Trefoil Factor Family (TFF) Peptides and Their Links to Inflammation: A Re-evaluation and New Medical Perspectives
Abstract
:1. Introduction
1.1. TFF Peptides: The “Classical” View
1.2. Exocrine TFF Peptides Occur in Different Molecular Forms and Have Diverse Molecular Functions
1.3. Pathological Expression of TFF Peptides: Links to Inflammation and Cancer
2. Regulation of TFF Expression by Inflammatory Mediators
2.1. Down-Regulation of TFF1 during Gastric Inflammation and Ectopic TFF1 Expression in Chronic Inflammatory Diseases
2.2. Regulation of TFF2 during Inflammation
2.3. Regulation of TFF3 during Inflammation
3. Role of TFF Peptides for Inflammatory Processes
3.1. Loss of TFF1 Is Linked to Antral Inflammation and Cancer
3.2. TFF2: Component of the Gastric Mucus Barrier (Lectin Binding to MUC6), Inhibition of Myeloid Cells (Anti-Inflammatory Factor), and Increased Synthesis of the Alarmin IL-33 (Promotion of Th2 Immunity)
3.3. Loss of Tff3 Is Linked to Increased DSS-Induced Colonic Inflammation
4. Conclusion and Medical Perspectives
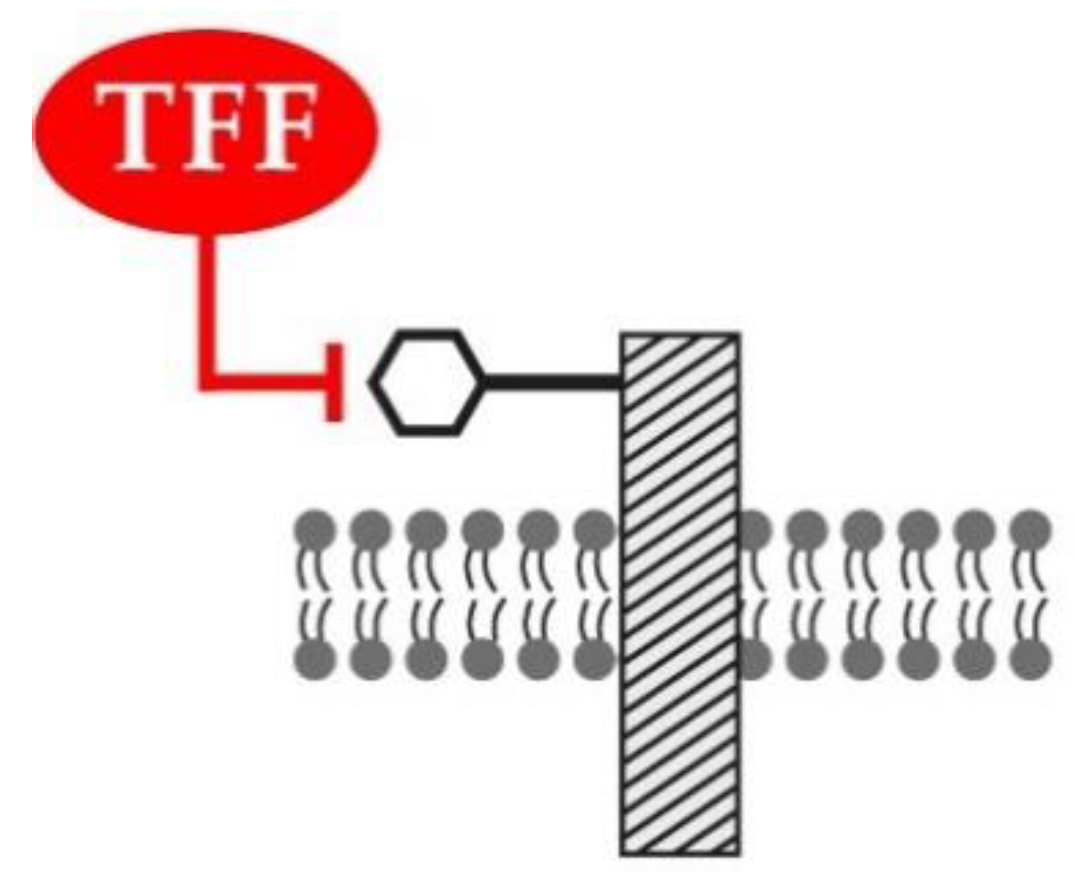
4.1. Lectin-Triggered Receptor Blocking by TFF Peptides: An Hypothesis
4.2. New Medical Perspectives
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CNS | Central nervous system |
| DAMPs | Danger-associated molecular patterns |
| DSS | Dextran sulfate sodium |
| ER | Endoplasmic reticulum |
| FCGBP | IgG Fc binding protein |
| FOX | Forkhead box |
| GKN | Gastrokine |
| IFN | Interferon |
| IL | Interleukin |
| NF-κB | Nuclear factor kappa B |
| PAMPs | Pathogen-associated molecular patterns |
| ROS | Reactive oxygen species |
| SDS | Sodium dodecyl sulfate |
| SMC | Surface mucous cell |
| TFF | Trefoil factor family |
| TGF | Transforming growth factor |
| TLR | Toll-like receptor |
| TNF | Tumor necrosis factor |
References
- Ribieras, S.; Tomasetto, C.; Rio, M.C. The pS2/TFF1 trefoil factor, from basic research to clinical applications. Biochim. Biophys. Acta 1998, 1378, F61–F77. [Google Scholar] [CrossRef]
- Thim, L.; May, F.E.B. Structure of mammalian trefoil factors and functional insights. Cell. Mol. Life Sci. 2005, 62, 2956–2973. [Google Scholar] [CrossRef]
- Kjellev, S. The trefoil factor family—small peptides with multiple functionalities. Cell. Mol. Life Sci. 2009, 66, 1350–1369. [Google Scholar] [CrossRef]
- Hoffmann, W. Trefoil factor family (TFF) peptides and their diverse molecular functions in mucus barrier protection and more: Changing the paradigm. Int. J. Mol. Sci. 2020, 21, 4535. [Google Scholar] [CrossRef] [PubMed]
- Wright, N.A.; Hoffmann, W.; Otto, W.R.; Rio, M.C.; Thim, L. Rolling in the clover: Trefoil factor family (TFF)-domain peptides, cell migration and cancer. FEBS Lett. 1997, 408, 121–123. [Google Scholar] [CrossRef] [Green Version]
- Thim, L. A new family of growth factor-like peptides. ‘Trefoil’ disulphide loop structures as a common feature in breast cancer associated peptide (pS2), pancreatic spasmolytic polypeptide (PSP), and frog skin peptides (spasmolysins). FEBS Lett. 1989, 250, 85–90. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, W.; Hauser, F. The P-domain or trefoil motif: A role in renewal and pathology of mucous epithelia? Trends Biochem. Sci. 1993, 18, 239–243. [Google Scholar] [CrossRef]
- Heuer, F.; Stürmer, R.; Heuer, J.; Kalinski, T.; Lemke, A.; Meyer, F.; Hoffmann, W. Different forms of TFF2, a lectin of the human gastric mucus barrier: In vitro binding studies. Int. J. Mol. Sci. 2019, 20, 5871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Znalesniak, E.B.; Salm, F.; Hoffmann, W. Molecular alterations in the stomach of Tff1-deficient mice: Early steps in antral carcinogenesis. Int. J. Mol. Sci. 2020, 21, 644. [Google Scholar] [CrossRef] [Green Version]
- Hanisch, F.-G.; Ragge, H.; Kalinski, T.; Meyer, F.; Kalbacher, H.; Hoffmann, W. Human gastric TFF2 peptide contains an N-linked fucosylated N,N′-diacetyllactosediamine (LacdiNAc) oligosaccharide. Glycobiology 2013, 23, 2–11. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, W.; Jagla, W. Cell type specific expression of secretory TFF peptides: Colocalization with mucins and synthesis in the brain. Int. Rev. Cytol. 2002, 213, 147–188. [Google Scholar]
- Hoffmann, W.; Jagla, W.; Wiede, A. Molecular medicine of TFF-peptides: From gut to brain. Histol. Histopathol. 2001, 16, 319–334. [Google Scholar]
- Madsen, J.; Nielsen, O.; Tornoe, I.; Thim, L.; Holmskov, U. Tissue localization of human trefoil factors 1, 2, and 3. J. Histochem. Cytochem. 2007, 55, 505–513. [Google Scholar] [CrossRef]
- Cook, G.A.; Familari, M.; Thim, L.; Giraud, A.S. The trefoil peptides TFF2 and TFF3 are expressed in rat lymphoid tissues and participate in the immune response. FEBS Lett. 1999, 456, 155–159. [Google Scholar] [CrossRef] [Green Version]
- Baus-Loncar, M.; Kayademir, T.; Takaishi, S.; Wang, T. Trefoil factor family 2 deficiency and immune response. Cell. Mol. Life Sci. 2005, 62, 2947–2955. [Google Scholar] [CrossRef]
- Kurt-Jones, E.A.; Cao, L.; Sandor, F.; Rogers, A.B.; Whary, M.T.; Nambiar, P.R.; Cerny, A.; Bowen, G.; Yan, J.; Takaishi, S.; et al. Trefoil family factor 2 is expressed in murine gastric and immune cells and controls both gastrointestinal inflammation and systemic immune responses. Infect. Immun. 2007, 75, 471–480. [Google Scholar] [CrossRef] [Green Version]
- Judd, L.M.; Chalinor, H.V.; Walduck, A.; Pavlic, D.I.; Däbritz, J.; Dubeykovskaya, Z.; Wang, T.C.; Menheniott, T.R.; Giraud, A.S. TFF2 deficiency exacerbates weight loss and alters immune cell and cytokine profiles in DSS colitis, and this cannot be rescued by wild-type bone marrow. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, G12–G24. [Google Scholar] [CrossRef] [PubMed]
- Dubeykovskaya, Z.; Si, Y.; Chen, X.; Worthley, D.L.; Renz, B.W.; Urbanska, A.M.; Hayakawa, Y.; Xu, T.; Westphalen, C.B.; Dubeykovskiy, A.; et al. Neural innervation stimulates splenic TFF2 to arrest myeloid cell expansion and cancer. Nat. Commun. 2016, 7, 10517. [Google Scholar] [CrossRef]
- Dignass, A.; Lynch-Devaney, K.; Kindon, H.; Thim, L.; Podolsky, D.K. Trefoil peptides promote epithelial migration through a transforming growth factor β-independent pathway. J. Clin. Investig. 1994, 94, 376–383. [Google Scholar] [CrossRef]
