Functional Analysis of Autophagy-Related Gene ATG12 in Potato Dry Rot Fungus Fusarium oxysporum
Abstract
1. Introduction
2. Results
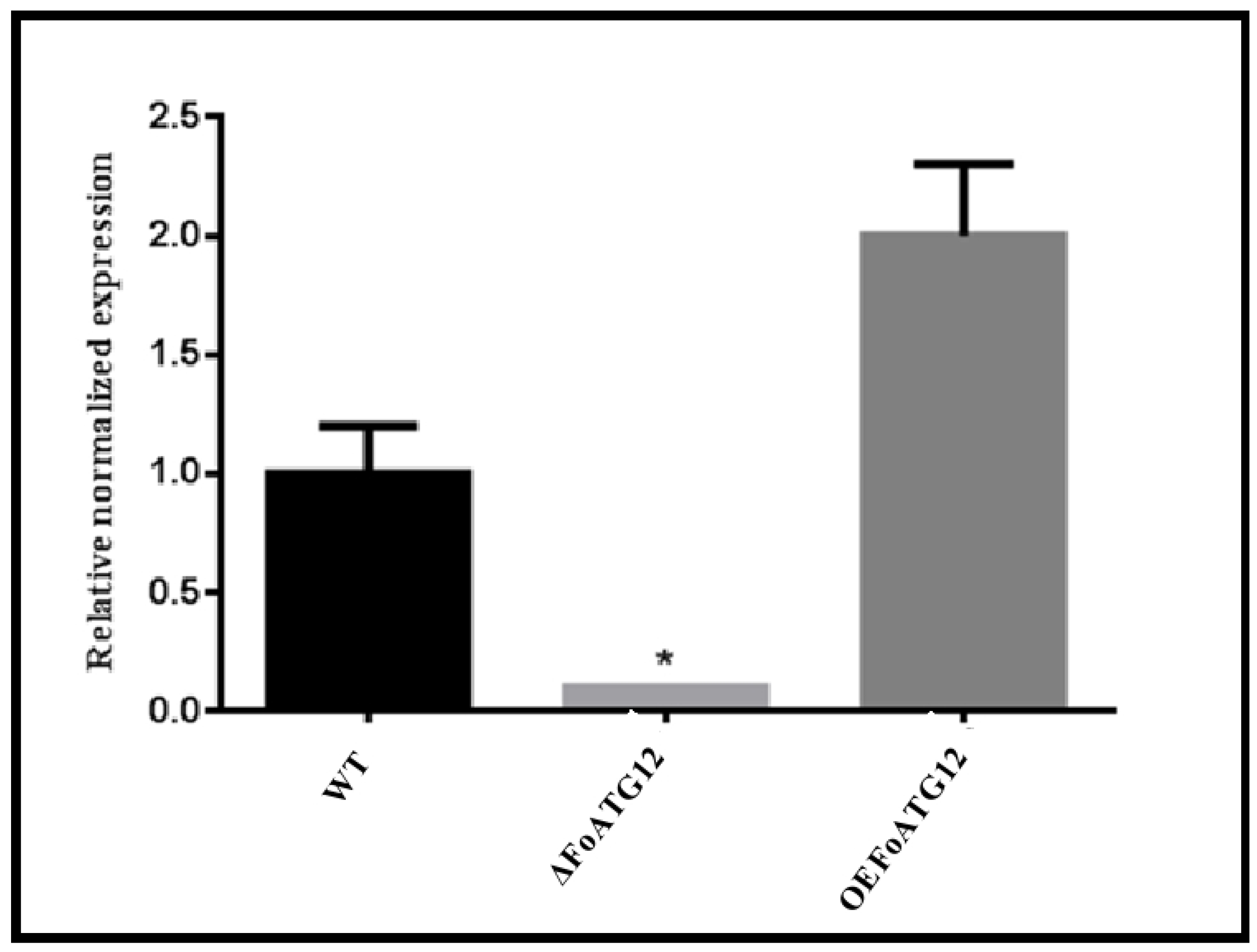
2.1. Gene Replacement of OEFoATG12 and ∆ATG12
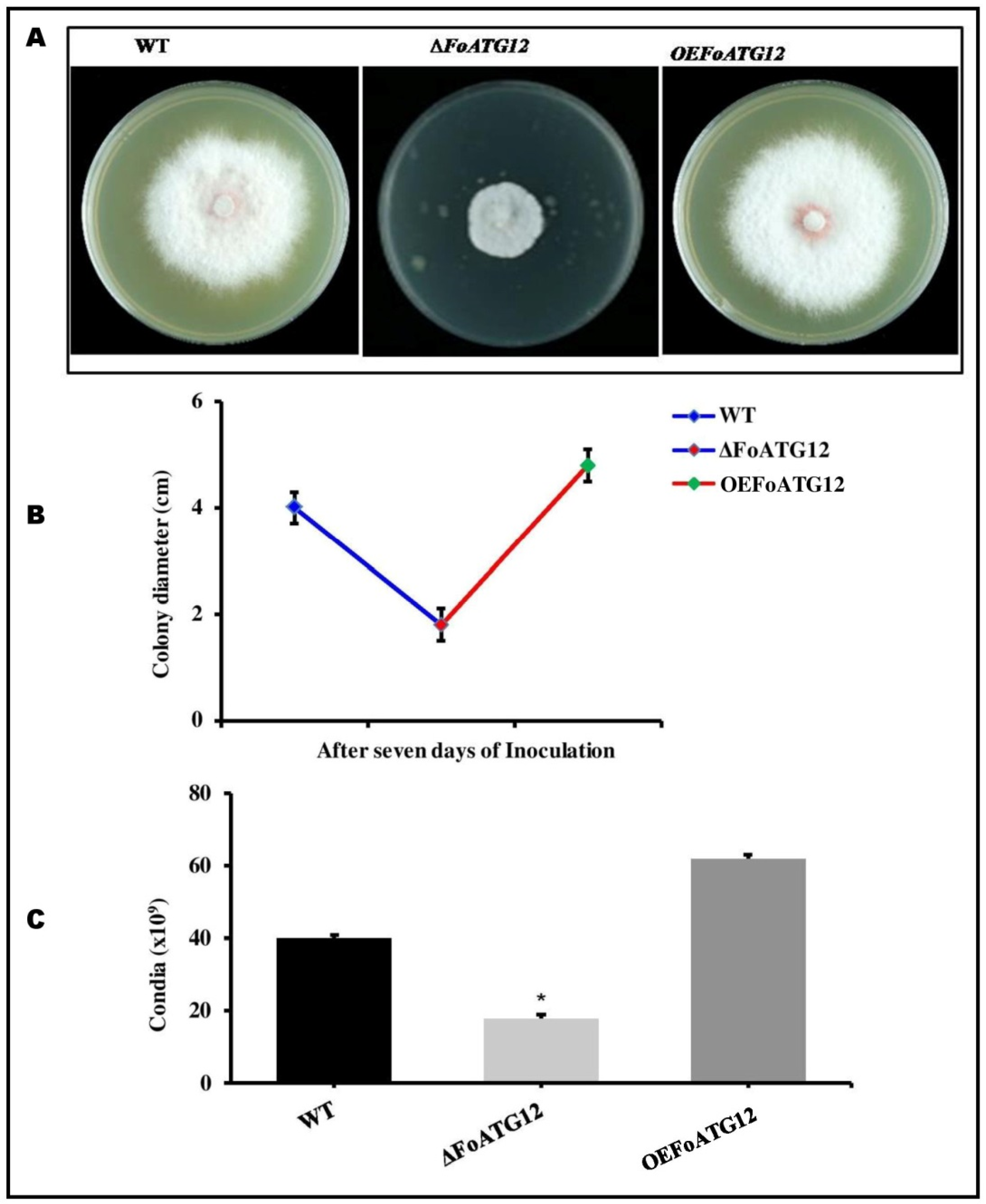
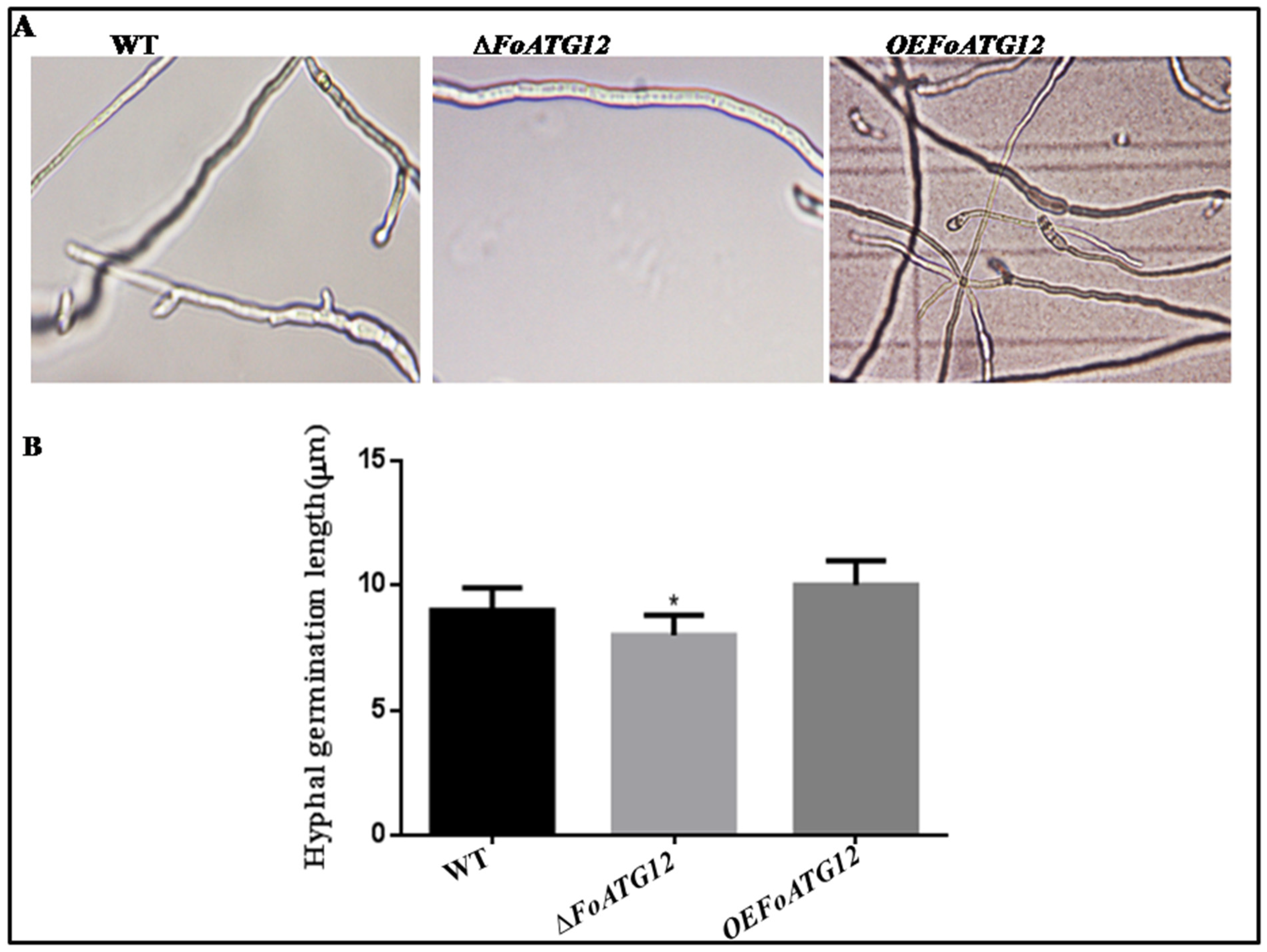
2.2. Impaired Fungal Development Exhibited in the ∆ATG12 Mutants
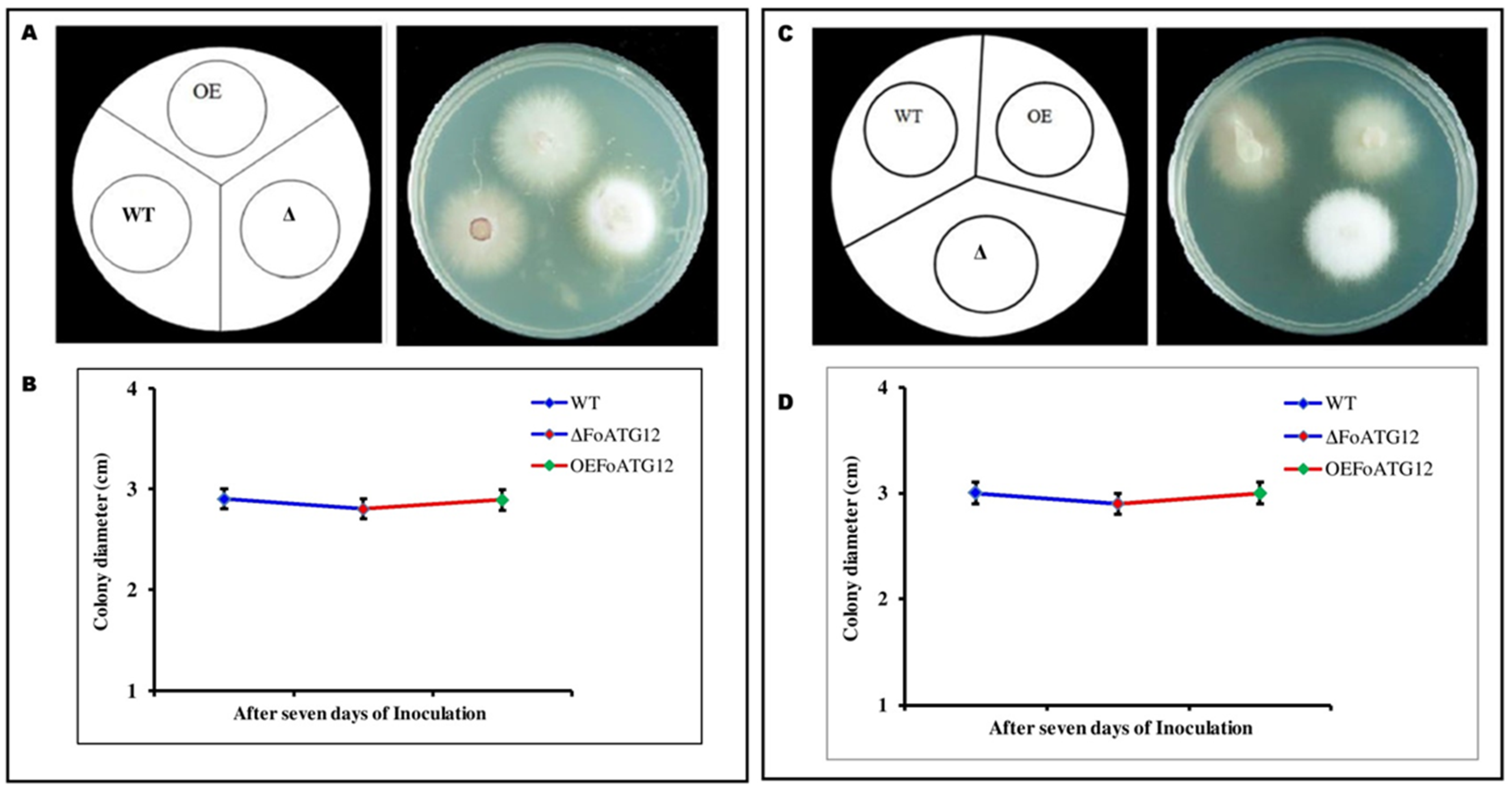
2.3. Response against Stress
2.4. Plant Infection Assay
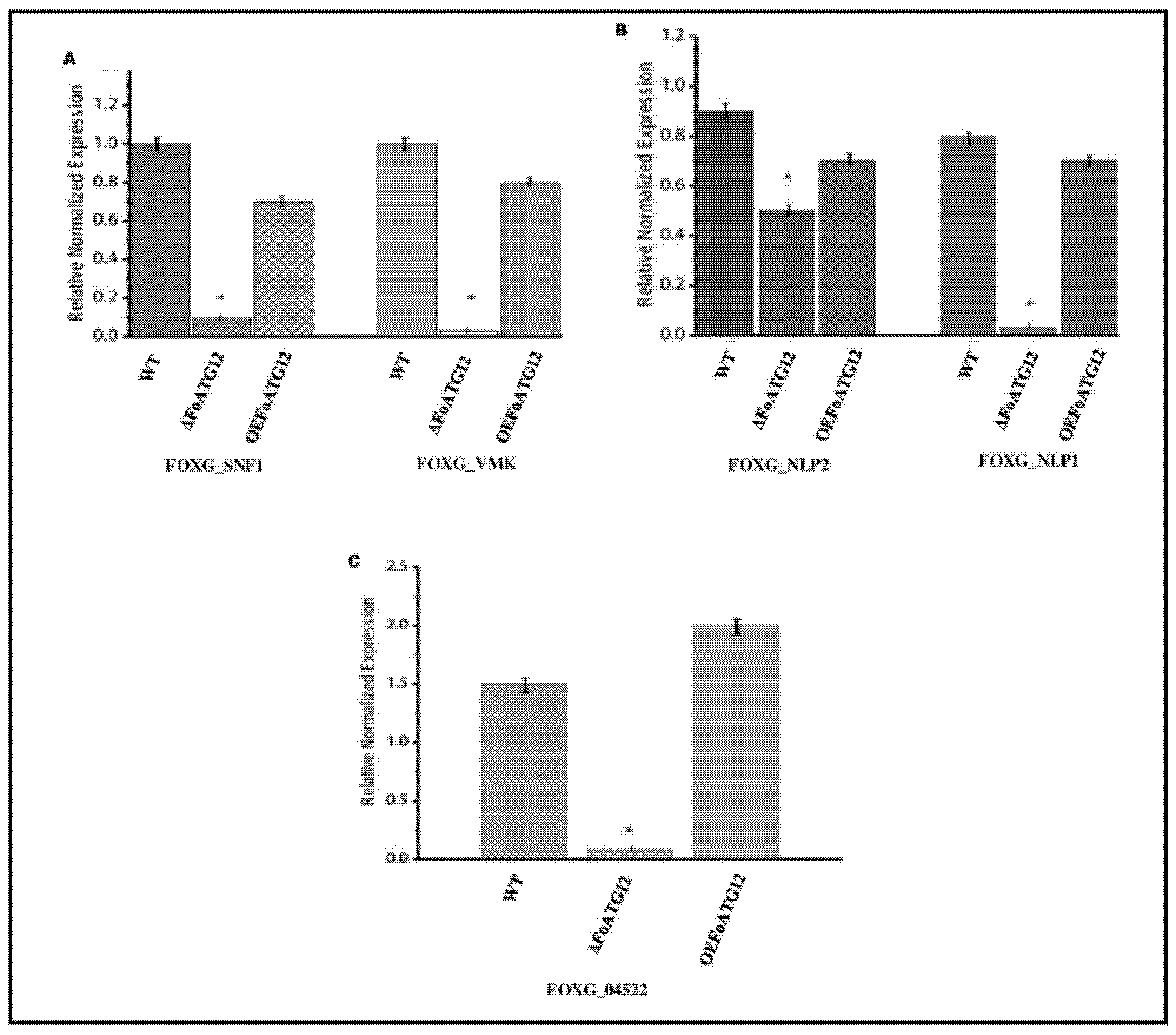
2.5. Expression of Pathogenicity and Vegetative Growth Related Genes
3. Discussion
4. Materials and Methods
4.1. Isolation of the Fungal Strain and the Culture Conditions
4.2. Fungal Transformation
4.3. Generation of FoATG12 Deletion Mutants
4.4. Overexpression (OE) of the Mutant FoATG12 Strains
4.5. Evaluation of Fungal Germination, Conidia Formation, and Radial Growth
4.6. Evaluation of the Fungal Sensitivity against Stress
4.7. Analysis of Gene Expression
4.8. Microscopic Analysis
4.9. Pathogenicity Test
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Yoshimoto, K.; Takano, Y.; Sakai, Y. Autophagy in plants and phytopathogens. FEBS Lett. 2010, 584, 1350–1358. [Google Scholar] [CrossRef] [PubMed]
- Reggiori, F.; Klionsky, D.J. Autophagy in the eukaryotic cell. Eukaryot. Cell 2002, 1, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.F.; Klionsky, D.J. The Atg8 and Atg12 ubiquitin-like conjugation systems in macroautophagy. EMBO Rep. 2008, 9, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.P.; Klionsky, D.J. Autophagosome formation: Core machinery and adaptations. Nat. Cell Biol. 2007, 9, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Veneault-Fourrey, C.; Barooah, M.; Egan, M.; Wakley, G.; Talbot, N.J. Autophagic fungal cell death is necessary for infection by the rice blast fungus. Science 2006, 312, 580–583. [Google Scholar] [CrossRef]
