Gene Expression Correlation Analysis Reveals MYC-NAC Regulatory Network in Cotton Pigment Gland Development
Abstract
:1. Introduction
2. Results
2.1. Identification and Phylogenetic Analysis of NAC Genes Family in Cotton
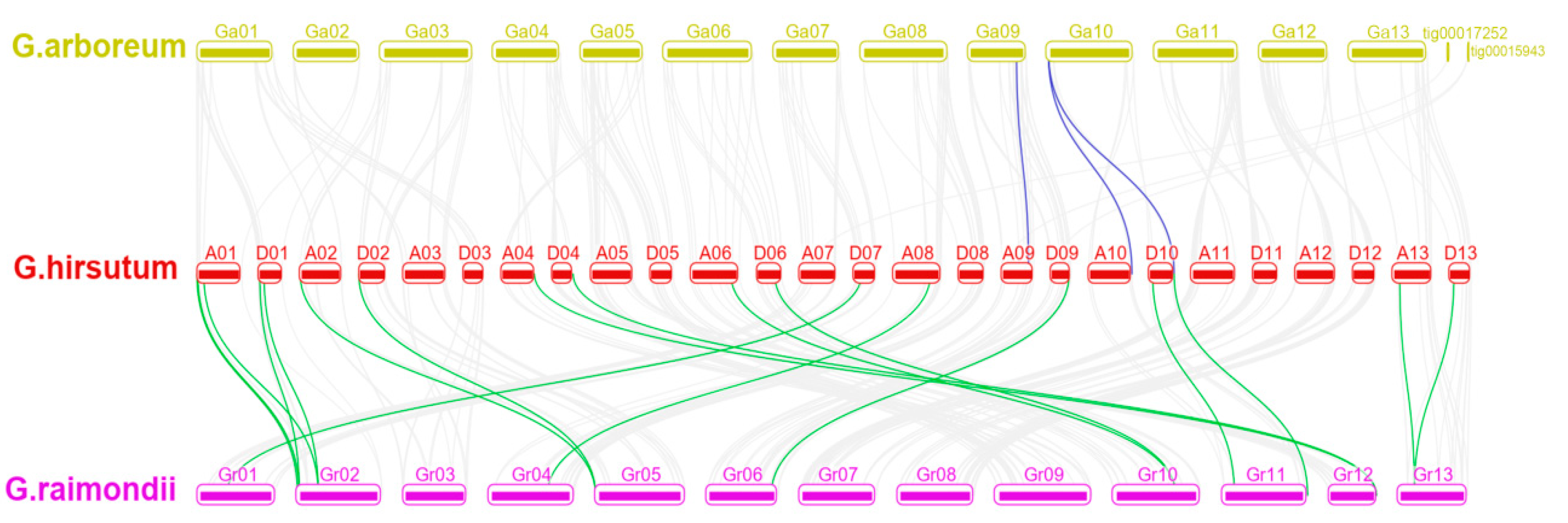
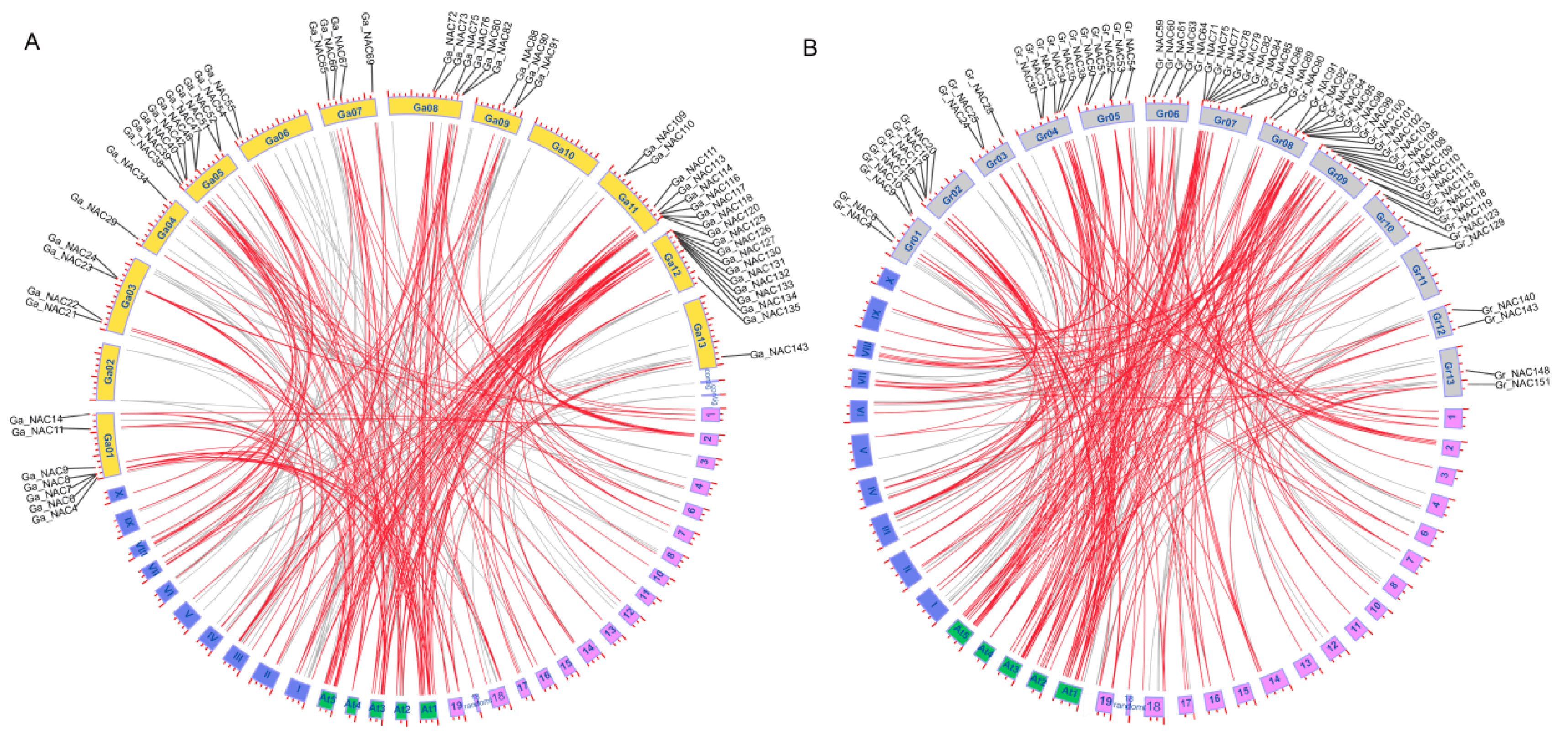
2.2. Gene Chromosomal Location, Duplication and Synteny