- Hoffmann, W. Trefoil factor family (TFF) peptides and their different roles in the mucosal innate immune defense and more: An update. Curr. Med. Chem. 2021, 28. in press. [Google Scholar] [CrossRef] [PubMed]
- Otto, W.R.; Thim, L. Trefoil factor family-interacting proteins. Cell. Mol. Life Sci. 2005, 62, 2939–2946. [Google Scholar] [CrossRef]
- Järvå, M.A.; Lingford, J.P.; John, A.; Soler, N.M.; Scott, N.E.; Goddard-Borger, E.D. Trefoil factors share a lectin activity that defines their role in mucus. Nat. Commun. 2020, 11, 2265. [Google Scholar] [CrossRef]
- Hoffmann, W. TFF2, a MUC6-binding lectin stabilizing the gastric mucus barrier and more. Int. J. Oncol. 2015, 47, 806–816. [Google Scholar] [CrossRef] [Green Version]
- Thim, L.; Mørtz, E. Isolation and characterization of putative trefoil peptide receptors. Regul. Pept. 2000, 90, 61–68. [Google Scholar] [CrossRef]
- Madsen, J.; Sorensen, G.L.; Nielsen, O.; Tornøe, I.; Thim, L.; Fenger, C.; Mollenhauer, J.; Holmskov, U. A variant form of the human deleted in malignant brain tumor 1 (DMBT1) gene shows increased expression in inflammatory bowel diseases and interacts with dimeric trefoil factor 3 (TFF3). PLoS ONE 2013, 8, e64441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubeykovskaya, Z.; Dubeykovskiy, A.; Solal-Cohen, J.; Wang, T.C. Secreted trefoil factor 2 activates the CXCR4 receptor in epithelial and lymphocytic cancer cell lines. J. Biol. Chem. 2009, 284, 3650–3662. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, W. Trefoil factor family (TFF) peptides and chemokine receptors: A promising relationship. J. Med. Chem. 2009, 52, 6505–6510. [Google Scholar] [CrossRef]
- Dieckow, J.; Brandt, W.; Hattermann, K.; Schob, S.; Schulze, U.; Mentlein, R.; Ackermann, P.; Sel, S.; Paulsen, F.P. CXCR4 and CXCR7 mediate TFF3-induced cell migration independently from the ERK1/2 signaling pathway. Investig. Ophthalmol. Sci. 2016, 57, 56–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrera Roa, G.J.; Sanchez Tortolero, G. Trefoil factor 3 (TFF3) from human breast milk activates PAR-2 receptors, of the intestinal epithelial cells HT-29, regulating cytokines and defensins. Bratisl. Med. J. 2016, 117, 332–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Yu, G.; Wang, Y.; Xiang, Y.; Gao, Q.; Jiang, P.; Zhang, J.; Lee, W.; Zhang, Y. Activation of protease-activated receptor (PAR) 1 by frog trefoil factor (TFF) 2 and PAR4 by human TFF2. Cell. Mol. Life Sci. 2011, 68, 3771–3780. [Google Scholar] [CrossRef]
- Belle, N.M.; Ji, Y.; Herbine, K.; Wei, Y.; Park, J.; Zullo, K.; Hung, L.Y.; Srivatsa, S.; Young, T.; Oniskey, T.; et al. TFF3 interacts with LINGO2 to regulate EGFR activation for protection against colitis and gastrointestinal helminths. Nat. Commun. 2019, 10, 4408. [Google Scholar] [CrossRef] [Green Version]
- Zullo, K.; Ji, Y.; Wei, Y.; Herbine, K.; Maloney, N.; Cohen, R.; Pastore, C.; Somsouk, M.; Srivatsa, S.; Hung, L.-Y. Lingo3 interacts with TFF2 to control mucosal integrity, type 1 inflammation, and colitic tissue repair. J. Immunol. 2019, 202, 192.8. [Google Scholar]
- Kouznetsova, I.; Laubinger, W.; Kalbacher, H.; Kalinski, T.; Meyer, F.; Roessner, A.; Hoffmann, W. Biosynthesis of gastrokine-2 in the human gastric mucosa: Restricted spatial expression along the antral gland axis and differential interaction with TFF1, TFF2 and mucins. Cell. Physiol. Biochem. 2007, 20, 899–908. [Google Scholar] [CrossRef] [Green Version]
- Albert, T.K.; Laubinger, W.; Müller, S.; Hanisch, F.-G.; Kalinski, T.; Meyer, F.; Hoffmann, W. Human intestinal TFF3 forms disulfide-linked heteromers with the mucus-associated FCGBP protein and is released by hydrogen sulfide. J. Proteome Res. 2010, 9, 3108–3117. [Google Scholar] [CrossRef]
- Stürmer, R.; Müller, S.; Hanisch, F.-G.; Hoffmann, W. Porcine gastric TFF2 is a mucus constituent and differs from pancreatic TFF2. Cell. Physiol. Biochem. 2014, 33, 895–904. [Google Scholar] [CrossRef]
- Stürmer, R.; Harder, S.; Schlüter, H.; Hoffmann, W. Commercial porcine gastric mucin preparations, also used as artificial saliva, are a rich source for the lectin TFF2: In vitro binding studies. ChemBioChem 2018, 19, 2598–2608. [Google Scholar] [CrossRef] [PubMed]
- Heuer, J.; Heuer, F.; Stürmer, R.; Harder, S.; Schlüter, H.; Braga Emidio, N.; Muttenthaler, M.; Jechorek, D.; Meyer, F.; Hoffmann, W. The Tumor suppressor TFF1 occurs in different forms and interacts with multiple partners in the human gastric mucus barrier: Indications for diverse protective functions. Int. J. Mol. Sci. 2020, 21, 2508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stürmer, R.; Reising, J.; Hoffmann, W. The TFF peptides xP1 and xP4 appear in distinctive forms in the Xenopus laevis gastric mucosa: Indications for different protective functions. Int. J. Mol. Sci. 2019, 20, 6052. [Google Scholar] [CrossRef] [Green Version]
- Fra, A.M.; Fagioli, C.; Finazzi, D.; Sitia, R.; Alberini, C.M. Quality control of ER synthesized proteins: An exposed thiol group as a three-way switch mediating assembly, retention and degradation. EMBO J. 1993, 12, 4755–4761. [Google Scholar] [CrossRef]
- Reddy, P.; Sparvoli, A.; Fagioli, C.; Fassina, G.; Sitia, R. Formation of reversible disulfide bonds with the protein matrix of the endoplasmic reticulum correlates with the retention of unassembled Ig light chains. EMBO J. 1996, 15, 2077–2085. [Google Scholar] [CrossRef] [PubMed]
- Torres, L.-F.; Karam, S.M.; Wendling, C.; Chenard, M.-P.; Kershenobich, D.; Tomasetto, C.; Rio, M.-C. Trefoil factor 1 (TFF1/pS2) deficiency activates the unfolded protein response. Mol. Med. 2002, 8, 273–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westley, B.R.; Griffin, S.M.; May, F.E.B. Interaction between TFF1, a gastric tumor suppressor trefoil protein, and TFIZ1, a brichos domain-containing protein with homology to SP-C. Biochemistry 2005, 44, 7967–7975. [Google Scholar] [CrossRef]
- Reeves, E.P.; Ali, T.; Leonard, P.; Hearty, S.; O’Kennedy, R.; May, F.E.B.; Westley, B.R.; Josenhans, C.; Rust, M.; Suerbaum, S.; et al. Helicobacter pylori lipopolysaccharide interacts with TFF1 in a pH-dependent manner. Gastroenterology 2008, 135, 2043–2054. [Google Scholar] [CrossRef]