- Asakura, M.; Ninomiya, S.; Oku, M.; Okuno, T.; Sakai, Y.; Takano, Y. Atg26-mediated pexophagy is required for host invasion by the plant pathogenic fungus Colletotrichum orbiculare. Autophagy 2009, 5, 903. [Google Scholar] [CrossRef]
- Dong, B.; Liu, X.H.; Lu, J.P.; Zhang, F.S.; Gao, H.M.; Wang, H.K.; Lin, F.C. MgAtg9 trafficking in Magnaporthe oryzae. Autophagy 2009, 5, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.P.; Liu, X.H.; Feng, X.X.; Min, H.; Lin, F.C. An autophagy gene, MgATG5, is required for cell differentiation and pathogenesis in Magnaporthe oryzae. Curr. Genet. 2009, 55, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Nadal, M.; Gold, S.E. The autophagy genes atg8 and atg1 affect morphogenesis and pathogenicity in Ustilago maydis. Mol. Plant Pathol. 2010, 11, 463–478. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Lu, J.P.; Zhang, L.; Dong, B.; Min, H.; Lin, F.C. Involvement of a Magnaporthe grisea serine/threonine kinase gene, MgATG1, in appressorium turgor and pathogenesis. Eukaryot. Cell 2007, 6, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.B.; Liu, X.H.; Lu, J.P.; Zhang, L.; Min, H.; Lin, F.C. The cysteine protease MoAtg4 interacts with MoAtg8 and is required for differentiation and pathogenesis in Magnaporthe oryzae. Autophagy 2010, 6, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Kershaw, M.J.; Talbot, N.J. Genome-wide functional analysis reveals that infection-associated fungal autophagy is necessary for rice blast disease. Proc. Natl. Acad. Sci. USA 2009, 106, 15967–15972. [Google Scholar] [CrossRef] [PubMed]
