Synthesis and Biological Activity of New Brassinosteroid Analogs of Type 24-Nor-5β-Cholane and 23-Benzoate Function in the Side Chain
Abstract
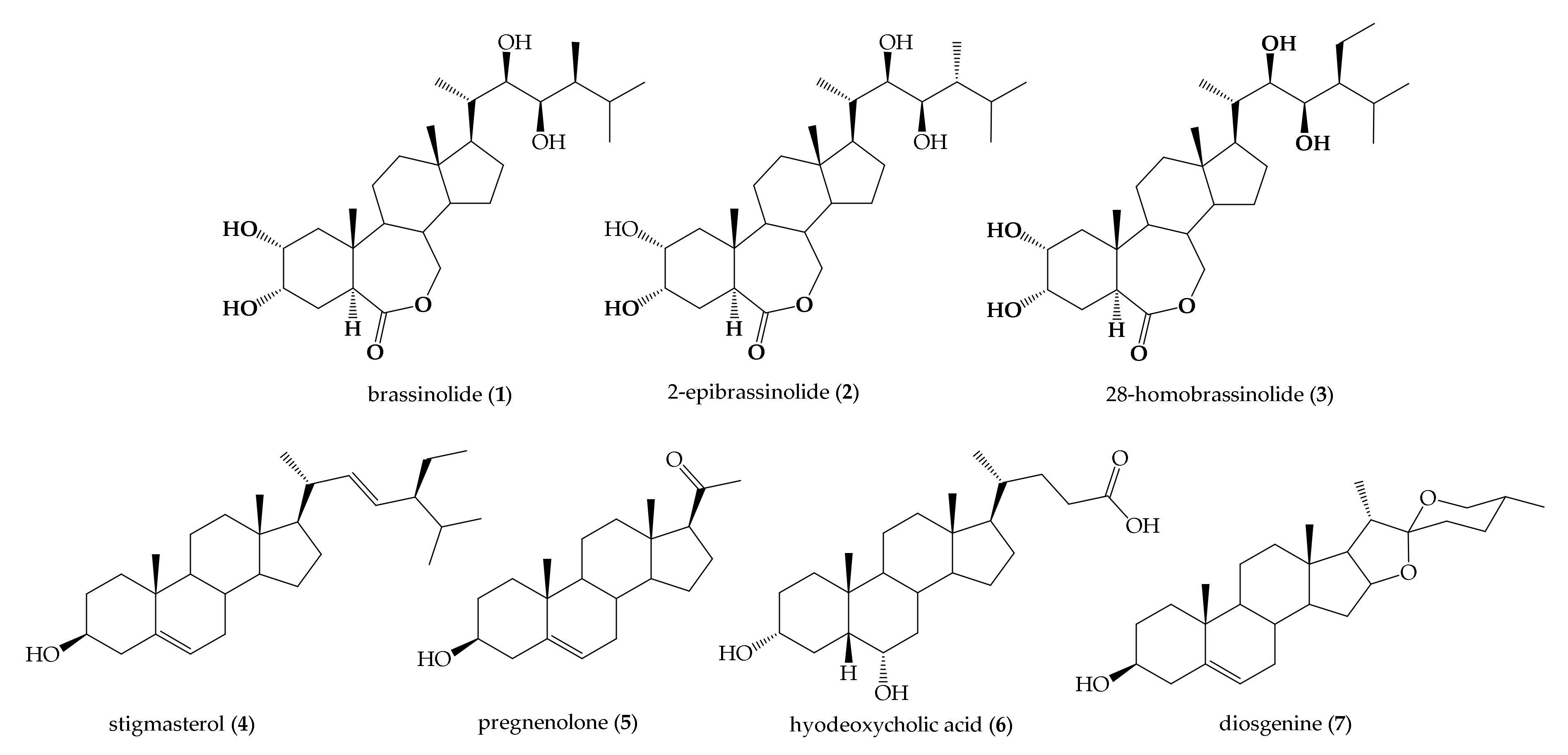
1. Introduction
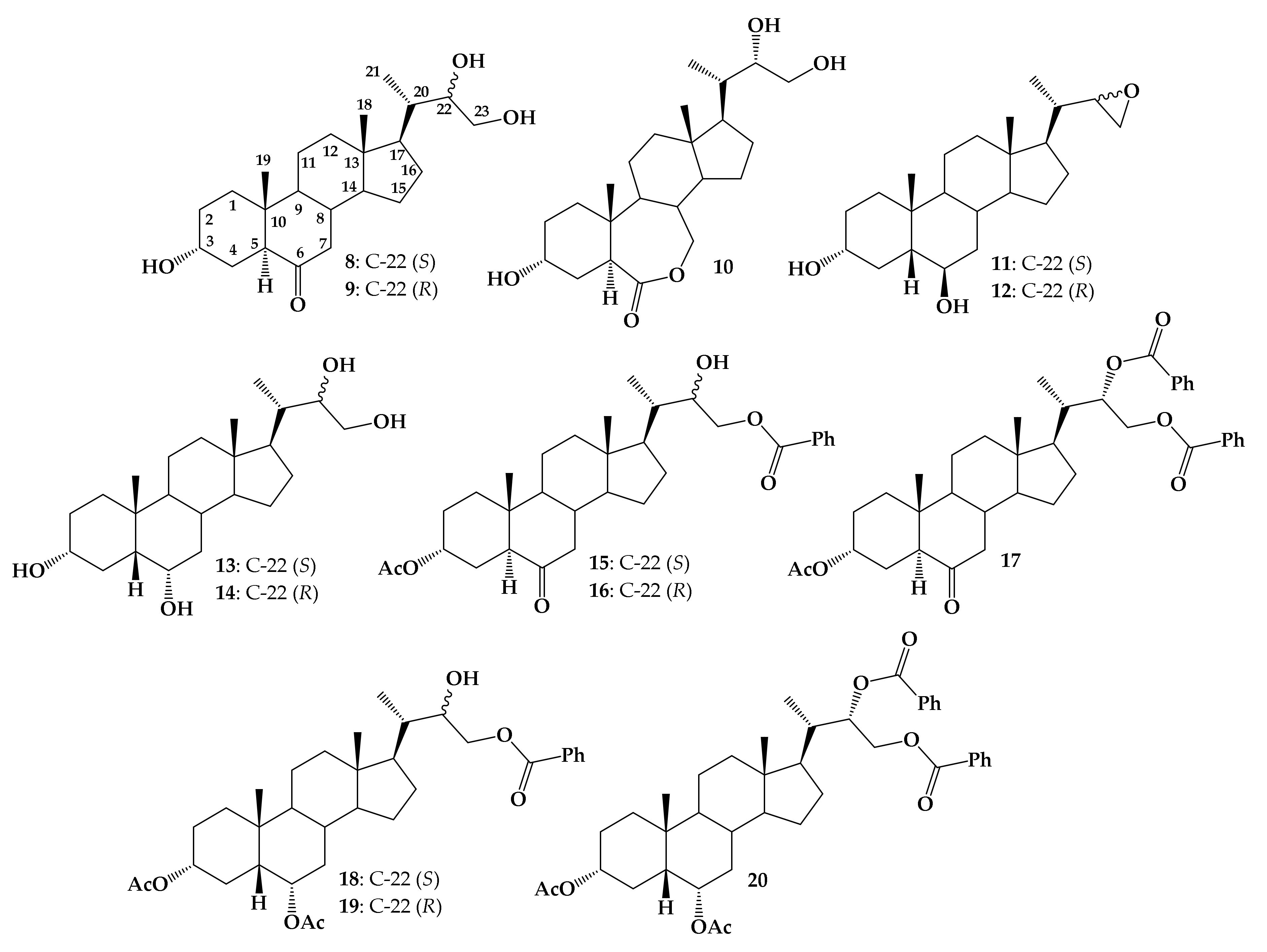
2. Results
2.1. Chemistry
2.2. Bioactivity of BR Analogs Determined by Rice Lamina Inclination Assay (RLIT)
3. Materials and Methods
3.1. Chemistry
3.1.1. General Experimental Methods
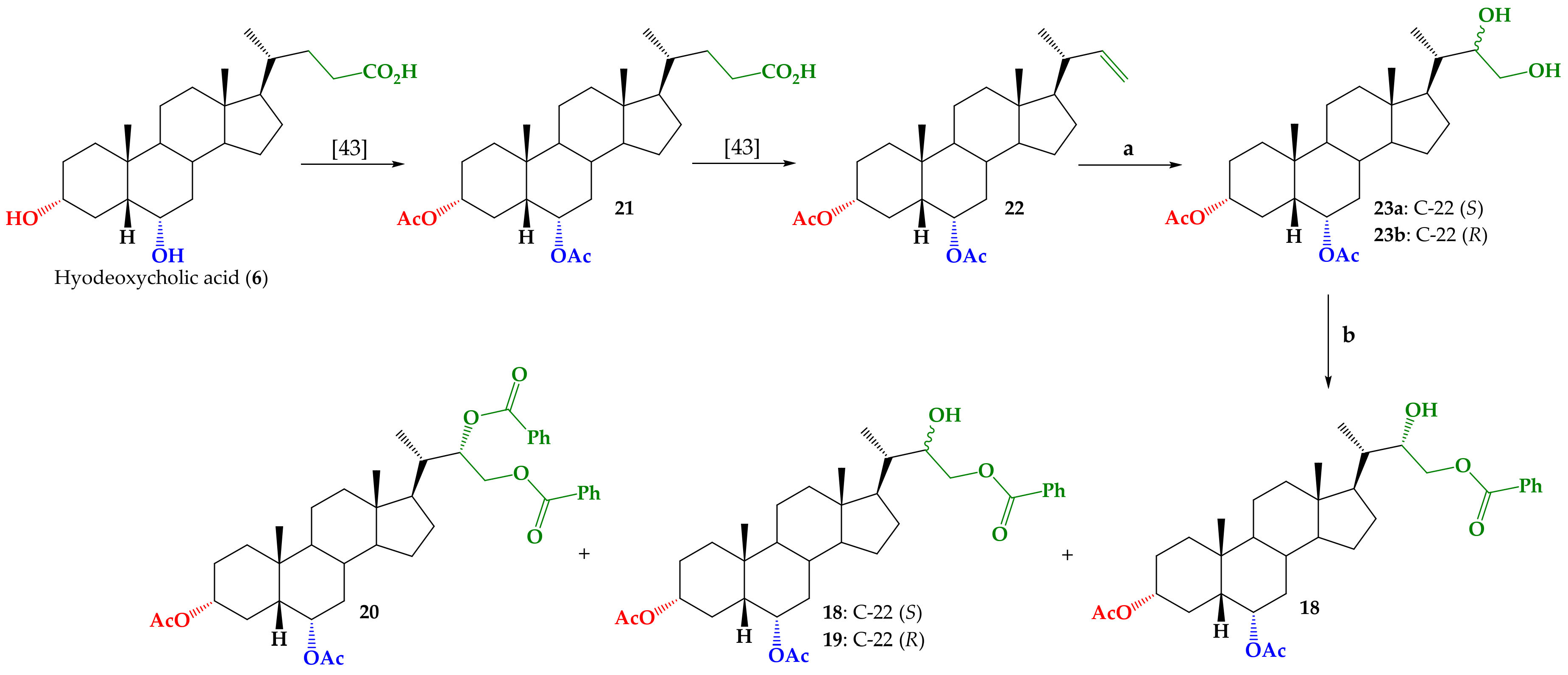
3.1.2. Synthesis of Mixture (22S)-22,23-Dihydroxy-24-nor-5β-cholan-3α,6α-diyl Diacetate (23a) and (22R)-22,23-Dihydroxy-24-nor-5β-cholan-3α,6α-diyl Diacetate (23b)
3.1.3. Synthesis of (22S)-22-Hydroxy-24-nor-5β-cholan-3α,6α-diyl Diacetate-23-benzoate (18), (22R)-22-Hydroxy-24-nor-5β-cholan-3α,6α-diyl Diacetate-23-benzoate (19) and (22S)-24-nor-5β-cholan-3α,6α-diyl diacetate-22,23-diyl dibenzoate (20)
3.1.4. Synthesis of (22S)-24-Nor-5β-cholan-3α,6α-diyl Diacetate-22,23-diyl Dibenzoate (20)
3.2. In Vitro Rice Lamina Inclination Test (RLIT)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mitchell, J.W.; Mandava, N.; Worley, J.F.; Plimmer, J.R.; Smith, M.V. Brassins—A New Family of Plant Hormones from Rape Pollen. Nature 1970, 225, 1065–1066. [Google Scholar] [CrossRef]
- Clouse, S.D.; Sasse, J.M. Brassinosteroids: Essential regulators of plant growth and development. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 427–451. [Google Scholar] [CrossRef]
- Clouse, S.D. A History of Brassinosteroid Research from 1970 through 2005: Thirty-Five Years of Phytochemistry, Physiology, Genes, and Mutants. J. Plant Growth Regul. 2015, 34, 828–844. [Google Scholar] [CrossRef]
- Bajguz, A. Brassinosteroids—Occurence and chemical structures in plants. In Brassinosteroids: A Class of Plant Hormone; Hayat, S., Ahmad, A., Eds.; Springer: Dordrecht, The Netherlands, 2016; pp. 1–27. [Google Scholar]
