Psoriasis: From Pathogenesis to Pharmacological and Nano-Technological-Based Therapeutics
Abstract
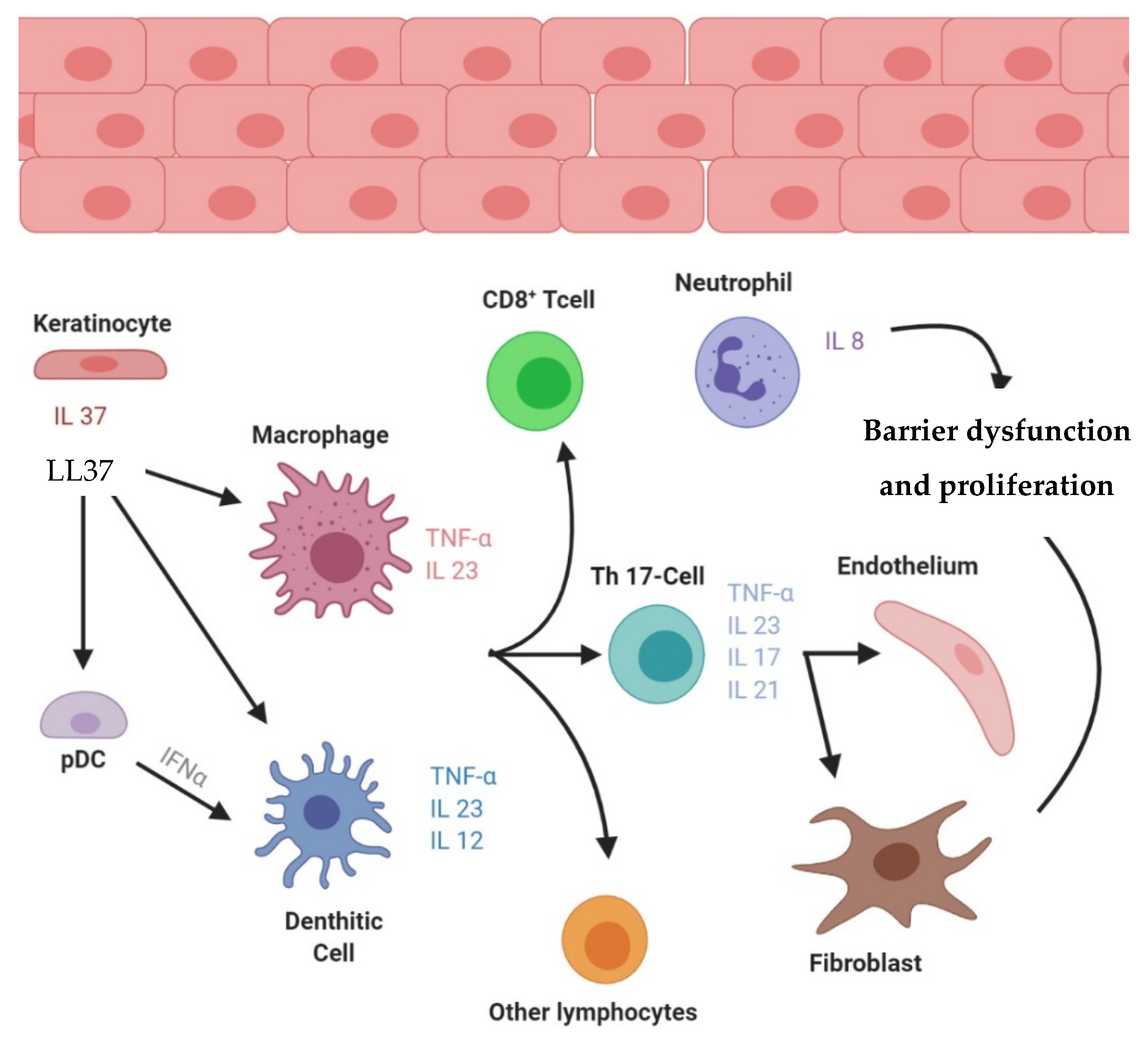
:1. Introduction
2. Methods
3. Current Marketed Therapies
4. Clinical Trials
5. Nanotechnological Approaches for Psoriasis
5.1. Polymeric Nanoparticles (PNPs)
5.1.1. Nanospheres
5.1.2. Nanocapsules
5.1.3. Dendrimers
5.1.4. Micelles
5.2. Lipid-Based Nanoparticles
5.2.1. Liposomes
5.2.2. Lipospheres
5.2.3. Ethosomes
5.2.4. Solid Lipid Nanoparticles
5.2.5. Nanostructured Lipid Carriers
5.3. Microneedles
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Samotij, D.; Nedoszytko, B.; Bartosińska, J.; Batycka-Baran, A.; Czajkowski, R.; Dobrucki, I.T.; Dobrucki, L.W.; Górecka-Sokołowska, M.; Janaszak-Jasienicka, A.; Krasowska, D.; et al. Pathogenesis of psoriasis in the “omic” era. Part I. Epidemiology, clinical manifestation, immunological and neuroendocrine disturbances. Postepy Dermatol. Alergol. 2020, 37, 135–153. [Google Scholar] [CrossRef]
- Rendon, A.; Schäkel, K. Psoriasis Pathogenesis and Treatment. Int. J. Mol. Sci. 2019, 20, 1475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortonne, J.; Chimenti, S.; Luger, T.; Puig, L.; Reid, F.; Trüeb, R.M. Scalp psoriasis: European consensus on grading and treatment algorithm. J. Eur. Acad. Dermatol. Venereol. JEADV 2009, 23, 1435–1444. [Google Scholar] [CrossRef] [PubMed]
- Harden, J.L.; Krueger, J.G.; Bowcock, A.M. The immunogenetics of Psoriasis: A comprehensive review. J. Autoimmun. 2015, 64, 66–73. [Google Scholar] [CrossRef] [Green Version]
- Howling, G.I.; Dettmar, P.W.; Goddard, P.A.; Hampson, F.C.; Dornish, M.; Wood, E.J. The effect of chitin and chitosan on the proliferation of human skin fibroblasts and keratinocytes in vitro. Biomaterials 2001, 22, 2959–2966. [Google Scholar] [CrossRef]
- Morizane, S.; Gallo, R.L. Antimicrobial peptides in the pathogenesis of psoriasis. J. Dermatol. 2012, 39, 225–230. [Google Scholar] [CrossRef]
- Morizane, S.; Yamasaki, K.; Mühleisen, B.; Kotol, P.F.; Murakami, M.; Aoyama, Y.; Iwatsuki, K.; Hata, T.; Gallo, R.L. Cathelicidin antimicrobial peptide LL-37 in psoriasis enables keratinocyte reactivity against TLR9 ligands. J. Investig. Dermatol. 2012, 132, 135–143. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Sarkar, M.K.; Tsoi, L.C.; Gudjonsson, J.E. Psoriasis: A mixed autoimmune and autoinflammatory disease. Curr. Opin. Immunol. 2017, 49, 1–8. [Google Scholar] [CrossRef]
- Girolomoni, G.; Strohal, R.; Puig, L.; Bachelez, H.; Barker, J.; Boehncke, W.H.; Prinz, J.C. The role of IL-23 and the IL-23/T(H) 17 immune axis in the pathogenesis and treatment of psoriasis. J. Eur. Acad. Dermatol. Venereol. JEADV 2017, 31, 1616–1626. [Google Scholar] [CrossRef] [Green Version]
- Fotiadou, C.; Lazaridou, E.; Sotiriou, E.; Ioannides, D. Targeting IL-23 in psoriasis: Current perspectives. Psoriasis 2018, 8, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Mansouri, M.; Mansouri, P.; Raze, A.A.; Jadali, Z. The potential role of Th17 lymphocytes in patients with psoriasis. An. Bras. Dermatol. 2018, 93, 63–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruan, Q.; Kameswaran, V.; Zhang, Y.; Zheng, S.; Sun, J.; Wang, J.; DeVirgiliis, J.; Liou, H.-C.; Beg, A.A.; Chen, Y.H. The Th17 immune response is controlled by the Rel-RORγ-RORγ T transcriptional axis. J. Exp. Med. 2011, 208, 2321–2333. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Luo, X.-Y.; Wu, D.-H.; Xu, Y. ROR nuclear receptors: Structures, related diseases, and drug discovery. Acta Pharm. Sin 2015, 36, 71–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ecoeur, F.; Weiss, J.; Kaupmann, K.; Hintermann, S.; Orain, D.; Guntermann, C. Antagonizing Retinoic Acid-Related-Orphan Receptor Gamma Activity Blocks the T Helper 17/Interleukin-17 Pathway Leading to Attenuated Pro-inflammatory Human Keratinocyte and Skin Responses. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Cyr, P.; Bronner, S.M.; Crawford, J.J. Recent progress on nuclear receptor RORγ modulators. Bioorganic Med. Chem. Lett. 2016, 26, 4387–4393. [Google Scholar] [CrossRef]
- Boutet, M.A.; Nerviani, A.; Gallo Afflitto, G.; Pitzalis, C. Role of the IL-23/IL-17 Axis in Psoriasis and Psoriatic Arthritis: The Clinical Importance of Its Divergence in Skin and Joints. Int. J. Mol. Sci. 2018, 19, 530. [Google Scholar] [CrossRef] [Green Version]
- Hänsel, A.; Günther, C.; Ingwersen, J.; Starke, J.; Schmitz, M.; Bachmann, M.; Meurer, M.; Rieber, E.P.; Schäkel, K. Human slan (6-sulfo LacNAc) dendritic cells are inflammatory dermal dendritic cells in psoriasis and drive strong TH17/TH1 T-cell responses. J. Allergy Clin. Immunol. 2011, 127, 787–794.e9. [Google Scholar] [CrossRef]
- Diluvio, L.; Vollmer, S.; Besgen, P.; Ellwart, J.W.; Chimenti, S.; Prinz, J.C. Identical TCR beta-chain rearrangements in streptococcal angina and skin lesions of patients with psoriasis vulgaris. J. Immunol. 2006, 176, 7104–7111. [Google Scholar] [CrossRef]
- Georgescu, S.R.; Tampa, M.; Caruntu, C.; Sarbu, M.I.; Mitran, C.I.; Mitran, M.I.; Matei, C.; Constantin, C.; Neagu, M. Advances in Understanding the Immunological Pathways in Psoriasis. Int. J. Mol. Sci. 2019, 20, 739. [Google Scholar] [CrossRef] [Green Version]
- Matsuzaki, G.; Umemura, M. Interleukin-17 family cytokines in protective immunity against infections: Role of hematopoietic cell-derived and non-hematopoietic cell-derived interleukin-17s. Microbiol. Immunol. 2018, 62, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Gaffen, S.L. Structure and signalling in the IL-17 receptor family. Nat. Rev. Immunol. 2009, 9, 556–567. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.S.; Tato, C.M.; Joyce-Shaikh, B.; Gulen, M.F.; Cayatte, C.; Chen, Y.; Blumenschein, W.M.; Judo, M.; Ayanoglu, G.; McClanahan, T.K.; et al. Interleukin-23-Independent IL-17 Production Regulates Intestinal Epithelial Permeability. Immunity 2015, 43, 727–738. [Google Scholar] [CrossRef] [Green Version]
- Schmitt, J.; Rosumeck, S.; Thomaschewski, G.; Sporbeck, B.; Haufe, E.; Nast, A. Efficacy and safety of systemic treatments for moderate-to-severe psoriasis: Meta-analysis of randomized controlled trials. Br. J. Dermatol. 2014, 170, 274–303. [Google Scholar] [CrossRef]