- Braga Emidio, N.; Baik, H.; Lee, D.; Stürmer, R.; Heuer, J.; Elliott, A.G.; Blaskovich, M.A.; Haupenthal, K.; Tegtmeyer, N.; Hoffmann, W.; et al. Chemical synthesis of human trefoil factor 1 (TFF1) and its homodimer provides novel insights into their mechanisms of action. Chem. Commun. 2020, 56, 6420–6423. [Google Scholar] [CrossRef] [PubMed]
- Hanisch, F.-G.; Bonar, D.; Schloerer, N.; Schroten, H. Human trefoil factor 2 is a lectin that binds α-GlcNAc-capped mucin glycans with antibiotic activity against Helicobacter pylori. J. Biol. Chem. 2014, 289, 27363–27375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oinuma, T.; Ide, S.; Kawano, J.; Suganuma, T. Purification and immunohistochemistry of Griffonia simplicifolia agglutinin-II-binding mucus glycoprotein in rat stomach. Glycobiology 1994, 4, 469–475. [Google Scholar] [CrossRef]
- Lang, T.; Klasson, S.; Larsson, E.; Johansson, M.E.; Hansson, G.C.; Samuelsson, T. Searching the evolutionary origin of epithelial mucus protein components - mucins and FCGBP. Mol. Biol. Evol. 2016, 33, 1921–1936. [Google Scholar] [CrossRef]
- Thim, L.; Madsen, F.; Poulsen, S.S. Effect of trefoil factors on the viscoelastic properties of mucus gels. Eur. J. Clin. Investig. 2002, 32, 519–527. [Google Scholar] [CrossRef]
- Schwarz, H.; Hoffmann, W. Subcellular localization of the TFF peptides xP1 and xP4 in the Xenopus laevis gastric/esophageal mucosa: Different secretion modes reflecting diverse protective functions. Int. J. Mol. Sci. 2020, 21, 761. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Wang, P.; Hoshino, H.; Ito, Y.; Kobayashi, M.; Nakayama, J.; Seeberger, P.H.; Fukuda, M. α1,4GlcNAc-capped mucin-type O-glycan inhibits cholesterol α-glucosyltransferase from Helicobacter pylori and suppresses H. pylori growth. Glycobiology 2008, 18, 549–558. [Google Scholar] [CrossRef] [Green Version]
- Houben, T.; Harder, S.; Schlüter, H.; Kalbacher, H.; Hoffmann, W. Different forms of TFF3 in the human saliva: Heterodimerization with IgG Fc binding protein (FCGBP). Int. J. Mol. Sci. 2019, 20, 5000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, K.; Ogata, H.; Morikawa, M.; Iijima, S.; Harada, N.; Yoshida, T.; Brown, W.R.; Inoue, N.; Hamada, Y.; Ishii, H.; et al. Distribution and partial characterisation of IgG Fc binding protein in various mucin producing cells and body fluids. Gut 2002, 51, 169–176. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Wang, R.; Su, B.; Luo, Y.; Terhune, J.; Beck, B.; Peatman, E. Evasion of mucosal defenses during Aeromonas hydrophila infection of channel catfish (Ictalurus punctatus) skin. Dev. Comp. Immunol. 2013, 39, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Y.; Liu, H.; Zhang, Q.; Song, H.; Tang, J.; Fu, J.; Wang, X. FcGBP was upregulated by HPV infection and correlated to longer survival time of HNSCC patients. Oncotarget 2017, 8, 86503–86514. [Google Scholar] [CrossRef] [PubMed]
- Lencer, W.I.; Blumberg, R.S. A passionate kiss, then run: Exocytosis and recycling of IgG by FcRn. Trends Cell Biol. 2005, 15, 5–9. [Google Scholar] [CrossRef]
- Schwartz, J.L. Fcgbp—A potential viral trap in RV144. Open AIDS J. 2014, 8, 21–24. [Google Scholar] [CrossRef] [Green Version]
- Wong, W.M.; Poulsom, R.; Wright, N.A. Trefoil peptides. Gut 1999, 44, 890–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wright, N.A. Aspects of the biology of regeneration and repair in the human gastrointestinal tract. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1998, 353, 925–933. [Google Scholar] [CrossRef] [Green Version]
- Rio, M.-C.; Chenard, M.P.; Wolf, C.; Marcellin, L.; Tomasetto, C.; Lathe, R.; Bellocq, J.P.; Chambon, P. Induction of pS2 and hSP genes as markers of mucosal ulceration of the digestive tract. Gastroenterology 1991, 100, 375–379. [Google Scholar] [CrossRef]
- Wong, W.M.; Playford, R.J.; Wright, N.A. Peptide gene expression in gastrointestinal mucosal ulceration: Ordered sequence or redundancy? Gut 2000, 46, 286–292. [Google Scholar] [CrossRef] [Green Version]
- Viby, N.-E.; Nexo, E.; Kissow, H.; Andreassen, H.; Clementsen, P.; Thim, L.; Poulsen, S.S. Trefoil factors (TFFs) are increased in bronchioalveolar lavage fluid from patients with chronic obstructive lung disease (COPD). Peptides 2015, 63, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, W. TFF (trefoil factor family) peptides and their potential roles for differentiation processes during airway remodeling. Curr. Med. Chem. 2007, 14, 2716–2719. [Google Scholar] [CrossRef] [PubMed]
- Goldenring, J.R.; Nam, K.T.; Wang, T.C.; Mills, J.C.; Wright, N.A. Spasmolytic polypeptide-expressing metaplasia and intestinal metaplasia: Time for reevaluation of metaplasias and the origins of gastric cancer. Gastroenterology 2010, 138, 2207–2210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- May, F.E.; Westley, B.R. Trefoil proteins: Their role in normal and malignant cells. J. Pathol. 1997, 183, 4–7. [Google Scholar] [CrossRef]
- Katoh, M. Trefoil factors and human gastric cancer. Int. J. Mol. Med. 2003, 12, 3–9. [Google Scholar] [CrossRef]
- Emami, S.; Rodrigues, S.; Rodrigue, C.M.; Le Floch, N.; Rivat, C.; Attoub, S.; Bruyneel, E.; Gespach, C. Trefoil factor family (TFF) peptides and cancer progression. Peptides 2004, 25, 885–898. [Google Scholar] [CrossRef]
- Regalo, G.; Wright, N.A.; Machado, J.C. Trefoil factors: From ulceration to neoplasia. Cell. Mol. Life Sci. 2005, 62, 2910–2915. [Google Scholar] [CrossRef]
- Perry, J.K.; Kannan, N.; Grandison, P.M.; Mitchell, M.D.; Lobie, P.E. Are trefoil factors oncogenic? Trends Endocrinol. Metab. 2008, 19, 74–81. [Google Scholar] [CrossRef]
- Mantovani, A.; Sica, A.; Sozziani, S.; Allavena, P.; Vecchi, A.; Locati, M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef]
- Neurath, M.F.; Finotto, S.; Glimcher, L.H. The role of Th1/Th2 polarization in mucosal immunity. Nat. Med. 2002, 8, 567–573. [Google Scholar] [CrossRef]
- Sallusto, F. Heterogeneity of human CD4+ T cells against microbes. Annu. Rev. Immunol. 2016, 34, 317–334. [Google Scholar] [CrossRef] [PubMed]