- Oklestkova, J.; Rarova, L.; Kvasnica, M.; Strnad, M. Brassinosteroids: Synthesis and biological activities. Phytochem. Rev. 2015, 14, 1053–1072. [Google Scholar] [CrossRef]
- Oh, M.-H.; Honey, S.H.; Tax, F.E. The Control of Cell Expansion, Cell Division, and Vascular Development by Brassinosteroids: A Historical Perspective. Int. J. Mol. Sci. 2020, 21, 1743. [Google Scholar] [CrossRef] [PubMed]
- Müssig, C. Brassinosteroid-promoted growth. Plant Biol. 2005, 7, 110–117. [Google Scholar] [CrossRef]
- Sasse, J.M. Physiological actions of brassinosteroids: An update. J. Plant Growth Regul. 2003, 22, 276–288. [Google Scholar] [CrossRef]
- Nolan, T.M.; Vukašinović, N.; Liu, D.; Russinova, E.; Yin, Y. Brassinosteroids: Multidimensional Regulators of Plant Growth, Development, and Stress Responses. Plant Cell 2020, 32, 295. [Google Scholar] [CrossRef]
- Bajguz, A.; Hayat, S. Effects of brassinosteroids on the plant responses to environmental stresses. Plant Physiol. Biochem. 2009, 47, 1–8. [Google Scholar] [CrossRef]
- Kaur, N.; Pati, P.K. Harnessing the Potential of Brassinosteroids in Abiotic Stress Tolerance in Plants. In Brassinosteroids: Plant Growth and Development; Hayat, S., Yusuf, M., Bhardwaj, R., Bajguz, A., Eds.; Springer: Singapore, 2019; pp. 407–423. [Google Scholar]
- Thompson, M.J.; Meudt, W.J.; Mandava, N.B.; Dutky, S.R.; Lusby, W.R.; Spaulding, D.W. Synthesis of Brassinosteroids and Relationship of Structure to Plant Growth-Promoting Effects. Steroids 1982, 39, 89–105. [Google Scholar] [CrossRef]
- Takatsuto, S.; Yazawa, N.; Ikekawa, N.; Takematsu, T.; Takeuchi, Y.; Koguchi, M. Structure Activity Relationship of Brassinosteroids. Phytochemistry 1983, 22, 2437–2441. [Google Scholar] [CrossRef]