- Leonardi, C.L.; Romiti, R.; Tebbey, P.W. Ten years on: The impact of biologics on the practice of dermatology. Dermatol. Clin. 2015, 33, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.; Van Voorhees, A.S.; Hsu, S.; Korman, N.J.; Lebwohl, M.G.; Bebo, B.F., Jr.; Kalb, R.E. Treatment of pustular psoriasis: From the Medical Board of the National Psoriasis Foundation. J. Am. Acad. Dermatol. 2012, 67, 279–288. [Google Scholar] [CrossRef]
- Johnston, A.; Xing, X.; Wolterink, L.; Barnes, D.H.; Yin, Z.; Reingold, L.; Kahlenberg, J.M.; Harms, P.W.; Gudjonsson, J.E. IL-1 and IL-36 are dominant cytokines in generalized pustular psoriasis. J. Allergy Clin. Immunol. 2017, 140, 109–120. [Google Scholar] [CrossRef] [Green Version]
- Onoufriadis, A.; Simpson, M.A.; Pink, A.E.; Di Meglio, P.; Smith, C.H.; Pullabhatla, V.; Knight, J.; Spain, S.L.; Nestle, F.O.; Burden, A.D.; et al. Mutations in IL36RN/IL1F5 are associated with the severe episodic inflammatory skin disease known as generalized pustular psoriasis. Am. J. Hum. Genet. 2011, 89, 432–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marrakchi, S.; Guigue, P.; Renshaw, B.R.; Puel, A.; Pei, X.Y.; Fraitag, S.; Zribi, J.; Bal, E.; Cluzeau, C.; Chrabieh, M.; et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N. Engl. J. Med. 2011, 365, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Navarini, A.A.; Burden, A.D.; Capon, F.; Mrowietz, U.; Puig, L.; Köks, S.; Kingo, K.; Smith, C.; Barker, J.N. European consensus statement on phenotypes of pustular psoriasis. J. Eur. Acad. Dermatol. Venereol. JEADV 2017, 31, 1792–1799. [Google Scholar] [CrossRef] [Green Version]
- Ko, H.C.; Jwa, S.W.; Song, M.; Kim, M.B.; Kwon, K.S. Clinical course of guttate psoriasis: Long-term follow-up study. J. Dermatol. 2010, 37, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.Y.; Travers, J.B.; Giorno, R.; Norris, D.A.; Skinner, R.; Aelion, J.; Kazemi, L.V.; Kim, M.H.; Trumble, A.E.; Kotb, M.; et al. Evidence for a streptococcal superantigen-driven process in acute guttate psoriasis. J. Clin. Investig. 1995, 96, 2106–2112. [Google Scholar] [CrossRef] [PubMed]
- Johnston, A.; Gudjonsson, J.E.; Sigmundsdottir, H.; Love, T.J.; Valdimarsson, H. Peripheral blood T cell responses to keratin peptides that share sequences with streptococcal M proteins are largely restricted to skin-homing CD8(+) T cells. Clin. Exp. Immunol. 2004, 138, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Micali, G.; Verzì, A.E.; Giuffrida, G.; Panebianco, E.; Musumeci, M.L.; Lacarrubba, F. Inverse Psoriasis: From Diagnosis to Current Treatment Options. Clin. Cosmet. Investig. Dermatol. 2019, 12, 953–959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Syed, Z.U.; Khachemoune, A. Inverse psoriasis: Case presentation and review. Am. J. Clin. Dermatol. 2011, 12, 143–146. [Google Scholar] [CrossRef]
- Mrowietz, U.; Kragballe, K.; Reich, K.; Spuls, P.; Griffiths, C.E.; Nast, A.; Franke, J.; Antoniou, C.; Arenberger, P.; Balieva, F.; et al. Definition of treatment goals for moderate to severe psoriasis: A European consensus. Arch. Dermatol. Res. 2011, 303, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torsekar, R.; Gautam, M.M. Topical Therapies in Psoriasis. Indian Dermatol. Online J. 2017, 8, 235–245. [Google Scholar] [CrossRef]
- Stein Gold, L.F. Topical Therapies for Psoriasis: Improving Management Strategies and Patient Adherence. Semin. Cutan. Med. Surg. 2016, 35, S36–S44. [Google Scholar] [CrossRef]
- Kim, W.B.; Jerome, D.; Yeung, J. Diagnosis and management of psoriasis. Can. Fam. Physician 2017, 63, 278–285. [Google Scholar]
- Psomadakis, C.E.; Han, G. New and Emerging Topical Therapies for Psoriasis and Atopic Dermatitis. J. Clin. Aesthetic Dermatol. 2019, 12, 28–34. [Google Scholar]
- Armstrong, A.W.; Read, C. Pathophysiology, Clinical Presentation, and Treatment of Psoriasis: A Review. JAMA 2020, 323, 1945–1960. [Google Scholar] [CrossRef]
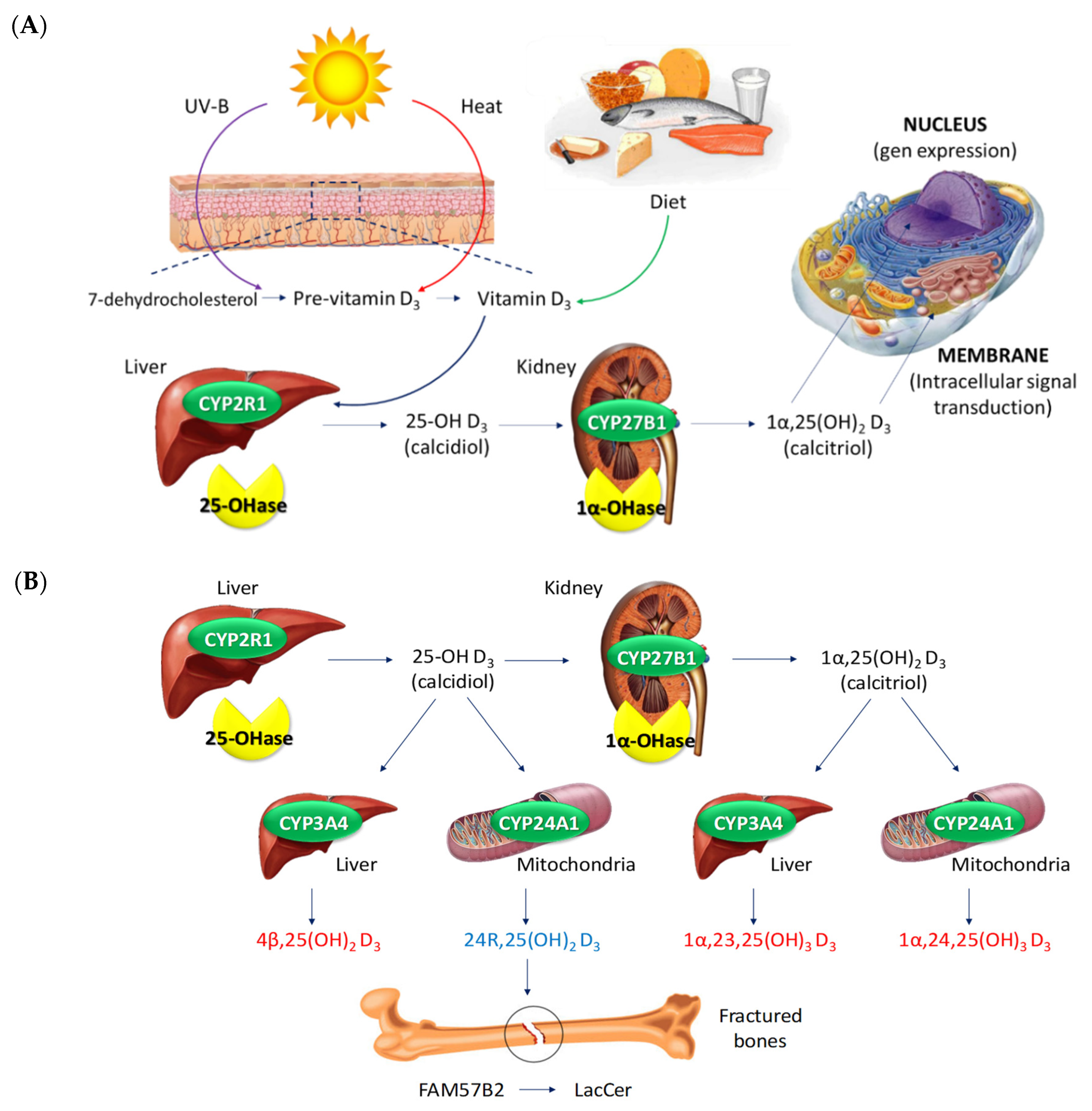
- Piotrowska, A.; Wierzbicka, J.; Żmijewski, M.A. Vitamin D in the skin physiology and pathology. Acta Biochim. Pol. 2016, 63, 17–29. [Google Scholar] [CrossRef] [Green Version]
- Wadhwa, B.; Relhan, V.; Goel, K.; Kochhar, A.M.; Garg, V.K. Vitamin D and skin diseases: A review. Indian J. Dermatol. Venereol. Leprol. 2015, 81, 344–355. [Google Scholar] [CrossRef]
- Barrea, L.; Savanelli, M.C.; Di Somma, C.; Napolitano, M.; Megna, M.; Colao, A.; Savastano, S. Vitamin D and its role in psoriasis: An overview of the dermatologist and nutritionist. Rev. Endocr. Metab. Disord. 2017, 18, 195–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattozzi, C.; Paolino, G.; Richetta, A.G.; Calvieri, S. Psoriasis, vitamin D and the importance of the cutaneous barrier’s integrity: An update. J. Dermatol. 2016, 43, 507–514. [Google Scholar] [CrossRef]
- Filoni, A.; Vestita, M.; Congedo, M.; Giudice, G.; Tafuri, S.; Bonamonte, D. Association between psoriasis and vitamin D: Duration of disease correlates with decreased vitamin D serum levels: An observational case-control study. Medicine 2018, 97, e11185. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Song, G.G. Association between circulating 25-hydroxyvitamin D levels and psoriasis, and correlation with disease severity: A meta-analysis. Clin. Exp. Dermatol. 2018, 43, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Giustina, A.; Bouillon, R.; Binkley, N.; Sempos, C.; Adler, R.A.; Bollerslev, J.; Dawson-Hughes, B.; Ebeling, P.R.; Feldman, D.; Heijboer, A.; et al. Controversies in Vitamin D: A Statement from the Third International Conference. JBMR Plus 2020, 4, e10417. [Google Scholar] [CrossRef] [PubMed]
- Hambly, R.; Kirby, B. The relevance of serum vitamin D in psoriasis: A review. Arch. Dermatol. Res. 2017, 309, 499–517. [Google Scholar] [CrossRef]
- Umar, M.; Sastry, K.S.; Al Ali, F.; Al-Khulaifi, M.; Wang, E.; Chouchane, A.I. Vitamin D and the Pathophysiology of Inflammatory Skin Diseases. Ski. Pharmacol. Physiol. 2018, 31, 74–86. [Google Scholar] [CrossRef]
- Jones, G. The discovery and synthesis of the nutritional factor vitamin D. Int. J. Paleopathol. 2018, 23, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Jarrett, P.; Scragg, R. A short history of phototherapy, vitamin D and skin disease. Photochem. Photobiol. Sci. Off. J. Eur. Photochem. Assoc. Eur. Soc. Photobiol. 2017, 16, 283–290. [Google Scholar] [CrossRef]