Analysis of NACs in Cotton
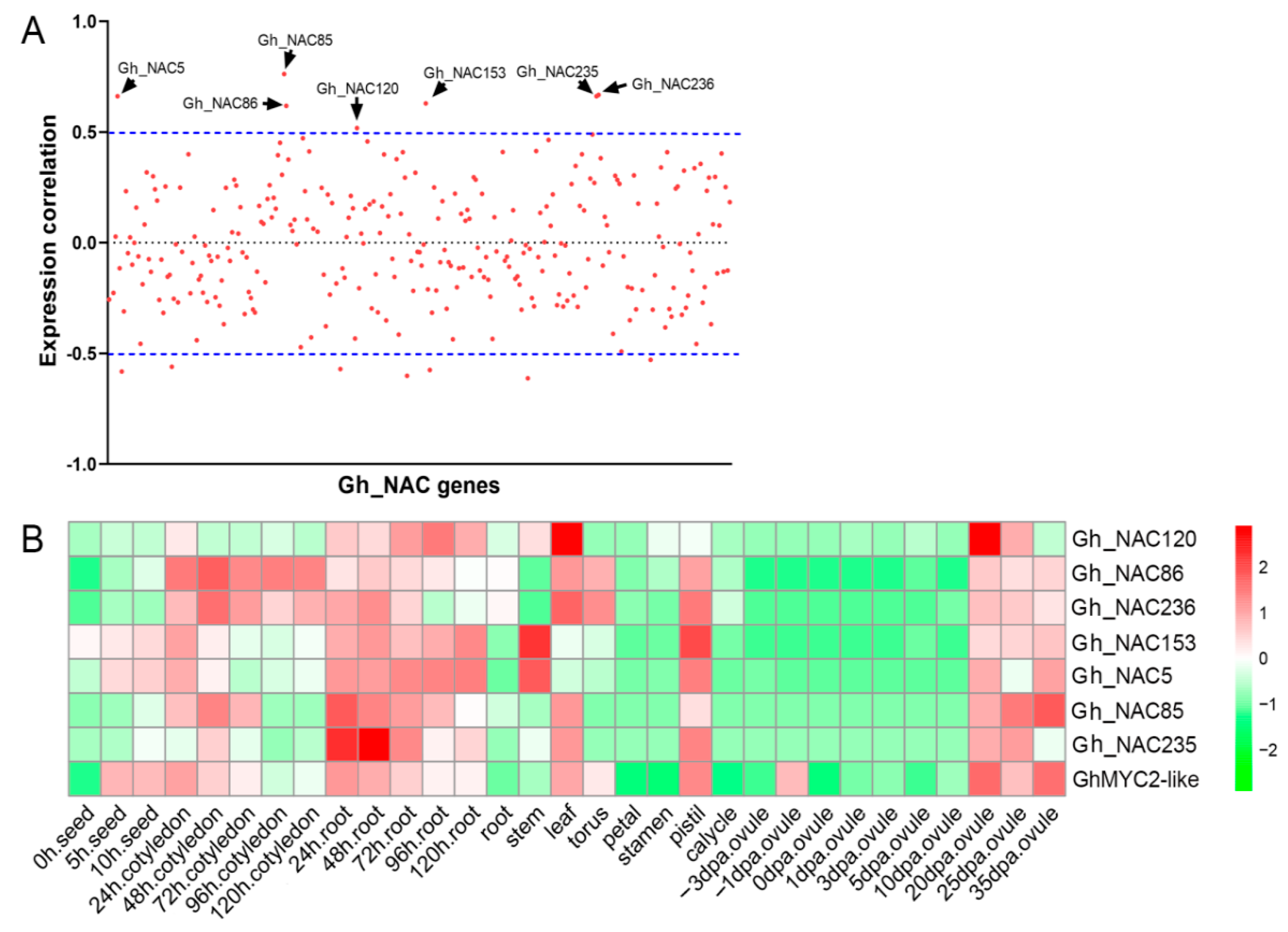
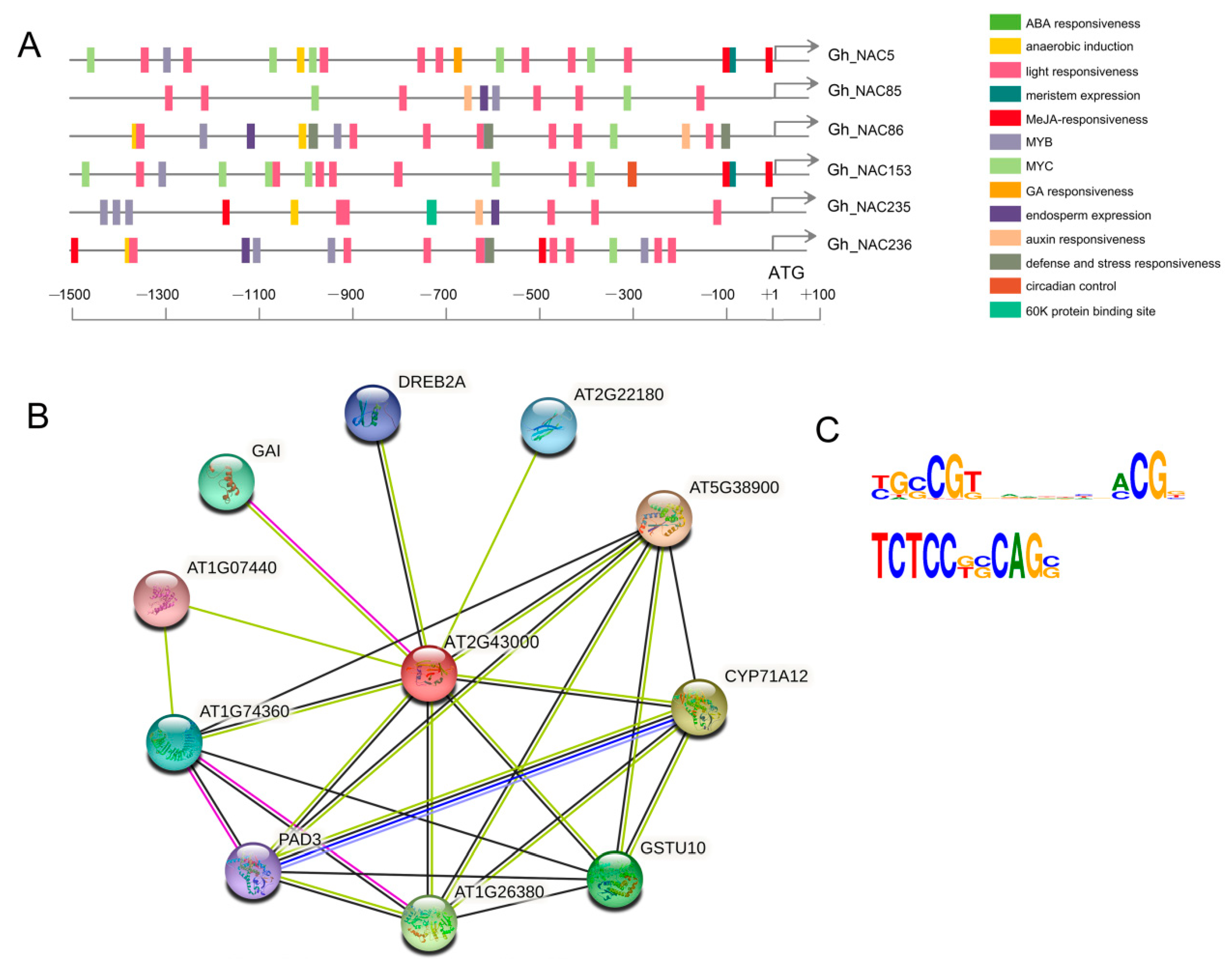