- Ruterbusch, M.; Pruner, K.B.; Shehata, L.; Pepper, M. In vivo CD4+ T cell differentiation and function: Revisiting the Th1/Th2 paradigm. Annu. Rev. Immunol. 2020, 38, 705–725. [Google Scholar] [CrossRef]
- Karin, M.; Clevers, H. Reparative inflammation takes charge of tissue regeneration. Nature 2016, 529, 307–315. [Google Scholar] [CrossRef]
- Karin, M.; Lawrence, T.; Nizet, V. Innate immunity gone awry: Linking microbial infections to chronic inflammation and cancer. Cell 2006, 124, 823–835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef]
- Mantovani, A. Cancer: Inflaming metastasis. Nature 2009, 457, 36–37. [Google Scholar] [CrossRef]
- Lin, W.W.; Karin, M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J. Clin. Investig. 2007, 117, 1175–1183. [Google Scholar] [CrossRef]
- West, N.R.; McCuaig, S.; Franchini, F.; Powrie, F. Emerging cytokine networks in colorectal cancer. Nat. Rev. Immunol. 2015, 15, 615–629. [Google Scholar] [CrossRef]
- Baus-Loncar, M.; Giraud, A.S. Multiple regulatory pathways for trefoil factor (TFF) genes. Cell. Mol. Life Sci. 2005, 62, 2921–2931. [Google Scholar] [CrossRef] [PubMed]
- Giraud, A.S.; Jackson, C.; Menheniott, T.R.; Judd, L.M. Differentiation of the gastric mucosa IV. role of trefoil peptides and IL-6 cytokine family signaling in gastric homeostasis. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G1–G5. [Google Scholar] [CrossRef]
- Beck, S.; Sommer, P.; Blin, N.; Gött, P. 5′-flanking motifs control cell-specific expression of trefoil factor genes (TFF). Int. J. Mol. Med. 1998, 2, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Van De Bovenkamp, J.H.; Korteland-Van Male, A.M.; Büller, H.A.; Einerhand, A.W.; Dekker, J. Infection with Helicobacter pylori affects all major secretory cell populations in the human antrum. Dig. Dis. Sci. 2005, 50, 1078–1086. [Google Scholar] [CrossRef]
- Esposito, R.; Morello, S.; Vllahu, M.; Eletto, D.; Porta, A.; Tosco, A. Gastric TFF1 expression from acute to chronic Helicobacter infection. Front. Cell. Infect. Microbiol. 2017, 7, 434. [Google Scholar] [CrossRef] [PubMed]
- Backert, S.; Naumann, M. What a disorder: Proinflammatory signaling pathways induced by Helicobacter pylori. Trends Microbiol. 2010, 18, 479–486. [Google Scholar] [CrossRef]
- Bornschein, J.; Malfertheiner, P. Helicobacter pylori and gastric cancer. Dig. Dis. 2014, 32, 249–264. [Google Scholar] [CrossRef]
- Dossinger, V.; Kayademir, T.; Blin, N.; Gött, P. Down-regulation of TFF expression in gastrointestinal cell lines by cytokines and nuclear factors. Cell. Physiol. Biochem. 2002, 12, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Franic, T.V.; van Driel, I.R.; Gleeson, P.A.; Giraud, A.S.; Judd, L.M. Reciprocal changes in trefoil 1 and 2 expression in stomachs of mice with gastric unit hypertrophy and inflammation. J. Pathol. 2005, 207, 43–52. [Google Scholar] [CrossRef]
- Soutto, M.; Belkhiri, A.; Piazuelo, M.B.; Schneider, B.G.; Peng, D.; Jiang, A.; Washington, M.K.; Kokoye, Y.; Crowe, S.E.; Zaika, A.; et al. Loss of TFF1 is associated with activation of NF-κB-mediated inflammation and gastric neoplasia in mice and humans. J. Clin. Investig. 2011, 121, 1753–1767. [Google Scholar] [CrossRef]
- Guillemin, K.; Salama, N.R.; Tompkins, L.S.; Falkow, S. Cag pathogenicity island-specific responses of gastric epithelial cells to Helicobacter pylori infection. Proc. Natl. Acad. Sci. USA 2002, 99, 15136–15141. [Google Scholar] [CrossRef] [Green Version]
- Tebbutt, N.C.; Giraud, A.S.; Inglese, M.; Jenkins, B.; Waring, P.; Clay, F.J.; Malki, S.; Alderman, B.M.; Grail, D.; Hollande, F. Reciprocal regulation of gastrointestinal homeostasis by SHP2 and STAT-mediated trefoil gene activation in gp130 mutant mice. Nat. Med. 2002, 8, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Soutto, M.; Chen, Z.; Katsha, A.M.; Romero-Gallo, J.; Krishna, U.S.; Piazuelo, M.B.; Washington, M.K.; Peek, R.M., Jr.; Belkhiri, A.; El-Rifai, W.M. Trefoil factor 1 expression suppresses Helicobacter pylori-induced inflammation in gastric carcinogenesis. Cancer 2015, 121, 4348–4358. [Google Scholar] [CrossRef] [Green Version]
- Wright, N.A.; Poulsom, R.; Stamp, G.; Van Noorden, S.; Sarraf, C.; Elia, G.; Ahnen, D.; Jeffery, R.; Longcroft, J.; Pike, C. Trefoil peptide gene expression in gastrointestinal epithelial cells in inflammatory bowel disease. Gastroenterology 1993, 104, 12–20. [Google Scholar] [CrossRef]
- Ebert, M.P.A.; Hoffmann, J.; Haeckel, C.; Rutkowski, K.; Schmid, R.M.; Wagner, M.; Adler, G.; Schulz, H.U.; Roessner, A.; Hoffmann, W.; et al. Induction of TFF1 gene expression in pancreas overexpressing transforming growth factor α. Gut 1999, 45, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Znalesniak, E.B.; Fu, T.; Guttek, K.; Händel, U.; Reinhold, D.; Hoffmann, W. Increased cerebral Tff1 expression in two murine models of neuroinflammation. Cell. Physiol. Biochem. 2016, 39, 2287–2296. [Google Scholar] [CrossRef]
- Kouznetsova, I.; Chwieralski, C.E.; Balder, R.; Hinz, M.; Braun, A.; Krug, N.; Hoffmann, W. Induced trefoil factor family 1 expression by trans-differentiating Clara cells in a murine asthma model. Am. J. Respir. Cell Mol. Biol. 2007, 36, 286–295. [Google Scholar] [CrossRef] [Green Version]
- Fu, T.; Znalesniak, E.B.; Kalinski, T.; Möhle, L.; Biswas, A.; Salm, F.; Dunay, I.R.; Hoffmann, W. TFF Peptides play a role in the immune response following oral infection of mice with Toxoplasma gondii. Eur. J. Microbiol. Immunol. 2015, 5, 221–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Znalesniak, E.B.; Fu, T.; Salm, F.; Händel, U.; Hoffmann, W. Transcriptional responses in the murine spleen after Toxoplasma gondii infection: Inflammasome and mucus-associated genes. Int. J. Mol. Sci. 2017, 18, 1245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koike, T.; Shimada, T.; Fujii, Y.; Chen, G.; Tabei, K.; Namatame, T.; Yamagata, M.; Tajima, A.; Yoneda, M.; Terano, A.; et al. Up-regulation of TFF1 (pS2) expression by TNF-α in gastric epithelial cells. Gastroenterol. Hepatol. 2007, 22, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Beck, S.; Sommer, P.; dos Santos Silva, E.; Blin, N.; Gött, P. Hepatocyte nuclear factor 3 (winged helix domain) activates trefoil factor gene TFF1 through a binding motif adjacent to the TATAA box. DNA Cell Biol. 1999, 18, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Hromas, R.; Costa, R. The hepatocyte nuclear factor-3/forkhead transcription regulatory family in development, inflammation, and neoplasia. Crit. Rev. Oncol. Hematol. 1995, 20, 129–140. [Google Scholar] [CrossRef]