- Juzeniene, A.; Grigalavicius, M.; Juraleviciute, M.; Grant, W.B. Phototherapy and vitamin D. Clin. Dermatol. 2016, 34, 548–555. [Google Scholar] [CrossRef]
- Lee, Y.H. Vitamin D receptor ApaI, TaqI, BsmI, and FokI polymorphisms and psoriasis susceptibility: An updated meta-analysis. Clin. Exp. Dermatol. 2019, 44, 498–505. [Google Scholar] [CrossRef]
- Liu, J.; Wang, W.; Liu, K.; Wan, D.; Wu, Z.; Cao, Z.; Luo, Y.; Xiao, C.; Yin, M. Vitamin D receptor gene polymorphisms are associated with psoriasis susceptibility and the clinical response to calcipotriol in psoriatic patients. Exp. Dermatol. 2020, 29, 1186–1190. [Google Scholar] [CrossRef]
- Slominski, A.T.; Kim, T.K.; Li, W.; Yi, A.K.; Postlethwaite, A.; Tuckey, R.C. The role of CYP11A1 in the production of vitamin D metabolites and their role in the regulation of epidermal functions. J. Steroid Biochem. Mol. Biol. 2014, 144 Pt A, 28–39. [Google Scholar] [CrossRef] [Green Version]
- Lang, P.O.; Aspinall, R. Vitamin D Status and the Host Resistance to Infections: What It Is Currently (Not) Understood. Clin. Ther. 2017, 39, 930–945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkinson, C. The vitamin D metabolome: An update on analysis and function. Cell Biochem. Funct. 2019, 37, 408–423. [Google Scholar] [CrossRef] [PubMed]
- Tuckey, R.C.; Li, W.; Shehabi, H.Z.; Janjetovic, Z.; Nguyen, M.N.; Kim, T.K.; Chen, J.; Howell, D.E.; Benson, H.A.; Sweatman, T.; et al. Production of 22-hydroxy metabolites of vitamin d3 by cytochrome p450scc (CYP11A1) and analysis of their biological activities on skin cells. Drug Metab. Dispos. Biol. Fate Chem. 2011, 39, 1577–1588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wierzbicka, J.; Piotrowska, A.; Żmijewski, M.A. The renaissance of vitamin D. Acta Biochim. Pol. 2014, 61, 679–686. [Google Scholar] [CrossRef]
- Gao, C.; Liao, M.Z.; Han, L.W.; Thummel, K.E.; Mao, Q. Hepatic Transport of 25-Hydroxyvitamin D(3) Conjugates: A Mechanism of 25-Hydroxyvitamin D(3) Delivery to the Intestinal Tract. Drug Metab. Dispos. Biol. Fate Chem. 2018, 46, 581–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farahnik, B.; Patel, V.; Beroukhim, K.; Zhu, T.H.; Abrouk, M.; Nakamura, M.; Singh, R.; Lee, K.; Bhutani, T.; Koo, J. Combining biologic and phototherapy treatments for psoriasis: Safety, efficacy, and patient acceptability. Psoriasis 2016, 6, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Young, M.; Aldredge, L.; Parker, P. Psoriasis for the primary care practitioner. J. Am. Assoc. Nurse Pract. 2017, 29, 157–178. [Google Scholar] [CrossRef]
- Perrone, V.; Sangiorgi, D.; Buda, S.; Degli Esposti, L. Topical medication utilization and health resources consumption in adult patients affected by psoriasis: Findings from the analysis of administrative databases of local health units. ClinicoEconomics Outcomes Res. 2017, 9, 181–188. [Google Scholar] [CrossRef] [Green Version]
- Pathak, S.N.; Scott, P.; West, C.E.; Feldman, S.R. Self-management in patients with psoriasis. Psoriasis Targets Ther. 2014, 4, 19–26. [Google Scholar]
- Dattola, A.; Silvestri, M.; Bennardo, L.; Passante, M.; Rizzuto, F.; Dastoli, S.; Patruno, C.; Bianchi, L.; Nisticò, S.P. A novel vehicle for the treatment of psoriasis. Dermatol. Ther. 2020, 33, e13185. [Google Scholar] [CrossRef] [Green Version]
- Pinzon, M.I.; Garcia, O.R.; Villa, C.C. The influence of Aloe vera gel incorporation on the physicochemical and mechanical properties of banana starch-chitosan edible films. J. Sci. Food Agric. 2018, 98, 4042–4049. [Google Scholar] [CrossRef]
- Fluhr, J.W.; Cavallotti, C.; Berardesca, E. Emollients, moisturizers, and keratolytic agents in psoriasis. Clin. Dermatol. 2008, 26, 380–386. [Google Scholar] [CrossRef]
- Nola, I.; Kostović, K.; Kotrulja, L.; Lugović, L. The use of emollients as sophisticated therapy in dermatology. Acta Dermatovenerol. Croat. Adc 2003, 11, 80–87. [Google Scholar] [PubMed]
- Arbiser, J.L.; Govindarajan, B.; Battle, T.E.; Lynch, R.; Frank, D.A.; Ushio-Fukai, M.; Perry, B.N.; Stern, D.F.; Bowden, G.T.; Liu, A.; et al. Carbazole is a naturally occurring inhibitor of angiogenesis and inflammation isolated from antipsoriatic coal tar. J. Investig. Dermatol. 2006, 126, 1396–1402. [Google Scholar] [CrossRef] [Green Version]
- Lebwohl, M. The role of salicylic acid in the treatment of psoriasis. Int. J. Dermatol. 1999, 38, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lin, A. Efficacy of topical calcineurin inhibitors in psoriasis. J. Cutan. Med. Surg. 2014, 18, 8–14. [Google Scholar] [CrossRef]
- Duvic, M.; Asano, A.T.; Hager, C.; Mays, S. The pathogenesis of psoriasis and the mechanism of action of tazarotene. J. Am. Acad. Dermatol. 1998, 39, S129–S133. [Google Scholar] [CrossRef]
- Weinstein, G.D.; Koo, J.Y.; Krueger, G.G.; Lebwohl, M.G.; Lowe, N.J.; Menter, M.A.; Lew-Kaya, D.A.; Sefton, J.; Gibson, J.R.; Walker, P.S. Tazarotene cream in the treatment of psoriasis: Two multicenter, double-blind, randomized, vehicle-controlled studies of the safety and efficacy of tazarotene creams 0.05% and 0.1% applied once daily for 12 weeks. J. Am. Acad. Dermatol. 2003, 48, 760–767. [Google Scholar] [CrossRef]
- McGill, A.; Frank, A.; Emmett, N.; Turnbull, D.M.; Birch-Machin, M.A.; Reynolds, N.J. The anti-psoriatic drug anthralin accumulates in keratinocyte mitochondria, dissipates mitochondrial membrane potential, and induces apoptosis through a pathway dependent on respiratory competent mitochondria. Faseb J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2005, 19, 1012–1014. [Google Scholar] [CrossRef] [Green Version]
- Hengge, U.R.; Ruzicka, T.; Schwartz, R.A.; Cork, M.J. Adverse effects of topical glucocorticosteroids. J. Am. Acad. Dermatol. 2006, 54, 1–15. [Google Scholar] [CrossRef]
- Van De Kerkhof, P.C.M.; Berth-Jones, J.; Griffiths, C.E.M.; Harrison, P.V.; Hönigsmann, H.; Marks, R.; Roelandts, R.; Schöpf, E.; Trompke, C. Long-term efficacy and safety of tacalcitol ointment in patients with chronic plaque psoriasis. Br. J. Dermatol. 2002, 146, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Li, K. A review of acitretin for the treatment of psoriasis. Expert Opin. Drug Saf. 2009, 8, 769–779. [Google Scholar] [CrossRef]