- Rauyaree, P.; Ospina-Giraldo, M.D.; Kang, S.; Bhat, R.G.; Subbarao, K.V.; Grant, S.J.; Dobinson, K.F. Mutations in VMK1, a mitogen-activated protein kinase gene, affect microsclerotia formation and pathogenicity in Verticillium dahliae. Curr. Genet. 2005, 48, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Tzima, A.; Paplomatas, E.J.; Rauyaree, P.; Kang, S. Roles of the catalytic subunit of cAMP-dependent protein kinase A in virulence and development of the soilborne plant pathogen Verticillium dahliae. Fungal Genet. Biol. 2010, 47, 406–415. [Google Scholar] [CrossRef]
- Tzima, A.K.; Paplomatas, E.J.; Rauyaree, P.; Ospina-Giraldo, M.D.; Kang, S. VdSNF1, the sucrose nonfermenting protein kinase gene of Verticillium dahliae, is required for virulence and expression of genes involved in cell-wall degradation. Mol. Plant Microbe Interact. 2011, 24, 129–142. [Google Scholar] [CrossRef]
- Tzima, A.K.; Paplomatas, E.J.; Tsitsigiannis, D.I.; Kang, S. The G protein beta subunit controls virulence and multiple growth- and development-related traits in Verticillium dahliae. Fungal Genet. Biol. 2012, 49, 271–283. [Google Scholar] [CrossRef]
- El-Kassas, H.Y.; Khairy, H.M. A trial for biological control of a pathogenic fungus (Fusarium solani) by some marine microorganisms. Am. Eurasian J. Agric. Environ. Sci. 2009, 5, 434–440. [Google Scholar]
