IL-10 in Mast Cell-Mediated Immune Responses: Anti-Inflammatory and Proinflammatory Roles
Abstract
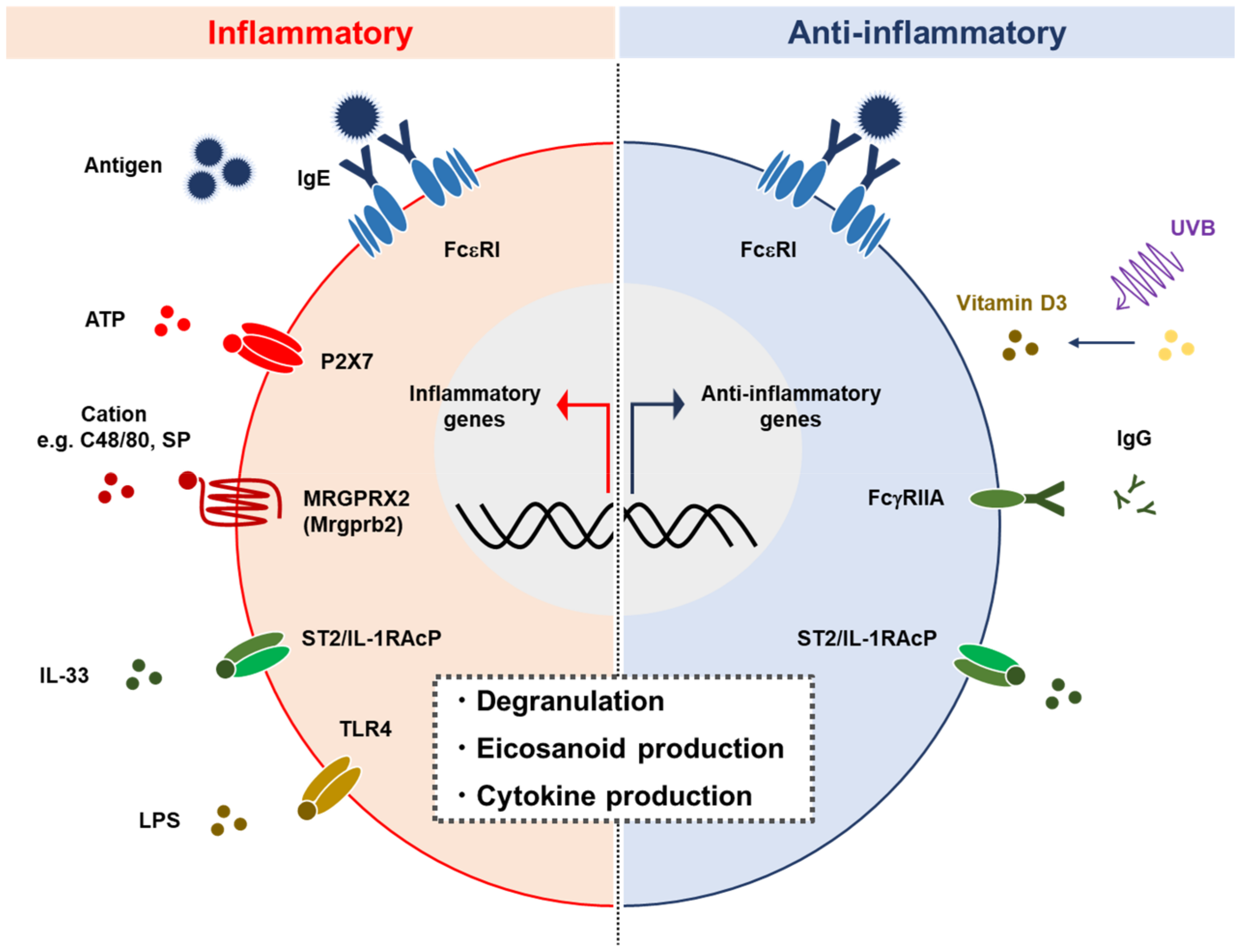
:1. Introduction
2. The Effects of IL-10 on Mast Cells
2.1. IL-10 Receptor Signaling
2.2. Proliferation and Apoptosis
2.3. Differentiation
2.4. Activation
3. The Roles of IL-10 in Mast Cell-Related Immune Diseases
3.1. Contact Hypersensitivity
3.2. Rheumatoid Arthritis
3.3. Antineutrophilic Cytoplasmic Antibody-Associated Vasculitis
3.4. Graft-Versus-Host Disease
3.5. Bladder Infection
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cairns, A.; Constantinides, P. Mast cells in human atherosclerosis. Science 1954, 120, 31–32. [Google Scholar] [CrossRef]
- Cass, R.; Riley, J.F.; West, G.B.; Head, K.W.; Stroud, S.W. Heparin and histamine in mast-cell tumours from dogs. Nature 1954, 174, 318–319. [Google Scholar] [CrossRef]
- Andrus, E.C.; Wilcox, H.B. The effects of anaphylaxis, and of histamine, upon the coronary arteries in the isolated heart. J. Exp. Med. 1939, 69, 546–553. [Google Scholar] [CrossRef] [Green Version]
- Riley, J.F.; West, G.B. Mast cells and histamine in normal and pathological tissues. J. Physiol. 1953, 119. [Google Scholar] [CrossRef]
- Ishizaka, K.; Ishizaka, T.; Hornbrook, M.M. Physicochemical properties of reaginic antibody: V. Correlation of reaginic activity wth gamma-E-globulin antibody. J. Immunol. 1966, 97, 840–853. [Google Scholar] [PubMed]
- Coleman, E.J.; Desalva, S.J. Mast cell response to cestode infection. Proc. Soc. Exp. Biol. Med. 1963, 112, 432–434. [Google Scholar] [CrossRef] [PubMed]
- Daëron, M.; Duc, H.T.; Kanellopoulos, J.; Le Bouteiller, P.; Kinsky, R.; Voisin, G.A. Allogenic mast cell degranulation induced by histocompatibility antibodies: An in vitro model of transplantation anaphylaxis. Cell. Immunol. 1975, 20, 133–155. [Google Scholar] [CrossRef]
- McCrea, P.C. Tissue mast cells in the bone marrow in rheumatoid arthritis. Ann. Rheum. Dis. 1961, 20, 83–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parish, W.E. Release of histamine and slow reacting substance with mast cell changes after challenge of human lung sensitized passively with reagin in vitro. Nature 1967, 215, 738–739. [Google Scholar] [CrossRef]
- Smith, R.O.; Wood, W.B. Cellular mechanisms of antibacterial defense in lymph nodes; pathogenesis of acute bacterial lymphadenitis. J. Exp. Med. 1949, 90, 555–566. [Google Scholar] [CrossRef] [Green Version]
- Sundberg, M.; Siurala, M. Mast cells in the gastric mucosa of patients with peptic ulcer and gastric cancer. Ann. Med. Exp. Biol. Fenn. 1959, 37, 175–179. [Google Scholar] [PubMed]
- Dudeck, A.; Dudeck, J.; Scholten, J.; Petzold, A.; Surianarayanan, S.; Köhler, A.; Peschke, K.; Vöhringer, D.; Waskow, C.; Krieg, T.; et al. Mast cells are key promoters of contact allergy that mediate the adjuvant effects of haptens. Immunity 2011, 34, 973–984. [Google Scholar] [CrossRef] [Green Version]
- Gimenez-Rivera, V.A.; Siebenhaar, F.; Zimmermann, C.; Siiskonen, H.; Metz, M.; Maurer, M. Mast cells limit the exacerbation of chronic allergic contact dermatitis in response to repeated allergen exposure. J. Immunol. 2016, 197, 4240–4246. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.S.; Lee, M.B.; Lee, D.; Min, K.Y.; Koo, J.; Kim, H.W.; Park, Y.H.; Kim, S.J.; Ikutani, M.; Takaki, S.; et al. The regulatory B cell-mediated peripheral tolerance maintained by mast cell IL-5 suppresses oxazolone-induced contact hypersensitivity. Sci. Adv. 2019, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiorentino, D.F.; Bond, M.W.; Mosmann, T.R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J. Exp. Med. 1989, 170, 2081–2095. [Google Scholar] [CrossRef]
