The Influence of an Adrenergic Antagonist Guanethidine on the Distribution Pattern and Chemical Coding of Caudal Mesenteric Ganglion Perikarya and Their Axons Supplying the Porcine Bladder
Abstract
1. Introduction
2. Results
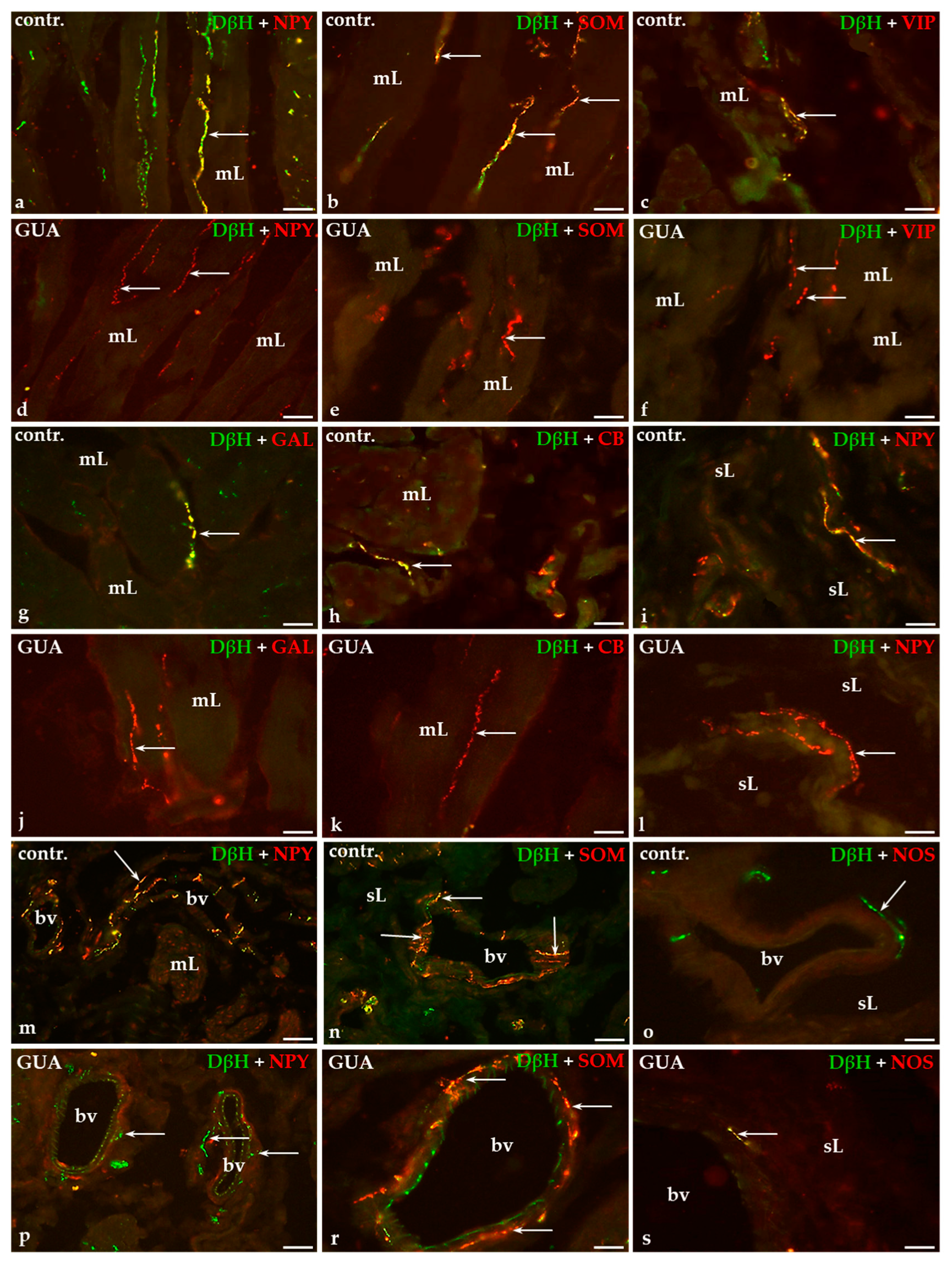
2.1. Distribution and Relative Frequency of Dopamine Beta Hydroxylase (DβH)-Immunoreactive (IR) Nerve Fibers in the Urinary Bladder Wall of Control and GUA-Treated Animals
2.2. Colocalization Pattern of DβH and Other Substances Studied in the Nerve Fibers of the Urinary Bladder Wall in Control and GUA-Treated Animals
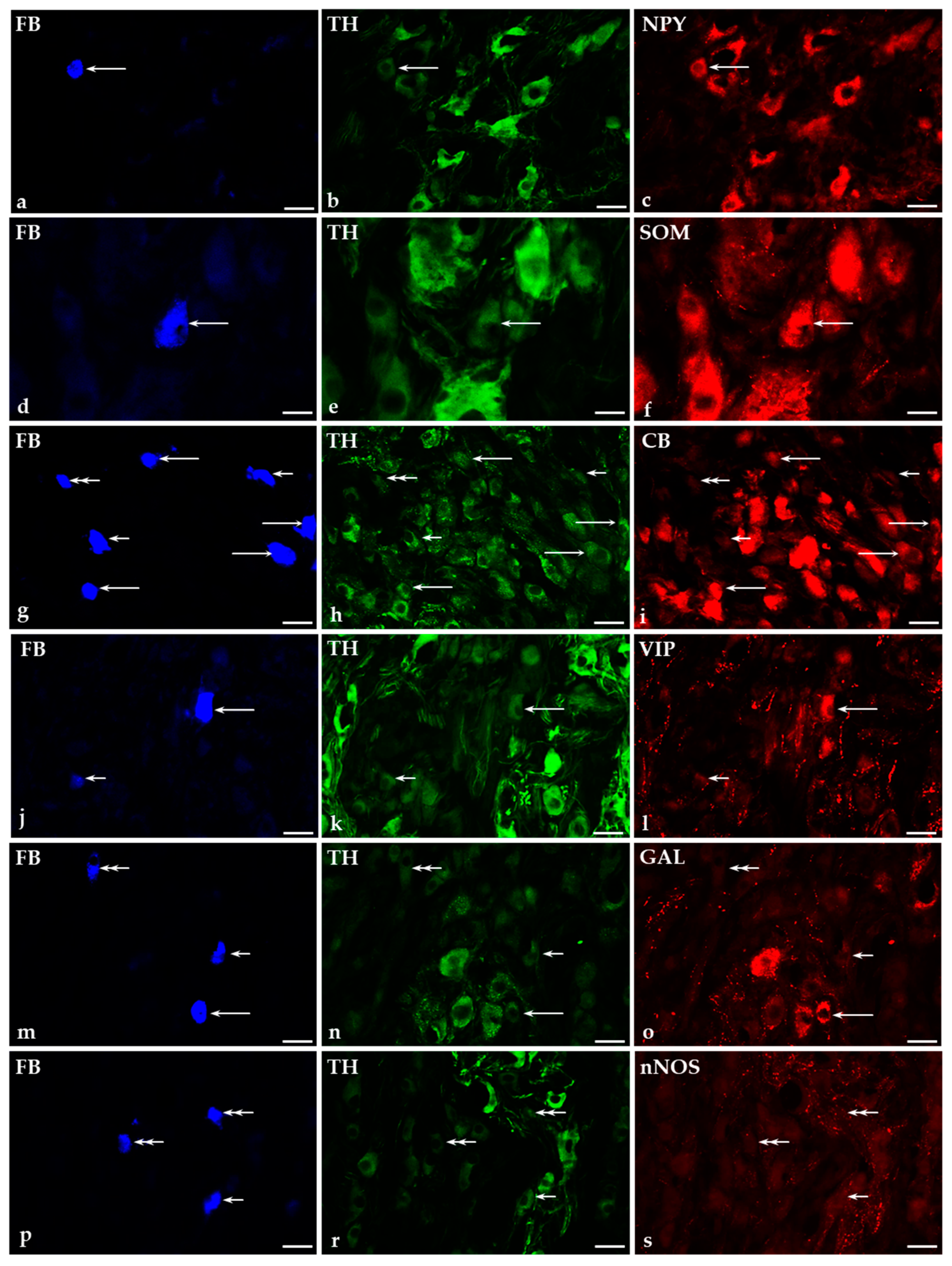
2.3. Distribution of Fast Blue-Positive (FB+) Neurons in the CaMG of Control Pigs
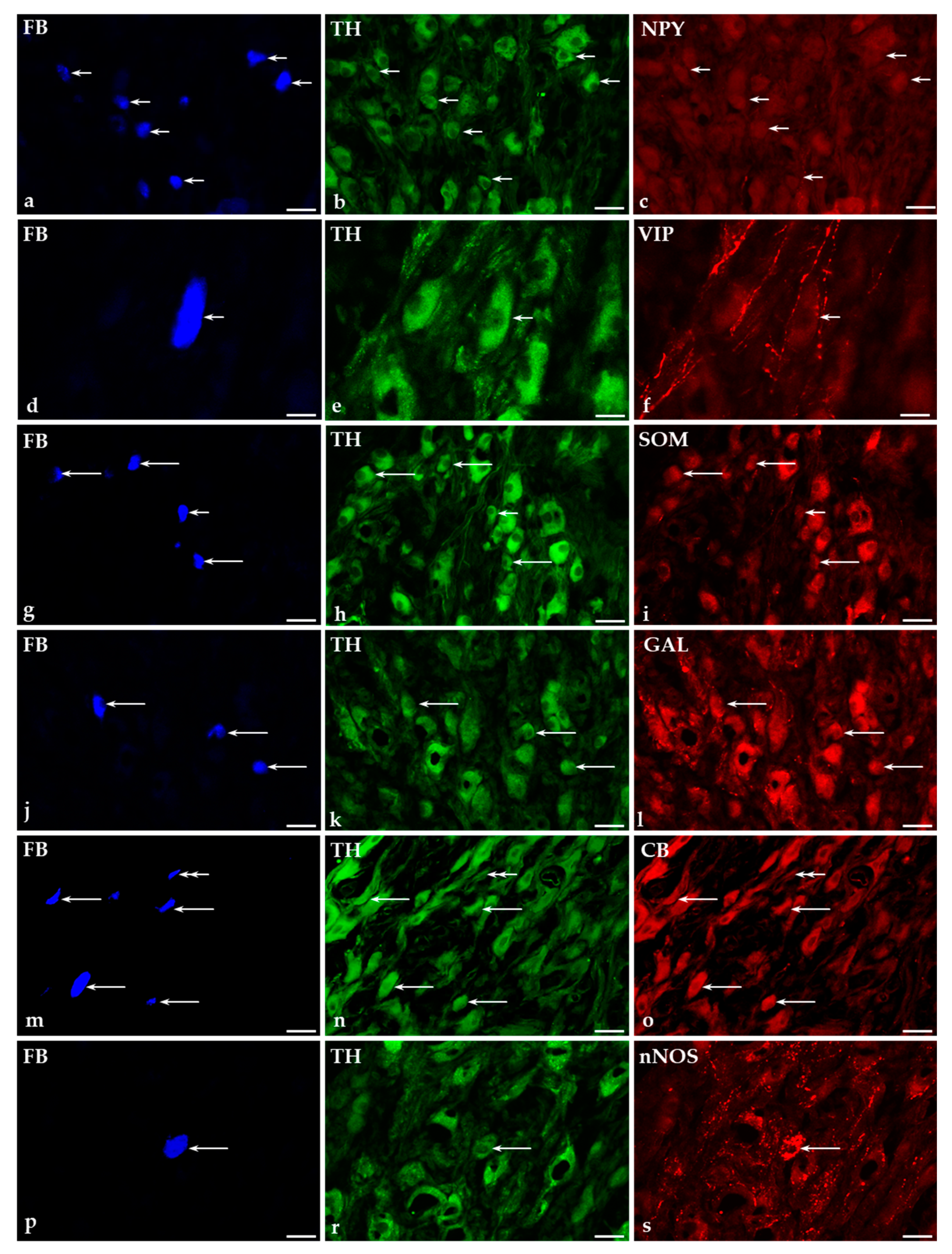
2.4. Distribution of FB+ Neurons in the CaMG of GUA-Treated Pigs
2.5. Immunohistochemical Characteristics of FB+ Neurons in the CaMG of Control and GUA-Treated Animals
2.5.1. Co-Transmitters of FB+/TH-Positive (TH+) Neurons in the CaMG of Control and GUA-Treated Animals
2.5.2. Immunohistochemical Characteristics of FB+/TH-Immunonegative Neurons in the CaMGs of Control and GUA-Treated Animals
3. Discussion
4. Materials and Methods
4.1. Laboratory Animals
4.2. Anesthesia and Surgical Procedures
4.3. Sectioning of the Tissue Samples and Estimation of the Total Number of CaMG-UBPN
4.4. Immunohistochemical Procedure
4.5. Estimation of the Density and Chemical Coding of Noradrenergic Nerve Fibers Supplying the Urinary Bladder and the CaMG-UBPN
4.6. Control of Specificity of the Tracer Staining and Immunohistochemical Procedures
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| Ach | acetylcholine |
| ATP | adenosine triphosphate |
| CaMG | caudal mesenteric ganglion |
| CB | calbindin |
| CGRP | calcitonin gene-related peptide |
| DRG | dorsal root ganglia |
| DβH | dopamine beta hydroxylase |
| FB | fast blue |
| FITC | fluorescein isothiocyanate |
| GAL | galanin |
| GUA | guanethidine |
| IMG | inferior mesenteric ganglion |
| IR | immunoreactive |
| L | lumbar |
| NA | noradrenaline |
| NET | norepinephrine transporter |
| NGF | nerve growth factor |
| nNOS | neuronal nitric oxide synthase |
| NO | nitric oxide |
| NPY | neuropeptide Y |
| SChG | sympathetic chain ganglia |
| SOM | somatostatin |
| Th | thoracic |
| TH | tyrosine hydroxylase |
| UBPN | urinary bladder-projecting neurons |
| VIP | vasoactive intestinal polypeptide |
References
- Andersson, K.E.; Arner, A. Urinary bladder contraction and relaxation: Physiology and pathophysiology. Physiol. Rev. 2004, 84, 935–986. [Google Scholar] [CrossRef]
- Fowler, C.J.; Griffiths, D.; De Groat, W.C. The neural control of micturition. Nat. Rev. Neurosci. 2008, 9, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Blok, B.F.; Holstege, G. The central control of micturition and continence: Implications for urology. BJU Int. 1999, 83 (Suppl. 2), 1–6. [Google Scholar] [CrossRef] [PubMed]
- Jobling, P. Autonomic control of the urogenital tract. Auton. Neurosci. 2011, 165, 113–126. [Google Scholar] [CrossRef]
- De Groat, W.C.; Griffiths, D.; Yoshimura, N. Neural control of the lower urinary tract. Compr. Physiol. 2015, 5, 327–396. [Google Scholar] [CrossRef] [PubMed]
- el-Badawi, A.; Schenk, E.A. Dual innervation of the mammalian urinary bladder. A histochemical study of the distribution of cholinergic and adrenergic nerves. Am. J. Anat. 1966, 119, 405–427. [Google Scholar] [CrossRef] [PubMed]
- Crowe, R.; Burnstock, G. A histochemical and immunohistochemical study of the autonomic innervation of the lower urinary tract of the female pig. Is the pig a good model for the human bladder and urethra? J. Urol. 1989, 141, 414–422. [Google Scholar] [CrossRef]
- Gabella, G. Intramural neurons in the urinary bladder of the guinea-pig. Cell Tissue Res. 1990, 261, 231–237. [Google Scholar] [CrossRef]
- Thuroff, J.W.; Bazeed, M.A.; Schmidt, R.A.; Luu, D.H.; Tanagho, E.A. Regional topography of spinal cord neurons innervating pelvic floor muscles and bladder neck in the dog: A study by combined horseradish peroxidase histochemistry and autoradiography. Urol. Int. 1982, 37, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Levin, R.M.; Ruggieri, M.R.; Wein, A.J. Functional effects of the purinergic innervation of the rabbit urinary bladder. J. Pharmacol. Exp. Ther. 1986, 236, 452–457. [Google Scholar] [PubMed]
- Ragionieri, L.; Botti, M.; Gazza, F.; Sorteni, C.; Chiocchetti, R.; Clavenzani, P.; Bo Minelli, L.; Panu, R. Localization of peripheral autonomic neurons innervating the boar urinary bladder trigone and neurochemical features of the sympathetic component. Eur. J. Histochem. 2013, 57, 93–105. [Google Scholar] [CrossRef]