- Kirk, W.; Wharton, P. Fusarium dry rot. In Extension Bullettin; E 2992; Department of Plant Pathology, Michighan State University: East Lansing, MI, USA, 2007. [Google Scholar]
- Wharton, P.S.; Kirk, W.W.; Berry, D.; Tumbalam, P. Seed treatment application-timing options for control of Fusarium decay and sprout rot of cut seedpieces. Am. J. Potato Res. 2007, 84, 237–244. [Google Scholar] [CrossRef]
- Slininger, P.J.; Burkhead, K.D.; Schisler, D.A. Antifungal and sprout regulatory bioactivities of phenylacetic acid, indole-3-acetic acid, and tyrosol isolated from the potato dry rot suppressive bacterium Enterobacter cloacae S11:T:07. J. Ind. Microbiol. Biotechnol. 2004, 31, 517–524. [Google Scholar] [CrossRef]
- Chełkowski, J. Chapter 25—Toxinogenicity of Fusarium species causing dry rot of potato tubers. In Fusarium; Chełkowski, J., Ed.; Elsevier: Amsterdam, The Netherlands, 1989; Volume 2, pp. 435–440. [Google Scholar]
- Secor, G.A.; Gudmestad, N.C. Managing fungal diseases of potato. Can. J. Plant Pathol. 1999, 21, 213–221. [Google Scholar] [CrossRef]
- Shattock, R. Compendium of Potato Diseases, Second Edition. W.R. Stevenson. Plant Pathol. 2002, 51, 520. [Google Scholar] [CrossRef]
- Secor, G.; Salas, B. Fusarium dry rot and Fusarium wilt. In Compendium of Potato Diseases; American Phytopathological Society: Saint Paul, MN, USA, 2001; pp. 23–25. [Google Scholar]
- Santhanam, P.; van Esse, H.P.; Albert, I.; Faino, L.; Nurnberger, T.; Thomma, B.P.H.J. Evidence for functional diversification within a fungal NEP1-like protein family. Mol. Plant Microbe Interact. 