- Moore, K.W.; Vieira, P.; Fiorentino, D.F.; Trounstine, M.L.; Khan, T.A.; Mosmann, T.R. Homology of cytokine synthesis inhibitory factor (IL-10) to the Epstein-Barr virus gene BCRFI. Science 1990, 248, 1230–1234. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, C.; Vodovotz, Y.; Nathan, C. Macrophage deactivation by interleukin 10. J. Exp. Med. 1991, 174, 1549–1555. [Google Scholar] [CrossRef] [Green Version]
- De Waal Malefyt, R.; Haanen, J.; Spits, H.; Roncarolo, M.G.; te Velde, A.; Figdor, C.; Johnson, K.; Kastelein, R.; Yssel, H.; de Vries, J.E. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J. Exp. Med. 1991, 174, 915–924. [Google Scholar] [CrossRef]
- Fiorentino, D.F.; Zlotnik, A.; Vieira, P.; Mosmann, T.R.; Howard, M.; Moore, K.W.; O’Garra, A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J. Immunol. 1991, 146, 3444–3451. [Google Scholar]
- Suda, T.; MacNeil, I.; Fischer, M.; Moore, K.W.; Zlotnik, A. Identification of a novel thymocyte growth factor derived from B cell lymphomas. Adv. Exp. Med. Biol. 1991, 292, 115–120. [Google Scholar] [CrossRef]
- Chen, W.F.; Zlotnik, A. IL-10: A novel cytotoxic T cell differentiation factor. J. Immunol. 1991, 147, 528–534. [Google Scholar]
- Carson, W.E.; Lindemann, M.J.; Baiocchi, R.; Linett, M.; Tan, J.C.; Chou, C.C.; Narula, S.; Caligiuri, M.A. The functional characterization of interleukin-10 receptor expression on human natural killer cells. Blood 1995, 85, 3577–3585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itoh, K.; Hirohata, S. The role of IL-10 in human B cell activation, proliferation, and differentiation. J. Immunol. 1995, 154, 4341–4350. [Google Scholar] [PubMed]
- Ghildyal, N.; McNeil, H.P.; Stechschulte, S.; Austen, K.F.; Silberstein, D.; Gurish, M.F.; Somerville, L.L.; Stevens, R.L. IL-10 induces transcription of the gene for mouse mast cell protease-1, a serine protease preferentially expressed in mucosal mast cells of Trichinella spiralis-infected mice. J. Immunol. 1992, 149, 2123–2129. [Google Scholar] [PubMed]
- Ghildyal, N.; McNeil, H.P.; Gurish, M.F.; Austen, K.F.; Stevens, R.L. Transcriptional regulation of the mucosal mast cell-specific protease gene, MMCP-2, by interleukin 10 and interleukin 3. J. Biol. Chem. 1992, 267, 8473–8477. [Google Scholar] [CrossRef]
- Gillespie, S.R.; DeMartino, R.R.; Zhu, J.; Chong, H.J.; Ramirez, C.; Shelburne, C.P.; Bouton, L.A.; Bailey, D.P.; Gharse, A.; Mirmonsef, P.; et al. IL-10 inhibits Fc epsilon RI expression in mouse mast cells. J. Immunol. 2004, 172, 3181–3188. [Google Scholar] [CrossRef] [Green Version]
- Chacón-Salinas, R.; Limón-Flores, A.Y.; Chávez-Blanco, A.D.; Gonzalez-Estrada, A.; Ullrich, S.E. Mast cell-derived IL-10 suppresses germinal center formation by affecting T follicular helper cell function. J. Immunol. 2011, 186, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Grimbaldeston, M.A.; Nakae, S.; Kalesnikoff, J.; Tsai, M.; Galli, S.J. Mast cell-derived interleukin 10 limits skin pathology in contact dermatitis and chronic irradiation with ultraviolet B. Nat. Immunol. 2007, 8, 1095–1104. [Google Scholar] [CrossRef]
- Chan, C.Y.; St. John, A.L.; Abraham, S.N. Mast cell interleukin-10 drives localized tolerance in chronic bladder infection. Immunity 2013, 38, 349–359. [Google Scholar] [CrossRef] [Green Version]
