liu, X.; Torres, L.; Chennakesavalu, M.; Huang, C.C.; Feng, L.; Zelka, R.; Lopez, J.; Sharma, M.; et al. Mesenchymal stem cell-derived extracellular vesicles and retinal ischemia-reperfusion. Biomaterials 2019, 197, 146–160. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Zhang, Q.; Zheng, Z.; Wang, J. Retinal ganglion cell-conditioned medium and surrounding pressure alters gene expression and differentiation of rat retinal progenitor cells. Mol. Med. Rep. 2018, 17, 7177–7183. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Nishida, H.; Yoshizaki, K.; Akiyoshi, H.; Hatoya, S.; Sugiura, K.; Inaba, T. Canine mesenchymal stromal cell-conditioned medium promotes survival and neurite outgrowth of neural stem cells. J. Vet. Med. Sci. 2020, 82, 668–672. [Google Scholar] [CrossRef] [PubMed]
- Jahanbazi Jahan-Abad, A.; Karima, S.; Sahab Negah, S.; Noorbakhsh, F.; Borhani-Haghighi, M.; Gorji, A. Therapeutic potential of conditioned medium derived from oligodendrocytes cultured in a self-assembling peptide nanoscaffold in experimental autoimmune encephalomyelitis. Brain Res. 2019, 1711, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Vikartovska, Z.; Kuricova, M.; Farbakova, J.; Liptak, T.; Mudronova, D.; Humenik, F.; Madari, A.; Maloveska, M.; Sykova, E.; Cizkova, D. Stem Cell Conditioned Medium Treatment for Canine Spinal Cord Injury: Pilot Feasibility Study. Int. J. Mol. Sci. 2020, 21, 5129. [Google Scholar] [CrossRef]
- Liu, A.; Zhang, X.; He, H.; Zhou, L.; Naito, Y.; Sugita, S.; Lee, J.W. Therapeutic potential of mesenchymal stem/stromal cell-derived secretome and vesicles for lung injury and disease. Expert Opin. Biol. Ther. 2020, 20, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Purushothaman, A. Exosomes from Cell Culture-Conditioned Medium: Isolation by Ultracentrifugation and Characterization. Methods Mol. Biol. 2019, 1952, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yucel, T.; Lu, Q.; Hu, X.; Kaplan, D.L. Silk nanospheres and microspheres from silk/pva blend films for drug delivery. Biomaterials 2010, 31, 1025–1035. [Google Scholar] [CrossRef]
- Dagsson-Waldhauserova, P.; Renard, J.B.; Olafsson, H.; Vignelles, D.; Berthet, G.; Verdier, N.; Duverger, V. Vertical distribution of aerosols in dust storms during the Arctic winter. Sci. Rep. 2019, 9, 16122. [Google Scholar] [CrossRef] [PubMed]
- Westerdahl, D.; Fruin, S.; Sax, T.; Fine, P.M.; Sioutas, C. Mobile platform measurements of ultrafine particles and associated pollutant concentrations on freeways and residential streets in Los Angeles. Atmos. Environ. 2005, 39, 3597–3610. [Google Scholar] [CrossRef]
- Sioutas, C.; Delfino, R.J.; Singh, M. Exposure assessment for atmospheric ultrafine particles (UFPs) and implications in epidemiologic research. Environ. Health Perspect. 2005, 113, 947–955. [Google Scholar] [CrossRef]
- Thompson, R.C.; Swan, S.H.; Moore, C.J.; vom Saal, F.S. Our plastic age. Philos. Trans. R Soc. Lond. B Biol. Sci. 2009, 364, 1973–1976. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.K.; Pal, S.; Ray, S. Study of microbes having potentiality for biodegradation of plastics. Environ. Sci. Pollut. Res. Int. 2013, 20, 4339–4355. [Google Scholar] [CrossRef]
- Song, Y.K.; Hong, S.H.; Jang, M.; Han, G.M.; Jung, S.W.; Shim, W.J. Corrections to “Combined Effects of UV Exposure Duration and Mechanical Abrasion on Microplastic Fragmentation by Polymer Type”. Environ. Sci. Technol. 2018, 52, 3831–3832. [Google Scholar] [CrossRef]
- Carney Almroth, B.M.; Astrom, L.; Roslund, S.; Petersson, H.; Johansson, M.; Persson, N.K. Quantifying shedding of synthetic fibers from textiles; a source of microplastics released into the environment. Environ. Sci. Pollut. Res. Int. 2018, 25, 1191–1199. [Google Scholar] [CrossRef]
- Sait, S.T.L.; Sorensen, L.; Kubowicz, S.; Vike-Jonas, K.; Gonzalez, S.V.; Asimakopoulos, A.G.; Booth, A.M. Microplastic fibres from synthetic textiles: Environmental degradation and additive chemical content. Environ. Pollut. 2021, 268, 115745. [Google Scholar] [CrossRef]
- Suaria, G.; Achtypi, A.; Perold, V.; Lee, J.R.; Pierucci, A.; Bornman, T.G.; Aliani, S.; Ryan, P.G. Microfibers in oceanic surface waters: A global characterization. Sci. Adv. 2020, 6, eaay8493. [Google Scholar] [CrossRef] [PubMed]
- Aldalbahi, A.; El-Naggar, M.E.; El-Newehy, M.H.; Rahaman, M.; Hatshan, M.R.; Khattab, T.A. Effects of Technical Textiles and Synthetic Nanofibers on Environmental Pollution. Polymers 2021, 13, 155. [Google Scholar] [CrossRef]
- Gago, J.; Carretero, O.; Filgueiras, A.V.; Vinas, L. Synthetic microfibers in the marine environment: A review on their occurrence in seawater and sediments. Mar. Pollut. Bull. 2018, 127, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Browne, M.A.; Crump, P.; Niven, S.J.; Teuten, E.; Tonkin, A.; Galloway, T.; Thompson, R. Accumulation of microplastic on shorelines woldwide: Sources and sinks. Environ. Sci. Technol. 2011, 45, 9175–9179. [Google Scholar] [CrossRef] [PubMed]
- Kole, P.J.; Lohr, A.J.; Van Belleghem, F.; Ragas, A.M.J. Wear and Tear of Tyres: A Stealthy Source of Microplastics in the Environment. Int. J. Environ. Res. Public Health 2017, 14, 1265. [Google Scholar] [CrossRef] [PubMed]
- Leads, R.R.; Weinstein, J.E. Occurrence of tire wear particles and other microplastics within the tributaries of the Charleston Harbor Estuary, South Carolina, USA. Mar. Pollut. Bull. 2019, 145, 569–582. [Google Scholar] [CrossRef]
- Zhang, Z.; Zulpiya, M.; Chen, Y. Current research and perspective of microplastics (MPs) in soils (dusts), rivers (lakes), and marine environments in China. Ecotoxicol. Environ. Saf. 2020, 202, 110976. [Google Scholar] [CrossRef] [PubMed]
- Unice, K.M.; Kreider, M.L.; Panko, J.M. Comparison of tire and road wear particle concentrations in sediment for watersheds in France, Japan, and the United States by quantitative pyrolysis GC/MS analysis. Environ. Sci. Technol. 2013, 47, 8138–8147. [Google Scholar] [CrossRef]
- Baensch-Baltruschat, B.; Kocher, B.; Stock, F.; Reifferscheid, G. Tyre and road wear particles (TRWP)-A review of generation, properties, emissions, human health risk, ecotoxicity, and fate in the environment. Sci. Total Environ. 2020, 733, 137823. [Google Scholar] [CrossRef]
- Klockner, P.; Seiwert, B.; Eisentraut, P.; Braun, U.; Reemtsma, T.; Wagner, S. Characterization of tire and road wear particles from road runoff indicates highly dynamic particle properties. Water Res. 2020, 185, 116262. [Google Scholar] [CrossRef]