2013, 26, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Corral-Ramos, C.; Roca, M.G.; Di Pietro, A.; Roncero, M.I.G.; Ruiz-Roldan, C. Autophagy contributes to regulation of nuclear dynamics during vegetative growth and hyphal fusion in Fusarium oxysporum. Autophagy 2015, 11, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Bartoszewska, M.; Kiel, J.A.K.W. The role of macroautophagy in development of filamentous fungi. Antioxid. Redox. Sign. 2011, 14, 2271–2287. [Google Scholar] [CrossRef]
- Josefsen, L.; Droce, A.; Sondergaard, T.E.; Sorensen, J.L.; Bormann, J.; Schafer, W.; Giese, H.; Olsson, S. Autophagy provides nutrients for nonassimilating fungal structures and is necessary for plant colonization but not for infection in the necrotrophic plant pathogen Fusarium graminearum. Autophagy 2012, 8, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, B.M.; Burggraaf-van Welzen, A.M.; Lamers, G.; Meyer, V.; Ram, A.F.J. Autophagy promotes survival in aging submerged cultures of the filamentous fungus Aspergillus niger. Appl. Microbiol. Biotechnol. 2013, 97, 8205–8218. [Google Scholar] [CrossRef]
- Zhou, L.; Zhao, J.; Guo, W.Z.; Zhang, T.Z. Functional analysis of autophagy genes via agrobacterium-mediated transformation in the vascular wilt fungus Verticillium dahliae. J. Genet. Genom. 2013, 40, 421–431. [Google Scholar] [CrossRef]
- Pinan-Lucarre, B.; Baiguerie, A.; Clave, C. Accelerated cell death in Podospora autophagy mutants. Eukaryot. Cell 2005, 4, 1765–1774. [Google Scholar] [CrossRef]
- Richie, D.L.; Fuller, K.K.; Fortwendel, J.; Miley, M.D.; McCarthy, J.W.; Feldmesser, M.; Rhodes, J.C.; Askew, D.S. Unexpected link between metal ion deficiency and autophagy in Aspergillus fumigatus. Eukaryot. Cell 2007, 6, 2437–2447. [Google Scholar] [CrossRef]