- Pidsudko, Z. Immunohistochemical characteristics and distribution of neurons in the paravertebral, prevertebral and pelvic ganglia supplying the urinary bladder in the male pig. J. Mol. Neurosci. 2014, 52, 56–70. [Google Scholar] [CrossRef]
- Lepiarczyk, E.; Bossowska, A.; Majewski, M. Changes in chemical coding of sympathetic chain ganglia (SChG) neurons supplying porcine urinary bladder after botulinum toxin (BTX) treatment. Cell Tissue Res. 2015, 360, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Langley, J.N.; Anderson, H.K. The constituents of the hypogastric nerves. J. Physiol. 1894, 17, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Downie, J.W.; Champion, J.A.; Nance, D.M. A quantitative analysis of the afferent and extrinsic efferent innervation of specific regions of the bladder and urethra in the cat. Brain Res. Bull. 1984, 12, 735–740. [Google Scholar] [CrossRef]
- Alm, P.; Elmer, M. Adrenergic and cholinergic innervation of the rat urinary bladder. Acta Physiol. Scand. 1975, 94, 36–45. [Google Scholar] [CrossRef]
- Crowe, R.; Noble, J.; Robson, T.; Soediono, P.; Milroy, E.J.; Burnstock, G. An increase of neuropeptide Y but not nitric oxide synthase immunoreactive nerves in the bladder neck from male patients with bladder neck dyssynergia. J. Urol. 1995, 154, 1231–1236. [Google Scholar] [CrossRef]
- Alm, P.; Uvelius, B.; Ekstrom, J.; Holmqvist, B.; Larsson, B.; Andersson, K.E. Nitric oxide synthase-containing neurons in rat parasympathetic, sympathetic and sensory ganglia: A comparative study. Histochem. J. 1995, 27, 819–831. [Google Scholar] [CrossRef]
- Alm, P.; Zygmunt, P.K.; Iselin, C.; Larsson, B.; Uvelius, B.; Werner, S.; Andersson, K.E. Nitric oxide synthase-immunoreactive, adrenergic, cholinergic, and peptidergic nerves of the female rat urinary tract: A comparative study. J. Auton. Nerv. Syst. 1995, 56, 105–114. [Google Scholar] [CrossRef]
- Hoyle, C.H. Non-adrenergic, non-cholinergic control of the urinary bladder. World J. Urol. 1994, 12, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Gustafson, E.L. Galanin receptor subtypes. Drug News Perspect. 1998, 11, 458–468. [Google Scholar]
- Abbs, E.T.; Dodd, M.G. The Relation between the adrenergic neurone-blocking and noradrenaline-depleting actions of some guanidine derivatives. Br. J. Pharm. 1974, 51, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, R.A.; Mull, R.P.; Plummer, A.J. 2-Octahydro-1-azocinyl)-ethyl]-guanidine sulfate (CIBA 5864-SU), a new synthetic antithypertensive agents. Experientia 1959, 15, 267. [Google Scholar] [CrossRef]
- Brodie, B.B.; Chang, C.C.; Costa, E. On the mechanism of action of guanethidine and bretylium. Br. J. Pharmacol. 1965, 25, 171–178. [Google Scholar] [CrossRef]
- Chang, C.C.; Costa, E.; Brodie, B.B. Interaction of guanethidine with adrenergic neurons. J. Pharmacol. Exp. Ther. 1965, 147, 303–312. [Google Scholar]
- Boullin, D.J. A calcium requirement for release of 3H-guanethidine by sympathetic nerve stimulation. J. Pharm. Pharmacol. 1966, 18, 709–712. [Google Scholar] [CrossRef]
- Oates, J.A.; Mitchell, J.R.; Feagin, O.T. Distribution of guanidium antihypertensive—Mechanism of their selective action. Ann. N. Y. Acad. Sci. 1971, 179, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, R.A. Guanethidine after twenty years: A pharmacologist’s perspective. Br. J. Clin. Pharmacol. 1982, 13, 35–44. [Google Scholar] [CrossRef]
- Johnson, E.M., Jr.; Manning, P.T. Guanethidine-induced destruction of sympathetic neurons. Int. Rev. Neurobiol. 1984, 25, 1–37. [Google Scholar] [CrossRef]
- Bustamante, S.; Orensanz, L.M.; Recio, P.; Carballido, J.; García-Sacristán, A.; Prieto, D.; Hernández, M. Functional evidence of nitrergic neurotransmission in the human urinary bladder neck. Neurosci. Lett. 2010, 477, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Creed, K.E.; Ishikawa, S.; Ito, Y. Electrical and mechanical activity recorded from rabbit urinary bladder in response to nerve stimulation. J. Physiol. 1983, 338, 149–164. [Google Scholar] [CrossRef]
- Koley, B.; Koley, J.; Saha, J.K. The effects of nicotine on spontaneous contractions of cat urinary bladder in situ. Br. J. Pharmacol. 1984, 83, 347–355. [Google Scholar] [CrossRef]
- Shinkai, M.; Takayanagi, I.; Kato, T. Tachykinin receptors of the NK2 type involved in the acetylcholine release by nicotine in guinea-pig bladder. Br. J. Pharmacol. 1993, 108, 759–762. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thornbury, K.D.; Donaghy, K.M.; Peake, J. Characteristics of the NANC post-stimulus (‘rebound’) contraction of the urinary bladder neck muscle in sheep. Br. J. Pharmacol. 1995, 116, 2451–2456. [Google Scholar] [CrossRef]