- Adamiec, E.; Jarosz-Krzeminska, E.; Wieszala, R. Heavy metals from non-exhaust vehicle emissions in urban and motorway road dusts. Environ. Monit. Assess. 2016, 188, 369. [Google Scholar] [CrossRef]
- Ning, Z.; Cheung, C.S.; Fu, J.; Liu, M.; Schnell, M. Experimental study of environmental tobacco smoke particles under actual indoor environment. Sci. Total Environ. 2006, 367, 822–830. [Google Scholar] [CrossRef] [PubMed]
- Alven, S.; Nqoro, X.; Aderibigbe, B.A. Polymer-Based Materials Loaded with Curcumin for Wound Healing Applications. Polymers 2020, 12, 2286. [Google Scholar] [CrossRef]
- Zarska, M.; Sramek, M.; Novotny, F.; Havel, F.; Babelova, A.; Mrazkova, B.; Benada, O.; Reinis, M.; Stepanek, I.; Musilek, K.; et al. Biological safety and tissue distribution of (16-mercaptohexadecyl)trimethylammonium bromide-modified cationic gold nanorods. Biomaterials 2018, 154, 275–290. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; He, N.; Zhao, Y.; Liu, T.; Deng, Y. Autophagy Modulated by Inorganic Nanomaterials. Theranostics 2020, 10, 3206–3222. [Google Scholar] [CrossRef] [PubMed]
- Boyles, M.S.; Ranninger, C.; Reischl, R.; Rurik, M.; Tessadri, R.; Kohlbacher, O.; Duschl, A.; Huber, C.G. Copper oxide nanoparticle toxicity profiling using untargeted metabolomics. Part. Fibre Toxicol. 2016, 13, 49. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.H.; Field, J.A.; Sierra-Alvarez, R. Microbial toxicity of gallium- and indium-based oxide and arsenide nanoparticles. J. Environ. Sci. Health A Tox Hazard. Subst. Environ. Eng. 2020, 55, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Neu-Baker, N.M.; Brenner, S.A. SEM analysis of particle size during conventional treatment of CMP process wastewater. Sci. Total Environ. 2015, 508, 1–6. [Google Scholar] [CrossRef]
- Nanotechnology versus coronavirus. Nat. Nanotechnol. 2020, 15, 617. [CrossRef] [PubMed]
- Patil, M.P.; Kim, G.D. Marine microorganisms for synthesis of metallic nanoparticles and their biomedical applications. Colloids Surf. B Biointerfaces 2018, 172, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Seib, F.P. Silk nanoparticles-an emerging anticancer nanomedicine. AIMS Bioeng. 2017, 42, 239–258. [Google Scholar] [CrossRef]
- Alebouyeh, S.; Assmar, M.; Mirpour, M. Effect of Chitosan Nanoparticle from Penaeus semisulcatus Shrimp on Salmonella typhi and Listeria monocytogenes. Iran. J. Public Health 2020, 49, 369. [Google Scholar] [CrossRef] [PubMed]
- Redondo-Hasselerharm, P.E.; de Ruijter, V.N.; Mintenig, S.M.; Verschoor, A.; Koelmans, A.A. Ingestion and Chronic Effects of Car Tire Tread Particles on Freshwater Benthic Macroinvertebrates. Environ. Sci. Technol. 2018, 52, 13986–13994. [Google Scholar] [CrossRef]
- Ekvall, M.T.; Lundqvist, M.; Kelpsiene, E.; Šileikis, E.; Gunnarsson, S.B.; Cedervall, T. Nanoplastics formed during the mechanical breakdown of daily-use polystyrene products. Nanoscale Adv. 2019, 1, 1055–1061. [Google Scholar] [CrossRef]
- Li, D.; Shi, Y.; Yang, L.; Xiao, L.; Kehoe, D.K.; Gun’ko, Y.K.; Boland, J.J.; Wang, J.J. Microplastic release from the degradation of polypropylene feeding bottles during infant formula preparation. Nat. Food 2020, 1, 746–754. [Google Scholar] [CrossRef]
- Yang, Y.F.; Chen, C.Y.; Lu, T.H.; Liao, C.M. Toxicity-based toxicokinetic/toxicodynamic assessment for bioaccumulation of polystyrene microplastics in mice. J. Hazard. Mater. 2019, 366, 703–713. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhang, Y.; Lemos, B.; Ren, H. Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure. Sci. Rep. 2017, 7, 46687. [Google Scholar] [CrossRef] [PubMed]
- Herzke, D.; Anker-Nilssen, T.; Nost, T.H.; Gotsch, A.; Christensen-Dalsgaard, S.; Langset, M.; Fangel, K.; Koelmans, A.A. Negligible Impact of Ingested Microplastics on Tissue Concentrations of Persistent Organic Pollutants in Northern Fulmars off Coastal Norway. Environ. Sci. Technol. 2016, 50, 1924–1933. [Google Scholar] [CrossRef]
- Abbasi, S.; Soltani, N.; Keshavarzi, B.; Moore, F.; Turner, A.; Hassanaghaei, M. Microplastics in different tissues of fish and prawn from the Musa Estuary, Persian Gulf. Chemosphere 2018, 205, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Van Cauwenberghe, L.; Janssen, C.R. Microplastics in bivalves cultured for human consumption. Environ. Pollut. 2014, 193, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Green, C.; Reynolds, A.; Shi, H.; Rotchell, J.M. Microplastics in mussels sampled from coastal waters and supermarkets in the United Kingdom. Environ. Pollut. 2018, 241, 35–44. [Google Scholar] [CrossRef]
- Kutralam-Muniasamy, G.; Perez-Guevara, F.; Elizalde-Martinez, I.; Shruti, V.C. Branded milks-Are they immune from microplastics contamination? Sci. Total Environ. 2020, 714, 136823. [Google Scholar] [CrossRef]
- Liebezeit, G.; Liebezeit, E. Synthetic particles as contaminants in German beers. Food Addit. Contam. Part. A Chem. Anal. Control. Expo. Risk Assess. 2014, 31, 1574–1578. [Google Scholar] [CrossRef]
- Kosuth, M.; Mason, S.A.; Wattenberg, E.V. Anthropogenic contamination of tap water, beer, and sea salt. PLoS ONE 2018, 13, e0194970. [Google Scholar] [CrossRef]
- Iniguez, M.E.; Conesa, J.A.; Fullana, A. Microplastics in Spanish Table Salt. Sci. Rep. 2017, 7, 8620. [Google Scholar] [CrossRef] [PubMed]
- Liebezeit, G.; Liebezeit, E. Non-pollen particulates in honey and sugar. Food Addit. Contam. Part. A Chem. Anal. Control. Expo. Risk Assess. 2013, 30, 2136–2140. [Google Scholar] [CrossRef]
- Hernandez, L.M.; Xu, E.G.; Larsson, H.C.E.; Tahara, R.; Maisuria, V.B.; Tufenkji, N. Plastic Teabags Release Billions of Microparticles and Nanoparticles into Tea. Environ. Sci. Technol. 2019, 53, 12300–12310. [Google Scholar] [CrossRef]
- Pivokonsky, M.; Cermakova, L.; Novotna, K.; Peer, P.; Cajthaml, T.; Janda, V. Occurrence of microplastics in raw and treated drinking water. Sci. Total Environ. 2018, 643, 1644–1651. [Google Scholar] [CrossRef] [PubMed]
- Schymanski, D.; Goldbeck, C.; Humpf, H.U.; Furst, P. Analysis of microplastics in water by micro-Raman spectroscopy: Release of plastic particles from different packaging into mineral water. Water Res. 2018, 129, 154–162. [Google Scholar] [CrossRef]
- Welle, F.; Franz, R. Microplastic in bottled natural mineral water-literature review and considerations on exposure and risk assessment. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2018, 35, 2482–2492. [Google Scholar] [CrossRef]
- Li, Y.; Ju, D. The Role of Autophagy in Nanoparticles-Induced Toxicity and Its Related Cellular and Molecular Mechanisms. Adv. Exp. Med. Biol. 2018, 1048, 71–84. [Google Scholar] [CrossRef]