- Shoji, J.Y.; Arioka, M.; Kitamoto, K. Possible involvement of pleiomorphic vacuolar networks in nutrient recycling in filamentous fungi. Autophagy 2006, 2, 226–227. [Google Scholar] [CrossRef]
- Kikuma, T.; Ohneda, M.; Arioka, M.; Kitamoto, K. Functional analysis of the ATG8 homologue Aoatg8 and role of autophagy in differentiation and germination in Aspergillus oryzae. Eukaryot. Cell 2006, 5, 1328–1336. [Google Scholar] [CrossRef]
- Duan, Z.B.; Chen, Y.X.; Huang, W.; Shang, Y.F.; Chen, P.L.; Wang, C.S. Linkage of autophagy to fungal development, lipid storage and virulence in Metarhizium robertsii. Autophagy 2013, 9, 538–549. [Google Scholar] [CrossRef]
- Molina, L.; Kahmann, R. An Ustilago maydis gene involved in H2O2 detoxification is required for virulence. Plant Cell 2007, 19, 2293–2309. [Google Scholar] [CrossRef]
- Guo, M.; Chen, Y.; Du, Y.; Dong, Y.H.; Guo, W.; Zhai, S.; Zhang, H.F.; Dong, S.M.; Zhang, Z.G.; Wang, Y.C.; et al. The bZIP transcription factor MoAP1 mediates the oxidative stress response and is critical for pathogenicity of the rice blast fungus Magnaporthe oryzae. PLoS Pathol. 2011, 7, e1001302. [Google Scholar] [CrossRef]
- Cessna, S.G.; Sears, V.E.; Dickman, M.B.; Low, P.S. Oxalic acid, a pathogenicity factor for Sclerotinia sclerotiorum, suppresses the oxidative burst of the host plant. Plant Cell 2000, 12, 2191–2199. [Google Scholar] [CrossRef]
- Lin, C.H.; Yang, S.L.; Chung, K.R. The YAP1 homolog-mediated oxidative stress tolerance is crucial for pathogenicity of the necrotrophic fungus Alternaria alternata in citrus. Mol. Plant Microbe Interact. 2009, 22, 942–952. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.G.; Li, J.Y.; Glass, N.L. Exploring the bZIP transcription factor regulatory network in Neurospora crassa. Microbiology 2011, 157, 747–759. [Google Scholar] [CrossRef]