- Takatsuto, S.; Yazawa, N.; Ikekawa, N.; Morishita, T.; Abe, H. Synthesis of (24R)-28-homobrassinolide analogues and structure-activity relationships of brassinosteroids in the rice-lamina inclination test. Phytochemistry 1983, 22, 1393–1397. [Google Scholar] [CrossRef]
- Fujioka, S. Natural Occurrence of Brassinosteroids in the Plant Kingdom. In Brassinosteroids: Steroidal Plant Hormones; Sakurai, A., Yokota, T., Clouse, S.D., Eds.; Springer: Tokyo, Japan, 1999; pp. 21–45. [Google Scholar]
- Liu, J.; Zhang, D.; Sun, X.; Ding, T.; Lei, B.; Zhang, C. Structure-activity relationship of brassinosteroids and their agricultural practical usages. Steroids 2017, 124, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Back, T.G. Stereoselective Synthesis of Brassinosteroids. In Stereoselective Synthesis (Part J); Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 1997; pp. 321–364. [Google Scholar]
- Back, T.G.; Krishna, M.V. Synthesis of castasterone and formal synthesis of brassinolide from stigmasterol via a selenosulfonation approach. J. Org. Chem. 1991, 56, 454–457. [Google Scholar] [CrossRef]
- Back, T.G.; Blazecka, P.G.; Krishna, M.V. A new synthesis of castasterone and brassinolide from stigmasterol—A concise and stereoselective elaboration of the side-chain from a C-22 aldehyde. Can. J. Chem. 1993, 71, 156–163. [Google Scholar] [CrossRef]
- Wada, K.; Marumo, S. Synthesis and Plant Growth-Promoting Activity of Brassinolide Analogs. Agric. Biol. Chem. 1981, 45, 2579–2585. [Google Scholar]
- Uesusuki, S.; Watanabe, B.; Yamamoto, S.; Otsuki, J.; Nakagawa, Y.; Miyagawa, H. Synthesis of Brassinosteroids of Varying Acyl Side Chains and Evaluation of Their Brassinolide-like Activity. Biosci. Biotechnol. Biochem. 2004, 68, 1097–1105. [Google Scholar] [CrossRef]