2.3. Identification of Gland Formation Associated NAC Genes in G. hirsutum
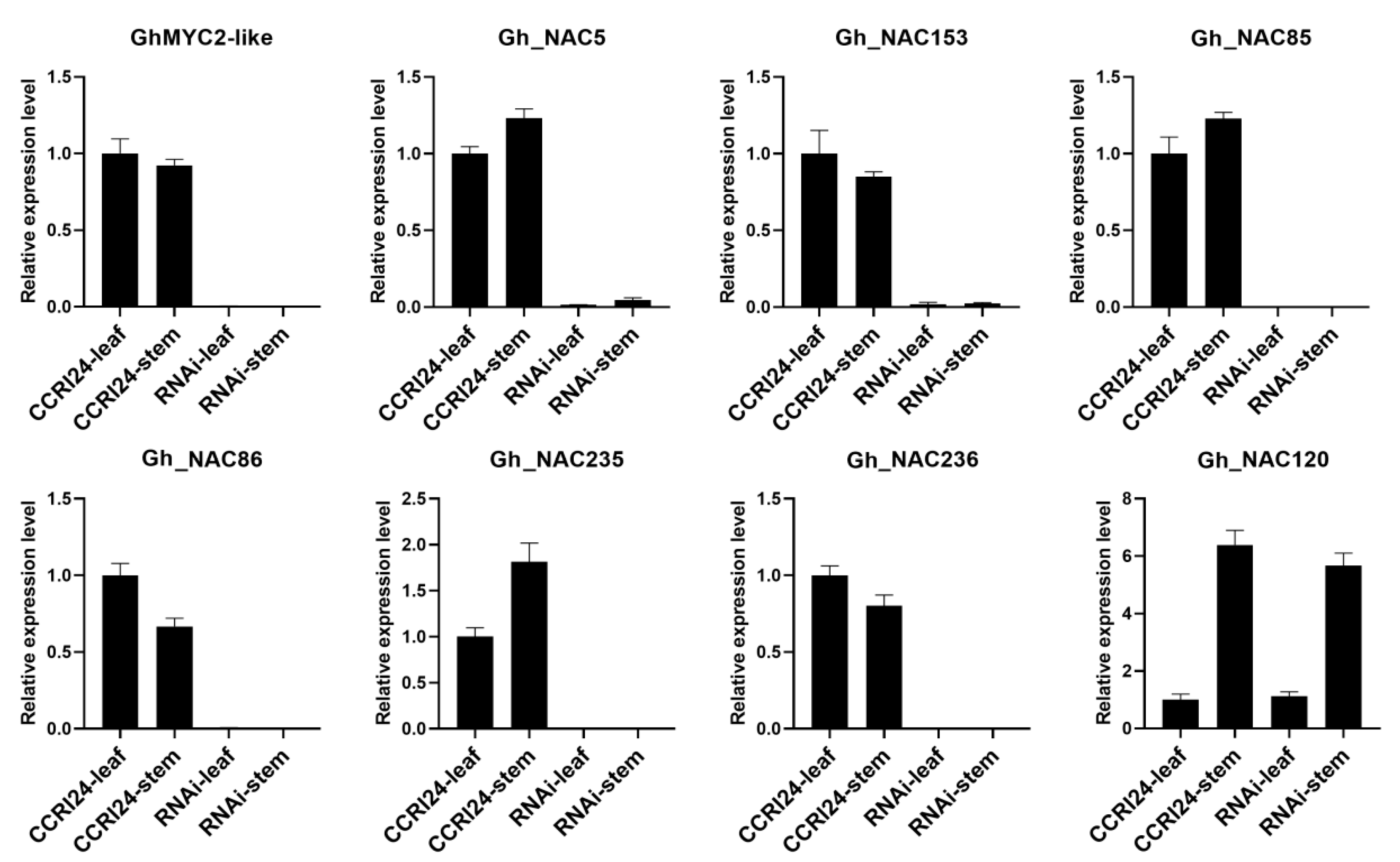