- Smith, D. Fumaric acid esters for psoriasis: A systematic review. Ir. J. Med. Sci. 2017, 186, 161–177. [Google Scholar] [CrossRef] [PubMed]
- Dogra, S.; Mahajan, R.; Narang, T.; Handa, S.; Dogra, S.; Mahajan, R.; Narang, T.; Handa, S. Systemic cyclosporine treatment in severe childhood psoriasis: A retrospective chart review Systemic cyclosporine treatment in severe childhood psoriasis: A retrospective chart review. J. Dermatolog. Treat. 2017, 6634. [Google Scholar] [CrossRef]
- Greb, J.E.; Goldminz, A.M.; Gottlieb, A.B. Insights on methotrexate in psoriatic disease. Clin. Immunol. 2016, 172, 61–64. [Google Scholar] [CrossRef]
- Keating, G.M. Apremilast: A Review in Psoriasis and Psoriatic Arthritis. Drugs 2017, 77, 459–472. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, A.; Torres, T. Tofacitinib: A New Oral Therapy for Psoriasis. Clin. Drug Investig. 2018, 38, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Dapavo, P. Vujic.I.; Fierro, M.T.; Quaglino, P.; Samlorenzo, M. The infliximab biosimilar in the treatment of moderate to severe plaque psoriasis. Pract. Nurs. 2008, 19, 560–565. [Google Scholar] [CrossRef]
- Leonardi, C.L.; Powers, J.L.; Matheson, R.T.; Goffe, B.S.; Zitnik, R.; Wang, A.; Gottlieb, A.B. Etanercept as Monotherapy in Patients with Psoriasis. N. Engl. J. Med. 2003, 349, 2014–2022. [Google Scholar] [CrossRef] [Green Version]
- Berends, M.A.M.; Driessen, R.J.B.; Langewouters, A.M.G.; Boezeman, J.B.; Van De Kerkhof, P.C.M.; De Jong, E.M.G.J. Etanercept and efalizumab treatment for high-need psoriasis. Effects and side effects in a prospective cohort study in outpatient clinical practice. J. Dermatolog. Treat. 2007, 18, 76–83. [Google Scholar] [CrossRef]
- Alwawi, E.A.; Mehlis, S.L.; Gordon, K.B. Treating psoriasis with adalimumab. Ther. Clin. Risk Manag. 2008, 4, 345–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chimenti, M.S.; Saraceno, R.; Chiricozzi, A.; Giunta, A.; Chimenti, S.; Perricone, R. Profile of certolizumab and its potential in the treatment of psoriatic arthritis. Open Access Rheumatol. Res. Rev. 2013, 6, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Fleischmann, R.; Vencovsky, J.; Van Vollenhoven, R.F.; Borenstein, D.; Box, J.; Coteur, G.; Goel, N.; Brezinschek, H.P.; Innes, A.; Strand, V. Efficacy and safety of certolizumab pegol monotherapy every 4 weeks in patients with rheumatoid arthritis failing previous disease-modifying antirheumatic therapy: The FAST4WARD study. Ann. Rheum. Dis. 2009, 68, 805–811. [Google Scholar] [CrossRef]
- Leonardi, C.L.; Kimball, A.B.; Papp, K.A. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-Week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet 2008, 24, 34. [Google Scholar] [CrossRef]
- Langley, R.G.; Elewski, B.E.; Lebwohl, M.; Reich, K.; Griffiths, C.E.M.; Papp, K.; Puig, L.; Nakagawa, H.; Spelman, L.; Sigurgeirsson, B.; et al. Secukinumab in plaque psoriasis—Results of two phase 3 trials. N. Engl. J. Med. 2014, 371, 326–338. [Google Scholar] [CrossRef] [Green Version]
- Glatt, S.; Helmer, E.; Haier, B.; Strimenopoulou, F.; Price, G.; Vajjah, P.; Harari, O.A.; Lambert, J.; Shaw, S. First-in-human randomized study of bimekizumab, a humanized monoclonal antibody and selective dual inhibitor of IL-17A and IL-17F, in mild psoriasis. Br. J. Clin. Pharmacol. 2017, 83, 991–1001. [Google Scholar] [CrossRef]
- Reich, K. Anti-interleukin-17 monoclonal antibody ixekizumab in psoriasis. N. Engl. J. Med. 2012, 367, 274–275. [Google Scholar] [CrossRef] [PubMed]
- Gordon, K.B.; Blauvelt, A.; Papp, K.A.; Langley, R.G.; Luger, T.; Ohtsuki, M.; Reich, K.; Amato, D.; Ball, S.G.; Braun, D.K.; et al. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N. Engl. J. Med. 2016, 375, 345–356. [Google Scholar] [CrossRef]
- Krueger, J.G.; Kricorian, G.; Aras, G.; Ph, D.; Li, J.; Russell, C.B.; Thompson, E.H.Z.; Baumgartner, S. Brodalumab, an Anti–Interleukin-17–Receptor Antibody for Psoriasis. N. Engl. J. Med. 2012, 366, 1181–1189. [Google Scholar]
- Papp, K.; Thaçi, D.; Reich, K.; Riedl, E.; Langley, R.G.; Krueger, J.G.; Gottlieb, A.B.; Nakagawa, H.; Bowman, E.P.; Mehta, A.; et al. Tildrakizumab (MK-3222), an anti-interleukin-23p19 monoclonal antibody, improves psoriasis in a phase IIb randomized placebo-controlled trial. Br. J. Dermatol. 2015, 173, 930–939. [Google Scholar] [CrossRef]
- Nakamura, M.; Lee, K.; Jeon, C.; Sekhon, S.; Afifi, L.; Yan, D.; Lee, K.; Bhutani, T. Guselkumab for the Treatment of Psoriasis: A Review of Phase III Trials. Dermatol. Ther. 2017, 7, 281–292. [Google Scholar] [CrossRef] [Green Version]
- Sofen, H.; Smith, S.; Matheson, R.T.; Leonardi, C.L.; Calderon, C.; Brodmerkel, C.; Li, K.; Campbell, K.; Marciniak, S.J.; Wasfi, Y.; et al. Guselkumab (an IL-23-specific mAb) demonstrates clinical and molecular response in patients with moderate-to-severe psoriasis. J. Allergy Clin. Immunol. 2014, 133, 1032–1040. [Google Scholar] [CrossRef] [Green Version]
- Gordon, K.B.; Strober, B.; Lebwohl, M.; Augustin, M.; Blauvelt, A.; Poulin, Y.; Papp, K.A.; Sofen, H.; Puig, L.; Foley, P.; et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): Results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet 2018, 392, 650–661. [Google Scholar] [CrossRef]
- Reich, K.; Rich, P.; Maari, C.; Bissonnette, R.; Leonardi, C.; Menter, A.; Igarashi, A.; Klekotka, P.; Patel, D.; Li, J.; et al. Efficacy and safety of mirikizumab (LY3074828) in the treatment of moderate-to-severe plaque psoriasis: Results from a randomized phase II study. Br. J. Dermatol. 2019, 181, 88–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iannone, L.F.; Bennardo, L.; Palleria, C.; Roberti, R.; De Sarro, C.; Naturale, M.D.; Dastoli, S.; Donato, L.; Manti, A.; Valenti, G.; et al. Safety profile of biologic drugs for psoriasis in clinical practice: An Italian prospective pharmacovigilance study. PLoS ONE 2020, 15, e0241575. [Google Scholar] [CrossRef] [PubMed]
- Boston Pharmaceuticals. Study to Evaluate the Safety, Tolerability, and Pharmacokinetics of Single and Repeat Topical Administration of BOS-475 in Healthy Subjects and Patients with Psoriasis; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2020.