- Zhou, W.S.; Huang, L.F. Studies on Steroidal Plant-Growth Regulator. 25. Concise Stereoselective Construction of Side-Chain of Brassinosteroid from the Intact Side-Chain of Hyodeoxycholic Acid: Formal Syntheses of Brassinolide, 25-Methylbrassinolide, 26,27-Bisnorbrassinolide and Their Related-Compounds. Tetrahedron 1992, 48, 1837–1852. [Google Scholar]
- Zhou, W.S.; Sun, L.Q.; Pan, X.F. Synthesis of Steroidal Plant-Growth Regulators. 23. Stereoselective Synthesis of Crinosterol and Brassicasterol from Hyodeoxycholic Acid. Acta Chim. Sin. 1992, 50, 1192–1199. [Google Scholar]
- Iglesias-Arteaga, M.; Gil, R.P.; Leliebre-Lara, V.; Martinez, C.S.P.; Manchado, F.; Perez, A.R.; Rios, L.P. Synthesis of (22R,25R)-3 beta,26-dihydroxy-5 alpha-furostan-6-one. Synth. Commun. 1998, 28, 1381–1386. [Google Scholar] [CrossRef]
- Iglesias-Arteaga, M.; Gil, R.P.; Leliebre-Lara, V.; Martinez, C.S.P.; Manchado, F. Synthesis of (22R,25R)-2 alpha,3 alpha,26-trihydroxy-5 alpha-furostanaone-6-one. Synth. Commun. 1998, 28, 1779–1784. [Google Scholar] [CrossRef]
- Zhou, W.S.; Tian, W.S. The Synthesis of Steroids Containing Structural Unit of A, B Ring of Brassinolide and Ecdysone from Hyodeoxycholic Acid. Acta Chim. Sin. 1984, 42, 1173–1177. [Google Scholar]
- Zhou, W.S.; Tian, W.S. Study on the Synthesis of Brassinolide and Related-Compounds. 3. Stereoselective Synthesis of Typhasterol from Hyodeoxycholic Acid. Tetrahedron 1987, 43, 3705–3712. [Google Scholar] [CrossRef]
- Zhou, W.S. The Synthesis of Brassinosteroid. Pure Appl. Chem. 1989, 61, 431–434. [Google Scholar] [CrossRef]
- Zhou, W.-S.; Biao, J.; Pan, X.-F. A Novel Synthesis of Brassinolide and Related Compounds. J. Chem. Soc. Chem. Commun. 1989, 612–614. [Google Scholar] [CrossRef]