- You, D.J.; Bonner, J.C. Susceptibility Factors in Chronic Lung Inflammatory Responses to Engineered Nanomaterials. Int. J. Mol. Sci. 2020, 21, 7310. [Google Scholar] [CrossRef]
- Alsaleh, N.B.; Brown, J.M. Engineered Nanomaterials and Type I Allergic Hypersensitivity Reactions. Front. Immunol. 2020, 11, 222. [Google Scholar] [CrossRef]
- Alsaleh, N.B.; Brown, J.M. Immune responses to engineered nanomaterials: Current understanding and challenges. Curr. Opin. Toxicol. 2018, 10, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Kim, H.; Kim, Y.; Yi, J.; Choi, K.; Park, K. Inflammatory responses may be induced by a single intratracheal instillation of iron nanoparticles in mice. Toxicology 2010, 275, 65–71. [Google Scholar] [CrossRef]
- Park, E.J.; Oh, S.Y.; Lee, S.J.; Lee, K.; Kim, Y.; Lee, B.S.; Kim, J.S. Chronic pulmonary accumulation of iron oxide nanoparticles induced Th1-type immune response stimulating the function of antigen-presenting cells. Environ. Res. 2015, 143, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Mohammad, A.; Patil, G.; Naqvi, S.A.; Chauhan, L.K.; Ahmad, I. Induction of ROS, mitochondrial damage and autophagy in lung epithelial cancer cells by iron oxide nanoparticles. Biomaterials 2012, 33, 1477–1488. [Google Scholar] [CrossRef] [PubMed]
- Petters, C.; Irrsack, E.; Koch, M.; Dringen, R. Uptake and metabolism of iron oxide nanoparticles in brain cells. Neurochem. Res. 2014, 39, 1648–1660. [Google Scholar] [CrossRef] [PubMed]
- Kornberg, T.G.; Stueckle, T.A.; Antonini, J.A.; Rojanasakul, Y.; Castranova, V.; Yang, Y.; Wang, L. Potential Toxicity and Underlying Mechanisms Associated with Pulmonary Exposure to Iron Oxide Nanoparticles: Conflicting Literature and Unclear Risk. Nanomaterials 2017, 7, 307. [Google Scholar] [CrossRef]
- Malhotra, N.; Lee, J.S.; Liman, R.A.D.; Ruallo, J.M.S.; Villaflores, O.B.; Ger, T.R.; Hsiao, C.D. Potential Toxicity of Iron Oxide Magnetic Nanoparticles: A Review. Molecules 2020, 25, 3159. [Google Scholar] [CrossRef]
- Afeseh Ngwa, H.; Kanthasamy, A.; Gu, Y.; Fang, N.; Anantharam, V.; Kanthasamy, A.G. Manganese nanoparticle activates mitochondrial dependent apoptotic signaling and autophagy in dopaminergic neuronal cells. Toxicol. Appl. Pharmacol. 2011, 256, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Nikazar, S.; Sivasankarapillai, V.S.; Rahdar, A.; Gasmi, S.; Anumol, P.S.; Shanavas, M.S. Revisiting the cytotoxicity of quantum dots: An in-depth overview. Biophys. Rev. 2020, 12, 703–718. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, M. Dysfunction of various organelles provokes multiple cell death after quantum dot exposure. Int. J. Nanomed. 2018, 13, 2729–2742. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, N.; Su, Y.; He, Y.; Yin, M.; Wei, M.; Wang, L.; Huang, W.; Fan, C.; Huang, Q. Autophagy-sensitized cytotoxicity of quantum dots in PC12 cells. Adv. Healthc Mater 2014, 3, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Stern, S.T.; Zolnik, B.S.; McLeland, C.B.; Clogston, J.; Zheng, J.; McNeil, S.E. Induction of autophagy in porcine kidney cells by quantum dots: A common cellular response to nanomaterials? Toxicol. Sci. 2008, 106, 140–152. [Google Scholar] [CrossRef]
- Ji, X.; Xu, B.; Yao, M.; Mao, Z.; Zhang, Y.; Xu, G.; Tang, Q.; Wang, X.; Xia, Y. Graphene oxide quantum dots disrupt autophagic flux by inhibiting lysosome activity in GC-2 and TM4 cell lines. Toxicology 2016, 374, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Feng, X.; He, L.; Zhang, Y.; Shao, L. The interrupted effect of autophagic flux and lysosomal function induced by graphene oxide in p62-dependent apoptosis of F98 cells. J. Nanobiotechnol. 2020, 18, 52. [Google Scholar] [CrossRef] [PubMed]
- Ihrie, M.D.; Bonner, J.C. The Toxicology of Engineered Nanomaterials in Asthma. Curr. Environ. Health Rep. 2018, 5, 100–109. [Google Scholar] [CrossRef]
- Roach, K.A.; Stefaniak, A.B.; Roberts, J.R. Metal nanomaterials: Immune effects and implications of physicochemical properties on sensitization, elicitation, and exacerbation of allergic disease. J. Immunotoxicol. 2019, 16, 87–124. [Google Scholar] [CrossRef] [PubMed]
- Dornhof, R.; Maschowski, C.; Osipova, A.; Giere, R.; Seidl, M.; Merfort, I.; Humar, M. Stress fibers, autophagy and necrosis by persistent exposure to PM2.5 from biomass combustion. PLoS ONE 2017, 12, e0180291. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, Q.; Wen, D.; Rule, A.; Koehler, K.; Li, M.; Gao, L. PM 2.5 Exposure Induced Autophagy Activation via the ROS/AMPK/mTOR/ULK1 Signaling Axis in Macrophages. J. Allergy Clin. Immunol. 2019, 143, AB230. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.; Li, J.; Han, L.; He, Q.; Wang, R.; Wang, X.; Liu, K. Developmental toxicity induced by PM2.5 through endoplasmic reticulum stress and autophagy pathway in zebrafish embryos. Chemosphere 2018, 197, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.M.; Wang, Q.; Xing, W.W.; Long, M.H.; Fu, W.L.; Xia, W.R.; Jin, C.; Guo, N.; Xu, D.Q.; Xu, D.G. PM2.5 induces autophagy-mediated cell death via NOS2 signaling in human bronchial epithelium cells. Int. J. Biol. Sci. 2018, 14, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Yuan, J.; Wang, Q.; Lei, T.; Shen, X.; Cui, B.; Zhang, F.; Ding, W.; Lu, Z. Metformin protects against PM2.5-induced lung injury and cardiac dysfunction independent of AMP-activated protein kinase alpha2. Redox Biol. 2020, 28, 101345. [Google Scholar] [CrossRef]
- Calderon-Garciduenas, L.; Leray, E.; Heydarpour, P.; Torres-Jardon, R.; Reis, J. Air pollution, a rising environmental risk factor for cognition, neuroinflammation and neurodegeneration: The clinical impact on children and beyond. Rev. Neurol. 2016, 172, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Zheng, Z.; Kim, H.; Yang, Z.; Zhang, G.; Shi, X.; Sun, F.; Peng, C.; Ding, Y.; Wang, A.; et al. Inhalation Exposure to PM2.5 Counteracts Hepatic Steatosis in Mice Fed High-fat Diet by Stimulating Hepatic Autophagy. Sci. Rep. 2017, 7, 16286. [Google Scholar] [CrossRef]
- Su, R.; Jin, X.; Lyu, L.; Tian, J.; Amin, S.; Li, Z. The potential immunotoxicity of fine particulate matter based on SD rat spleen. Environ. Sci. Pollut. Res. Int. 2019, 26, 23958–23966. [Google Scholar] [CrossRef]
- Huang, X.; Zhou, Z.; Liu, X.; Li, J.; Zhang, L. PM 2.5 exposure induced renal injury via the activation of the autophagic pathway in the rat and HK-2 cell. Environ. Sci. Eur. 2020, 32, 1–13. [Google Scholar] [CrossRef]