- AbbVie. A Study to Evaluate the Pharmacokinetics, Safety and Tolerability of ABBV-157 in Healthy Volunteers and in Participants with Chronic Plaque Psoriasis; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2019.
- Celgene. A Safety Study of CC-92252 in Healthy Adult Subjects and Adult Subjects with Psoriasis; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2018.
- Evelo Biosciences, I. A Study of EDP1066 in Healthy Participants and Participants with Mild to Moderate Psoriasis and Atopic Dermatitis; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2018.
- Evelo Biosciences, I. A Study of EDP1815 in Healthy Participants and Participants with Mild to Moderate Psoriasis and Atopic Dermatitis; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2018.
- Affibody. A Study to Evaluate ABY-035 in Subjects with Moderate-to-severe Plaque Psoriasis (AFFIRM-35); U.S. Food and Drug Administration: Silver Spring, MD, USA, 2018.
- Arcutis Biotherapeutics, I. Safety, Pharmacokinetics and Efficacy of ARQ-151 Cream in Adults with Mild to Moderate Chronic Plaque Psoriasis; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2018.
- Boehringer, I. A Study to Test. How Well Patients with Plaque Psoriasis Tolerate BI 730357 over a Longer Period and How Effective It Is; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2019.
- Santalis Pharmaceuticals Inc. A Trial of a Botanical Drug (EISO) for Treatment of Mild-to-Moderate Plaque Psoriasis; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2019.
- Akros Pharma Inc. Study to Evaluate the Efficacy and Safety of JTE-451 in Subjects with Moderate to Severe Plaque Psoriasis (IMPACT-PS); U.S. Food and Drug Administration: Silver Spring, MD, USA, 2019.
- Bond Avillion 2 Development LP. A Phase 2b Study of the Efficacy, Safety, and Tolerability of M1095 in Subjects with Moderate to Severe Psoriasis; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2017.
- Pfizer. Dose Ranging Study to Assess. Efficacy, Safety and Tolerability of PF-06700841 Topical Cream in Psoriasis; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2019.
- Pfizer. A Study to Evaluate Safety and Efficacy of PF-06826647 for Moderate to Severe Plaque Psoriasis; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2019.
- Jiangsu Hengrui Medicine Company Ltd. A Clinical Study of SHR-1314 Injection in the Treatment of Moderate to Severe Plaque Psoriasis in Adults; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2019.
- Bristol-Myers Squibb. An. Investigational Study to Evaluate Experimental Medication BMS-986165 Compared to Placebo and a Currently Available Treatment in Participants with Moderate-to-Severe Plaque Psoriasis (POETYK-PSO-2); U.S. Food and Drug Administration: Silver Spring, MD, USA, 2018.
- Biocad. Clinical Study of Efficacy and Safety of BCD-085 (Monoclonal Anti-IL-17 Antibody) in Psoriatic Arthritis (PATERA); U.S. Food and Drug Administration: Silver Spring, MD, USA, 2018.
- Boehringer, I. The VOLTAIRE-X Trial Looks at the Effect of Switching Between Humira® and BI 695501 in Patients with Plaque Psoriasis; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2017.
- Can-Fite BioPharma Ltd. CF101 Therapy in Patients with Moderate-to-Severe Plaque Psoriasis; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2017.
- Coherus Biosciences, Inc. Comparison of CHS-1420 Versus Humira in Subjects with Chronic Plaque Psoriasis (PsOsim); U.S. Food and Drug Administration: Silver Spring, MD, USA, 2019.
- Gilead Sciences. Study to Evaluate the Efficacy and Safety of Filgotinib in Participants with Active Psoriatic Arthritis Who Are Naive to Biologic DMARD Therapy (PENGUIN 1); U.S. Food and Drug Administration: Silver Spring, MD, USA, 2019.
- Pradhan, M.; Alexander, A.; Singh, M.R.; Singh, D.; Saraf, S.; Saraf, S.; Ajazuddin. Understanding the prospective of nano-formulations towards the treatment of psoriasis. Biomed. Pharmacother. 2018, 107, 447–463. [Google Scholar] [CrossRef]
- Menlo Therapeutics Inc. Study of the Long Term Safety of Serlopitant for the Treatment of Pruritus (Itch); U.S. Food and Drug Administration: Silver Spring, MD, USA, 2018.
- Dermavant Sciences GmbH. Long Term Extension Study of Tapinarof for Plaque Psoriasis in Adults; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2019.
- Sun Pharma Global Fze. A Study to Evaluate the Efficacy and Safety/Tolerability of Subcutaneous Tildrakizumab (SCH 900222/MK-3222) in Participants with Moderate-to-Severe Chronic Plaque Psoriasis Followed by a Long-term Extension Study (MK-3222-011) (reSURFACE 2); U.S. Food and Drug Administration: Silver Spring, MD, USA, 2018.
- AbbVie. A Study Comparing Upadacitinib (ABT-494) to Placebo in Participants with Active Psoriatic Arthritis Who Have a History of Inadequate Response to at Least One Biologic Disease Modifying Anti-Rheumatic Drug (SELECT—PsA 2); U.S. Food and Drug Administration: Silver Spring, MD, USA, 2017.