- Zhou, W.S.; Huang, L.F.; Sun, L.Q.; Pan, X.F. Studies on A Steroidal Plant-Growth Regulator. Part 26. Stereoselective Construction of the Brassinolide Side-Chain: New Practical Syntheses of Brassinolide Analogs from Hyodeoxycholic Acid. J. Chem. Soc. Perkin Trans. 1 1992, 2039–2043. [Google Scholar] [CrossRef]
- Brosa, C.; Capdevila, J.M.; Zamora, I. Brassinosteroids: A new way to define the structural requirements. Tetrahedron 1996, 52, 2435–2448. [Google Scholar] [CrossRef]
- Pérez Gil, R.; Pérez Martínez, C.S.; Coll Manchado, F. Synthesis of Analogues of Brassinosteroids with 5β-Cholanic Acid Skeleton. Synth. Commun. 1998, 28, 3387–3396. [Google Scholar]
- Espinoza, L.; Bulat, F.; Coll, D.; Coll, F.; Preite, M.D.; Cortes, M. Synthesis and plant growth-activity of three new brassinosteroids analogues. Synth. Commun. 2000, 30, 1963–1974. [Google Scholar] [CrossRef]
- Espinoza, L.; Cortes, M. Synthesis and biological activities of two new brassinosteroids functionalized in ring C. Bol. Soc. Chil. Quim. 2002, 47, 335–347. [Google Scholar]
- Soto, N.; González, C.; Mellado, M.; Olea, A.F.; Coll, Y.; Díaz, K.; Espinoza, L. Epimeric Mixtures of Brassinosteroid Analogs: Synthesis, Plant Growth, and Germination Effects in Tomato (Lycopersicum esculentum Mill.). Agronomy 2020, 10, 808. [Google Scholar] [CrossRef]
- Herrera, H.; Carvajal, R.; Olea, A.F.; Espinoza, L. Structural modifications of deoxycholic acid to obtain three known brassinosteroid analogues and full NMR spectroscopic characterization. Molecules 2016, 21, 1139. [Google Scholar] [CrossRef] [PubMed]
- Duran, M.I.; Gonzalez, C.; Acosta, A.; Olea, A.F.; Diaz, K.; Espinoza, L. Synthesis of Five Known Brassinosteroid Analogs from Hyodeoxycholic Acid and Their Activities as Plant-Growth Regulators. Int. J. Mol. Sci. 2017, 18, 516. [Google Scholar] [CrossRef]