- Min, C.H.; Wang, Y.; Bae, J.; Han, J.H.; Sohn, U.D. The Inhibitory Mechanism of Gentamicin on Electrical Field Stimulation Response in Rat Bladder Smooth Muscle. Korean J. Physiol. Pharmacol. 2015, 19, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.H.; Chang, S.-J.; Hsieh, C.-H.; Hsu, C.-K.; Yang, S.S.-D. Capsaicin-Sensitive Sensory Nerves Indirectly Modulate Motor Function of the Urinary Bladder. Int. Neurourol. J. 2018, 22, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Hernández, M.; Knight, G.E.; Wildman, S.S.P.; Burnstock, G. Role of ATP and related purines in inhibitory neurotransmission to the pig urinary bladder neck. Br. J. Pharmacol. 2009, 157, 1463–1473. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Sáenz, A.; Recio, P.; Orensanz, L.M.; Fernandes, V.S.; Pilar Martínez, M.; Bustamante, S.; Carballido, J.; García-Sacristán, A.; Prieto, D.; Hernández, M. Role of Calcitonin Gene-Related Peptide in Inhibitory Neurotransmission to the Pig Bladder Neck. J. Urol. 2011, 186, 728–735. [Google Scholar] [CrossRef]
- Moro, C.; Chess-Williams, R. Non-adrenergic, non-cholinergic, non-purinergic contractions of the urothelium/lamina propria of the pig bladder. Auton. Autacoid Pharmacol. 2012, 32, 53–59. [Google Scholar] [CrossRef]
- Dalmose, A.L.; Hvistendahl, J.J.; Olsen, L.H.; Eskild-Jensen, A.; Djurhuus, J.C.; Swindle, M.M. Surgically induced urologic models in swine. J. Investig. Surg. 2000, 13, 133–145. [Google Scholar] [CrossRef]
- Swindle, M.M.; Makin, A.; Herron, A.J.; Clubb, F.J., Jr.; Frazier, K.S. Swine as models in biomedical research and toxicology testing. Vet. Pathol. 2012, 49, 344–356. [Google Scholar] [CrossRef]
- Lepiarczyk, E.; Bossowska, A.; Kaleczyc, J.; Majewski, M. The influence of botulinum toxin type A (BTX) on the immunohistochemical characteristics of noradrenergic and cholinergic nerve fibers supplying the porcine urinary bladder wall. Pol. J. Vet. Sci. 2011, 14, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Lepiarczyk, E.; Dudek, A.; Kaleczyc, J.; Majewski, M.; Markiewicz, W.; Radziszewski, P.; Bossowska, A. The influence of resiniferatoxin on the chemical coding of caudal mesenteric ganglion neurons supplying the urinary bladder in the pig. J. Physiol. Pharmacol. 2016, 67, 625–632. [Google Scholar]
- Shepherd, J.T.; Semler, H.J.; Helmholz, H.F., Jr.; Wood, E.H. Effects of infusion of acetylcholine on pulmonary vascular resistance in patients with pulmonary hypertension and congenital heart disease. Circulation 1959, 20, 381–390. [Google Scholar] [CrossRef]
- Cass, R.; Kuntzman, R.; Brodie, B.B. Norepinephrine depletion as a possible mechanism of action of guanethidine (SU 5864), a new hypotensive agent. Proc. Soc. Exp. Biol. Med. 1960, 103, 871–872. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, J.L.; Richardson, J.A. Acute effects of guanethidine on myocardial contractility and catecholamine levels. Proc. Soc. Exp. Biol. Med. 1961, 106, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Bogaert, M.; de Schaepdryver, A.F.; de Vleeschhouwer, G.R. On the pharmacology of guanethidine. Arch. Int. Pharmacodyn. Ther. 1961, 134, 224–236. [Google Scholar] [PubMed]
- Cass, R.; Spriggs, T.L. Tissue amine levels and sympathetic blockade after guanethidine and bretylium. Br. J. Pharmacol. Chemother. 1961, 17, 442–450. [Google Scholar] [CrossRef]
- Johnson, E.M., Jr.; O’Brien, F.; Werbitt, R. Modification and characterization of the permanent sympathectomy produced by the administration of guanethidine to newborn rats. Eur. J. Pharmacol. 1976, 37, 45–54. [Google Scholar] [CrossRef]
- Hartman, B.K. Immunofluorescence of dopoamine-β-hydroxylase. Application of improved methodology to the localization of the peripheral and central noradrenergic nervous system. J. Histochem. Cytochem. 1973, 21, 312–332. [Google Scholar] [CrossRef]
- Hashitani, H.; Takano, H.; Fujita, K.; Mitsui, R.; Suzuki, H. Functional properties of suburothelial microvessels in the rat bladder. J. Urol. 2011, 185, 2382–2391. [Google Scholar] [CrossRef]
- Abercrombie, G.F.; Davies, B.N. The action of guanethidine with particular reference to the sympathetic nervous system. Br. J. Pharmacol. Chemother. 1963, 20, 171–177. [Google Scholar] [CrossRef]
- Collier, B.; Johnson, G.; Quik, M.; Welner, S. Effect of chemical destruction of adrenergic neurones on some cholinergic mechanisms in adult rat sympathetic ganglia. Br. J. Pharm. 1984, 82, 827–832. [Google Scholar] [CrossRef]
- Loh, L.; Nathan, P.W.; Schott, G.D.; Wilson, P.G. Effects of regional guanethidine infusion in certain painful states. J. Neurol. Neurosurg. Psychiatry 1980, 43, 446–451. [Google Scholar] [CrossRef][Green Version]
- Vera, P.L.; Nadelhaft, I. Afferent and sympathetic innervation of the dome and the base of the urinary bladder of the female rat. Brain Res. Bull. 1992, 29, 651–658. [Google Scholar] [CrossRef]