- Tseng, C.Y.; Wang, J.S.; Chao, M.W. Causation by Diesel Exhaust Particles of Endothelial Dysfunctions in Cytotoxicity, Pro-inflammation, Permeability, and Apoptosis Induced by ROS Generation. Cardiovasc. Toxicol. 2017, 17, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Lawal, A.O. Diesel Exhaust Particles and the Induction of Macrophage Activation and Dysfunction. Inflammation 2018, 41, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Molfino, A.; Amabile, M.I.; Muscaritoli, M.; Germano, A.; Alfano, R.; Ramaccini, C.; Spagnoli, A.; Cavaliere, L.; Marseglia, G.; Nardone, A.; et al. Association between Metabolic and Hormonal Derangements and Professional Exposure to Urban Pollution in a High Intensity Traffic Area. Front. Endocrinol. 2020, 11, 509. [Google Scholar] [CrossRef]
- Hsu, Y.H.; Chuang, H.C.; Lee, Y.H.; Lin, Y.F.; Chen, Y.J.; Hsiao, T.C.; Wu, M.Y.; Chiu, H.W. Traffic-related particulate matter exposure induces nephrotoxicity in vitro and in vivo. Free Radic. Biol. Med. 2019, 135, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Zhou, H.; Gong, X.; Gao, J. Silica sub-microspheres induce autophagy in an endocytosis dependent manner. RSC Adv. 2017, 7, 12496–12502. [Google Scholar] [CrossRef]
- Popp, L.; Segatori, L. Zinc Oxide Particles Induce Activation of the Lysosome–Autophagy System. ACS Omega 2019, 4, 573–581. [Google Scholar] [CrossRef]
- Bai, D.P.; Zhang, X.F.; Zhang, G.L.; Huang, Y.F.; Gurunathan, S. Zinc oxide nanoparticles induce apoptosis and autophagy in human ovarian cancer cells. Int. J. Nanomed. 2017, 12, 6521–6535. [Google Scholar] [CrossRef] [PubMed]
- Gautam, A.; Rakshit, M.; Nguyen, K.T.; Kathawala, M.H.; Nguyen, L.T.H.; Tay, C.Y.; Wong, E.; Ng, K.W. Understanding the implications of engineered nanoparticle induced autophagy in human epidermal keratinocytes in vitro. NanoImpact 2019, 15, 100177. [Google Scholar] [CrossRef]
- Johnson, B.M.; Fraietta, J.A.; Gracias, D.T.; Hope, J.L.; Stairiker, C.J.; Patel, P.R.; Mueller, Y.M.; McHugh, M.D.; Jablonowski, L.J.; Wheatley, M.A.; et al. Acute exposure to ZnO nanoparticles induces autophagic immune cell death. Nanotoxicology 2015, 9, 737–748. [Google Scholar] [CrossRef]
- Liu, Z.; Lv, X.; Xu, L.; Liu, X.; Zhu, X.; Song, E.; Song, Y. Zinc oxide nanoparticles effectively regulate autophagic cell death by activating autophagosome formation and interfering with their maturation. Part. Fibre Toxicol. 2020, 17, 46. [Google Scholar] [CrossRef] [PubMed]
- Song, W.J.; Jeong, M.S.; Choi, D.M.; Kim, K.N.; Wie, M.B. Zinc Oxide Nanoparticles Induce Autophagy and Apoptosis via Oxidative Injury and Pro-Inflammatory Cytokines in Primary Astrocyte Cultures. Nanomaterials 2019, 9, 1043. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Kim, J.H.; Lee, J.C.; Won, M.H.; Yang, S.R.; Kim, H.C.; Wie, M.B. Zinc Oxide Nanoparticles Exhibit Both Cyclooxygenase- and Lipoxygenase-Mediated Apoptosis in Human Bone Marrow-Derived Mesenchymal Stem Cells. Toxicol. Res. 2019, 35, 83–91. [Google Scholar] [CrossRef]
- Lozano, O.; Silva-Platas, C.; Chapoy-Villanueva, H.; Perez, B.E.; Lees, J.G.; Ramachandra, C.J.A.; Contreras-Torres, F.F.; Lazaro-Alfaro, A.; Luna-Figueroa, E.; Bernal-Ramirez, J.; et al. Amorphous SiO2 nanoparticles promote cardiac dysfunction via the opening of the mitochondrial permeability transition pore in rat heart and human cardiomyocytes. Part. Fibre Toxicol. 2020, 17, 15. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Beltran, C.E.; Bernal-Ramirez, J.; Lozano, O.; Oropeza-Almazan, Y.; Castillo, E.C.; Garza, J.R.; Garcia, N.; Vela, J.; Garcia-Garcia, A.; Ortega, E.; et al. Silica nanoparticles induce cardiotoxicity interfering with energetic status and Ca(2+) handling in adult rat cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H645–H661. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Q.; Xue, S.M.; Zhang, P.; Xu, L.N.; Wang, D.P.; Li, G.; Cao, J.M. Silica Nanoparticles Disturb Ion Channels and Transmembrane Potentials of Cardiomyocytes and Induce Lethal Arrhythmias in Mice. Int. J. Nanomed. 2020, 15, 7397–7413. [Google Scholar] [CrossRef]
- Lee, K.; Lee, J.; Kwak, M.; Cho, Y.L.; Hwang, B.; Cho, M.J.; Lee, N.G.; Park, J.; Lee, S.H.; Park, J.G.; et al. Two distinct cellular pathways leading to endothelial cell cytotoxicity by silica nanoparticle size. J. Nanobiotechnol. 2019, 17, 24. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.P.; Wang, Z.J.; Zhao, R.; Lin, C.X.; Sun, Q.Y.; Yan, C.P.; Zhou, X.; Cao, J.M. Silica nanomaterials induce organ injuries by Ca(2+)-ROS-initiated disruption of the endothelial barrier and triggering intravascular coagulation. Part. Fibre Toxicol. 2020, 17, 12. [Google Scholar] [CrossRef]
- Yu, Y.; Duan, J.; Yu, Y.; Li, Y.; Liu, X.; Zhou, X.; Ho, K.-f.; Tian, L.; Sun, Z. Silica nanoparticles induce autophagy and autophagic cell death in HepG2 cells triggered by reactive oxygen species. J. Hazard. Mater. 2014, 270, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.I.; Su, C.C.; Fang, K.M.; Wu, C.C.; Wu, C.T.; Chen, Y.W. Ultrafine silicon dioxide nanoparticles cause lung epithelial cells apoptosis via oxidative stress-activated PI3K/Akt-mediated mitochondria- and endoplasmic reticulum stress-dependent signaling pathways. Sci. Rep. 2020, 10, 9928. [Google Scholar] [CrossRef]
- Wang, M.; Li, J.; Dong, S.; Cai, X.; Simaiti, A.; Yang, X.; Zhu, X.; Luo, J.; Jiang, L.H.; Du, B.; et al. Silica nanoparticles induce lung inflammation in mice via ROS/PARP/TRPM2 signaling-mediated lysosome impairment and autophagy dysfunction. Part. Fibre Toxicol. 2020, 17, 23. [Google Scholar] [CrossRef]
- Wu, Y.; Jin, Y.; Sun, T.; Zhu, P.; Li, J.; Zhang, Q.; Wang, X.; Jiang, J.; Chen, G.; Zhao, X. p62/SQSTM1 accumulation due to degradation inhibition and transcriptional activation plays a critical role in silica nanoparticle-induced airway inflammation via NF-kappaB activation. J. Nanobiotechnol. 2020, 18, 77. [Google Scholar] [CrossRef]
- Guo, C.; Yang, M.; Jing, L.; Wang, J.; Yu, Y.; Li, Y.; Duan, J.; Zhou, X.; Li, Y.; Sun, Z. Amorphous silica nanoparticles trigger vascular endothelial cell injury through apoptosis and autophagy via reactive oxygen species-mediated MAPK/Bcl-2 and PI3K/Akt/mTOR signaling. Int. J. Nanomed. 2016, 11, 5257–5276. [Google Scholar] [CrossRef] [PubMed]
- Flores-Lopez, L.Z.; Espinoza-Gomez, H.; Somanathan, R. Silver nanoparticles: Electron transfer, reactive oxygen species, oxidative stress, beneficial and toxicological effects. Mini review. J. Appl. Toxicol. 2019, 39, 16–26. [Google Scholar] [CrossRef]