- Oyarce, J.; Aitken, V.; Gonzalez, C.; Ferrer, K.; Olea, A.F.; Parella, T.; Espinoza, L. Synthesis and structural determination of new brassinosteroid 24-nor-5α-cholane type analogs. Molecules 2019, 24, 4612. [Google Scholar] [CrossRef]
- Carvajal, R.; Gonzalez, C.; Olea, A.F.; Fuentealba, M.; Espinoza, L. Synthesis of 2-Deoxybrassinosteroids Analogs with 24-nor, 22(S)-23-Dihydroxy-Type Side Chains from Hyodeoxycholic Acid. Molecules 2018, 23, 1306. [Google Scholar] [CrossRef] [PubMed]
- Diaz, K.; Espinoza, L.; Carvajal, R.; Conde-Gonzalez, M.; Niebla, V.; Olea, A.F.; Coll, Y. Biological Activities and Molecular Docking of Brassinosteroids 24-Norcholane Type Analogs. Int. J. Mol. Sci. 2020, 21, 1832. [Google Scholar] [CrossRef]
- Zhou, W.S.; Tian, W.S. Studies on Steroidal Plant-Growth Hormones. 2. Stereoselective Synthesis of (22S, 23S)-Typhasterol from Hyodeoxycholic Acid. Acta Chim. Sin. 1985, 43, 1060–1067. [Google Scholar]
- Tian, W.S.; Zhou, W.S.; Jiang, B.; Pan, X.F. Studies on Steroidal Plant-Growth Regulator. 9. The Preparation of 22R-Penta-Nor-Brassinolides and 22S-24,25,26,27,28-Penta-Nor-Brassinolides. Acta Chim. Sin. 1989, 47, 1017–1021. [Google Scholar]
- Ferrer, K.; Díaz, K.; Kvasnica, M.; Olea, A.F.; Cuellar, M.; Espinoza, L. Synthesis of New Brassinosteroid 24-Norcholane Type Analogs Conjugated in C-3 with Benzoate Groups. Molecules 2021, 26, 1173. [Google Scholar] [CrossRef]
- Kvasnica, M.; Oklestkova, J.; Bazgier, V.; Rárová, L.; Korinkova, P.; Mikulík, J.; Budesinsky, M.; Béres, T.; Berka, K.; Lu, Q.; et al. Design, synthesis and biological activities of new brassinosteroid analogues with a phenyl group in the side chain. Org. Biomol. Chem. 2016, 14, 8691–8701. [Google Scholar] [CrossRef]