- Yamanishi, T.; Chapple, C.R.; Yasuda, K.; Yoshida, K.; Chess-Williams, R. The role of beta(3)-adrenoceptors in mediating relaxation of porcine detrusor muscle. Br. J. Pharmacol. 2002, 135, 129–134. [Google Scholar] [CrossRef]
- Yamanishi, T.; Chapple, C.R.; Yasuda, K.; Yoshida, K.; Chess-Williams, R. Role of beta-adrenoceptor subtypes in mediating relaxation of the pig bladder trigonal muscle in vitro. Neurourol. Urodyn. 2003, 22, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Trevisani, M.; Campi, B.; Gatti, R.; André, E.; Materazzi, S.; Nicoletti, P.; Gazzieri, D.; Geppetti, P. The influence of alpha1-adrenoreceptors on neuropeptide release from primary sensory neurons of the lower urinary tract. Eur. Urol. 2007, 52, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, N.; Sugiyama, R.; Ichihara, K.; Fujimura, T.; Fukuhara, H.; Homma, Y.; Igawa, Y. Functional roles of bladder α1-adrenoceptors in the activation of single-unit primary bladder afferent activity in rats. BJU Int. 2016, 117, 993–1001. [Google Scholar] [CrossRef]
- Giuliani, S.; Santicioli, P.; Lippi, A.; Lecci, A.; Tramontana, M.; Maggi, C.A. The role of sensory neuropeptides in motor innervation of the hamster isolated urinary bladder. Naunyn Schmiedebergs Arch. Pharmacol. 2001, 364, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Somogyi, G.T.; Tanowitz, M.; de Groat, W.C. Prejunctional facilitatory alpha 1-adrenoceptors in the rat urinary bladder. Br. J. Pharmacol. 1995, 114, 1710–1716. [Google Scholar] [CrossRef]
- Yamamoto, T.; Ghosh, R.; De Groat, W.C.; Somogyi, G.T. Facilitation of transmitter release in the urinary bladders of neonatal and adult rats via alpha1-adrenoceptors. Eur. J. Pharmacol. 2001, 414, 31–35. [Google Scholar] [CrossRef]
- Michel, M.C.; Vrydag, W. Alpha1-, alpha2- and beta-adrenoceptors in the urinary bladder, urethra and prostate. Br. J. Pharmacol. 2006, 147 (Suppl. 2), S88–S119. [Google Scholar] [CrossRef]
- Yanase, H.; Wang, X.; Momota, Y.; Nimura, T.; Kawatani, M. The in-volvement of urothelial alpha1A adrenergic receptor in controlling the micturition reflex. Biomed. Res. 2008, 29, 239–244. [Google Scholar] [CrossRef]
- Hernández, M.; Recio, P.; Barahona, M.V.; Bustamante, S.; Peña, L.; Martínez, A.C.; García-Sacristán, A.; Prieto, D.; Orensanz, L.M. Prejunctional alpha(2)-adrenoceptors modulation of the nitrergic transmission in the pig urinary bladder neck. Neurourol. Urodyn. 2007, 26, 578–583. [Google Scholar] [CrossRef]
- Schuschke, D.A.; Reed, M.W.; Wingren, U.F.; Miller, F.N. The rat urinary bladder: Vasoactivity and macromolecular leakage in a new model. Microvasc. Res. 1989, 38, 23–35. [Google Scholar] [CrossRef]
- Shiraki, H.; Kawasaki, H.; Tezuka, S.; Nakatsuma, A.; Kurosaki, Y. Endogenous calcitonin gene-related peptide (CGRP) mediates adrenergic-dependent vasodilation induced by nicotine in mesenteric resistance arteries of the rat. Br. J. Pharmacol. 2000, 130, 1083–1091. [Google Scholar] [CrossRef]
- Nielson, G.D. Guanethidine induced sympathectomy in the adult rat. II Functional effects following chronic administration. Acta Pharmacol. Toxicol. 1977, 41, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Favre-Maurice, R.; De Haut, M.; Dalmaz, Y.; Peyrin, L. Differential effect of guanethidine on dopamine and norepinephrine in rat peripheral tissues. J. Neural Transm. Gen. Sect. 1992, 88, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Manning, P.T.; Powers, C.W.; Schmidt, R.E.; Johnson, E.M., Jr. Guanethidine-induced destruction of peripheral sympathetic neurons occurs by an immune-mediated mechanism. J. Neurosci. 1983, 3, 714–724. [Google Scholar] [CrossRef]
- Benarroch, E.E.; Zollman, P.J.; Smithson, I.L.; Schmelzer, J.D.; Low, P.A. Different reinnervation patterns in the celiac/mesenteric and superior cervical ganglia following guanethidine sympathectomy in adult rats. Brain Res. 1994, 644, 322–326. [Google Scholar] [CrossRef]
- Heath, J.W.; Burnstock, G. Selectivity of neuronal degeneration produced by chronic guanethidine treatment. J. Neurocytol. 1977, 6, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Heath, J.W.; Evans, B.K.; Gannon, B.J.; Burnstock, G.; James, V.B. Degeneration of adrenergic neurons following guanethidine treatment: An ultrastructural study. Virchows Arch. B Cell Pathol. 1972, 11, 182–197. [Google Scholar] [CrossRef]
- Johnson, E.M.; Aloe, L. Suppression of the in vitro and in vivo cytotoxic effects of guanethidine in sympathetic neurons by nerve growth factor. Brain Res. 1974, 81, 519–532. [Google Scholar] [CrossRef]
- Johnson, E.M., Jr. Destruction of the sympathetic nervous system in neonatal rats and hamsters by vinblastine: Prevention by concomitant administration of nerve growth factor. Brain Res. 1978, 141, 105–118. [Google Scholar] [CrossRef]