- Moore, K.W.; de Waal Malefyt, R.; Coffman, R.L.; O’Garra, A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001, 19, 683–765. [Google Scholar] [CrossRef]
- Ho, A.S.; Liu, Y.; Khan, T.A.; Hsu, D.H.; Bazan, J.F.; Moore, K.W. A receptor for interleukin 10 is related to interferon receptors. Proc. Natl. Acad. Sci. USA 1993, 90, 11267–11271. [Google Scholar] [CrossRef] [Green Version]
- Motakis, E.; Guhl, S.; Ishizu, Y.; Itoh, M.; Kawaji, H.; de Hoon, M.; Lassmann, T.; Carninci, P.; Hayashizaki, Y.; Zuberbier, T.; et al. Redefinition of the human mast cell transcriptome by deep-CAGE sequencing. Blood 2014, 123, e58–e67. [Google Scholar] [CrossRef]
- Sonoda, T.; Ohno, T.; Kitamura, Y. Concentration of mast-cell progenitors in bone marrow, spleen, and blood of mice determined by limiting dilution analysis. J. Cell. Physiol. 1982, 112, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Gentek, R.; Ghigo, C.; Hoeffel, G.; Bulle, M.J.; Msallam, R.; Gautier, G.; Launay, P.; Chen, J.; Ginhoux, F.; Bajénoff, M. Hemogenic endothelial fate mapping reveals dual developmental origin of mast cells. Immunity 2018, 48, 1160–1171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Liu, S.; Xu, J.; Zhang, X.; Han, D.; Liu, J.; Xia, M.; Yi, L.; Shen, Q.; Xu, S.; et al. Adult connective tissue-Resident mast cells originate from late erythro-myeloid progenitors. Immunity 2018, 49, 640–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Razin, E.; Ihle, J.N.; Seldin, D.; Mencia-Huerta, J.M.; Katz, H.R.; LeBlanc, P.A.; Hein, A.; Caulfield, J.P.; Austen, K.F.; Stevens, R.L. Interleukin 3: A differentiation and growth factor for the mouse mast cell that contains chondroitin sulfate E proteoglycan. J. Immunol. 1984, 132, 1479–1486. [Google Scholar]
- Broxmeyer, H.E.; Cooper, S.; Lu, L.; Hangoc, G.; Anderson, D.; Cosman, D.; Lyman, S.D.; Williams, D.E. Effect of murine mast cell growth factor (c-kit proto-oncogene ligand) on colony formation by human marrow hematopoietic progenitor cells. Blood 1991, 77, 2142–2149. [Google Scholar] [CrossRef] [Green Version]
- Rennick, D.; Hunte, B.; Holland, G.; Thompson-Snipes, L. Cofactors are essential for stem cell factor-dependent growth and maturation of mast cell progenitors: Comparative effects of interleukin-3 (IL-3), IL-4, IL-10, and fibroblasts. Blood 1995, 85, 57–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Z.Q.; Zenda, N.; Shimamura, T. Down-regulation by IL-4 and up-regulation by IFN-gamma of mast cell induction from mouse spleen cells. J. Immunol. 1996, 156, 3925–3931. [Google Scholar]
- Lukacs, N.W.; Kunkel, S.L.; Strieter, R.M.; Evanoff, H.L.; Kunkel, R.G.; Key, M.L.; Taub, D.D. The role of stem cell factor (c-kit ligand) and inflammatory cytokines in pulmonary mast cell activation. Blood 1996, 87, 2262–2268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Speiran, K.; Bailey, D.P.; Fernando, J.; Macey, M.; Barnstein, B.; Kolawole, M.; Curley, D.; Watowich, S.S.; Murray, P.J.; Oskeritzian, C.; et al. Endogenous suppression of mast cell development and survival by IL-4 and IL-10. J. Leukoc. Biol. 2009, 85, 826–836. [Google Scholar] [CrossRef] [Green Version]