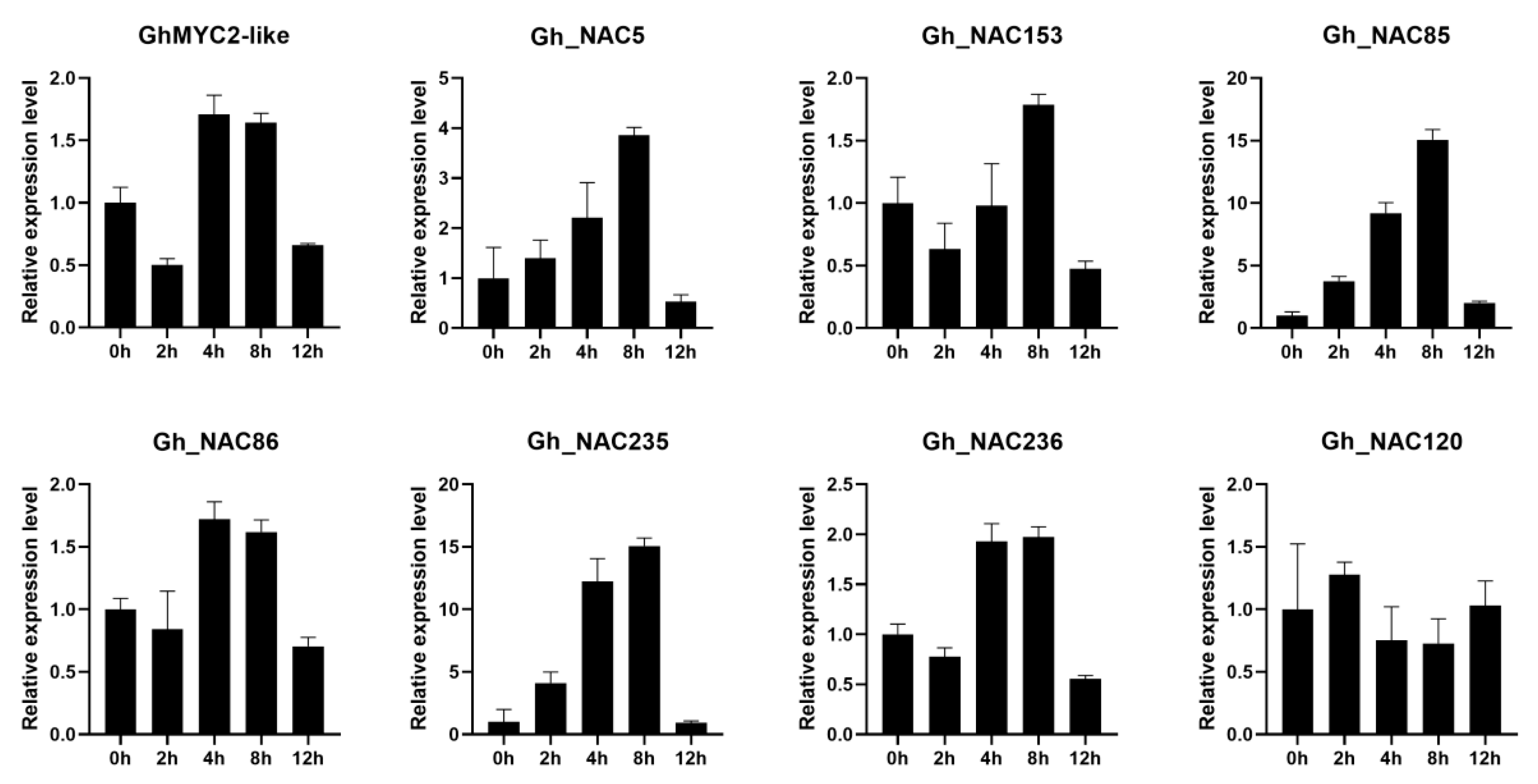
2.4. The Gland-Related NACs Function Downstream of GhMYC2-Like
2.5. Silencing Gland-Related NACs