- Johnson, E.M.; Taylor, A.S. Inhibition of S-adenosyl methionine decarboxylase by guanethidine. Biochem. Pharmacol. 1980, 29, 113–115. [Google Scholar] [CrossRef]
- Johnson, E.M.; O’Brien, F. Evaluation of the permanent sympathectomy produced by the administration of guanethidine to adult rats. J. Pharmacol. Exp. Ther. 1976, 196, 53–61. [Google Scholar]
- Milner, P.; Lincoln, J.; Corr, L.A.; Aberdeen, J.A.; Burnstock, G. Neuropeptide Y in non-sympathetic nerves of the rat: Changes during maturation but not after guanethidine sympathectomy. Neuroscience 1991, 43, 661–669. [Google Scholar] [CrossRef]
- Aberdeen, J.; Milner, P.; Lincoln, J.; Burnstock, G. Guanethidine sympathectomy of mature rats leads to increases in calcitonin gene-related peptide and vasoactive intestinal polypeptide-containing nerves. Neuroscience 1992, 47, 453–461. [Google Scholar] [CrossRef]
- Mattiasson, A.; Ekblad, E.; Sundler, F.; Uvelius, B. Origin and distribution of neuropeptide Y, vasoactive intestinal polypeptide and substance P- containing fibers in the urinary bladder of the rat. Cell Tissue Res. 1985, 239, 141–146. [Google Scholar] [CrossRef]
- Lundberg, J.M.; Tatemoto, K. Pancreatic polypeptide family (APP, BPP, NPY and PYY) in relation to aadrenoceptor-resistant sympathetic vasoconstriction. Acta Physiol. Scand. 1982, 116, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Rudehill, A.; Olcén, M.; Sollevi, A.; Hamberger, B.; Lundberg, J.M. Release of neuropeptide Y upon hemorrhagic hypovolemia in relation to vasoconstrictor effects in the pig. Acta Physiol. Scand. 1987, 131, 517–523. [Google Scholar] [CrossRef]
- Pernow, J.; Lundberg, J.M.; Kaijser, L. Vasoconstrictor effects in vivo and plasma disappearance rate of neuropeptide Y in man. Life Sci. 1987, 40, 47–54. [Google Scholar] [CrossRef]
- Stjärne, L.; Astrand, P. Relative pre- and postjunctional roles of noradrenaline and adenosine 5′-triphosphate as neurotransmitters of the sympathetic nerves of guinea-pig and mouse vas deferens. Neuroscience 1985, 14, 929–946. [Google Scholar] [CrossRef]
- Ellis, J.L.; Burnstock, G. Neuropeptide Y neuromodulation of sympathetic co-transmission in the guinea-pig vas deferens. Br. J. Pharmacol. 1990, 100, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Prieto, D.; Hernández, M.; Rivera, L.; García-Sacristán, A.; Simonsen, U. Distribution and functional effects of neuropeptide Y on equine ureteral smooth muscle and resistance arteries. Regul. Pept. 1997, 69, 155–165. [Google Scholar] [CrossRef]
- Iravani, M.M.; Zar, M.A. Neuropeptide Y in rat detrusor and its effect on nerve-mediated and acetylcholine-evoked contractions. Br. J. Pharmacol. 1994, 113, 95–102. [Google Scholar] [CrossRef][Green Version]
- Zoubek, J.; Somogyi, G.T.; De Groat, W.C. A comparison of inhibitory effects of neuropeptide Y on rat urinary bladder, urethra, and vas deferens. Am. J. Physiol. 1993, 265, R537–R543. [Google Scholar] [CrossRef]
- Tran, L.V.; Somogyi, G.T.; De Groat, W.C. Inhibitory effect of neuropeptide Y on adrenergic and cholinergic transmission in rat urinary bladder and urethra. Am. J. Physiol. 1994, 266, R1411–R1417. [Google Scholar] [CrossRef] [PubMed]
- Majewski, M.; Heym, C. Immunohistochemical localization of calcitonin gene-related peptide and cotransmitters in a subpopulation of postganglionic neurons in the porcine inferior mesenteric ganglion. Acta Histochem. 1992, 92, 138–146. [Google Scholar] [CrossRef]
- Lacroix, J.S.; Auberson, S.; Morel, D.R.; Theodorsson, E.; Hökfelt, T.; Lundberg, J.M. Vascular control of the pig nasal mucosa: Distribution and effect of somatostatin in relation to noradrenaline and neuropeptide Y. Regul. Pept. 1992, 40, 373–387. [Google Scholar] [CrossRef]
- Majewski, M. Synaptogenesis and structure of the autonomic ganglia. Folia Morphol. 1999, 58, 65–99. [Google Scholar]
- Sjögren, C.; Andersson, K.E.; Husted, S. Contractile effects of some polypeptides on the isolated urinary bladder of guinea-pig, rabbit, and rat. Acta Pharmacol. Toxicol. 1982, 50, 175–184. [Google Scholar] [CrossRef]
- Reubi, J.C.; Mazzucchelli, L.; Laissue, J.A. Intestinal vessels express a high density of somatostatin receptors in human inflammatory bowel disease. Gastroenterology 1994, 106, 951–959. [Google Scholar] [CrossRef]
- Bjorling, D.E.; Saban, M.R.; Saban, R. Effect of octreotide, a somatostatin analogue, on release of inflammatory mediators from isolated guinea pig bladder. J. Urol. 1997, 158, 258–264. [Google Scholar] [CrossRef]
- Patel, Y.C. Somatostatin and its receptor family. Front. Neuroendocrinol. 1999, 20, 157–198. [Google Scholar] [CrossRef]