- Polukort, S.H.; Rovatti, J.; Carlson, L.; Thompson, C.; Ser-Dolansky, J.; Kinney, S.R.; Schneider, S.S.; Mathias, C.B. IL-10 enhances IgE-mediated mast cell responses and is essential for the development of experimental food allergy in IL-10-deficient MICE. J. Immunol. 2016, 196, 4865–4876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helmby, H.; Grencis, R.K. Contrasting roles for IL-10 in protective immunity to different life cycle stages of intestinal nematode parasites. Eur. J. Immunol. 2003, 33, 2382–2390. [Google Scholar] [CrossRef]
- Yeatman, C.F.; Jacobs-Helber, S.M.; Mirmonsef, P.; Gillespie, S.R.; Bouton, L.A.; Collins, H.A.; Sawyer, S.T.; Shelburne, C.P.; Ryan, J.J. Combined stimulation with the T helper cell type 2 cytokines interleukin (IL)-4 and IL-10 induces mouse mast cell apoptosis. J. Exp. Med. 2000, 192, 1093–1103. [Google Scholar] [CrossRef]
- Bailey, D.P.; Kashyap, M.; Bouton, L.A.; Murray, P.J.; Ryan, J.J. Interleukin-10 induces apoptosis in developing mast cells and macrophages. J. Leukoc. Biol. 2006, 80, 581–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kambe, M.; Kambe, N.; Oskeritzian, C.A.; Schechter, N.; Schwartz, L.B. IL-6 attenuates apoptosis, while neither IL-6 nor IL-10 affect the numbers or protease phenotype of fetal liver-derived human mast cells. Clin. Exp. Allergy 2001, 31, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Mirmonsef, P.; Shelburne, C.P.; Fitzhugh Yeatman, C.; Chong, H.J.; Ryan, J.J. Inhibition of Kit expression by IL-4 and IL-10 in murine mast cells: Role of STAT6 and phosphatidylinositol 3’-kinase. J. Immunol. 1999, 163, 2530–2539. [Google Scholar]
- Bouton, L.A.; Ramirez, C.D.; Bailey, D.P.; Yeatman, C.F.; Yue, J.; Wright, H.V.; Domen, J.; Rosato, R.R.; Grant, S.; Fischer-Stenger, K.; et al. Costimulation with interleukin-4 and interleukin-10 induces mast cell apoptosis and cell-cycle arrest: The role of p53 and the mitochondrion. Exp. Hematol. 2004, 32, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Befus, A.D.; Pearce, F.L.; Goodacre, R.; Bienenstock, J. Unique functional characteristics of mucosal mast cells. Adv. Exp. Med. Biol. 1982, 149, 521–527. [Google Scholar] [CrossRef]
- Befus, A.D.; Pearce, F.L.; Gauldie, J.; Horsewood, P.; Bienenstock, J. Mucosal mast cells. I. Isolation and functional characteristics of rat intestinal mast cells. J. Immunol. 1982, 128, 2475–2480. [Google Scholar]
- Nakahata, T.; Kobayashi, T.; Ishiguro, A.; Tsuji, K.; Naganuma, K.; Ando, O.; Yagi, Y.; Tadokoro, K.; Akabane, T. Extensive proliferation of mature connective-tissue type mast cells in vitro. Nature 1986, 324, 65–67. [Google Scholar] [CrossRef]
- Schwartz, L.B.; Lewis, R.A.; Austen, K.F. Tryptase from human pulmonary mast cells. Purification and characterization. J. Biol. Chem. 1981, 256, 11939–11943. [Google Scholar] [CrossRef]
- Schwartz, L.B.; Irani, A.M.; Roller, K.; Castells, M.C.; Schechter, N.M. Quantitation of histamine, tryptase, and chymase in dispersed human T and TC mast cells. J. Immunol. 1987, 138, 2611–2615. [Google Scholar]
- Ghildyal, N.; Friend, D.S.; Nicodemus, C.F.; Austen, K.F.; Stevens, R.L. Reversible expression of mouse mast cell protease 2 mRNA and protein in cultured mast cells exposed to IL-10. J. Immunol. 1993, 151, 3206–3214. [Google Scholar] [PubMed]
- Kennedy Norton, S.; Barnstein, B.; Brenzovich, J.; Bailey, D.P.; Kashyap, M.; Speiran, K.; Ford, J.; Conrad, D.; Watowich, S.; Moralle, M.R.; et al. IL-10 suppresses mast cell IgE receptor expression and signaling in vitro and in vivo. J. Immunol. 2008, 180, 2848–2854. [Google Scholar] [CrossRef] [Green Version]