- Luo, X.M.; Mao, H.Q.; Wei, Y.M.; Cai, J.; Xie, C.J.; Sui, A.P.; Yang, X.Y.; Dong, J.Y. The fungal-specific transcription factor Vdpf influences conidia production, melanized microsclerotia formation and pathogenicity in Verticillium dahliae. Mol. Plant Pathol. 2016, 17, 1364–1381. [Google Scholar] [CrossRef] [PubMed]
- Maruthachalam, K.; Klosterman, S.J.; Kang, S.; Hayes, R.J.; Subbarao, K.V. Identification of pathogenicity-related genes in the vascular wilt fungus verticillium dahliae by agrobacterium tumefaciens-mediated T-DNA insertional mutagenesis. Mol. Biotechnol. 2011, 49, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, E.; Nayak, T.; Oakley, C.E.; Edgerton, H.; Xiong, Y.; Taheri-Talesh, N.; Osmani, S.A.; Oakley, B.R. Fusion PCR and gene targeting in Aspergillus nidulans. Nat. Protoc. 2006, 1, 3111–3120. [Google Scholar] [CrossRef]
- Lam, S.W.; Cleton-Jansen, A.M.; Cleven, A.H.G.; Ruano, D.; van Wezel, T.; Szuhai, K.; Bovee, J.V.M.G. Molecular analysis of gene fusions in bone and soft tissue tumors by anchored multiplex PCR-based targeted next-generation sequencing. J. Mol. Diagn. 2018, 20, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, A.; Roncero, M.I.G. Cloning, expression, and role in pathogenicity of pg1 encoding the major extracellular endopolygalacturonase of the vascular wilt pathogen Fusarium oxysporum. Mol. Plant Microbe Interact. 