- Erol, K.; Ulak, G.; Dönmez, T.; Cingi, M.I.; Alpan, R.S.; Ozdemir, M. Effects of vasoactive intestinal polypeptide on isolated rat urinary bladder smooth muscle. Urol. Int. 1992, 49, 151–153. [Google Scholar] [CrossRef]
- Igawa, Y.; Persson, K.; Andersson, K.E.; Uvelius, B.; Mattiasson, A. Facilitatory effect of vasoactive intestinal polypeptide on spinal and peripheral micturition reflex pathways in conscious rats with and without detrusor instability. J. Urol. 1993, 149, 884–889. [Google Scholar] [CrossRef]
- Hernández, M.; Barahona, M.V.; Recio, P.; Benedito, S.; Martínez, A.C.; Rivera, L.; García-Sacristán, A.; Prieto, D.; Orensanz, L.M. Neuronal and smooth muscle receptors involved in the PACAP- and VIP-induced relaxations of the pig urinary bladder neck. Br. J. Pharmacol. 2006, 149, 100–109. [Google Scholar] [CrossRef]
- Uckert, S.; Stief, C.G.; Lietz, B.; Burmester, M.; Jonas, U.; Machtens, S.A. Possible role of bioactive peptides in the regulation of human detrusor smooth muscle-functional effects in vitro and immunohistochemical presence. World J. Urol. 2002, 20, 244–249. [Google Scholar] [CrossRef]
- Levin, R.M.; Wein, A.J. Effect of vasoactive intestinal peptide on the contractility of the rabbit urinary bladder. Urol. Res. 1981, 9, 217–218. [Google Scholar] [CrossRef]
- Callahan, S.M.; Creed, K.E. Non-cholinergic neurotransmission and the effects of peptides on the urinary bladder of guinea-pigs and rabbits. J. Physiol. 1986, 374, 103–115. [Google Scholar] [CrossRef]
- Saito, M.; Kondo, A.; Gotoh, M.; Kato, K.; Levin, R.M. Age-related changes in the response of the rat urinary bladder to neurotransmitters. Neurourol. Urodyn. 1993, 12, 191–200. [Google Scholar] [CrossRef]
- Klarskov, P.; Gerstenberg, T.; Hald, T. Vasoactive intestinal polypeptide influence on lower urinary tract smooth muscle from human and pig. J. Urol. 1984, 131, 1000–1004. [Google Scholar] [CrossRef]
- Hills, J.; Meldrum, L.A.; Klarskov, P.; Burnstock, G. A novel non-adrenergic, non-cholinergic nerve-mediated relaxation of the pig bladder neck: An examination of possible neurotransmitter candidates. Eur. J. Pharmacol. 1984, 99, 287–293. [Google Scholar] [CrossRef]
- Yoshiyama, M.; de Groat, W.C. The role of vasoactive intestinal polypeptide and pituitary adenylate cyclase-activating polypeptide in the neural pathways controlling the lower urinary tract. J. Mol. Neurosci. 2008, 36, 227–240. [Google Scholar] [CrossRef]
- Hernández, M.; Barahona, M.V.; Recio, P.; Rivera, L.; Benedito, S.; Martínez, A.C.; García-Sacristán, A.; Orensanz, L.M.; Prieto, D. Heterogeneity of neuronal and smooth muscle receptors involved in the VIP- and PACAP-induced relaxations of the pig intravesical ureter. Br. J. Pharmacol. 2004, 141, 123–131. [Google Scholar] [CrossRef]
- Hosokawa, H.; Kaseda, M. Experimental studies on VIP as noncholinergic and non-adrenergic neurotransmitter in bladder neck and posterior urethra. Nippon Hinyokika Gakkai Zasshi 1993, 84, 440–449. [Google Scholar] [CrossRef][Green Version]
- Werkström, V.; Persson, K.; Andersson, K.E. NANC transmitters in the female pig urethra—localization and modulation of release via alpha 2-adrenoceptors and potassium channels. Br. J. Pharmacol. 1997, 121, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Maggi, C.A.; Santicioli, P.; Patacchini, R.; Turini, D.; Barbanti, G.; Beneforti, P.; Giuliani, S.; Meli, A. Galanin: A potent modulator of excitatory neurotransmission in the human urinary bladder. Eur. J. Pharmacol. 1987, 143, 135–137. [Google Scholar] [CrossRef]
- Honda, M.; Yoshimura, N.; Inoue, S.; Hikita, K.; Muraoka, K.; Saito, M.; Chancellor, M.B.; Takenaka, A. Inhibitory role of the spinal galanin system in the control of micturition. Urology 2013, 82, 1188.e9–1188.e14. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Andrade, J.M.; Zhou, S.; Du, J.; Yamani, A.; Grady, J.J.; Castaneda-Hernandez, G.; Carlton, S.M. Pro-nociceptive role of peripheral galanin in inflammatory pain. Pain 2004, 110, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Elliott-Hunt, C.R.; Pope, R.J.; Vanderplank, P.; Wynick, D. Activation of the galanin receptor 2 (GalR2) protects the hippocampus from neuronal damage. J. Neurochem. 2007, 100, 780–789. [Google Scholar] [CrossRef]
- Colvin, L.A.; Mark, M.A.; Duggan, A.W. The effect of a peripheral mononeuropathy on immunoreactive (ir)-galanin release in the spinal cord of the rat. Brain Res. 1997, 766, 259–261. [Google Scholar] [CrossRef]
- Callsen-Cencic, P.; Mense, S. Expression of neuropeptides and nitric oxide synthase in neurons innervating the inflamed rat urinary bladder. J. Auton. Nerv. Syst. 1997, 65, 33–44. [Google Scholar] [CrossRef]