- Marshall, J.S.; Leal-Berumen, I.; Nielsen, L.; Glibetic, M.; Jordana, M. Interleukin (IL)-10 inhibits long-term IL-6 production but not preformed mediator release from rat peritoneal mast cells. J. Clin. Investig. 1996, 97, 1122–1128. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.J.; Befus, A.D. Differential regulation of mast cell function by IL-10 and stem cell factor. J. Immunol. 1997, 159, 4015–4023. [Google Scholar]
- Royer, B.; Varadaradjalou, S.; Saas, P.; Gabiot, A.C.; Kantelip, B.; Féger, F.; Guillosson, J.J.; Kantelip, J.P.; Arock, M. Autocrine regulation of cord blood-derived human mast cell activation by IL-10. J. Allergy Clin. Immunol. 2001, 108, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Qayum, A.A.; Paranjape, A.; Abebayehu, D.; Kolawole, E.M.; Haque, T.T.; McLeod, J.J.; Spence, A.J.; Caslin, H.L.; Taruselli, M.T.; Chumanevich, A.P.; et al. IL-10-induced miR-155 targets SOCS1 to enhance IgE-mediated mast cell function. J. Immunol. 2016, 196, 4457–4467. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.; Qin, L.; Zamarin, D.; Kotenko, S.V.; Pestka, S.; Moore, K.W.; Bromberg, J.S. Differential IL-10R1 expression plays a critical role in IL-10-mediated immune regulation. J. Immunol. 2001, 167, 6884–6892. [Google Scholar] [CrossRef] [Green Version]
- Askenase, P.W.; Van Loveren, H.; Kraeuter-Kops, S.; Ron, Y.; Meade, R.; Theoharides, T.C.; Nordlund, J.J.; Scovern, H.; Gerhson, M.D.; Ptak, W. Defective elicitation of delayed-type hypersensitivity in W/Wv and SI/SId mast cell-deficient mice. J. Immunol. 1983, 131, 2687–2694. [Google Scholar]
- Alard, P.; Kurimoto, I.; Niizeki, H.; Doherty, J.M.; Streilein, J.W. Hapten-specific tolerance induced by acute, low-dose ultraviolet B radiation of skin requires mast cell degranulation. Eur. J. Immunol. 2001, 31, 1736–1746. [Google Scholar] [CrossRef]
- Depinay, N.; Hacini, F.; Beghdadi, W.; Peronet, R.; Mécheri, S. Mast cell-dependent down-regulation of antigen-specific immune responses by mosquito bites. J. Immunol. 2006, 176, 4141–4146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hershko, A.Y.; Suzuki, R.; Charles, N.; Alvarez-Errico, D.; Sargent, J.L.; Laurence, A.; Rivera, J. Mast cell interleukin-2 production contributes to suppression of chronic allergic dermatitis. Immunity 2011, 35, 562–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Fujisawa, H.; Zhuang, L.; Freed, I.; Howell, B.G.; Shahid, S.; Shivji, G.M.; Mak, T.W.; Sauder, D.N. CD4+ Th1 and CD8+ type 1 cytotoxic T cells both play a crucial role in the full development of contact hypersensitivity. J. Immunol. 2000, 165, 6783–6790. [Google Scholar] [CrossRef] [Green Version]
- Kissenpfennig, A.; Henri, S.; Dubois, B.; Laplace-Builhé, C.; Perrin, P.; Romani, N.; Tripp, C.H.; Douillard, P.; Leserman, L.; Kaiserlian, D.; et al. Dynamics and function of langerhans cells in vivo: Dermal dendritic cells colonize lymph node areas distinct from slower migrating langerhans cells. Immunity 2005, 22, 643–654. [Google Scholar] [CrossRef] [Green Version]
- Thomas, W.R.; Schrader, J.W. Delayed hypersensitivity in mast-cell-deficient mice. J. Immunol. 1983, 130, 2565–2567. [Google Scholar] [PubMed]
- Reber, L.L.; Sibilano, R.; Starkl, P.; Roers, A.; Grimbaldeston, M.A.; Tsai, M.; Gaudenzio, N.; Galli, S.J. Imaging protective mast cells in living mice during severe contact hypersensitivity. JCI Insight 2017, 2. [Google Scholar] [CrossRef] [Green Version]