- Lombardo, D.; Kiselev, M.A.; Caccamo, M.T. Smart Nanoparticles for Drug Delivery Application: Development of Versatile Nanocarrier Platforms in Biotechnology and Nanomedicine. J. Nanomater. 2019, 2019, 3702518. [Google Scholar] [CrossRef]
- Batheja, P.; Sheihet, L.; Kohn, J.; Singer, A.J.; Michniak-Kohn, B. Topical drug delivery by a polymeric nanosphere gel: Formulation optimization and in vitro and in vivo skin distribution studies. J. Control. Release 2011, 149, 159–167. [Google Scholar] [CrossRef]
- Diering. Polymeric Nanospheres for Topical Delivery of Vitamin D3. Physiol. Behav. 2018, 176, 139–148. [Google Scholar] [CrossRef]
- Mora-Huertas, C.E.; Fessi, H.; Elaissari, A. Polymer-based nanocapsules for drug delivery. Int. J. Pharm. 2010, 385, 113–142. [Google Scholar] [CrossRef]
- Barbosa, T.C.; Nascimento, L.E.D.; Bani, C.; Almeida, T.; Nery, M.; Santos, R.S.; Menezes, L.R.O.; Zielinska, A.; Fernandes, A.R.; Cardoso, J.C.; et al. Development, Cytotoxicity and Eye Irritation Profile of a New Sunscreen Formulation Based on Benzophenone-3-poly(epsilon-caprolactone) Nanocapsules. Toxics 2019, 7, 51. [Google Scholar] [CrossRef] [Green Version]
- Marchiori, M.L.; Lubini, G.; Dalla Nora, G.; Friedrich, R.B.; Fontana, M.C.; Ourique, A.F.; Bastos, M.O.; Rigo, L.A.; Silva, C.B.; Tedesco, S.B.; et al. Hydrogel containing dexamethasone-loaded nanocapsules for cutaneous administration: Preparation, characterization, and in vitro drug release study. Drug Dev. Ind. Pharm. 2010, 36, 962–971. [Google Scholar] [CrossRef] [PubMed]
- Mignani, S.; El Kazzouli, S.; Bousmina, M.; Majoral, J.P. Expand classical drug administration ways by emerging routes using dendrimer drug delivery systems: A concise overview. Adv. Drug Deliv. Rev. 2013, 65, 1316–1330. [Google Scholar] [CrossRef] [PubMed]
- Sikwal, D.R.; Kalhapure, R.S.; Govender, T. An emerging class of amphiphilic dendrimers for pharmaceutical and biomedical applications: Janus amphiphilic dendrimers. Eur. J. Pharm. Sci. 2017, 97, 113–134. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, U.; Mehra, N.K.; Gupta, U.; Jain, N.K. Hyperbranched dendritic nano-carriers for topical delivery of dithranol. J. Drug Target. 2013, 21, 497–506. [Google Scholar] [CrossRef]
- Damiani, G.; Pacifico, A.; Linder, D.M.; Pigatto, P.D.M.; Conic, R.; Grada, A.; Bragazzi, N.L. Nanodermatology-based solutions for psoriasis: State-of-the art and future prospects. Dermatol. Ther. 2019, 32, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Lapteva, M.; Mondon, K.; Möller, M.; Gurny, R.; Kalia, Y.N. Polymeric micelle nanocarriers for the cutaneous delivery of tacrolimus: A targeted approach for the treatment of psoriasis. Mol. Pharm. 2014, 11, 2989–3001. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.C.; Carbone, C.; Souto, E.B. Beyond liposomes: Recent advances on lipid based nanostructures for poorly soluble/poorly permeable drug delivery. Prog. Lipid Res. 2017, 68, 1–11. [Google Scholar] [CrossRef]
- Doktorovova, S.; Kovacevic, A.B.; Garcia, M.L.; Souto, E.B. Preclinical safety of solid lipid nanoparticles and nanostructured lipid carriers: Current evidence from in vitro and in vivo evaluation. Eur. J. Pharm. Biopharm. 2016, 108, 235–252. [Google Scholar] [CrossRef] [PubMed]
- Clares, B.; Calpena, A.C.; Parra, A.; Abrego, G.; Alvarado, H.; Fangueiro, J.F.; Souto, E.B. Nanoemulsions (NEs), liposomes (LPs) and solid lipid nanoparticles (SLNs) for retinyl palmitate: Effect on skin permeation. Int. J. Pharm. 2014, 473, 591–598. [Google Scholar] [CrossRef]
- Wadhwa, S.; Singh, B.; Sharma, G.; Raza, K.; Katare, O.P. Liposomal fusidic acid as a potential delivery system: A new paradigm in the treatment of chronic plaque psoriasis. Drug Deliv. 2016, 23, 1204–1213. [Google Scholar] [CrossRef] [Green Version]
- Hua, S. Lipid-based nano-delivery systems for skin delivery of drugs and bioactives. Front. Pharmacol. 2015, 6, 2011–2015. [Google Scholar] [CrossRef]
- Jain, A.; Doppalapudi, S.; Domb, A.J.; Khan, W. Tacrolimus and curcumin co-loaded liposphere gel: Synergistic combination towards management of psoriasis. J. Control. Release 2016, 243, 132–145. [Google Scholar] [CrossRef]
- Ainbinder, D.; Paolino, D.; Fresta, M.; Touitou, E. Drug delivery applications with ethosomes. J. Biomed. Nanotechnol. 2010, 6, 558–568. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.T.; Shen, L.N.; Zhao, J.H.; Feng, N.P. Evaluation of psoralen ethosomes for topical delivery in rats by using in vivo microdialysis. Int. J. Nanomed. 2014, 9, 669–678. [Google Scholar] [CrossRef] [Green Version]
- Mueller, R.H.; Mehnert, W.; Souto, E.B. Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers for Dermal Delivery. Adv. Drug Deliv. Rev. 2006, 37. [Google Scholar] [CrossRef]
- Almeida, A.J.; Souto, E. Solid lipid nanoparticles as a drug delivery system for peptides and proteins. Adv. Drug Deliv Rev. 2007, 59, 478–490. [Google Scholar] [CrossRef] [PubMed]
- Teeranachaideekul, V.; Souto, E.B.; Muller, R.H.; Junyaprasert, V.B. Physicochemical characterization and in vitro release studies of ascorbyl palmitate-loaded semi-solid nanostructured lipid carriers (NLC gels). J. Microencapsul. 2008, 25, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.R.; Martins-Gomes, C.; Santini, A.; Silva, A.M.; Souto, E.B. Chapter 9—Psoriasis vulgaris—Pathophysiology of the disease and its classical treatment versus new drug delivery systems. In Design of Nanostructures for Versatile Therapeutic Applications; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 379–406. [Google Scholar] [CrossRef]
- Pradhan, M.; Singh, D.; Singh, M.R. Development characterization and skin permeating potential of lipid based novel delivery system for topical treatment of psoriasis. Chem. Phys. Lipids 2015, 186, 9–16. [Google Scholar] [CrossRef]
- Shimojo, A.A.M.; Fernandes, A.R.V.; Ferreira, N.R.E.; Sanchez-Lopez, E.; Santana, M.H.A.; Souto, E.B. Evaluation of the Influence of Process Parameters on the Properties of Resveratrol-Loaded NLC Using 2(2) Full Factorial Design. Antioxidants 2019, 8, 272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souto, E.B.; Doktorovova, S. Chapter 6—Solid lipid nanoparticle formulations pharmacokinetic and biopharmaceutical aspects in drug delivery. Methods Enzym. 2009, 464, 105–129. [Google Scholar] [CrossRef]
- Shrotriya, S.N.; Ranpise, N.S.; Vidhate, B.V. Skin targeting of resveratrol utilizing solid lipid nanoparticle-engrossed gel for chemically induced irritant contact dermatitis. Drug Deliv. Transl. Res. 2017, 7, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Avasatthi, V.; Pawar, H.; Dora, C.P.; Bansod, P.; Gill, M.S.; Suresh, S. A novel nanogel formulation of methotrexate for topical treatment of psoriasis: Optimization, in vitro and in vivo evaluation. Pharm. Dev. Technol. 2016, 21, 554–562. [Google Scholar] [CrossRef]
- Donnelly, R.F.; Raghu, T.; Singh, R.; Woolfson, A.D. Microneedle-based drug delivery systems: Microfabrication, drug delivery, and safety. Drug Deliv. 2010, 17, 187–207. [Google Scholar] [CrossRef] [Green Version]
- Larrañeta, E.; Lutton, R.E.M.; Woolfson, A.D.; Donnelly, R.F. Microneedle arrays as transdermal and intradermal drug delivery systems: Materials science, manufacture and commercial development. Mater. Sci. Eng. R Rep. 2016, 104, 1–32. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Chen, Y.; Shi, Y. Microneedles: A potential strategy in transdermal delivery and application in the management of psoriasis. RSC Adv. 2020, 10, 14040–14049. [Google Scholar] [CrossRef]
- Ishihara, T.; Kubota, T.; Choi, T.; Takahashi, M.; Ayano, E.; Kanazawa, H.; Higaki, M. Polymeric nanoparticles encapsulating betamethasone phosphate with different release profiles and stealthiness. Int. J. Pharm. 2009, 375, 148–154. [Google Scholar] [CrossRef]
- Ourique, A.F.; Pohlmann, A.R.; Guterres, S.S.; Beck, R.C.R. Tretinoin-loaded nanocapsules: Preparation, physicochemical characterization, and photostability study. Int. J. Pharm. 2008, 352, 1–4. [Google Scholar] [CrossRef]
- Pandi, P.; Jain, A.; Kommineni, N.; Ionov, M.; Bryszewska, M.; Khan, W. Dendrimer as a new potential carrier for topical delivery of siRNA: A comparative study of dendriplex vs. lipoplex for delivery of TNF-α siRNA. Int. J. Pharm. 2018, 550, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Lapteva, M.; Santer, V.; Mondon, K.; Patmanidis, I.; Chiriano, G.; Scapozza, L.; Gurny, R.; Möller, M.; Kalia, Y.N. Targeted cutaneous delivery of ciclosporin A using micellar nanocarriers and the possible role of inter-cluster regions as molecular transport pathways. J. Control. Release 2014, 196, 9–18. [Google Scholar] [CrossRef]