- Wąsowicz, K. Effect of total or partial uterus extirpation on sympathetic uterus-projecting neurons in porcine inferior mesenteric ganglion. B. Changes in expression of neuropeptide Y, galanin, vasoactive intestinal polypeptide, pituitary adenylate-cyclase activating peptide, somatostatin and substance P. Pol. J. Vet. Sci. 2003, 6, 147–160. [Google Scholar]
- Schwaller, B.; Meyer, M.; Schiffmann, S. ‘New’ functions for ‘old’ proteins: The role of the calcium binding proteins calbindin D-28k, calretinin and parvalbumin, in celebellar physiology. Studies with knockout mice. Cerebellum 2002, 1, 241–258. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.S.; Lee, E.; Lim, J.; Oh, Y.J. Calbindin-D28K prevents drug-induced dopaminergic neuronal death by inhibiting caspase and calpain activity. Biochem. Biophys. Res. Commun. 2008, 371, 127–131. [Google Scholar] [CrossRef]
- Choi, W.S.; Oh, Y.J. Calbindin-D28K prevents staurosporininduced Bax cleavage and membrane permeabilization. Exp. Neurobiol. 2014, 23, 173–177. [Google Scholar] [CrossRef]
- Kozlowski, R.; Siroky, M.B.; Krane, R.J.; Azadzoi, K.M. Regulation of blood flow and microcirculation resistance in rabbit bladder. J. Urol. 2002, 168, 1608–1614. [Google Scholar] [CrossRef]
- Persson, K.; Andersson, K.E. Nitric oxide and relaxation of pig lower urinary tract. Br. J. Pharmacol. 1992, 106, 416–422. [Google Scholar] [CrossRef]
- Persson, K.; Alm, P.; Johansson, K.; Larsson, B.; Andersson, K.E. Nitric oxide synthase in pig lower urinary tract: Immunohistochemistry, NADPH diaphorase histochemistry and functional effects. Br. J. Pharmacol. 1993, 110, 521–530. [Google Scholar] [CrossRef]
- Vanhatalo, S.; Soinila, S. NADPH-diaphorase activity and its colocalization with transmitters and neuropeptides in the postganglionic neurons of the rat superior cervical ganglion. Brain Res. 1994, 652, 107–112. [Google Scholar] [CrossRef]
- Wojtkiewicz, J.; Równiak, M.; Crayton, R.; Barczewska, M.; Bladowski, M.; Robak, A.; Pidsudko, Z.; Majewski, M. Inflammation-Induced Changes in the Chemical Coding Pattern of Colon-Projecting Neurons in the Inferior Mesenteric Ganglia of the Pig. J. Mol. Neurosci. 2012, 46, 450–458. [Google Scholar] [CrossRef]
- Wojtkiewicz, J.; Równiak, M.; Crayton, R.; Gonkowski, S.; Robak, A.; Zalecki, M.; Majewski, M.; Klimaschewski, L. Axotomy-induced changes in the chemical coding pattern of colon projecting calbindin-positive neurons in the inferior mesenteric ganglia of the pig. J. Mol. Neurosci. 2013, 51, 99–108. [Google Scholar] [CrossRef]
- Purves-Tyson, T.D.; Keast, J.R. Rapid actions of estradiol on cyclic amp response-element binding protein phosphorylation in dorsal root ganglion neurons. Neuroscience 2004, 129, 629–637. [Google Scholar] [CrossRef]
- Koszykowska, M.; Całka, J.; Gańko, M.; Jana, B. Long-term estradiol-17β administration reduces population of neurons in the sympathetic chain ganglia supplying the ovary in adult gilts. Exp. Mol. Pathol. 2011, 91, 353–361. [Google Scholar] [CrossRef]
- Kaleczyc, J.; Timmermans, J.P.; Majewski, M.; Lakomy, M.; Mayer, B.; Scheuermann, D.W. NO-synthase-containing neurons of the pig inferior mesenteric ganglion, part of them innervating the ductus deferens. Acta Anat. 1994, 151, 62–67. [Google Scholar] [CrossRef]
- Bossowska, A.; Majewski, M. Botulinum toxin type A-induced changes in the chemical coding of dorsal root ganglion neurons supplying the porcine urinary bladder. Pol. J. Vet. Sci. 2012, 15, 345–353. [Google Scholar] [CrossRef]
- Lepiarczyk, E.; Bossowska, A.; Kaleczyc, J.; Skowrońska, A.; Majewska, M.; Majewski, M.; Majewski, M. The Influence of Resiniferatoxin (RTX) and Tetrodotoxin (TTX) on the Distribution, Relative Frequency, and Chemical Coding of Noradrenergic and Cholinergic Nerve Fibers Supplying the Porcine Urinary Bladder Wall. Toxins 2017, 9, 310. [Google Scholar] [CrossRef]
- Lepiarczyk, E.; Bossowska, A.; Kaleczyc, J.; Majewska, M.; Gonkowski, S.; Majewski, M. The Influence of Tetrodotoxin (TTX) on the Distribution and Chemical Coding of Caudal Mesenteric Ganglion (CaMG) Neurons Supplying the Porcine Urinary Bladder. Mar. Drugs 2017, 15, 101. [Google Scholar] [CrossRef] [PubMed]
- Kaleczyc, J.; Timmermans, J.P.; Majewski, M.; Lakomy, M.; Scheuermann, D.W. Immunohistochemical properties of nerve fibres supplying accessory male genital glands in the pig. A colocalisation study. Histochem. Cell Biol. 1999, 111, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Gonkowski, S.; Kamińska, B.; Landowski, P.; Całka, J. Immunohistochemical distribution of cocaine- and amphetamine-regulated transcript peptide-like immunoreactive (CART-LI) nerve fibers and various degree of co-localization with other neuronal factors in the circular muscle layer of human descending colon. Histol. Histopathol. 2013, 28, 851–858. [Google Scholar] [CrossRef] [PubMed]