- Zhen, G.; Park, S.W.; Nguyenvu, L.T.; Rodriguez, M.W.; Barbeau, R.; Paquet, A.C.; Erle, D.J. IL-13 and epidermal growth factor receptor have critical but distinct roles in epithelial cell mucin production. Am. J. Respir. Cell Mol. Biol. 2007, 36, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.C.; Goldenring, J.R. Inflammation intersection: gp130 balances gut irritation and stomach cancer. Nat. Med. 2002, 8, 1080–1082. [Google Scholar] [CrossRef] [PubMed]
- Judd, L.M.; Alderman, B.M.; Howlett, M.; Shulkes, A.; Dow, C.; Moverley, J.; Grail, D.; Jenkins, B.J.; Ernst, M.; Giraud, A.S. Gastric cancer development in mice lacking the SHP2 binding site on the IL-6 family co-receptor gp130. Gastroenterology 2004, 126, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Tomasetto, C.; Rio, M.C. Pleiotropic effects of trefoil factor 1 deficiency. Cell. Mol. Life Sci. 2005, 62, 2916–2920. [Google Scholar] [CrossRef]
- Hirota, M.; Awatsuji, H.; Furukawa, Y.; Hayashi, K. Cytokine regulation of PS2 gene expression in mouse astrocytes. Biochem. Mol. Biol. Int. 1994, 33, 515–520. [Google Scholar]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Trefoil factor family member 2 (TFF2) as an inflammatory-induced and anti-inflammatory tissue repair factor. Animals 2020, 10, 1646. [Google Scholar] [CrossRef]
- Royce, S.G.; Lim, C.; Muljadi, R.C.; Samuel, C.S.; Ververis, K.; Karagiannis, T.C.; Giraud, A.S.; Tang, M.L. Trefoil factor-2 reverses airway remodeling changes in allergic airways disease. Am. J. Respir. Cell Mol. Biol. 2013, 48, 135–144. [Google Scholar] [CrossRef]
- Kang, W.; Rathinavelu, S.; Samuelson, L.C.; Merchant, J.L. Interferon gamma induction of gastric mucous neck cell hypertrophy. Lab. Investig. 2005, 85, 702–715. [Google Scholar] [CrossRef] [Green Version]
- Shimada, T.; Fujii, Y.; Koike, T.; Tabei, K.; Namatame, T.; Yamagata, M.; Tajima, A.; Yoneda, M.; Terano, A.; Hraishi, H. Peroxisome proliferator-activated receptor γ (PPARγ) regulates trefoil factor family 2 (TFF2) expression in gastric epithelial cells. Int. J. Biochem. Cell Biol. 2007, 39, 626–637. [Google Scholar] [CrossRef] [PubMed]
- Nikolaidis, N.M.; Zimmermann, N.; King, N.E.; Mishra, A.; Pope, S.M.; Finkelman, F.D.; Rothenberg, M.E. Trefoil factor-2 is an allergen-induced gene regulated by Th2 cytokines and STAT6 in the lung. Am. J. Respir. Cell Mol. Biol. 2003, 29, 458–464. [Google Scholar] [CrossRef]
- Blanchard, C.; Durual, S.; Estienne, M.; Bouzakri, K.; Heim, M.H.; Blin, N.; Cuber, J.C. IL-4 and IL-13 up-regulate intestinal trefoil factor expression: Requirement for STAT6 and de novo protein synthesis. J. Immunol. 2004, 172, 3775–3783. [Google Scholar] [CrossRef] [Green Version]
- Steenwinckel, V.; Louahed, J.; Lemaire, M.M.; Sommereyns, C.; Warnier, G.; McKenzie, A.; Brombacher, F.; Van Snick, J.; Renauld, J.C. IL-9 promotes IL-13-dependent paneth cell hyperplasia and up-regulation of innate immunity mediators in intestinal mucosa. J. Immunol. 2009, 182, 4737–4743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamauchi, J.; Kawai, Y.; Yamada, M.; Uchikawa, R.; Tegoshi, T.; Arizono, N. Altered expression of goblet cell- and mucin glycosylation-related genes in the intestinal epithelium during infection with the nematode Nippostrongylus brasiliensis in rat. APMIS 2006, 114, 270–278. [Google Scholar] [CrossRef]
- Engevik, M.A.; Luk, B.; Chang-Graham, A.L.; Hall, A.; Herrmann, B.; Ruan, W.; Endres, B.T.; Shi, Z.; Garey, K.W.; Hyser, J.M.; et al. Bifidobacterium dentium fortifies the intestinal mucus layer via autophagy and calcium signaling pathways. mBio 2019, 10, e01087-19. [Google Scholar] [CrossRef] [Green Version]
- Manko-Prykhoda, A.; Allain, T.; Motta, J.P.; Cotton, J.A.; Feener, T.; Oyeyemi, A.; Bindra, S.; Vallance, B.A.; Wallace, J.L.; Beck, P.; et al. Giardia spp. promote the production of antimicrobial peptides and attenuate disease severity induced by attaching and effacing enteropathogens via the induction of the NLRP3 inflammasome. Int. J. Parasitol. 2020, 50, 263–275. [Google Scholar] [CrossRef]
- Bergstrom, K.S.; Guttman, J.A.; Rumi, M.; Ma, C.; Bouzari, S.; Khan, M.A.; Gibson, D.L.; Vogl, A.W.; Vallance, B.A. Modulation of intestinal goblet cell function during infection by an attaching and effacing bacterial pathogen. Infect. Immun. 2008, 76, 796–811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baus-Loncar, M.; Al-azzeh, E.D.; Sommer, P.S.; Marinovic, M.; Schmehl, K.; Kruschewski, M.; Blin, N.; Stohwasser, R.; Gött, P.; Kayademir, T. Tumour necrosis factor α and nuclear factor κB inhibit transcription of human TFF3 encoding a gastrointestinal healing peptide. Gut 2003, 52, 1297–1303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baus-Loncar, M.; Al-azzeh, E.D.; Romanska, H.; Lalani el, N.; Stamp, G.W.; Blin, N.; Kayademir, T. Transcriptional control of TFF3 (intestinal trefoil factor) via promoter binding sites for the nuclear factor κB and C/EBPβ. Peptides 2004, 25, 849–854. [Google Scholar] [CrossRef]
- Mashimo, H.; Wu, D.C.; Podolsky, D.K.; Fishman, M.C. Impaired defense of intestinal mucosa in mice lacking intestinal trefoil factor. Science 1996, 274, 262–265. [Google Scholar] [CrossRef]
- Podolsky, D.K.; Gerken, G.; Eyking, A.; Cario, E. Colitis-associated variant of TLR2 causes impaired mucosal repair because of TFF3 deficiency. Gastroenterology 2009, 137, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Pierik, M.; Joossens, S.; Van Steen, K.; Van Schuerbeek, N.; Vlietinck, R.; Rutgeerts, P.; Vermeire, S. Toll-like receptor-1, -2, and -6 polymorphisms influence disease extension in inflammatory bowel diseases. Inflamm. Bowel Dis. 2006, 12, 1–8. [Google Scholar] [CrossRef]