- Alard, P.; Niizeki, H.; Hanninen, L.; Streilein, J.W. Local ultraviolet B irradiation impairs contact hypersensitivity induction by triggering release of tumor necrosis factor-alpha from mast cells. Involvement of mast cells and langerhans cells in susceptibility to ultraviolet B. J. Investig. Dermatol. 1999, 113, 983–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biggs, L.; Yu, C.; Fedoric, B.; Lopez, A.F.; Galli, S.J.; Grimbaldeston, M.A. Evidence that vitamin D(3) promotes mast cell-dependent reduction of chronic UVB-induced skin pathology in mice. J. Exp. Med. 2010, 207, 455–463. [Google Scholar] [CrossRef]
- Mion, F.; D’Incà, F.; Danelli, L.; Toffoletto, B.; Guarnotta, C.; Frossi, B.; Burocchi, A.; Rigoni, A.; Gerdes, N.; Lutgens, E.; et al. Mast cells control the expansion and differentiation of IL-10-competent B cells. J. Immunol. 2014, 193, 4568–4579. [Google Scholar] [CrossRef] [Green Version]
- Crisp, A.J.; Chapman, C.M.; Kirkham, S.E.; Schiller, A.L.; Krane, S.M. Articular mastocytosis in rheumatoid arthritis. Arthritis Rheum. 1984, 27, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Van der Velden, D.; Lagraauw, H.M.; Wezel, A.; Launay, P.; Kuiper, J.; Huizinga, T.W.; Toes, R.E.; Bot, I.; Stoop, J.N. Mast cell depletion in the preclinical phase of collagen-induced arthritis reduces clinical outcome by lowering the inflammatory cytokine profile. Arthritis Res. Ther. 2016, 18. [Google Scholar] [CrossRef] [Green Version]
- Marcelletti, J.F.; Ohara, J.; Katz, D.H. Collagen-induced arthritis in mice. Relationship of collagen-specific and total IgE synthesis to disease. J. Immunol. 1991, 147, 4185–4191. [Google Scholar]
- Lee, E.J.; So, M.W.; Hong, S.; Kim, Y.G.; Yoo, B.; Lee, C.K. Interleukin-33 acts as a transcriptional repressor and extracellular cytokine in fibroblast-like synoviocytes in patients with rheumatoid arthritis. Cytokine 2016, 77, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Rivellese, F.; Suurmond, J.; Habets, K.; Dorjée, A.L.; Ramamoorthi, N.; Townsend, M.J.; de Paulis, A.; Marone, G.; Huizinga, T.W.; Pitzalis, C.; et al. Ability of interleukin-33- and immune complex-triggered activation of human mast cells to down-regulate monocyte-mediated immune responses. Arthritis Rheumatol. 2015, 67, 2343–2353. [Google Scholar] [CrossRef]
- Otsubo, S.; Nitta, K.; Uchida, K.; Yumura, W.; Nihei, H. Mast cells and tubulointerstitial fibrosis in patients with ANCA-associated glomerulonephritis. Clin. Exp. Nephrol. 2003, 7, 41–47. [Google Scholar] [CrossRef]
- Falk, R.J.; Jennette, J.C. Anti-neutrophil cytoplasmic autoantibodies with specificity for myeloperoxidase in patients with systemic vasculitis and idiopathic necrotizing and crescentic glomerulonephritis. N. Engl. J. Med. 1988, 318, 1651–1657. [Google Scholar] [CrossRef]
- Gan, P.Y.; O’Sullivan, K.M.; Ooi, J.D.; Alikhan, M.A.; Odobasic, D.; Summers, S.A.; Kitching, A.R.; Holdsworth, S.R. Mast cell stabilization ameliorates autoimmune anti-myeloperoxidase glomerulonephritis. J. Am. Soc. Nephrol. 2016, 27, 1321–1333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gan, P.Y.; Summers, S.A.; Ooi, J.D.; O’Sullivan, K.M.; Tan, D.S.; Muljadi, R.C.; Odobasic, D.; Kitching, A.R.; Holdsworth, S.R. Mast cells contribute to peripheral tolerance and attenuate autoimmune vasculitis. J. Am. Soc. Nephrol. 2012, 23, 1955–1966. [Google Scholar] [CrossRef] [Green Version]