| Part of the Urinary Bladder Wall | Control Pigs | GUA-Treated Pigs |
|---|---|---|
| Muscle layer | + | −↓ |
| Submucosal layer | ++ | −↓ |
| Under the urothelium | +/− | −↓ |
| Around blood vessels | ++++ | +↓ |
| Substances | Control Animals | GUA-Treated Animals | ||||||
|---|---|---|---|---|---|---|---|---|
| mL | sL | u | bv | mL | sL | u | bV | |
| DβH/NPY | ++++ | + | + | ++++ | −↓ | −↓ | −↓ | +/−↓ |
| DβH/SOM | ++ | + | + | +/− | −↓ | −↓ | −↓ | ++++↑ |
| DβH/VIP | +/− | − | − | − | −↓ | − | − | − |
| DβH/GAL | +/− | − | − | − | −↓ | − | − | − |
| DβH/CB | +/− | − | − | − | −↓ | − | − | − |
| DβH/NOS | − | − | − | − | − | − | − | −/+↑ |
| Experimental Groups | FB+/TH+ % | FB+/TH+/ NPY+ % | FB+/TH+/ SOM+ % | FB+/TH+/ CB+ % | FB+/TH+/ VIP+ % | FB+/TH+/ GAL+ % | FB+/TH+/ nNOS+ % |
|---|---|---|---|---|---|---|---|
| Control pigs | 94.3 ± 1.8 | 89.6 ± 0.7 | 3.6 ± 0.4 | 2.06 ± 0.2 | 1.6 ± 0.2 | 1.6 ± 0.3 | 0 |
| GUA-treated pigs | 73.3 ± 1.4 *** | 27.8 ±0.9 **** | 68.7 ± 1.9 **** | 9.1 ± 1.2 *** | 0 *** | 28.2 ± 1.3 **** | 4.5 ± 0.6 *** |
| Experimental Groups | FB+/TH- % | FB+/TH-/ NPY+ % | FB+/TH-/ SOM+ % | FB+/TH-/ CB+ % | FB+/TH-/ VIP+ % | FB+/TH-/ GAL+ % | FB+/TH-/ nNOS+ % |
|---|---|---|---|---|---|---|---|
| Control pigs | 5.7 ± 1.8 | 38.9 ± 6.7 | 0 | 0 | 14.8 ± 6.2 | 0 | 0 |
| GUA-treated pigs | 26.7 ± 1.4 *** | 28.9 ± 0.8 *** | 0 | 0 | 0 **** | 0 | 0 |
| Antigen | Code | Dilution | Host | Supplier |
|---|---|---|---|---|
| Primary Antibodies | ||||
| DβH | MAB 308 | 1:1000 | Mouse | Millipore; Temecula; CA; USA |
| TH | MAB318 | 1:800 | Mouse | Millipore; Temecula; CA; USA |
| NPY | NA 1233 | 1:8000 | Rabbit | Enzo Life Sciences; Farmingdale; NY; USA |
| SOM | T-4103 | 1:4000 | Rabbit | Peninsula; San Carlos; CA; USA |
| CB | CB-38a | 1:2000 | Rabbit | Swant; Marly; Fribourg; Switzerland |
| VIP | VA 1285-0025 | 1:3000 | Rabbit | Enzo Life Sciences; Farmingdale;NY; USA |
| GAL | AB 5909 | 1:2000 | Rabbit | Millipore; Temecula; CA; USA |
| nNOS | AB5380 | 1:4000 | Rabbit | Millipore; Temecula; CA; USA |
| Secondary Reagents | ||||
| Biotinylated anti-rabbit immunoglobulins | E 0432 | 1:1000 | Goat | Dako; Hamburg; Germany |
| CY3-conjugated streptavidin | 711-165-152 | 1:12,000 | - | Jackson I.R.; West Grove; PA; USA |
| FITC-conjugated anti-mouse IgG | 715-096-151 | 1:600 | Donkey | Jackson I.R.; West Grove; PA; USA |
| Density Assessment Scale | Density Description |
|---|---|
| − | nerve fibers not found |
| +/− | single nerve fibers |
| + | few nerve fibers |
| ++ | moderate number of nerve fibers |
| +++ | numerous nerve fibers |
| ++++ | a very dense meshwork of fibers |
| Antigen | Code | Supplier |
|---|---|---|
| DβH | MBS9218238 | MyBioSource; CA; USA |
| TH | AC21-0699-P | Abcore, Ramona, CA, USA |
| NPY | N3266 | Sigma, St. Louis, MO, USA |
| SOM | S9129 | Sigma, St. Louis, MO, USA |
| CB | AC21-2748-P | Abcore, Ramona, CA, USA |
| VIP | V6130 | Sigma, St. Louis, MO, USA |
| GAL | G5773 | Sigma, St. Louis, MO, USA |
| nNOS | N3033 | Sigma, St. Louis, MO, USA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bossowska, A.; Lepiarczyk, E.; Janikiewicz, P.; Wasilewska, B.; Mazur, U.; Markiewicz, W.; Majewski, M. The Influence of an Adrenergic Antagonist Guanethidine on the Distribution Pattern and Chemical Coding of Caudal Mesenteric Ganglion Perikarya and Their Axons Supplying the Porcine Bladder. Int. J. Mol. Sci. 2021, 22, 4896. https://doi.org/10.3390/ijms22094896
Bossowska A, Lepiarczyk E, Janikiewicz P, Wasilewska B, Mazur U, Markiewicz W, Majewski M. The Influence of an Adrenergic Antagonist Guanethidine on the Distribution Pattern and Chemical Coding of Caudal Mesenteric Ganglion Perikarya and Their Axons Supplying the Porcine Bladder. International Journal of Molecular Sciences. 2021; 22(9):4896. https://doi.org/10.3390/ijms22094896
Chicago/Turabian StyleBossowska, Agnieszka, Ewa Lepiarczyk, Paweł Janikiewicz, Barbara Wasilewska, Urszula Mazur, Włodzimierz Markiewicz, and Mariusz Majewski. 2021. "The Influence of an Adrenergic Antagonist Guanethidine on the Distribution Pattern and Chemical Coding of Caudal Mesenteric Ganglion Perikarya and Their Axons Supplying the Porcine Bladder" International Journal of Molecular Sciences 22, no. 9: 4896. https://doi.org/10.3390/ijms22094896
APA StyleBossowska, A., Lepiarczyk, E., Janikiewicz, P., Wasilewska, B., Mazur, U., Markiewicz, W., & Majewski, M. (2021). The Influence of an Adrenergic Antagonist Guanethidine on the Distribution Pattern and Chemical Coding of Caudal Mesenteric Ganglion Perikarya and Their Axons Supplying the Porcine Bladder. International Journal of Molecular Sciences, 22(9), 4896. https://doi.org/10.3390/ijms22094896