- Kont, V.; Laan, M.; Kisand, K.; Merits, A.; Scott, H.S.; Peterson, P. Modulation of Aire regulates the expression of tissue-restricted antigens. Mol. Immunol. 2008, 45, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Probst, J.C.; Skutella, T.; Müller-Schmid, A.; Jirikowski, G.F.; Hoffmann, W. Molecular and cellular analysis of rP1.B in the rat hypothalamus: In situ hybridization and immunohisdtochemistry of a new P-domain neuropeptide. Mol. Brain Res. 1995, 33, 269–276. [Google Scholar] [CrossRef]
- Hinz, M.; Schwegler, H.; Chwieralski, C.E.; Laube, G.; Linke, R.; Pohle, W.; Hoffmann, W. Trefoil factor family (TFF) expression in the mouse brain and pituitary: Changes in the developing cerebellum. Peptides 2004, 25, 827–832. [Google Scholar] [CrossRef]
- Fu, T.; Stellmacher, A.; Znalesniak, E.B.; Dieterich, D.C.; Kalbacher, H.; Hoffmann, W. Tff3 is expressed in neurons and microglial cells. Cell. Physiol. Biochem. 2014, 34, 1912–1919. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, H.G.; Dobrowolny, H.; Trübner, K.; Steiner, J.; Bogerts, B.; Hoffmann, W. Differential regional and cellular distribution of TFF3 peptide in the human brain. Amino Acids 2015, 47, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Kettenmann, H.; Kirchhoff, F.; Verkhratsky, A. Microglia: New roles for the synaptic stripper. Neuron 2013, 77, 10–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lefebvre, O.; Chenard, M.P.; Masson, R.; Linares, J.; Dierich, A.; LeMeur, M.; Wendling, C.; Tomasetto, C.; Chambon, P.; Rio, M.C. Gastric mucosa abnormalities and tumorigenesis in mice lacking the pS2 trefoil protein. Science 1996, 274, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Karam, S.M.; Tomasetto, C.; Rio, M.-C. Amplification and invasiveness of epithelial progenitors during gastric carcinogenesis in trefoil factor 1 knockout mice. Cell Prolif. 2008, 41, 923–935. [Google Scholar] [CrossRef] [PubMed]
- Hertel, S.C.; Chwieralski, C.E.; Hinz, M.; Rio, M.-C.; Tomasetto, C.; Hoffmann, W. Profiling trefoil factor family (TFF) expression in the mouse: Identification of an antisense TFF1-related transcript in the kidney and liver. Peptides 2004, 25, 755–762. [Google Scholar] [CrossRef]
- Peterson, A.J.; Menheniott, T.R.; O’Connor, L.; Walduck, A.K.; Fox, J.G.; Kawakami, K.; Minamoto, T.; Ong, E.K.; Wang, T.C.; Judd, L.M.; et al. Helicobacter pylori infection promotes methylation and silencing of trefoil factor 2, leading to gastric tumor development in mice and humans. Gastroenterology 2010, 139, 2005–2017. [Google Scholar] [CrossRef] [Green Version]
- Soutto, M.; Saleh, M.; Arredouani, M.S.; Piazuelo, B.; Belkhiri, A.; El-Rifai, W. Loss of Tff1 promotes pro-inflammatory phenotype with increase in the levels of RORγt+ T lymphocytes and Il-17 in mouse gastric neoplasia. J. Cancer 2017, 8, 2424–2435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saukkonen, K.; Tomasetto, C.; Narko, K.; Rio, M.-C.; Ristimaki, A. Cyclooxygenase-2 expression and effect of celecoxib in gastric adenomas of trefoil factor 1-deficient mice. Cancer Res. 2003, 63, 3032–3036. [Google Scholar]
- Bossenmeyer-Pourie, C.; Kannan, R.; Ribieras, S.; Wendling, C.; Stoll, I.; Thim, L.; Tomasetto, C.; Rio, M.-C. The trefoil factor 1 participates in gastrointestinal cell differentiation by delaying G1-S phase transition and reducing apoptosis. J. Cell Biol. 2002, 157, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Buache, E.; Etique, N.; Alpy, F.; Stoll, I.; Muckensturm, M.; Reina-San-Martin, B.; Chenard, M.P.; Tomasetto, C.; Rio, M.C. Deficiency in trefoil factor 1 (TFF1) increases tumorigenicity of human breast cancer cells and mammary tumor development in TFF1-knockout mice. Oncogene 2011, 30, 3261–3273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinoshita, H.; Hayakawa, Y.; Konishi, M.; Hata, M.; Tsuboi, M.; Hayata, Y.; Hikiba, Y.; Ihara, S.; Nakagawa, H.; Ikenoue, T.; et al. Three types of metaplasia model through Kras activation, Pten deletion, or Cdh1 deletion in the gastric epithelium. J. Pathol. 2019, 247, 35–47. [Google Scholar] [CrossRef]
- Thiem, S.; Eissmann, M.F.; Elzer, J.; Jonas, A.; Putoczki, T.L.; Poh, A.; Nguyen, P.; Preaudet, A.; Flanagan, D.; Vincan, E.; et al. Stomach-specific activation of oncogenic KRAS and STAT3-dependent inflammation cooperatively promote gastric tumorigenesis in a preclinical model. Cancer Res. 2016, 76, 2277–2287. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, W. Current status on stem cells and cancers of the gastric epithelium. Int. J. Mol. Sci. 2015, 16, 19153–19169. [Google Scholar] [CrossRef]
- Bjerknes, M.; Cheng, H. Multipotential stem cells in adult mouse gastric epithelium. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 283, G767–G777. [Google Scholar] [CrossRef] [Green Version]
- Stange, D.E.; Koo, B.K.; Huch, M.; Sibbel, G.; Basak, O.; Lyubimova, A.; Kujala, P.; Bartfeld, S.; Koster, J.; Geahlen, J.H.; et al. Differentiated Troy+ chief cells act as reserve stem cells to generate all lineages of the stomach epithelium. Cell 2013, 155, 357–368. [Google Scholar] [CrossRef] [Green Version]
- Barker, N.; Huch, M.; Kujala, P.; van de Wetering, M.; Snippert, H.J.; van Es, J.H.; Sato, T.; Stange, D.E.; Begthel, H.; van den Born, M.; et al. Lgr5+ve stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 2010, 6, 25–36. [Google Scholar] [CrossRef] [Green Version]
- Hayakawa, Y.; Jin, G.; Wang, H.; Chen, X.; Westphalen, C.B.; Asfaha, S.; Renz, B.W.; Ariyama, H.; Dubeykovskaya, Z.A.; Takemoto, Y.; et al. CCK2R identifies and regulates gastric antral stem cell states and carcinogenesis. Gut 2015, 64, 544–553. [Google Scholar] [CrossRef] [Green Version]
- Shishkin, S.S.; Eremina, L.S.; Kovalev, L.I.; Kovaleva, M.A. AGR2, ERp57/GRP58, and some other human protein disulfide isomerases. Biochemistry 2013, 78, 1415–1430. [Google Scholar] [CrossRef] [PubMed]
- Hauri, H.-P.; Appenzeller, C.; Kuhn, F.; Nufer, O. Lectins and traffic in the secretory pathway. FEBS Lett. 2000, 476, 32–37. [Google Scholar] [CrossRef] [Green Version]
- Karasawa, F.; Shiota, A.; Goso, Y.; Kobayashi, M.; Sato, Y.; Masumoto, J.; Fujiwara, M.; Yokosawa, S.; Muraki, T.; Miyagawa, S.; et al. Essential role of gastric gland mucin in preventing gastric cancer in mice. J. Clin. Investig. 2012, 122, 923–934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soutto, M.; Chen, Z.; Bhat, A.A.; Wang, L.; Zhu, S.; Gomaa, A.; Bates, A.; Bhat, N.S.; Peng, D.; Belkhiri, A.; et al. Activation of STAT3 signaling is mediated by TFF1 silencing in gastric neoplasia. Nat. Commun. 2019, 10, 3039. [Google Scholar] [CrossRef]