- Nagle, A.; Goyal, A.K.; Kesarla, R.; Murthy, R.R.; Nagle, A.; Goyal, A.K.; Kesarla, R.; Murthy, R.R. Efficacy study of vesicular gel containing methotrexate and menthol combination on parakeratotic rat skin model Efficacy study of vesicular gel containing methotrexate and menthol combination on parakeratotic rat skin model. J. Liposome Res. 2011, 2104. [Google Scholar] [CrossRef]
- Kumar, R.; Dogra, S.; Amarji, B.; Singh, B.; Kumar, S.; Sharma; Vinay, K.; Mahajan, R.; Katare, O.P. Efficacy of Novel Topical Liposomal Formulation of Cyclosporine in Mild to Moderate Stable Plaque Psoriasis: A Randomized Clinical Trial. JAMA Dermatol. 2016, 152, 807–815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Østergaard, N.; Jorgensen, L.; Hansen, J.; Vermehren, C.; Frokjaer, S.; Foged, C. Targeting of liposome-associated calcipotriol to the skin: Effect of liposomal membrane fluidity and skin barrier integrity. Int. J. Pharm. 2011, 416, 478–485. [Google Scholar] [CrossRef]
- Chen, M.; Kumar, S.; Anselmo, A.C.; Gupta, V.; Slee, D.H.; Muraski, J.A.; Mitragotri, S. Topical delivery of Cyclosporine A into the skin using SPACE-peptide. J. Control. Release 2015, 199, 190–197. [Google Scholar] [CrossRef]
- Li, G.; Fan, Y.; Fan, C.; Li, X.; Wang, X.; Li, M.; Liu, Y. Tacrolimus-loaded ethosomes: Physicochemical characterization and in vivo evaluation. Eur. J. Pharm. Biopharm. 2012, 82, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, U.; Gupta, M.; Vyas, S.P. Capsaicin delivery into the skin with lipidic nanoparticles for the treatment of psoriasis. Artif. Cells Nanomed. Biotechnol. 2015, 43, 33–39. [Google Scholar] [CrossRef]
- Sonawane, R.; Harde, H.; Katariya, M.; Agrawal, S.; Sonawane, R.; Harde, H.; Katariya, M.; Agrawal, S. Solid lipid nanoparticles-loaded topical gel containing combination drugs: An approach to offset psoriasis Solid lipid nanoparticles-loaded topical gel containing combination drugs: An approach to offset psoriasis. Expert Opin. Drug Deliv. 2014, 5247. [Google Scholar] [CrossRef]
- Doktorovová, S.; Araújo, J.; Garcia, M.L.; Rakovský, E.; Souto, E.B. Formulating fluticasone propionate in novel PEG-containing nanostructured lipid carriers (PEG-NLC). Colloids Surf. B Biointerfaces 2010, 75, 538–542. [Google Scholar] [CrossRef]
- Lin, Y.-K. Combination of calcipotriol and methotrexate in nanostructured lipid carriers for topical delivery. Int. J. Nanomed. 2010, 5, 117–128. [Google Scholar]
- Tekko, I.A.; Permana, A.D.; Vora, L.; Hatahet, T.; McCarthy, H.O.; Donnelly, R.F. Localised and sustained intradermal delivery of methotrexate using nanocrystal-loaded microneedle arrays: Potential for enhanced treatment of psoriasis. Eur. J. Pharm. Sci. 2020, 152, 105469. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jung, Y.S.; Kim, G.M.; Bae, J.M. A hyaluronic acid-based microneedle patch to treat psoriatic plaques: A pilot open trial. Br. J. Dermatol. 2018, 178, e24–e25. [Google Scholar] [CrossRef] [PubMed]


| Active Ingredient | Effects | Drawbacks | References |
|---|---|---|---|
| Moisturizers | Reduces hyperproliferation, differentiation, and apoptosis. Moreover, anti-inflammatory effects and improving barrier function. | Irritant dermatitis, allergic contact dermatitis, fragrance allergy, stinging, and acne. | [67,68] |
| Coal Tar | Suppresses DNA synthesis, reducing the hyperproliferation of keratinocytes. | Odour, staining, irritant contact dermatitis, erythema, stinging, folliculitis, and formation of keratoacanthomas. | [69] |
| Salicylic acid | Reduces intercellular cohesiveness of the horny cells by dissolving the intercellular cement material. Furthermore, it reduces the pH of the stratum corneum, increasing hydration and softening. | Potential chronic or acute systemic intoxication, oral mucosa burning, frontal headache, central nervous system symptoms, metabolic acidosis, tinnitus, nausea, and vomiting. | [70] |
| Topical calcineurin inhibitors (TCIs) | It inhibits the action of calcineurin phosphatase and block the production of inflammatory substances that are thought to be important in causing skin lesions. | Stinging sensation and skin irritation. | [71] |
| Tazarotene | It binds to β and γ retinoic acid on the cell membrane of keratinocytes and is then transported to the nucleus, altering transcription of genes in keratinocytes. | The most common side effect of tazarotene is localized irritation. | [72,73] |
| Anthralin (Dithranol) | It reduces keratinocyte proliferation, prevents T-cell activation, and restores cell differentiation, probably through mitochondrial dysfunction. | Skin irritation, stains lesioned, and adjoining skin, hair, nails, clothing, and other objects, with which the patients come into contact. | [74] |
| Topical corticosteroids | Corticosteroids are vasoconstrictive, antiproliferative, anti-inflammatory, and immunosuppressive. They bind to the intracellular corticosteroid receptor and regulate gene transcription of numerous genes, particularly those that code for proinflammatory cytokines. | Skin atrophy striae, telangiectasia, or secondary infection. Therefore, potent TCS should not be used on the face or intertriginous sites. Systemic adverse events occur when TCS is used for prolonged periods of time or at doses higher than commonly prescribed. Prolonged use of potent TCS may result in its significant systemic absorption, which can lead to HPA axis suppression, Cushing’s syndrome, and hyperglycaemia. | [36,75] |
| Vitamin D analogues | Vitamin D analogues bind to the intracellular Vitamin D receptor, which then binds to and regulates the genes involved in epidermal proliferation, inflammation, and keratinization. | Skin irritation, hypercalcemia, hypercalciuria, and parathyroid hormone suppression, but these are very rare. | [76] |
| Type of Treatment | Drug | Effects | Drawbacks | References |
|---|---|---|---|---|
| Conventional treatments | Acitretin | It binds to nuclear receptors on genes controlling cellular differentiation, anti-proliferation, anti-inflammation, anti-keratinization, and inhibition of neutrophil chemotaxis. It is the only systemic treatment that is not immunosuppressive. | Depression, hypertriglyceridemia and hypercholesterolemia, Myalgias, cheilitis, skin peeling, alopecia, xerosis, rhinitis, nail dystrophy, epistaxis, sticky skin, retinoid dermatitis, and xerophthalmia. | [77] |
| Fumaric Acid Esthers (FAEs) | It has immunomodulatory, anti-inflammatory, and antiproliferative properties and apoptotic actions on activated T cells. | Warmth, reddening of the face, and headaches, proteinuria, reversible renal insufficiency, microscopic haematuria, and proximal tubular damage. | [78] | |
| Cyclosporine | It inhibits Interleukin synthesis, such as IL-2 and T cell differentiation. | Hypertension, arrythmia, hypertension, anxiety, headaches, fever, hypomagnesemia, hyperkalaemia, dyslipidaemia, and encephalopathy. | [79] | |
| Methotrexate | It reduces interleukin (IL)-17 mRNA and IL-17 protein expression in CD3- and CD28-stimulated peripheral blood mononuclear cells. It modulates pro-inflammatory mediators and its effects on atherogenic gene expression in psoriatic lesion skin. | Nausea, stomach pain, and diarrhoea. | [80] | |
| Small molecules | Apremilast | It inhibits the expression and/or production of TNF-α, IFN- c, IL-12, and IL-23 and the chemokines CXCL9, CXCL10, CCL2, and CCL3, IL-2, IL-5, IL-13, IL-17, TNF-α, and IFN-c by stimulated T cells and IFN-a by dendritic cells. | Nausea, diarrhoea, and headaches. | [81] |
| Small molecules | Tofacitinib | It is a potent inhibitor of JAK1 and JAK3 and has some activity against JAK2 and Tyk2. | Nasopharyngitis, upper respiratory tract infection, headache, urinary tract infection, and diarrhoea. | [82] |
| Anti-TNF alpha | Infliximab | It interferes with the actions of TNF-α by directly binding to soluble and transmembrane TNF-α molecules in the plasma and the diseased tissue. | Dyspnoea, urticaria, hypotension, flushing, and headache. | [83] |
| Etanercept | It is a recombinant human TNF-recipient by subcutaneous injection. Tor fusion protein that antagonizes the effects of endogenous TNF by competitively inhibiting its in- study disintegration with cell-surface receptors. | Respiratory infections, flu-like symptoms, and gastrointestinal symptoms. | [84,85] | |