- Li, Y.; Qin, T.; Ingle, T.; Yan, J.; He, W.; Yin, J.J.; Chen, T. Differential genotoxicity mechanisms of silver nanoparticles and silver ions. Arch. Toxicol. 2017, 91, 509–519. [Google Scholar] [CrossRef]
- Mishra, A.R.; Zheng, J.; Tang, X.; Goering, P.L. Silver Nanoparticle-Induced Autophagic-Lysosomal Disruption and NLRP3-Inflammasome Activation in HepG2 Cells Is Size-Dependent. Toxicol. Sci. 2016, 150, 473–487. [Google Scholar] [CrossRef]
- Miyayama, T.; Fujiki, K.; Matsuoka, M. Silver nanoparticles induce lysosomal-autophagic defects and decreased expression of transcription factor EB in A549 human lung adenocarcinoma cells. Toxicol. In Vitro 2018, 46, 148–154. [Google Scholar] [CrossRef]
- Skalska, J.; Dabrowska-Bouta, B.; Frontczak-Baniewicz, M.; Sulkowski, G.; Struzynska, L. A Low Dose of Nanoparticulate Silver Induces Mitochondrial Dysfunction and Autophagy in Adult Rat Brain. Neurotox Res. 2020, 38, 650–664. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-H.; Fang, C.-Y.; Chiu, H.-W.; Cheng, F.-Y.; Tsai, J.-C.; Chen, C.-W.; Wang, Y.-J. Endoplasmic reticulum stress-triggered autophagy and lysosomal dysfunction contribute to the cytotoxicity of amine-modified silver nanoparticles in NIH 3T3 cells. J. Biomed. Nanotechnol. 2017, 13, 778–794. [Google Scholar] [CrossRef]
- Demoy, M.; Andreux, J.-P.; Weingarten, C.; Gouritin, B.; Guilloux, V.; Couvreur, P. Spleen capture of nanoparticles: Influence of animal species and surface characteristics. Pharm. Res. 1999, 16, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Cataldi, M.; Vigliotti, C.; Mosca, T.; Cammarota, M.; Capone, D. Emerging Role of the Spleen in the Pharmacokinetics of Monoclonal Antibodies, Nanoparticles and Exosomes. Int. J. Mol. Sci. 2017, 18, 1249. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yu, H.; Zhang, X.; Wang, Y.; Song, Z.; Zhao, J.; Shi, H.; Li, R.; Wang, Y.; Zhang, L.W. The protective role of autophagy in nephrotoxicity induced by bismuth nanoparticles through AMPK/mTOR pathway. Nanotoxicology 2018, 12, 586–601. [Google Scholar] [CrossRef]
- Alamer, A.; Ali, D.; Alarifi, S.; Alkahtane, A.; Al-Zharani, M.; Abdel-Daim, M.M.; Albasher, G.; Almeer, R.; Al-Sultan, N.K.; Almalik, A.; et al. Bismuth oxide nanoparticles induce oxidative stress and apoptosis in human breast cancer cells. Environ. Sci. Pollut. Res. Int. 2021, 28, 7379–7389. [Google Scholar] [CrossRef]
- Hao, B.M.; Liu, Y.N.; Zhang, C.Y.; Li, G.Q.; Wang, W.N.; Xu, W.D.; Zha, Z.B.; Wang, F.; Li, C.; Miao, Z.H.; et al. Autophagic blockage by bismuth sulfide nanoparticles inhibits migration and invasion of HepG2 cells. Nanotechnology 2020, 31, 465102. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhuang, J.; Zhang, X.; Yue, C.; Zhu, N.; Yang, L.; Wang, Y.; Chen, T.; Wang, Y.; Zhang, L.W. Autophagy associated cytotoxicity and cellular uptake mechanisms of bismuth nanoparticles in human kidney cells. Toxicol. Lett. 2017, 275, 39–48. [Google Scholar] [CrossRef]
- Hong, F.; Zhou, Y.; Ye, L.; Ze, Y.; Ji, J.; Zhuang, J.; Wang, L. Wnt Pathway-Mediated Nano TiO2-Induced Toxic Effects on Rat Primary Cultured Sertoli Cells. J. Biomed. Nanotechnol. 2018, 14, 2124–2134. [Google Scholar] [CrossRef]
- Hong, F.; Ji, J.; Ze, X.; Zhou, Y.; Ze, Y. Liver Inflammation and Fibrosis Induced by Long-Term Exposure to Nano Titanium Dioxide (TiO2) Nanoparticles in Mice and Its Molecular Mechanism. J. Biomed. Nanotechnol. 2020, 16, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Ji, J.; Ji, L.; Wang, L.; Hong, F. Respiratory exposure to nano-TiO2 induces pulmonary toxicity in mice involving reactive free radical-activated TGF-beta/Smad/p38MAPK/Wnt pathways. J. Biomed. Mater. Res. A 2019, 107, 2567–2575. [Google Scholar] [CrossRef] [PubMed]
- Hong, F.; Wu, N.; Ge, Y.; Zhou, Y.; Shen, T.; Qiang, Q.; Zhang, Q.; Chen, M.; Wang, Y.; Wang, L.; et al. Nanosized titanium dioxide resulted in the activation of TGF-beta/Smads/p38MAPK pathway in renal inflammation and fibration of mice. J. Biomed. Mater. Res. A 2016, 104, 1452–1461. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yin, H.; Li, Z.; Zhang, T.; Yang, Z. Nano-TiO2 induces autophagy to protect against cell death through antioxidative mechanism in podocytes. Cell Biol. Toxicol. 2016, 32, 513–527. [Google Scholar] [CrossRef]
- Lopes, V.R.; Loitto, V.; Audinot, J.N.; Bayat, N.; Gutleb, A.C.; Cristobal, S. Dose-dependent autophagic effect of titanium dioxide nanoparticles in human HaCaT cells at non-cytotoxic levels. J. Nanobiotechnol. 2016, 14, 22. [Google Scholar] [CrossRef] [PubMed]
- Popp, L.; Tran, V.; Patel, R.; Segatori, L. Autophagic response to cellular exposure to titanium dioxide nanoparticles. Acta Biomater. 2018, 79, 354–363. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, B.; Yao, M.; Dong, T.; Mao, Z.; Hang, B.; Han, X.; Lin, Z.; Bian, Q.; Li, M.; et al. Titanium dioxide nanoparticles induce proteostasis disruption and autophagy in human trophoblast cells. Chem. Biol. Interact. 2018, 296, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Borisova, T. Nervous System Injury in Response to Contact with Environmental, Engineered and Planetary Micro- and Nano-Sized Particles. Front. Physiol. 2018, 9, 728. [Google Scholar] [CrossRef]
- Sun, T.; Yan, Y.; Zhao, Y.; Guo, F.; Jiang, C. Copper oxide nanoparticles induce autophagic cell death in A549 cells. PLoS ONE 2012, 7, e43442. [Google Scholar] [CrossRef]
- Zhang, Y.; Sha, R.; Zhang, L.; Zhang, W.; Jin, P.; Xu, W.; Ding, J.; Lin, J.; Qian, J.; Yao, G.; et al. Harnessing copper-palladium alloy tetrapod nanoparticle-induced pro-survival autophagy for optimized photothermal therapy of drug-resistant cancer. Nat. Commun. 2018, 9, 4236. [Google Scholar] [CrossRef]
- Shepard, M.N.; Brenner, S. An occupational exposure assessment for engineered nanoparticles used in semiconductor fabrication. Ann. Occup. Hyg. 2014, 58, 251–265. [Google Scholar] [CrossRef]
- Debia, M.; Bakhiyi, B.; Ostiguy, C.; Verbeek, J.H.; Brouwer, D.H.; Murashov, V. A Systematic Review of Reported Exposure to Engineered Nanomaterials. Ann. Occup. Hyg. 2016, 60, 916–935. [Google Scholar] [CrossRef] [PubMed]
- Alimba, C.G.; Faggio, C. Microplastics in the marine environment: Current trends in environmental pollution and mechanisms of toxicological profile. Environ. Toxicol. Pharmacol. 2019, 68, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.; Krauth, T.; Wagner, S. Export of Plastic Debris by Rivers into the Sea. Environ. Sci. Technol. 2017, 51, 12246–12253. [Google Scholar] [CrossRef] [PubMed]