- Farrell, J.J.; Taupin, D.; Koh, T.J.; Chen, D.; Zhao, C.M.; Podolsky, D.K.; Wang, T.C. TFF2/SP-deficient mice show decreased gastric proliferation, increased acid secretion, and increased susceptibility to NSAID injury. J. Clin. Investig. 2002, 109, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Aihara, E.; Podolsky, D.K.; Wang, T.C.; Montrose, M.H. In vivo action of trefoil factor 2 (TFF2) to speed gastric repair is independent of cyclooxygenase. Gut 2010, 59, 1184–1191. [Google Scholar] [CrossRef]
- Fox, J.G.; Rogers, A.B.; Whary, M.T.; Ge, Z.; Ohtani, M.; Jones, E.K.; Wang, T.C. Accelerated progression of gastritis to dysplasia in the pyloric antrum of TFF2−/− C57BL6 x Sv129 Helicobacter pylori-infected mice. Am. J. Pathol. 2007, 171, 1520–1528. [Google Scholar] [CrossRef] [Green Version]
- Soriano-Izquierdo, A.; Gironella, M.; Massaguer, A.; May, F.E.; Salas, A.; Sans, M.; Poulsom, R.; Thim, L.; Piqué, J.M.; Panés, J. Trefoil peptide TFF2 treatment reduces VCAM-1 expression and leukocyte recruitment in experimental intestinal inflammation. J. Leukoc. Biol. 2004, 75, 214–223. [Google Scholar] [CrossRef]
- Vandenbroucke, K.; Hans, W.; Van Huysse, J.; Neirynck, S.; Demetter, P.; Remaut, E.; Rottiers, P.; Steidler, L. Active delivery of trefoil factors by genetically modified Lactococcus lactis prevents and heals acute colitis in mice. Gastroenterology 2004, 127, 502–513. [Google Scholar] [CrossRef]
- McBerry, C.; Egan, C.E.; Rani, R.; Yang, Y.; Wu, D.; Boespflug, N.; Boon, L.; Butcher, B.; Mirpuri, J.; Hogan, S.P.; et al. Trefoil factor 2 negatively regulates type 1 immunity against Toxoplasma gondii. J. Immunol. 2012, 189, 3078–3084. [Google Scholar] [CrossRef] [Green Version]
- Shah, A.A.; Mihalj, M.; Ratkay, I.; Lubka-Pathak, M.; Balogh, P.; Klingel, K.; Bohn, E.; Blin, N.; Baus-Lončar, M. Increased susceptibility to Yersinia enterocolitica Infection of Tff2 deficient mice. Cell. Physiol. Biochem. 2012, 30, 853–862. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, A.J.; Birchenough, G.M.H.; Taylor, P.W. Loss of trefoil factor 2 sensitizes rat pups to systemic infection with the neonatal pathogen Escherichia coli K1. Infect. Immun. 2019, 87, e00878-18. [Google Scholar] [CrossRef] [Green Version]
- Birchenough, G.M.; Johansson, M.E.; Stabler, R.A.; Dalgakiran, F.; Hansson, G.C.; Wren, B.W.; Luzio, J.P.; Taylor, P.W. Altered innate defenses in the neonatal gastrointestinal tract in response to colonization by neuropathogenic Escherichia coli. Infect. Immun. 2013, 81, 3264–3275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hung, L.-Y.; Sen, D.; Oniskey, T.K.; Katzen, J.; Cohen, N.A.; Vaughan, A.E.; Nieves, W.; Urisman, A.; Beers, M.F.; Krummel, M.F.; et al. Macrophages promote epithelial proliferation following infectious and non-infectious lung injury through a Trefoil factor 2-dependent mechanism. Mucosal Immunol. 2019, 12, 64–76. [Google Scholar] [CrossRef]
- Wills-Karp, M.; Rani, R.; Dienger, K.; Lewkowich, I.; Fox, J.G.; Perkins, C.; Lewis, L.; Finkelman, F.D.; Smith, D.E.; Bryce, P.J.; et al. Trefoil factor 2 rapidly induces interleukin 33 to promote type 2 immunity during allergic asthma and hookworm infection. J. Exp. Med. 2012, 209, 607–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buzzelli, J.N.; Chalinor, H.V.; Pavlic, D.I.; Sutton, P.; Menheniott, T.R.; Giraud, A.S.; Judd, L.M. IL33 is a stomach alarmin that initiates a skewed Th2 response to injury and infection. Cell. Mol. Gastroenterol. Hepatol. 2015, 1, 203–221.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vermeer, P.D.; Einwalter, L.A.; Moninger, T.O.; Rokhlina, T.; Kern, J.A.; Zabner, J.; Welsh, M.J. Segregation of receptor and ligand regulates activation of epithelial growth factor receptor. Nature 2003, 422, 322–326. [Google Scholar] [CrossRef]
- Katz-Kiriakos, E.; Steinberg, D.F.; Kluender, C.E.; Osorio, O.A.; Newsom-Stewart, C.; Baronia, A.; Byers, D.E.; Holtzman, M.J.; Katafiasz, D.; Bailey, K.L.; et al. Epithelial IL-33 appropriates exosome trafficking for secretion in chronic airway disease. JCI Insight 2021, 6, e136166. [Google Scholar]
- Engevik, K.A.; Hanyu, H.; Matthis, A.L.; Zhang, T.; Frey, M.R.; Oshima, Y.; Aihara, E.; Montrose, M.H. Trefoil factor 2 activation of CXCR4 requires calcium mobilization to drive epithelial repair in gastric organoids. J. Physiol. 2019, 597, 2673–2690. [Google Scholar] [CrossRef] [PubMed]
- Händel, T.M.; Dyer, D.P. Perspectives on the biological role of chemokine:glycosaminoglycan interactions. J. Histochem. Cytochem. 2021, 69, 87–91. [Google Scholar] [CrossRef]
- Park, S.-W.; Zhen, G.; Verhaeghe, C.; Nakagami, Y.; Nguyenvu, L.T.; Barczak, A.J.; Killeen, N.; Erle, D.J. The protein disulfide isomerase AGR2 is essential for production of intestinal mucus. Proc. Natl. Acad. Sci. USA 2009, 106, 6950–6955. [Google Scholar] [CrossRef] [Green Version]
- Johansson, M.E.; Larsson, J.M.; Hansson, G.C. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host-microbial interactions. Proc. Natl. Acad. Sci. USA 2011, 108 Suppl 1, 4659–4665. [Google Scholar] [CrossRef] [Green Version]
- Johansson, M.E.; Hansson, G.C. Immunological aspects of intestinal mucus and mucins. Nat. Rev. Immunol. 2016, 16, 639–649. [Google Scholar] [CrossRef]
- Johansson, M.E.; Gustafsson, J.K.; Sjöberg, K.E.; Petersson, J.; Holm, L.; Sjövall, H.; Hansson, G.C. Bacteria penetrate the inner mucus layer before inflammation in the dextran sulfate colitis model. PLoS ONE 2010, 5, e12238. [Google Scholar] [CrossRef] [Green Version]
- Hoebler, C.; Gaudier, E.; De Coppet, P.; Rival, M.; Cherbut, C. MUC genes are differently expressed during onset and maintenance of inflammation in dextran sodium sulfate-treated mice. Dig. Dis. Sci. 2006, 51, 381–389. [Google Scholar] [CrossRef]
- Kjellev, S.; Thim, L.; Pyke, C.; Poulsen, S.S. Cellular localization, binding sites, and pharmacologic effects of TFF3 in experimental colitis in mice. Dig. Dis. Sci. 2007, 52, 1050–1059. [Google Scholar] [CrossRef]