1998, 11, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Berges, M.S.; Capilla, J.; Turra, D.; Schafferer, L.; Matthijs, S.; Jochl, C.; Cornelis, P.; Guarro, J.; Haas, H.; Di Pietro, A. HapX-mediated iron homeostasis is essential for rhizosphere competence and virulence of the soilborne pathogen Fusarium oxysporum. Plant Cell 2012, 24, 3805–3822. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalid, A.R.; Zhang, S.; Luo, X.; Shaheen, H.; Majeed, A.; Maqbool, M.; Zahid, N.; Rahim, J.; Ren, M.; Qiu, D. Functional Analysis of Autophagy-Related Gene ATG12 in Potato Dry Rot Fungus Fusarium oxysporum. Int. J. Mol. Sci. 2021, 22, 4932. https://doi.org/10.3390/ijms22094932
Khalid AR, Zhang S, Luo X, Shaheen H, Majeed A, Maqbool M, Zahid N, Rahim J, Ren M, Qiu D. Functional Analysis of Autophagy-Related Gene ATG12 in Potato Dry Rot Fungus Fusarium oxysporum. International Journal of Molecular Sciences. 2021; 22(9):4932. https://doi.org/10.3390/ijms22094932
Chicago/Turabian StyleKhalid, A. Rehman, Shumin Zhang, Xiumei Luo, Hamayun Shaheen, Afshan Majeed, Mehdi Maqbool, Noosheen Zahid, Junaid Rahim, Maozhi Ren, and Dan Qiu. 2021. "Functional Analysis of Autophagy-Related Gene ATG12 in Potato Dry Rot Fungus Fusarium oxysporum" International Journal of Molecular Sciences 22, no. 9: 4932. https://doi.org/10.3390/ijms22094932
APA StyleKhalid, A. R., Zhang, S., Luo, X., Shaheen, H., Majeed, A., Maqbool, M., Zahid, N., Rahim, J., Ren, M., & Qiu, D. (2021). Functional Analysis of Autophagy-Related Gene ATG12 in Potato Dry Rot Fungus Fusarium oxysporum. International Journal of Molecular Sciences, 22(9), 4932. https://doi.org/10.3390/ijms22094932