- Gigault, J.; Halle, A.T.; Baudrimont, M.; Pascal, P.Y.; Gauffre, F.; Phi, T.L.; El Hadri, H.; Grassl, B.; Reynaud, S. Current opinion: What is a nanoplastic? Environ. Pollut. 2018, 235, 1030–1034. [Google Scholar] [CrossRef]
- Wang, J.; Tan, Z.; Peng, J.; Qiu, Q.; Li, M. The behaviors of microplastics in the marine environment. Mar. Environ. Res. 2016, 113, 7–17. [Google Scholar] [CrossRef]
- Wright, S.L.; Thompson, R.C.; Galloway, T.S. The physical impacts of microplastics on marine organisms: A review. Environ. Pollut. 2013, 178, 483–492. [Google Scholar] [CrossRef]
- Jin, Y.; Lu, L.; Tu, W.; Luo, T.; Fu, Z. Impacts of polystyrene microplastic on the gut barrier, microbiota and metabolism of mice. Sci. Total Environ. 2019, 649, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.L.; Ng, C.T.; Zou, L.; Lu, Y.; Chen, J.; Bay, B.H.; Shen, H.M.; Ong, C.N. Targeted metabolomics reveals differential biological effects of nanoplastics and nanoZnO in human lung cells. Nanotoxicology 2019, 13, 1117–1132. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Liu, Y.; Li, Y.; Li, G.; Wang, D. Effect of chronic exposure to nanopolystyrene on nematode Caenorhabditis elegans. Chemosphere 2020, 256, 127172. [Google Scholar] [CrossRef] [PubMed]
- Han, S.W.; Choi, J.; Ryu, K.Y. Stress Response of Mouse Embryonic Fibroblasts Exposed to Polystyrene Nanoplastics. Int. J. Mol. Sci. 2021, 22, 2094. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.W.; Xia, T.; Lee, Y.H.; Chen, C.W.; Tsai, J.C.; Wang, Y.J. Cationic polystyrene nanospheres induce autophagic cell death through the induction of endoplasmic reticulum stress. Nanoscale 2015, 7, 736–746. [Google Scholar] [CrossRef]
- Huang, N.C.; Wann, S.R.; Chang, H.T.; Lin, S.L.; Wang, J.S.; Guo, H.R. Arsenic, vinyl chloride, viral hepatitis, and hepatic angiosarcoma: A hospital-based study and review of literature in Taiwan. BMC Gastroenterol. 2011, 11, 142. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.H.; Chuang, H.C.; Lee, Y.H.; Lin, Y.F.; Chiu, Y.J.; Wang, Y.L.; Wu, M.S.; Chiu, H.W. Induction of Fibrosis and Autophagy in Kidney Cells by Vinyl Chloride. Cells 2019, 8, 601. [Google Scholar] [CrossRef] [PubMed]
- Hours, M.; Fevotte, J.; Lafont, S.; Bergeret, A. Cancer mortality in a synthetic spinning plant in Besancon, France. Occup. Environ. Med. 2007, 64, 575–581. [Google Scholar] [CrossRef]
- Ates, I.; Yucesoy, B.; Yucel, A.; Suzen, S.H.; Karakas, Y.; Karakaya, A. Possible effect of gene polymorphisms on the release of TNFα and IL1 cytokines in coal workers’ pneumoconiosis. Exp. Toxicol. Pathol. 2011, 63, 175–179. [Google Scholar] [CrossRef]
- Landrigan, P.J.; Cherniack, M.G.; Lewis, F.A.; Catlett, L.R.; Hornung, R.W. Silicosis in a grey iron foundry: The persistence of an ancient disease. Scand. J. Work Environ. Health 1986, 12, 32–39. [Google Scholar] [CrossRef]
- Lauridsen, H.L.; Bonlokke, J.H.; Davidsen, J.R.; Eldahl, F.; Huremovic, J.; Kruger, K.; Omland, O.; Shaker, S.B.; Sherson, D. [Asbestosis and pleural plaques]. Ugeskr. Laeger 2018, 180, V10170773. [Google Scholar] [PubMed]
- Yang, H.; Rivera, Z.; Jube, S.; Nasu, M.; Bertino, P.; Goparaju, C.; Franzoso, G.; Lotze, M.T.; Krausz, T.; Pass, H.I. Programmed necrosis induced by asbestos in human mesothelial cells causes high-mobility group box 1 protein release and resultant inflammation. Proc. Natl. Acad. Sci. USA 2010, 107, 12611–12616. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Patergnani, S.; Giorgi, C.; Suarez, J.; Goto, K.; Bononi, A.; Tanji, M.; Novelli, F.; Pastorino, S.; Xu, R. Asbestos induces mesothelial cell transformation via HMGB1-driven autophagy. Proc. Natl. Acad. Sci. USA 2020, 117, 25543–25552. [Google Scholar] [CrossRef]
- Dehghani, S.; Moore, F.; Akhbarizadeh, R. Microplastic pollution in deposited urban dust, Tehran metropolis, Iran. Environ. Sci. Pollut. Res. Int. 2017, 24, 20360–20371. [Google Scholar] [CrossRef]
- Abbasi, S.; Keshavarzi, B.; Moore, F.; Turner, A.; Kelly, F.J.; Dominguez, A.O.; Jaafarzadeh, N. Distribution and potential health impacts of microplastics and microrubbers in air and street dusts from Asaluyeh County, Iran. Environ. Pollut. 2019, 244, 153–164. [Google Scholar] [CrossRef]
- Su, L.; Nan, B.; Craig, N.J.; Pettigrove, V. Temporal and spatial variations of microplastics in roadside dust from rural and urban Victoria, Australia: Implications for diffuse pollution. Chemosphere 2020, 252, 126567. [Google Scholar] [CrossRef]
- Chung, Y.-S.; Kim, S.-H.; Jong-Hwa, M.; Kim, Y.-J.; Lim, J.-M.; Lee, J.-H. Analysis of urban dust (PM2/PM10-2) in Daejeon city by instrumental neutron activation analysis. J. Radioanal. Nucl. Chem. 2005, 267, 95–107. [Google Scholar] [CrossRef]
- Kirwale, S.; Pooladanda, V.; Thatikonda, S.; Murugappan, S.; Khurana, A.; Godugu, C. Selenium nanoparticles induce autophagy mediated cell death in human keratinocytes. Nanomedicine 2019, 14, 1991–2010. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.K.; Qiu, W.R.; Naveen Raj, E.; Liu, H.F.; Huang, H.S.; Lin, Y.W.; Chang, C.J.; Chen, T.H.; Chen, C.; Chang, H.C.; et al. Ubiquitin-coated nanodiamonds bind to autophagy receptors for entry into the selective autophagy pathway. Autophagy 2017, 13, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Liu, Y.; Wu, H.; Huang, Z.; Ma, J.; Guo, C.; Gao, F.; Jin, P.; Wei, P.; Zhang, Y.; et al. Key Role of TFEB Nucleus Translocation for Silver Nanoparticle-Induced Cytoprotective Autophagy. Small 2018, 14, e1703711. [Google Scholar] [CrossRef] [PubMed]
- Lorente, J.; Velandia, C.; Leal, J.A.; Garcia-Mayea, Y.; Lyakhovich, A.; Kondoh, H.; ME, L.L. The interplay between autophagy and tumorigenesis: Exploiting autophagy as a means of anticancer therapy. Biol. Rev. Camb. Philos. Soc. 2018, 93, 152–165. [Google Scholar] [CrossRef]
- Kisen, G.O.; Tessitore, L.; Costelli, P.; Gordon, P.B.; Schwarze, P.E.; Baccino, F.M.; Seglen, P.O. Reduced autophagic activity in primary rat hepatocellular carcinoma and ascites hepatoma cells. Carcinogenesis 1993, 14, 2501–2505. [Google Scholar] [CrossRef] [PubMed]
- Dikic, I.; Elazar, Z. Mechanism and medical implications of mammalian autophagy. Nat. Rev. Mol. Cell Biol. 2018, 19, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Nassour, J.; Radford, R.; Correia, A.; Fuste, J.M.; Schoell, B.; Jauch, A.; Shaw, R.J.; Karlseder, J. Autophagic cell death restricts chromosomal instability during replicative crisis. Nature 2019, 565, 659–663. [Google Scholar] [CrossRef] [PubMed]