- Khripach, V.; Zhabinskii, V.; de Groot, A. Twenty years of brassinosteroids: Steroidal plant hormones warrant better crops for the XXI century. Ann. Bot. 2000, 86, 441–447. [Google Scholar] [CrossRef]
- Wada, K.; Marumo, S.; Ikekawa, N.; Morisaki, M.; Mori, K. Brassinolide and Homobrassinolide Promotion of Lamina Inclination of Rice Seedlings. Plant Cell Physiol 1981, 22, 323–325. [Google Scholar]
- Han, K.S.; Ko, K.W.; Nam, S.J.; Park, S.H.; Kim, S.K. Optimization of a rice lamina inclination assay for detection of brassinosteroids: I. effect of phytohormones on the inclination activity. J. Plant Biol. 1997, 40, 240–244. [Google Scholar] [CrossRef]
- Wada, K.; Marumo, S.; Abe, H.; Morishita, T.; Nakamura, K.; Uchiyama, M.; Mori, K. A Rice Lamina Inclination Test—A Micro-Quantitative Bioassay for Brassinosteroids. Agric. Biol. Chem. 1984, 48, 719–726. [Google Scholar]
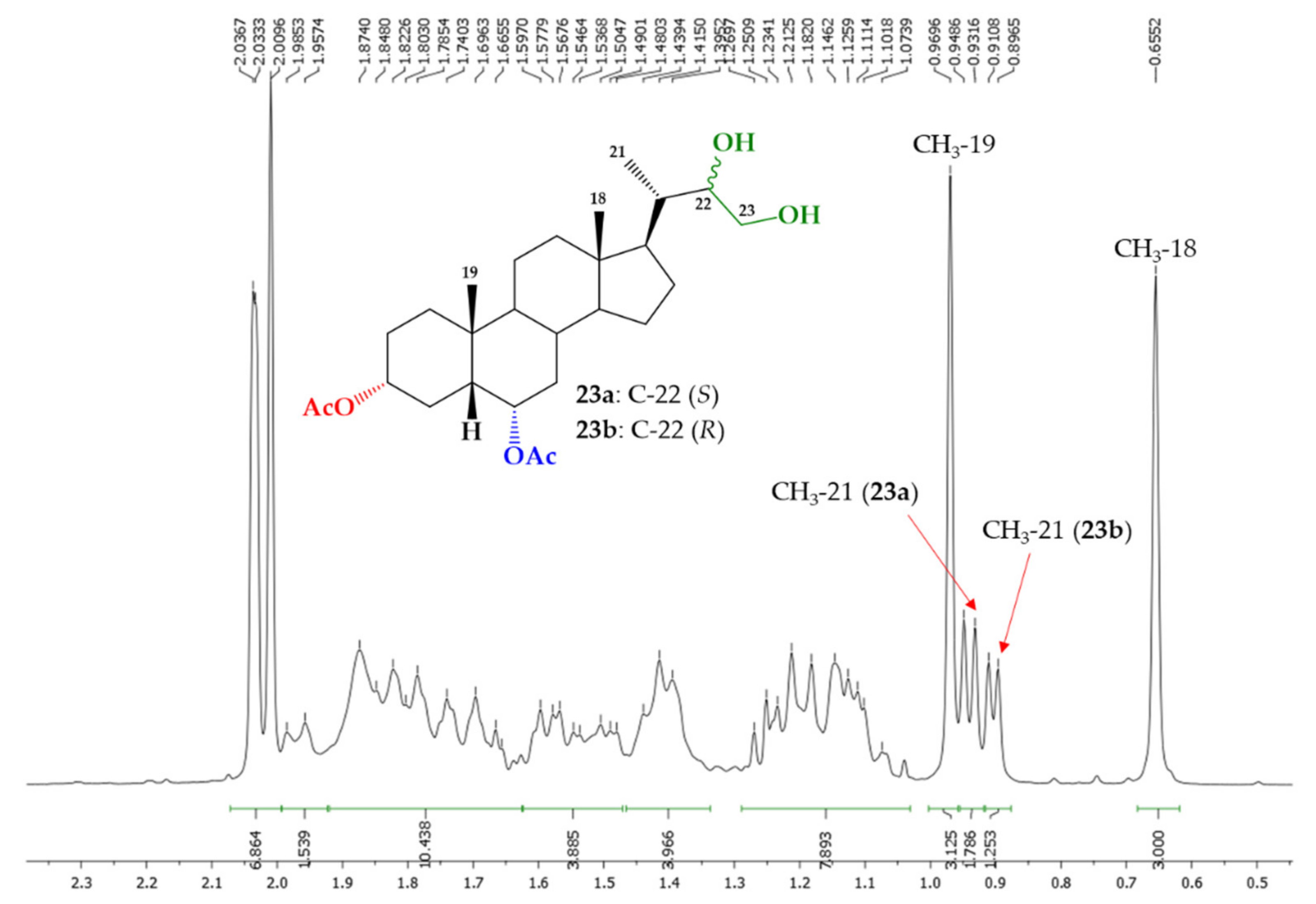
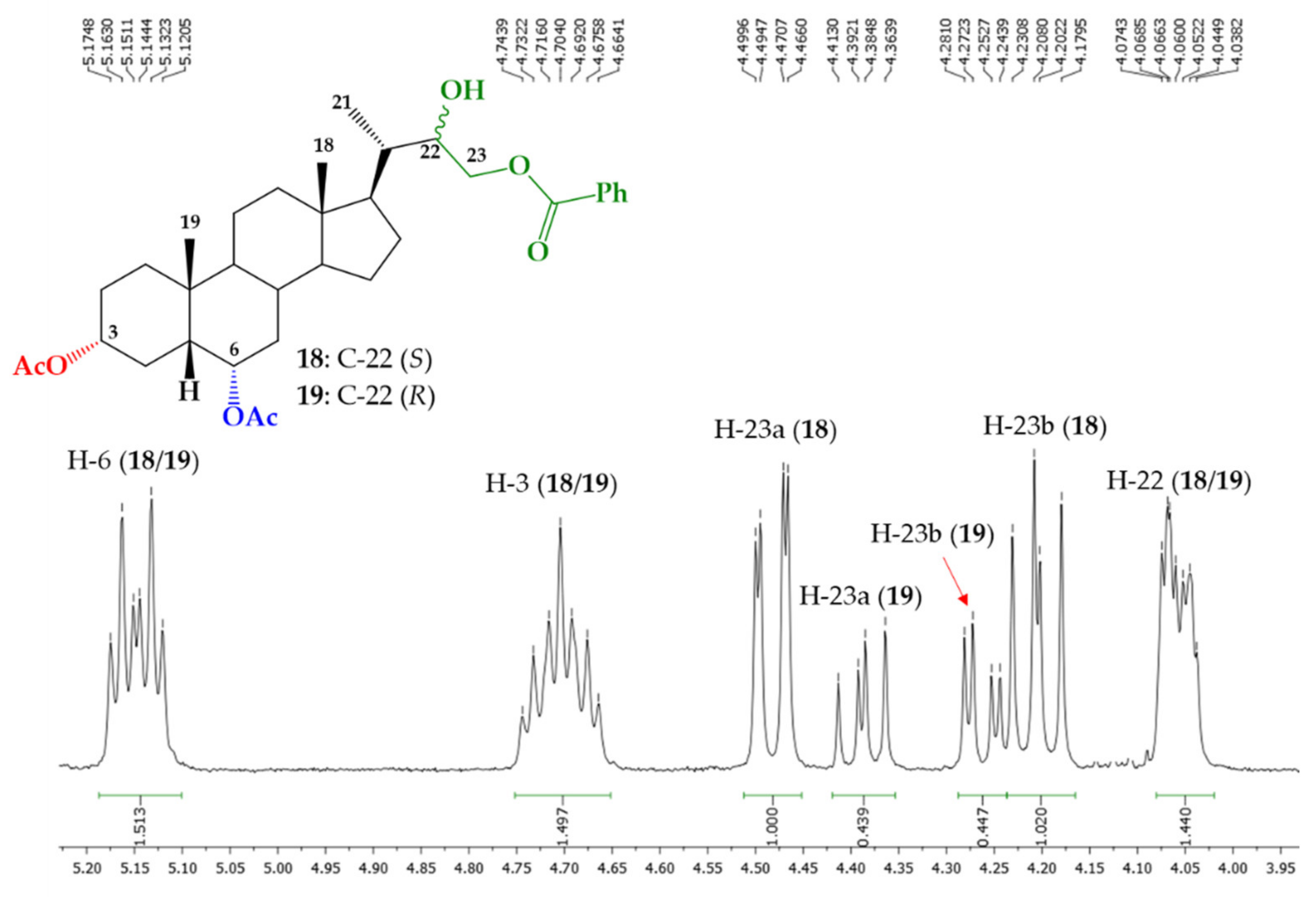
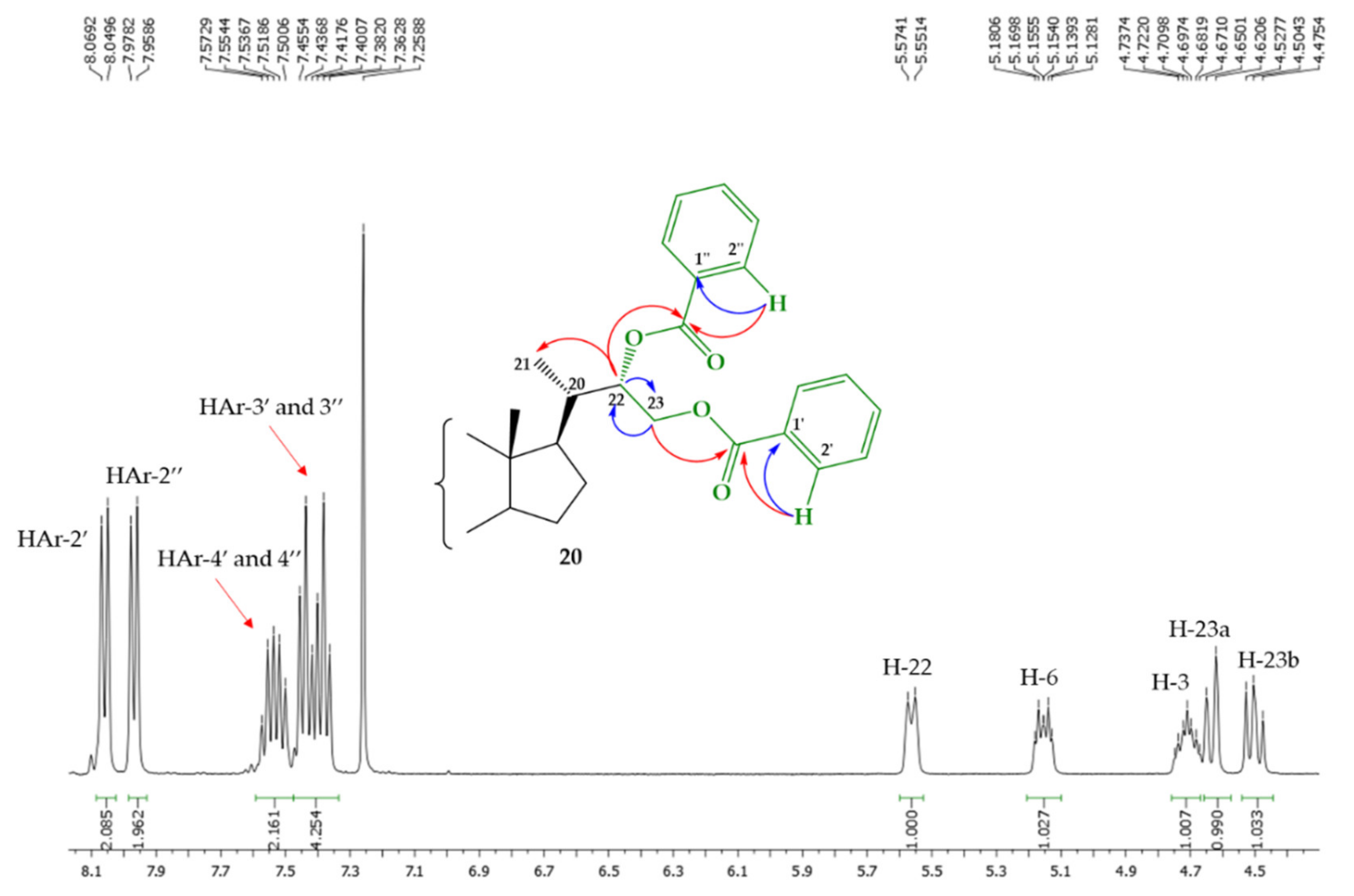
| H/C Signal | Compound 23a | Compound 23b |
|---|---|---|
| H-21 | 0.94 ppm (d, J = 6.8 Hz) | 0.90 (d, J = 5.7 Hz) |
| H-22 | 3.81–3.79 ppm (m) | 3.81–3.79 ppm (m) |
| H-23a | 3.63 (dd, J = 9.9 and 9.9 Hz) | 3.63 (dd, J = 9.9 and 9.9 Hz) |
| H-23b | 3.51 (m) | 3.51 (m) |
| C21 | 12.62 ppm | 13.01 ppm |
| C22 | 74.11 ppm | 73.88 ppm |
| C23 | 62.47 ppm | 66.04 ppm |
| H/C Signal | Compound 18 | Compound 19 |
|---|---|---|
| H-21 | 1.04 ppm (d, J = 6.8 Hz) | 1.00 (d, J = 6.4 Hz) |
| H-22 | 4.07-4.04 ppm (m) | 4.07–4.04 ppm (m) |
| H-23a | 4.48 (dd, J = 11.4 and 2.0 Hz) | 4.39 (dd, J = 11.4 and 8.4 Hz) |
| H-23b | 4.21 (dd, J = 11.4 and 10.2 Hz) | 4.26 (dd, J = 11.4 and 3.3 Hz) |
| C21 | 12.90 ppm | 12.39 ppm |
| C22 | 71.79 ppm | 71.86 ppm |
| C23 | 68.95 ppm | 68.88 ppm |
| Bending Angle between Lamina and Sheaths (Degrees ± Standard Error) | ||||
|---|---|---|---|---|