- Murphy, G.F.; Sueki, H.; Teuscher, C.; Whitaker, D.; Korngold, R. Role of mast cells in early epithelial target cell injury in experimental acute graft-versus-host disease. J. Invest. Dermatol. 1994, 102, 451–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korngold, R.; Jameson, B.A.; McDonnell, J.M.; Leighton, C.; Sutton, B.J.; Gould, H.J.; Murphy, G.F. Peptide analogs that inhibit IgE-Fc epsilon RI alpha interactions ameliorate the development of lethal graft-versus-host disease. Biol. Blood Marrow Transplant. 1997, 3, 187–193. [Google Scholar]
- Leveson-Gower, D.B.; Sega, E.I.; Kalesnikoff, J.; Florek, M.; Pan, Y.; Pierini, A.; Galli, S.J.; Negrin, R.S. Mast cells suppress murine GVHD in a mechanism independent of CD4+ CD25+ regulatory T cells. Blood 2013, 122, 3659–3665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mellor, A.L.; Munn, D.H. Creating immune privilege: Active local suppression that benefits friends, but protects foes. Nat. Rev. Immunol. 2008, 8, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Mulvey, M.A.; Schilling, J.D.; Martinez, J.J.; Hultgren, S.J. Bad bugs and beleaguered bladders: Interplay between uropathogenic Escherichia coli and innate host defenses. Proc. Natl. Acad. Sci. USA 2000, 97, 8829–8835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, H.W.; Abraham, S.N. Why serological responses during cystitis are limited. Pathogens 2016, 5, 19. [Google Scholar] [CrossRef] [Green Version]
- Foxman, B. Recurring urinary tract infection: Incidence and risk factors. Am. J. Public Health 1990, 80, 331–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babina, M.; Guhl, S.; Stärke, A.; Kirchhof, L.; Zuberbier, T.; Henz, B.M. Comparative cytokine profile of human skin mast cells from two compartments--strong resemblance with monocytes at baseline but induction of IL-5 by IL-4 priming. J. Leukoc. Biol. 2004, 75, 244–252. [Google Scholar] [CrossRef]
- Ishizuka, T.; Okayama, Y.; Kobayashi, H.; Mori, M. Interleukin-10 is localized to and released by human lung mast cells. Clin. Exp. Allergy 1999, 29, 1424–1432. [Google Scholar] [CrossRef]
- Kasakura, K.; Nagata, K.; Miura, R.; Iida, M.; Nakaya, H.; Okada, H.; Arai, T.; Kawakami, Y.; Kawakami, T.; Yashiro, T.; et al. Cooperative regulation of the mucosal mast cell-specific protease genes. J. Immunol. 2020, 204, 1641–1649. [Google Scholar] [CrossRef]


| Effects | in | Strain | MC Type | Condition | Response | Ref. |
|---|---|---|---|---|---|---|
| Promotive | vitro | C57BL/6 | Nb-infected MLN SCF+IL-10 | 7 days | Proliferation ↑ | [38,39,40,41,42] |
| BMMC IL-3+SCF | IL-10, 24 h | |||||
| Upper airway tract IL-3+SCF | IL-10, 24 h | |||||
| C3H/HeN | Splenocytes IL-3 | IL-10, 2–4 days | ||||
| BALB/c | BMMC IL-3+SCF+IL-10 | 8 days | ||||
| 21 days | ||||||
| IL-10-/- (Balb) | BMMC IL-3+SCF | 8 days | Proliferation ↓ | |||
| vivo | IL-10-/- (B6) | MMCs (T. spiralis-infected MLN) | 8 days | MC number ↓ | [42,43] | |
| NIH | Anti-IL-10 Ab i.p. 11–15 days | |||||
| IL-10-/- (Balb) | Jejunal MC | OVA-induced FA | ||||
| Suppressive | vitro | C57BL/6 | BMMC W+IL-10 | 14–21 days | Apoptosis ↑ | [41,44,45,46,47] |
| BMMC W | IL-10, 14 days | |||||
| IL-10, 6 days + IgE-XL | ||||||
| Human | Fetal liver monocytes SCF | IL-10, 7 days | ||||
| C57BL/6 | BMMC W | IL-10, 2–7 days | ||||
| BALB/c | IL-10, 3 days | |||||
| IL-10-/- (B6) | BMMC IL-3+SCF | 21 days | Proliferation ↑ |
| Effects | Strain | MC Type | Condition | Stimuli | Response | Ref. | |
|---|---|---|---|---|---|---|---|
| Suppression | C57BL/6 | BMMC IL-3 or BMMC W | IL-10 | 4 days | - | FcεRI ↓ | [26,55] |
| BALB/c | BMMC IL-3 | ||||||