Inffluences Gland Development
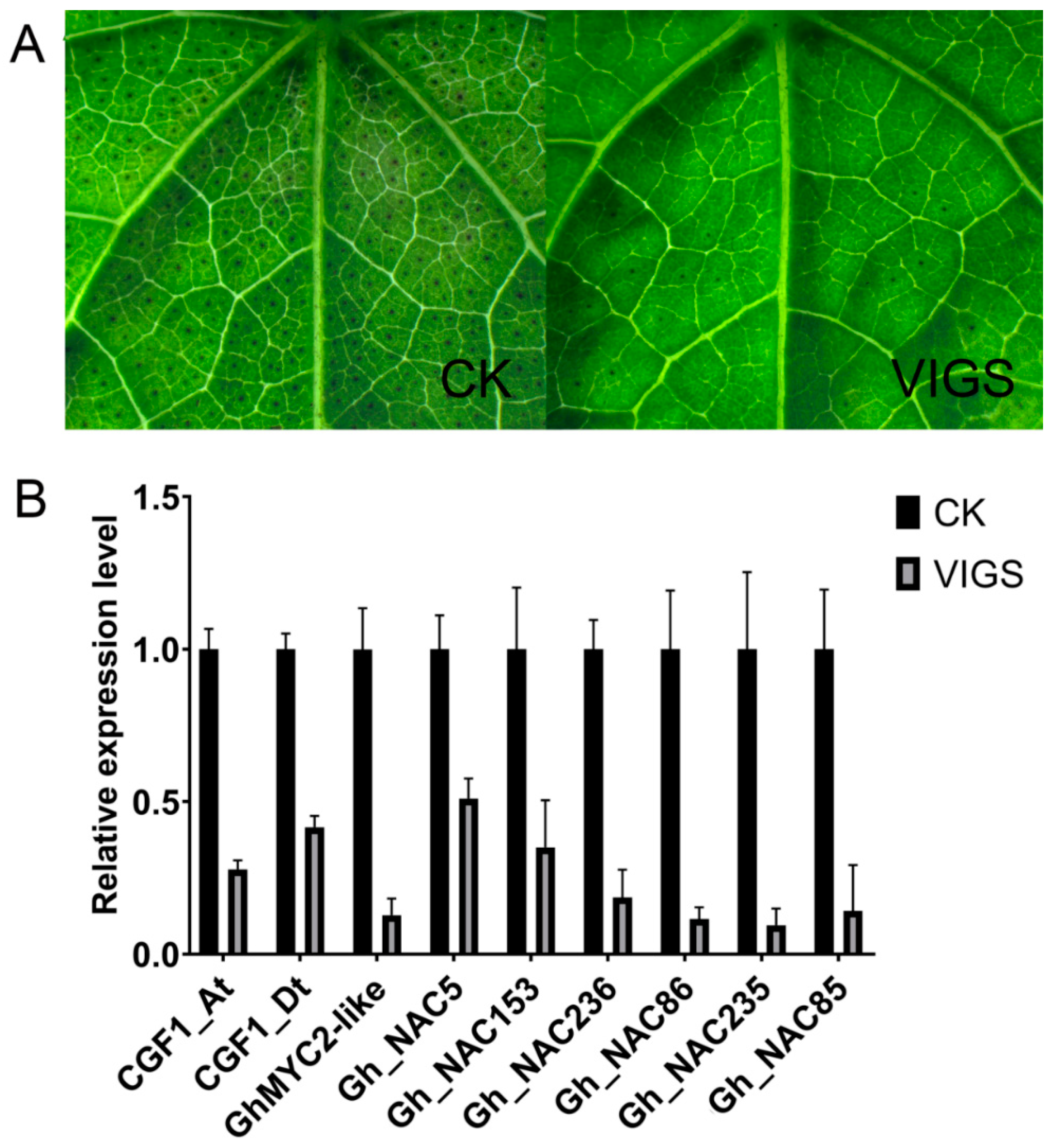
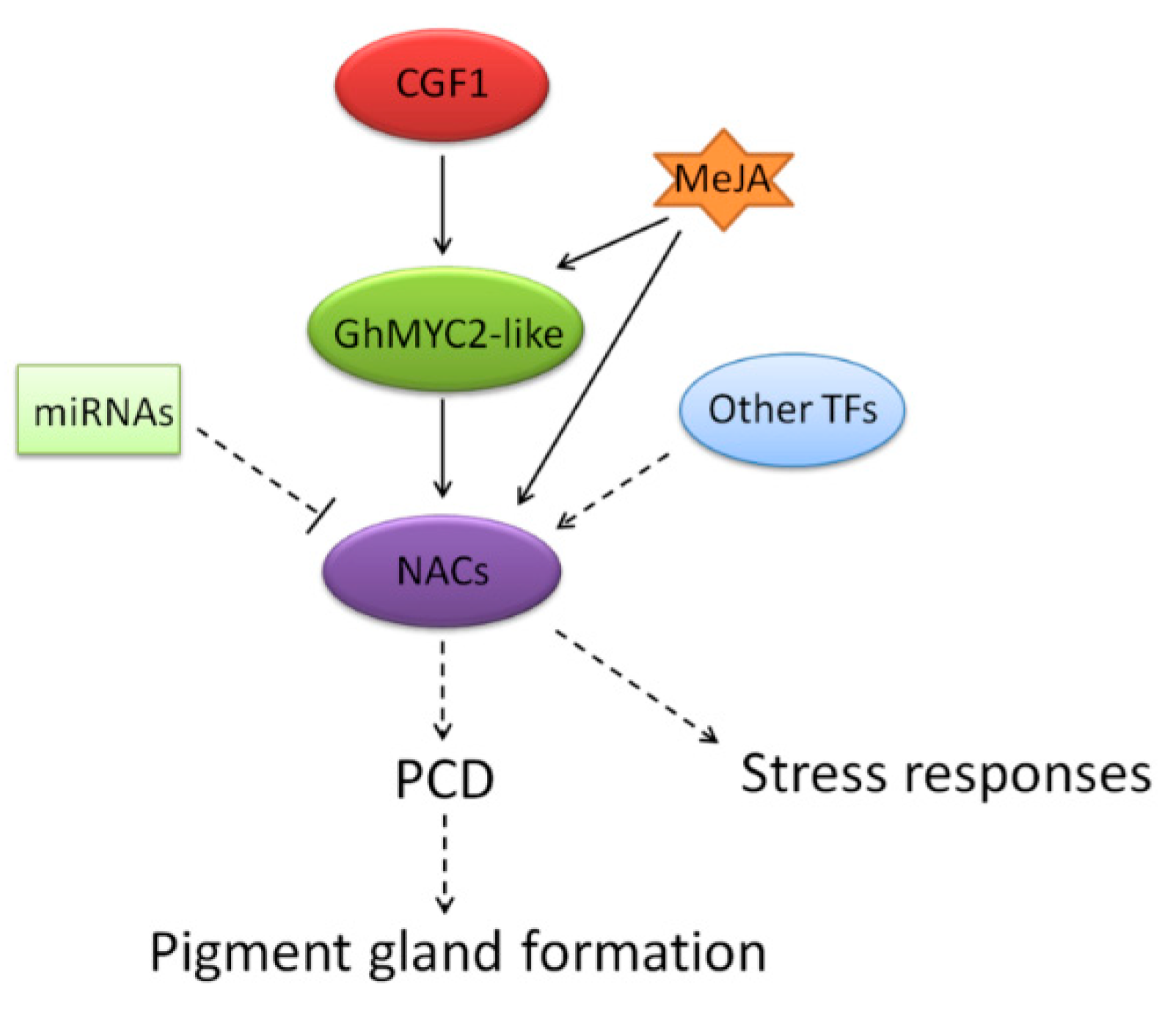