| Compound | Structure | Concentration [M] | ||
| 1 × 10−8 | 1 × 10−7 | 1 × 10−6 | ||
| 18 |  | 20 ± 1.0 | 48 ± 2.9 | 49 ± 2.5 |
| 18/19 (1.0:0.44) |  | 68 ± 2.5 | 50 ± 2.5 | 46 ± 2.9 |
| 20 | 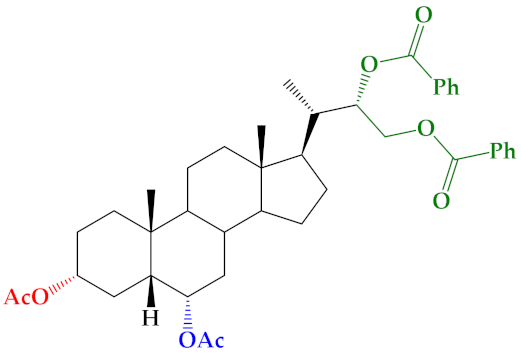 | 6 ± 2.5 | 25 ± 1.0 | 23 ± 2.1 |
| 1 (C+) * | 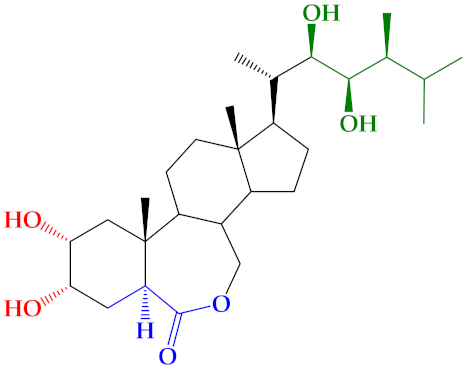 | 31 ± 1.1 | 41 ± 4.5 | 70 ± 7.6 |
| Water (C−) | 7 ± 4.5 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soto, N.; Ferrer, K.; Díaz, K.; González, C.; Taborga, L.; Olea, A.F.; Carrasco, H.; Espinoza, L. Synthesis and Biological Activity of New Brassinosteroid Analogs of Type 24-Nor-5β-Cholane and 23-Benzoate Function in the Side Chain. Int. J. Mol. Sci. 2021, 22, 4808. https://doi.org/10.3390/ijms22094808
Soto N, Ferrer K, Díaz K, González C, Taborga L, Olea AF, Carrasco H, Espinoza L. Synthesis and Biological Activity of New Brassinosteroid Analogs of Type 24-Nor-5β-Cholane and 23-Benzoate Function in the Side Chain. International Journal of Molecular Sciences. 2021; 22(9):4808. https://doi.org/10.3390/ijms22094808
Chicago/Turabian StyleSoto, Nitza, Karoll Ferrer, Katy Díaz, César González, Lautaro Taborga, Andrés F. Olea, Héctor Carrasco, and Luis Espinoza. 2021. "Synthesis and Biological Activity of New Brassinosteroid Analogs of Type 24-Nor-5β-Cholane and 23-Benzoate Function in the Side Chain" International Journal of Molecular Sciences 22, no. 9: 4808. https://doi.org/10.3390/ijms22094808
APA StyleSoto, N., Ferrer, K., Díaz, K., González, C., Taborga, L., Olea, A. F., Carrasco, H., & Espinoza, L. (2021). Synthesis and Biological Activity of New Brassinosteroid Analogs of Type 24-Nor-5β-Cholane and 23-Benzoate Function in the Side Chain. International Journal of Molecular Sciences, 22(9), 4808. https://doi.org/10.3390/ijms22094808