| C3H | |||||||
| 129 | |||||||
| C57BL/6 | BMMC IL-3 | 5–14 days | - or IgE | ||||
| Human | Skin MC or Lung MC | 4 days | - | ||||
| C57BL/6 | BMMC IL-3 or W | 4 days | IgE-XL | TNF-α ↓ | [26,42,47,55,56,57] | ||
| BMMC IL-3+SCF | 16 h | ||||||
| BMMC W | 24 h | SCF | TNF-α, IL-13 ↓ | ||||
| Brown North rat | PMC | 1–18 h | IgE-XL, LPS, PGE1, 2 | Histamine, IL-6 ↓ | |||
| Lewis rat | LPS | TNF-α, IL-6 ↓ | |||||
| Sprague-Dawley rat | PMC (Nb-infected) | 24 h | IgE-XL | TNF-α, Histamine, Nitrite ↓ | |||
| Human | Skin MC | 4 days | Degranulation, GM-CSF ↓ | ||||
| BALB/c | BMMC IL-3+SCF | 24 h | TNF-α, IL-4↓ | ||||
| Sprague-Dawley rat | PMC (Nb-infected) | Anti-IL-10 Ab 24 h | 6 h | - or IgE-XL | TNF-α ↑ | [57] | |
| Human | CBMC | 24 h | IgE-XL | LTC4, D4, E4 IL-5, IL-8 ↑ | [58] | ||
| Human | CBMC | 12 h, IgE-XL | TNF-α ↑ | ||||
| CD68-IL-10 Tg (B6) | - | PSA | - | TNF-α, MIP-1α ↓, Δ of body temperature ↓ | [55] | ||
| Promotion | C57BL/6 | BMMC IL-3 or W | IL-10 | 4 d | IgE-XL | Degranulation ↑ | [26,40,42,59] |
| BALB/c | BMMC IL-3+SCF | 7 d | TNF-α, IL-6, IL-13 ↑ | ||||
| C57BL/6 | BMMC IL-3+SCF | 24 h | IL-6, IL-13, MCPT-1 ↑ | ||||
| C57BL/6 | PMC IL-3+SCF | IL-6, IL-13 ↑ | |||||
| C57BL/6 | Upper airway tract IL-3+SCF | Degranulation ↑ | |||||
| Human | Skin MC | MCP-1 ↑ | |||||
| C57BL/6 | - | PSA (IL-10 i.p.) | - | Histamine, IL-6, MIP-1α ↑, Δ of body temperature ↑ | |||
| BALB/c | BMMC IL-3+SCF | IL-10 | 24 h | LgE-XL | IL-6, IL-13 ↑ | [42] | |
| IL-10-/- (Balb) | - | OVA-induced FA | - | Diarrhea, MC number, Activation ↓ | |||
| Model | Strain | Treatment | Symptom | Ref. |
|---|---|---|---|---|
| Picryl chloride | WBB6F-KitW/W-v | None | Attenuated | [61] |
| WCB6F-SI/SId | ||||
| Oxazolone | WBB6F-KitW/W-v | No significance | [67] | |
| Picryl chloride | KitWf/Wf | |||
| DNFB | MCPT-5-Cre DTA | Attenuated | [12] | |
| FITC | ||||
| Urushiol | B6-KitW-sh/W-sh | Exacerbated | [14,28,68] | |
| DNFB | WBB6F-KitW/W-v | |||
| Oxazolone | B6-KitW-sh/W-sh | |||
| DNFB | KitW-sh/W-sh | |||
| Mcpt5-Cre DTA | ||||
| Cpa3-Cre Mcl-1fl/fl | ||||
| Mcpt5-Cre Il10fl/fl | ||||
| chronic low-dose UVB | WBB6F-KitW/W-v | |||
| DNFB | B6-KitW-sh/W-sh | BMMC reconstitution | Attenuated | [14,28,68] |
| Oxazolone | ||||
| chronic low-dose UVB | WBB6F-KitW/W-v | |||
| Oxazolone | C3H/HeN | pre-IgE-XL | Attenuated | [62] |
| WCB6F-SI/SId | pre-UVB irradiation | Loss of UVB-induced imunosuppression | [27,62] | |
| DNP-KLH | B6-KitW-sh/W-sh | |||
| BMMC reconstitution and pre-UVB irradiation |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagata, K.; Nishiyama, C. IL-10 in Mast Cell-Mediated Immune Responses: Anti-Inflammatory and Proinflammatory Roles. Int. J. Mol. Sci. 2021, 22, 4972. https://doi.org/10.3390/ijms22094972
Nagata K, Nishiyama C. IL-10 in Mast Cell-Mediated Immune Responses: Anti-Inflammatory and Proinflammatory Roles. International Journal of Molecular Sciences. 2021; 22(9):4972. https://doi.org/10.3390/ijms22094972
Chicago/Turabian StyleNagata, Kazuki, and Chiharu Nishiyama. 2021. "IL-10 in Mast Cell-Mediated Immune Responses: Anti-Inflammatory and Proinflammatory Roles" International Journal of Molecular Sciences 22, no. 9: 4972. https://doi.org/10.3390/ijms22094972
APA StyleNagata, K., & Nishiyama, C. (2021). IL-10 in Mast Cell-Mediated Immune Responses: Anti-Inflammatory and Proinflammatory Roles. International Journal of Molecular Sciences, 22(9), 4972. https://doi.org/10.3390/ijms22094972