- Madsen, J.; Mollenhauer, J.; Holmskov, U. Review: Gp-340/DMBT1 in mucosal innate immunity. Innate Immun. 2010, 16, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Arnold, P.; Rickert, U.; Helmers, A.K.; Spreu, J.; Schneppenheim, J.; Lucius, R. Trefoil factor 3 shows anti-inflammatory effects on activated microglia. Cell Tissue Res. 2016, 365, 3–11. [Google Scholar] [CrossRef]
- Leclaire, C.; Lecointe, K.; Gunning, P.A.; Tribolo, S.; Kavanaugh, D.W.; Wittmann, A.; Latousakis, D.; MacKenzie, D.A.; Kawasaki, N.; Juge, N. Molecular basis for intestinal mucin recognition by galectin-3 and C-type lectins. FASEB J. 2018, 32, 3301–3320. [Google Scholar] [CrossRef] [Green Version]
- García Caballero, G.; Kaltner, H.; Kutzner, T.J.; Ludwig, A.K.; Manning, J.C.; Schmidt, S.; Sinowatz, F.; Gabius, H.J. How galectins have become multifunctional proteins. Histol. Histopathol. 2020, 35, 509–539. [Google Scholar] [PubMed]
- Wesener, D.A.; Dugan, A.; Kiessling, L.L. Recognition of microbial glycans by soluble human lectins. Curr. Opin. Struct. Biol. 2017, 44, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Meanwatthana, J.; Majam, T. Interleukin-6 antagonists: Lessons from cytokine release syndrome to the therapeutic application in severe COVID-19 infection. J. Pharm. Pract. 2021. [Google Scholar] [CrossRef] [PubMed]
- Omar, O.M.; Suotto, M.; Bhat, N.S.; Bhat, A.A.; Lu, H.; Chen, Z.; El-Rifai, W. TFF1 antagonizes TIMP-1 mediated proliferative functions in gastric cancer. Mol. Carcinogen. 2018, 57, 1577–1587. [Google Scholar] [CrossRef]
- Rabinovich, G.A.; Rubinstein, N.; Toscano, M.A. Role of galectins in inflammatory and immunomodulatory processes. Biochim. Biophys. Acta 2002, 1572, 274–284. [Google Scholar] [CrossRef]
- Johannssen, T.; Lepenies, B. Glycan-Based Cell Targeting To Modulate Immune Responses. Trends Biotechnol. 2017, 35, 334–346. [Google Scholar] [CrossRef] [Green Version]
- Sacchettini, J.C.; Baum, L.G.; Brewer, C.F. Multivalent protein-carbohydrate interactions. a new paradigm for supermolecular assembly and signal transduction. Biochemistry 2001, 40, 3009–3015. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich, G.A.; Toscano, M.A.; Jackson, S.S.; Vasta, G.R. Functions of cell surface galectin-glycoprotein lattices. Curr. Opin. Struct. Biol. 2007, 17, 513–520. [Google Scholar] [CrossRef] [Green Version]
- Patel, U.; Rajasingh, S.; Samanta, S.; Cao, T.; Dawn, B.; Rajasingh, J. Macrophage polarization in response to epigenetic modifiers during infection and inflammation. Drug Discov. Today 2017, 22, 186–193. [Google Scholar] [CrossRef] [Green Version]
- Bulitta, C.J.; Fleming, J.V.; Raychowdhury, R.; Taupin, D.; Rosenberg, I.; Wang, T.C. Autoinduction of the trefoil factor 2 (TFF2) promoter requires an upstream cis-acting element. Biochem. Biophys. Res. Commun. 2002, 293, 366–374. [Google Scholar] [CrossRef]
- Martin, V.; Ribieras, S.; Rio, M.-C.; Dante, R. The estrogen responsive element of the pS2 gene is recognized by a methylation sensitive DNA binding protein. Biol. Chem. 1998, 379, 409–416. [Google Scholar] [CrossRef]
- Ribieras, S.; Lefèbvre, O.; Tomasetto, C.; Rio, M.-C. Mouse Trefoil Factor genes: Genomic organization, sequences and methylation analyses. Gene 2001, 266, 67–75. [Google Scholar] [CrossRef]
- Carvalho, R.; Kayademir, T.; Soares, P.; Canedo, P.; Sousa, S.; Oliveira, C.; Leistenschneider, P.; Seruca, R.; Gött, P.; Blin, N.; et al. Loss of heterozygosity and promoter methylation, but not mutation, may underlie loss of TFF1 in gastric carcinoma. Lab. Investig. 2002, 82, 1319–1326. [Google Scholar] [CrossRef] [Green Version]
- Braga Emidio, N.; Hoffmann, W.; Brierley, S.M.; Muttenthaler, M. Trefoil factor family: Unresolved questions and clinical perspectives. Trends Biochem. Sci. 2019, 44, 387–390. [Google Scholar] [CrossRef]
- Royce, S.G.; Lim, C.; Muljadi, R.C.; Tang, M.L.K. Trefoil factor 2 regulates airway remodeling in animal models of asthma. J. Asthma 2011, 48, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Popp, J.; Schicht, M.; Garreis, F.; Klinger, P.; Gelse, K.; Sesselmann, S.; Tsokos, M.; Etzold, S.; Stiller, D.; Claassen, H.; et al. Human synovia contains trefoil factor family (TFF) peptides 1-3 although synovial membrane only produces TFF3: Implications in osteoarthritis and rheumatoid arthritis. Int. J. Mol. Sci. 2019, 20, 6105. [Google Scholar] [CrossRef] [Green Version]
- Paterson, R.W.; Bartlett, J.W.; Blennow, K.; Fox, N.C.; Shaw, L.M.; Trojanowski, J.Q.; Zetterberg, H.; Schott, J.M. Cerebrospinal fluid markers including trefoil factor 3 are associated with neurodegeneration in amyloid-positive individuals. Transl. Psychiatry 2014, 4, e419. [Google Scholar] [CrossRef]



| Loss of TFF | Impaired Functions | Inflammatory Phenotypes |
|---|---|---|
| TFF1 | Dysregulated self-renewal of gastric antral units | Antral inflammation and cancer |
| TFF2 | Gastric barrier defect | Enhanced gastric inflammation after H. pylori infection Changed inflammatory responses after infections |
| Dysregulated immune reactions | ||
| TFF3 | Intestinal barrier defect | Increased inflammation after DSS challenge |
| Dysregulated immune reactions? | Changed inflammatory responses? |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoffmann, W. Trefoil Factor Family (TFF) Peptides and Their Links to Inflammation: A Re-evaluation and New Medical Perspectives. Int. J. Mol. Sci. 2021, 22, 4909. https://doi.org/10.3390/ijms22094909
Hoffmann W. Trefoil Factor Family (TFF) Peptides and Their Links to Inflammation: A Re-evaluation and New Medical Perspectives. International Journal of Molecular Sciences. 2021; 22(9):4909. https://doi.org/10.3390/ijms22094909
Chicago/Turabian StyleHoffmann, Werner. 2021. "Trefoil Factor Family (TFF) Peptides and Their Links to Inflammation: A Re-evaluation and New Medical Perspectives" International Journal of Molecular Sciences 22, no. 9: 4909. https://doi.org/10.3390/ijms22094909
APA StyleHoffmann, W. (2021). Trefoil Factor Family (TFF) Peptides and Their Links to Inflammation: A Re-evaluation and New Medical Perspectives. International Journal of Molecular Sciences, 22(9), 4909. https://doi.org/10.3390/ijms22094909