| Adalimumab | It blocks its interaction with the p55 and p75 cell surface TNF receptors. | Headache, nausea, elevated triglycerides, cough, sinus congestion, and fatigue most common. | [86] | |
| Certolizumab | It inhibits lipopolysaccharide-induced IL-1-β release from monocytes and provokes nonapoptotic cell death in tmTNF- α-expressing cells. | Headache, nasopharyngitis, upper respiratory tract infections, diarrhoea, and sinusitis. | [87,88] | |
| Anti-IL12/23 | Ustekinumab | It is a human monoclonal antibody that binds to the shared p40 protein subunit of human interleukins 12 and 23 with high affinity and specificity (unpublished data), thereby preventing interaction with their cell surface IL12Rβ1 receptor. | Headache, nasopharyngitis, arthralgia, and upper respiratory tract system. | [89] |
| Anti-IL17 | Secukinumab | It is a recombinant, high-affinity, fully human immunoglobulin G1κ monoclonal antibody that selectively binds and neutralizes interleukin-17A. | Nasopharyngitis, headache, and upper respiratory tract infection. | [90] |
| Bimekizumab | It is a monoclonal antibody of the immunoglobulin G1 isotype, rationally designed to be able to bind at a similar site on both IL-17A and IL-17F, conveying dual inhibition of both isoforms. | Nasopharyngitis, oropharyngeal pain, and headache. | [91] | |
| Ixekizumab | It is recombinant, high-affinity, humanized IgG4-κ monoclonal antibody, which selectively binds and neutralizes interleukin 17A (IL-17A), the proinflammatory and primary effector cytokine of type 17 helper T (Th17) cells. | Nasopharyngitis, upper respiratory infection, injection-site reaction, and headache. | [92,93] | |
| IL17 R antagonist | Brodalumab | It binds with high affinity to human interleukin-17RA and blocks the biologic activity of interleukins 17A, 17F, 17A/F heterodimer, and 17E (interleukin-25). | Nasopharyngitis, upper respiratory tract infection, arthralgia, and erythema at the injection site. | [94] |
| Anti-IL23 | Tildrakizumab | It is a novel, high-affinity humanized IgG1/j monoclonal antibody that specifically binds to the p19 subunit of human IL-23 without binding IL-12. | Nasopharyngitis and headache. | [95] |
| Guselkumab | It is a fully human IgG1 lambda monoclonal antibody that binds to the p19 subunit of IL-23. | Nasopharyngitis and upper respiratory tract infection. | [96,97] | |
| Risankizumab | It is a humanised IgG1 monoclonal anti- body that binds the p19 subunit of IL-23, thus inhibiting this key cytokine and its role in psoriatic inflammation. | Upper respiratory tract infection, urinary tract infection, influenza, and headache. | [98] | |
| Mirikizumab | It is a humanized IgG4-variant monoclonal antibody that binds to the p19 subunit of IL-23 and does not bind IL-12. | Viral upper and other respiratory tract infections, injection-site pain, hypertension, and diarrhoea. | [99] |
| Clinical Trial Phase | Drug Name | Administration Via | Targeting | References |
|---|---|---|---|---|
| Phase 1 | BOS-475 | Topical | Bromodomain and extraterminal domain protein inhibitors | [101] |
| ABBV-157 | Oral | RORγt inhibitor | [102] | |
| CC-92252 | Oral | Interleukin-2 receptor agonists; Regulatory T-lymphocyte stimulants | [103] | |
| EDP 1066 | Oral | Immunomodulators | [104] | |
| EDP 1815 | Oral | Immunomodulator | [105] | |
| Phase II | ABY-035 | Parenteral | IL-17A inhibitor | [106] |
| ARQ-151 | Topical | PDE4 Enzyme inhibitor | [107] | |
| BI 730357 | Oral | Nuclear receptor antagonist | [108] | |
| EISO (SAN021) | Topical | PDE4 blocker | [109] | |
| JTE-451 | Oral | ROR inhibitor | [110] | |
| M1095 | Parenteral | Trivalent monomeric nanobody that neutralizes interleukins IL-17A, IL-17F, and IL-17A/F | [111] | |
| PF-06700841 | Topical | JAK1 and TYK2 inhibitor | [112] | |
| Phase II | PF-06826647 | Oral | TYK2 inhibitor | [113] |
| SHR-1314 | Parenteral | IL-17A Antagonist | [114] | |
| Phase III | BMS- 986165 | Oral | Tyk2 inhibitor | [115] |
| BCD-085 | Parenteral | IL-17 inhibitor | [116] | |
| BI695502 | Parenteral | TNF-α inhibitor | [117] | |
| CF101 | Oral | Adosine A3 receptor inhibitor | [118] | |
| CHS-1420 | Parenteral | TNF-α inhibitor | [119] | |
| Filgotinib | Oral | JAK 1 inhibitor | [120] | |
| Mirikizumab | Parenteral | IL-23 inhibitor | [121] | |
| Serlopitant | Oral | Neurokinin-1 receptor antagonist | [122] | |
| Tapinarof | Topical | AHR agonist | [123] | |
| Tikdrakizumab | Parenteral | IL-23 inhibitor | [124] | |
| Upadacitinib | Oral | JAK inhibitor | [125] |
| Nanocarrier | Advantages | Limitations | Drug Released | Administration Via | References |
|---|---|---|---|---|---|
| Nanospheres | Enhanced solubility, extended release of drug, and improve absorption. | Poor drug loading, agglomeration, storage issues, problems in large scale production. | Vitamin D3 | Topical | [128] |
| Bethamethasone bisodium 21-phosphate | Intravenous | [157] | |||
| Nanocapsules | Improved skin permeation, sustained and controlled release, improved selectivity, and biocompatibility. | Poor drug loading, agglomeration, storage issues, problems in large scale production. | Tretinoin | Topical | [158] |
| Dexamethasone | Topical | [131] | |||
| Dendrimers | Ease of preparation and modification. | Polymer dependent biocompatibility. | TNF-α Si RNA | Topical | [159] |
| Dithranol | Topical | [134] | |||
| Micelles | Self-assembling, thermodynamic, stability, and targeting potential. | Not good for hydrophilic drugs. | Tacrolimus | Topical | [136] |
| Cyclosporine A | Topical | [160] | |||
| Liposomes | Biocompatible, ease of surface modification, and amphiphilic nature. | Weak loading capacity, rapid drug leakage, limited physical and chemical stability during storage. | Fusidic acid | Topical | [140] |
| Methotrexate | Topical | [161] | |||
| Cyclosporine | Topical | [162] | |||
| Calcipotriol | Topical | [163] | |||
| Lipospheres | Biocompatible, amphiphilic nature, and surface modification is easy. | Weak loading capacity, rapid drug leakage, limited physical and chemical stability during storage. | Tacrolimus and curcumin | Topical | [142] |
| Ethosomes | Very good permeation power, high patient compliance. Composition is safe for dermal and pharmaceutical use. | Poor yield | Cyclosporine | Topical | [164] |
| Psoralen | Topical | [144] | |||
| Tacrolimus | Topical | [165] | |||
| Solid lipid nanoparticles | Biocompatible, biodegradable, higher efficacy, flexibility of size, and surface manipulation | Poor stability, poor batch to batch reproducibility, sterilization difficulties, and low drug loading | Fluocinolone acetonide | Topical | [149] |
| Capsaicin | Topical | [166] | |||
| Betamethasone dipropionate and Calcipotriol | Topical | [167] | |||
| Nanostructured lipid carriers | Biodegradable, biocompatible, reduces expulsion of drug during storage, good drug load | Sterilization difficulties | Methotrexate | Topical | [153] |
| Fluticasone propionate | Topical | [168] | |||
| Calcipotriol and methotrexate | Topical | [169] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petit, R.G.; Cano, A.; Ortiz, A.; Espina, M.; Prat, J.; Muñoz, M.; Severino, P.; Souto, E.B.; García, M.L.; Pujol, M.; et al. Psoriasis: From Pathogenesis to Pharmacological and Nano-Technological-Based Therapeutics. Int. J. Mol. Sci. 2021, 22, 4983. https://doi.org/10.3390/ijms22094983
Petit RG, Cano A, Ortiz A, Espina M, Prat J, Muñoz M, Severino P, Souto EB, García ML, Pujol M, et al. Psoriasis: From Pathogenesis to Pharmacological and Nano-Technological-Based Therapeutics. International Journal of Molecular Sciences. 2021; 22(9):4983. https://doi.org/10.3390/ijms22094983
Chicago/Turabian StylePetit, Robert Gironés, Amanda Cano, Alba Ortiz, Marta Espina, Josefina Prat, Montserrat Muñoz, Patrícia Severino, Eliana B. Souto, Maria L. García, Montserrat Pujol, and et al. 2021. "Psoriasis: From Pathogenesis to Pharmacological and Nano-Technological-Based Therapeutics" International Journal of Molecular Sciences 22, no. 9: 4983. https://doi.org/10.3390/ijms22094983
APA StylePetit, R. G., Cano, A., Ortiz, A., Espina, M., Prat, J., Muñoz, M., Severino, P., Souto, E. B., García, M. L., Pujol, M., & Sánchez-López, E. (2021). Psoriasis: From Pathogenesis to Pharmacological and Nano-Technological-Based Therapeutics. International Journal of Molecular Sciences, 22(9), 4983. https://doi.org/10.3390/ijms22094983