- Takamura, A.; Komatsu, M.; Hara, T.; Sakamoto, A.; Kishi, C.; Waguri, S.; Eishi, Y.; Hino, O.; Tanaka, K.; Mizushima, N. Autophagy-deficient mice develop multiple liver tumors. Genes Dev. 2011, 25, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Yu, J.; Bhagat, G.; Furuya, N.; Hibshoosh, H.; Troxel, A.; Rosen, J.; Eskelinen, E.L.; Mizushima, N.; Ohsumi, Y.; et al. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J. Clin. Investig. 2003, 112, 1809–1820. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Jeong, E.G.; Ahn, C.H.; Kim, S.S.; Lee, S.H.; Yoo, N.J. Frameshift mutation of UVRAG, an autophagy-related gene, in gastric carcinomas with microsatellite instability. Hum. Pathol. 2008, 39, 1059–1063. [Google Scholar] [CrossRef] [PubMed]
- Marino, G.; Salvador-Montoliu, N.; Fueyo, A.; Knecht, E.; Mizushima, N.; Lopez-Otin, C. Tissue-specific autophagy alterations and increased tumorigenesis in mice deficient in Atg4C/autophagin-3. J. Biol. Chem. 2007, 282, 18573–18583. [Google Scholar] [CrossRef]
- Komata, T.; Kanzawa, T.; Nashimoto, T.; Aoki, H.; Endo, S.; Nameta, M.; Takahashi, H.; Yamamoto, T.; Kondo, S.; Tanaka, R. Mild heat shock induces autophagic growth arrest, but not apoptosis in U251-MG and U87-MG human malignant glioma cells. J. Neurooncol. 2004, 68, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Maiuri, M.C.; Tasdemir, E.; Criollo, A.; Morselli, E.; Vicencio, J.M.; Carnuccio, R.; Kroemer, G. Control of autophagy by oncogenes and tumor suppressor genes. Cell Death Differ. 2009, 16, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Bialik, S.; Kimchi, A. Lethal weapons: DAP-kinase, autophagy and cell death: DAP-kinase regulates autophagy. Curr. Opin. Cell Biol. 2010, 22, 199–205. [Google Scholar] [CrossRef]
- Rusten, T.E.; Lindmo, K.; Juhasz, G.; Sass, M.; Seglen, P.O.; Brech, A.; Stenmark, H. Programmed autophagy in the Drosophila fat body is induced by ecdysone through regulation of the PI3K pathway. Dev. Cell 2004, 7, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Kung, C.P.; Budina, A.; Balaburski, G.; Bergenstock, M.K.; Murphy, M. Autophagy in tumor suppression and cancer therapy. Crit. Rev. Eukaryot. Gene Expr. 2011, 21, 71–100. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.R.; Neves, S.P.; Santos, L.S.; Dias, R.B.; Bezerra, D.P. Challenges and Therapeutic Opportunities of Autophagy in Cancer Therapy. Cancers 2020, 12, 3461. [Google Scholar] [CrossRef]
- Park, S.M.; Ou, J.; Chamberlain, L.; Simone, T.M.; Yang, H.; Virbasius, C.M.; Ali, A.M.; Zhu, L.J.; Mukherjee, S.; Raza, A.; et al. U2AF35(S34F) Promotes Transformation by Directing Aberrant ATG7 Pre-mRNA 3’ End Formation. Mol. Cell 2016, 62, 479–490. [Google Scholar] [CrossRef]
- Nazio, F.; Bordi, M.; Cianfanelli, V.; Locatelli, F.; Cecconi, F. Autophagy and cancer stem cells: Molecular mechanisms and therapeutic applications. Cell Death Differ. 2019, 26, 690–702. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhou, Y.; Li, Y.; Yang, L.; Ma, Y.; Peng, X.; Yang, S.; Liu, J.; Li, H. Autophagy: A novel mechanism of chemoresistance in cancers. Biomed. Pharmacother. 2019, 119, 109415. [Google Scholar] [CrossRef] [PubMed]
- Sanchez Galan, L. Asbestos. Laryngeal cancer. Acta Otorrinolaringol. Esp. 2017, 68, 250. [Google Scholar] [CrossRef] [PubMed]
- Muzammil, A.; Miandad, R.; Waqas, M.; Gehany, F.; Barakat, M. Remediation of wastewater using various nanomaterials. Arab. J. Chem. 2016, 12, 4897–4919. [Google Scholar]
- Ziajahromi, S.; Neale, P.A.; Rintoul, L.; Leusch, F.D. Wastewater treatment plants as a pathway for microplastics: Development of a new approach to sample wastewater-based microplastics. Water Res. 2017, 112, 93–99. [Google Scholar] [CrossRef]
- Mason, S.A.; Garneau, D.; Sutton, R.; Chu, Y.; Ehmann, K.; Barnes, J.; Fink, P.; Papazissimos, D.; Rogers, D.L. Microplastic pollution is widely detected in US municipal wastewater treatment plant effluent. Environ. Pollut. 2016, 218, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Gies, E.A.; LeNoble, J.L.; Noel, M.; Etemadifar, A.; Bishay, F.; Hall, E.R.; Ross, P.S. Retention of microplastics in a major secondary wastewater treatment plant in Vancouver, Canada. Mar. Pollut. Bull. 2018, 133, 553–561. [Google Scholar] [CrossRef]
- Gundogdu, S.; Cevik, C.; Guzel, E.; Kilercioglu, S. Microplastics in municipal wastewater treatment plants in Turkey: A comparison of the influent and secondary effluent concentrations. Environ. Monit. Assess. 2018, 190, 626. [Google Scholar] [CrossRef]
- Enfrin, M.; Dumee, L.F.; Lee, J. Nano/microplastics in water and wastewater treatment processes—Origin, impact and potential solutions. Water Res. 2019, 161, 621–638. [Google Scholar] [CrossRef]
- Masia, P.; Sol, D.; Ardura, A.; Laca, A.; Borrell, Y.J.; Dopico, E.; Laca, A.; Machado-Schiaffino, G.; Diaz, M.; Garcia-Vazquez, E. Bioremediation as a promising strategy for microplastics removal in wastewater treatment plants. Mar. Pollut. Bull. 2020, 156, 111252. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, L.; Qi, P.; Liu, X.; Wei, G. Synthesis of Three-Dimensional Graphene-Based Hybrid Materials for Water Purification: A Review. Nanomaterials 2019, 9, 1123. [Google Scholar] [CrossRef]
- Yang, J.; Hou, B.; Wang, J.; Tian, B.; Bi, J.; Wang, N.; Li, X.; Huang, X. Nanomaterials for the Removal of Heavy Metals from Wastewater. Nanomaterials 2019, 9, 424. [Google Scholar] [CrossRef] [PubMed]
- Poerio, T.; Piacentini, E.; Mazzei, R. Membrane Processes for Microplastic Removal. Molecules 2019, 24, 4148. [Google Scholar] [CrossRef]
- Urbanek, A.K.; Rymowicz, W.; Mironczuk, A.M. Degradation of plastics and plastic-degrading bacteria in cold marine habitats. Appl. Microbiol. Biotechnol. 2018, 102, 7669–7678. [Google Scholar] [CrossRef]
- Brandon, A.M.; Gao, S.H.; Tian, R.; Ning, D.; Yang, S.S.; Zhou, J.; Wu, W.M.; Criddle, C.S. Biodegradation of Polyethylene and Plastic Mixtures in Mealworms (Larvae of Tenebrio molitor) and Effects on the Gut Microbiome. Environ. Sci. Technol. 2018, 52, 6526–6533. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, A.; Hecht, K.; Buller, R. Enzymatic PET Degradation. Chimia 2019, 73, 743–749. [Google Scholar] [CrossRef]
- Gambarini, V.; Pantos, O.; Kingsbury, J.M.; Weaver, L.; Handley, K.M.; Lear, G. Phylogenetic Distribution of Plastic-Degrading Microorganisms. mSystems 2021, 6. [Google Scholar] [CrossRef] [PubMed]
- Satti, S.M.; Shah, A.A. Polyester-based biodegradable plastics: An approach towards sustainable development. Lett. Appl. Microbiol. 2020, 70, 413–430. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wang, C. Biodegradable plastics: Green hope or greenwashing? Mar. Pollut. Bull. 2020, 161, 111774. [Google Scholar] [CrossRef] [PubMed]
- Senturk, G.; Dumludag, D. An evaluation of the effect of plastic bag fee on consumer behavior: Case of Turkey. Waste Manag. 2021, 120, 748–754. [Google Scholar] [CrossRef]
- Martinho, G.; Balaia, N.; Pires, A. The Portuguese plastic carrier bag tax: The effects on consumers’ behavior. Waste Manag. 2017, 61, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Wagner, T.P. Reducing single-use plastic shopping bags in the USA. Waste Manag. 2017, 70, 3–12. [Google Scholar] [CrossRef]
- Clayton, C.A.; Walker, T.R.; Bezerra, J.C.; Adam, I. Policy responses to reduce single-use plastic marine pollution in the Caribbean. Mar. Pollut. Bull. 2021, 162, 111833. [Google Scholar] [CrossRef] [PubMed]


| MSs & NSs | Autophagy-Related Responses or Increasing Markers | Cells or Tissues | Reference |
|---|---|---|---|
| Magnetic nanoparticles | Autophagy markers: Atg5, Atg12, and LC3 | In vitro: Human lung adenocarcinoma cells (A549) and human lung fibroblast cells (IMR-90) | [154] |
| Manganese nanoparticles | Autophagy markers: Beclin 1, and LC3 | In vitro: Rat mesencephalic dopaminergic cells (N27) | [158] |
| Quantum dots | Autophagy markers: p62 and LC3 | In vitro: Rat adrenal medulla pheochromocytoma cells (PC12) | [161] |
| Autophagy marker: LC3 | In vitro: Porcine renal proximal cell line (LLC-PK1) | [162] | |
| Graphene oxide quantum dots | Autophagy markers: p62 and LC3 | In vitro: Mouse reproductive cells (GC-2 and TM4 cells) | [163] |
| Graphene oxide | Autophagy markers: p62 and LC3 | In vitro: Rat glioblastoma cells (F98) | [164] |
| Particulate matter 2.5 (PM2.5) | Autophagy markers: Beclin 1, ULK-1, and LC3 | In vitro: Human bronchial epithelial cells (BEAS-2B) | [167] |
| Autophagy markers: Beclin 1, ATG5,ULK-1, and LC3 | In vitro: Monocytic leukemia cells (THP-1) | [168] | |
| Autophagy-mediated cell death | In vitro: Human bronchial epithelium cells (BEAS-2B) | [170] | |
| Autophagy markers: p62 and LC3 | In vivo: Liver of C57BL/6 mice | [173] | |
| Autophagy markers: ATG5, VSP34, Beclin 1, and LC3 | In vivo: Spleen of Sprague Dawley (SD) rats | [174] | |
| Autophagy markers: p62, Beclin 1, and LC3 | In vitro: Human kidney tubular epithelial cells (HK-2) | [175] | |
| In vivo: Kidney of SD rat | |||
| Diesel exhaust particles (DEP) | Autophagy markers: p62, Beclin 1, and LC3 | In vitro: Human kidney tubular epithelial cells (HK-2) | [179] |
| In vivo: Kidney of SD rat | |||
| Zinc oxide (ZnO) nanoparticles | Autophagy markers: p62 and LC3 | In vitro: Human cervical cancer cells (HeLa cells) | [180] |
| Autophagy marker: LC3 | [181] | ||
| Autophagy marker: LC3 | In vitro: Human ovarian cancer cells (SKOV3) | [182] | |
| Autophagy markers: p62 and LC3 | In vitro: Human epidermal keratinocytes (HEKn) | [183] | |
| Autophagy marker LC3A and autophagic cell death | In vitro: Human T lymphoblast cells (SupT1 and Jurkat cells), C57BL/6 mouse primary splenocytes and primary human T-cells | [184] | |
| Autophagic cell death | In vitro: Rat adrenal medulla pheochromocytoma cells (PC12 cells) | [185] | |
| Autophagy marker: LC3 | In vitro: Primary murine astrocytes | [186] | |
| Silica nanoparticles | Autophagy marker: LC3 and autophagic cell death | In vitro: Human liver cancer cells (HepG2 cells) | [193] |
| Autophagy marker: P62 | In vitro: Human bronchial epithelial cells (BEAS-2B) | [196] | |
| In vivo: Lung of Bltw:CD1 (ICR) mice | |||
| Autophagy markers: p62 and LC3 | Human umbilical vein endothelial cells (HUVECs) | [197] | |
| Silver nanoparticles | Autophagy markers: LC3 | In vitro: Human liver cancer cells (HepG2 cells) | [200] |
| Autophagy markers: p62 and LC3 | In vitro: Human lung adenocarcinoma cells (A549) | [201] | |
| Autophagy markers: Beclin 1 and LC3 | In vivo: Adult brain of Wistar rat | [202] | |
| Autophagy markers: P62 and LC3 | In vitro: Mouse embryonic fibroblast cells (NIH 3T3 cells) | [203] | |
| Bismuth nanoparticles | Autophagy markers: Atg12, Beclin 1, and LC3 | In vitro: Human embryonic kidney 293 cells (HEK293) | [204] |
| In vivo: Kidney of BALB/c mice | |||
| Autophagy marker: p62 | In vitro: Human liver cancer cells (HepG2 cells) | [208] | |
| Autophagy associated cytotoxicity | In vitro: Human embryonic kidney 293 cells (HEK293) | [209] | |
| Nanosized titanium dioxide(Nano TiO2) | Autophagy markers: Beclin 1, p62, and LC3 | In vitro: Mouse podocyte cells (MPCs) | [214] |
| Autophagy marker: LC3 | In vitro: Human keratinocytes (HaCaT cells) | [215] | |
| Autophagy marker: LC3 | In vitro: Human cervical cancer cells (HeLa cells) | [216] | |
| Autophagy markers: p62, LC3 and autophagic cell death | In vitro: Human trophoblast cells (HTR-8/SVneo cells) | [217] | |
| Copper oxide nanoparticles | Autophagic cell death | In vitro: Human lung adenocarcinoma cells (A549) | [219] |
| Polystyrene (PS) nanoplastics | Endoplasmic Reticulum(ER) stress-mediated autophagy marker: LC3 | In vitro: Human bronchial epithelial cells (BEAS-2B) | [229] |
| Autophagic marker: LC3B | In vitro: Mouse embryonic fibroblasts (MEFs) | [231] | |
| Autophagy markers: p62, Beclin 1, and LC3 | In vitro: Mouse macrophage-like cells (RAW 264.7) and human bronchial epithelial cells (BEAS-2B) | [232] | |
| Vinyl chloride (VC) | Autophagy markers: Beclin 1 and LC3 | In vitro: Human kidney tubular epithelial cells (HK-2) | [234] |
| In vivo: Kidney of C57BL/6 mice | |||
| Asbestos | Autophagy markers: ATG5, p62, Beclin 1, and LC3 | In vitro: Primary human mesothelial cells (HM) | [240] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.-L.; Zheng, C.-M.; Lee, Y.-H.; Cheng, Y.-Y.; Lin, Y.-F.; Chiu, H.-W. Micro- and Nanosized Substances Cause Different Autophagy-Related Responses. Int. J. Mol. Sci. 2021, 22, 4787. https://doi.org/10.3390/ijms22094787
Wang Y-L, Zheng C-M, Lee Y-H, Cheng Y-Y, Lin Y-F, Chiu H-W. Micro- and Nanosized Substances Cause Different Autophagy-Related Responses. International Journal of Molecular Sciences. 2021; 22(9):4787. https://doi.org/10.3390/ijms22094787
Chicago/Turabian StyleWang, Yung-Li, Cai-Mei Zheng, Yu-Hsuan Lee, Ya-Yun Cheng, Yuh-Feng Lin, and Hui-Wen Chiu. 2021. "Micro- and Nanosized Substances Cause Different Autophagy-Related Responses" International Journal of Molecular Sciences 22, no. 9: 4787. https://doi.org/10.3390/ijms22094787
APA StyleWang, Y.-L., Zheng, C.-M., Lee, Y.-H., Cheng, Y.-Y., Lin, Y.-F., & Chiu, H.-W. (2021). Micro- and Nanosized Substances Cause Different Autophagy-Related Responses. International Journal of Molecular Sciences, 22(9), 4787. https://doi.org/10.3390/ijms22094787