2.6. CGF1 Influences Gland Formation by Regulating GhMYC2-Like and GLAND-Related NACs
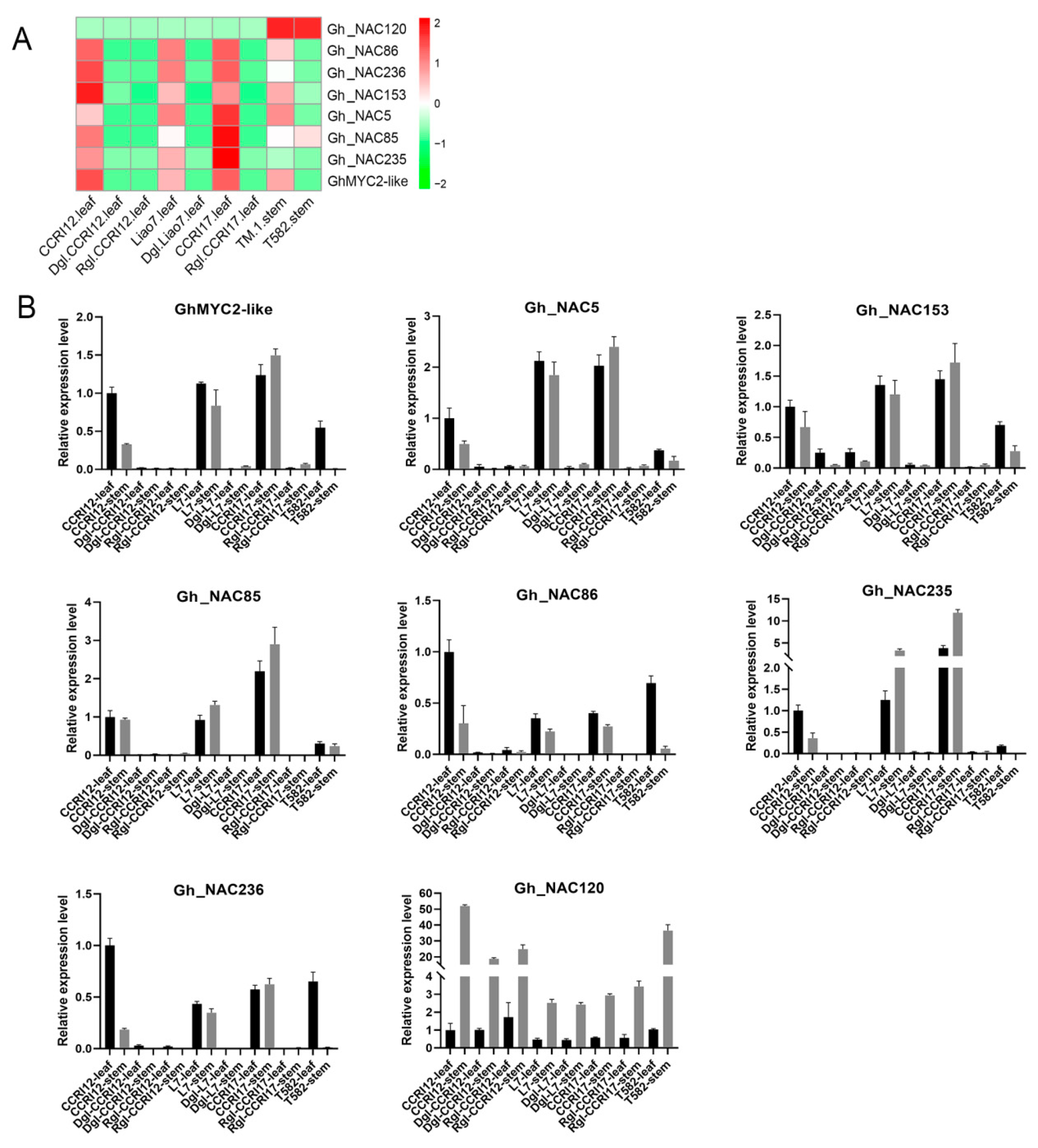
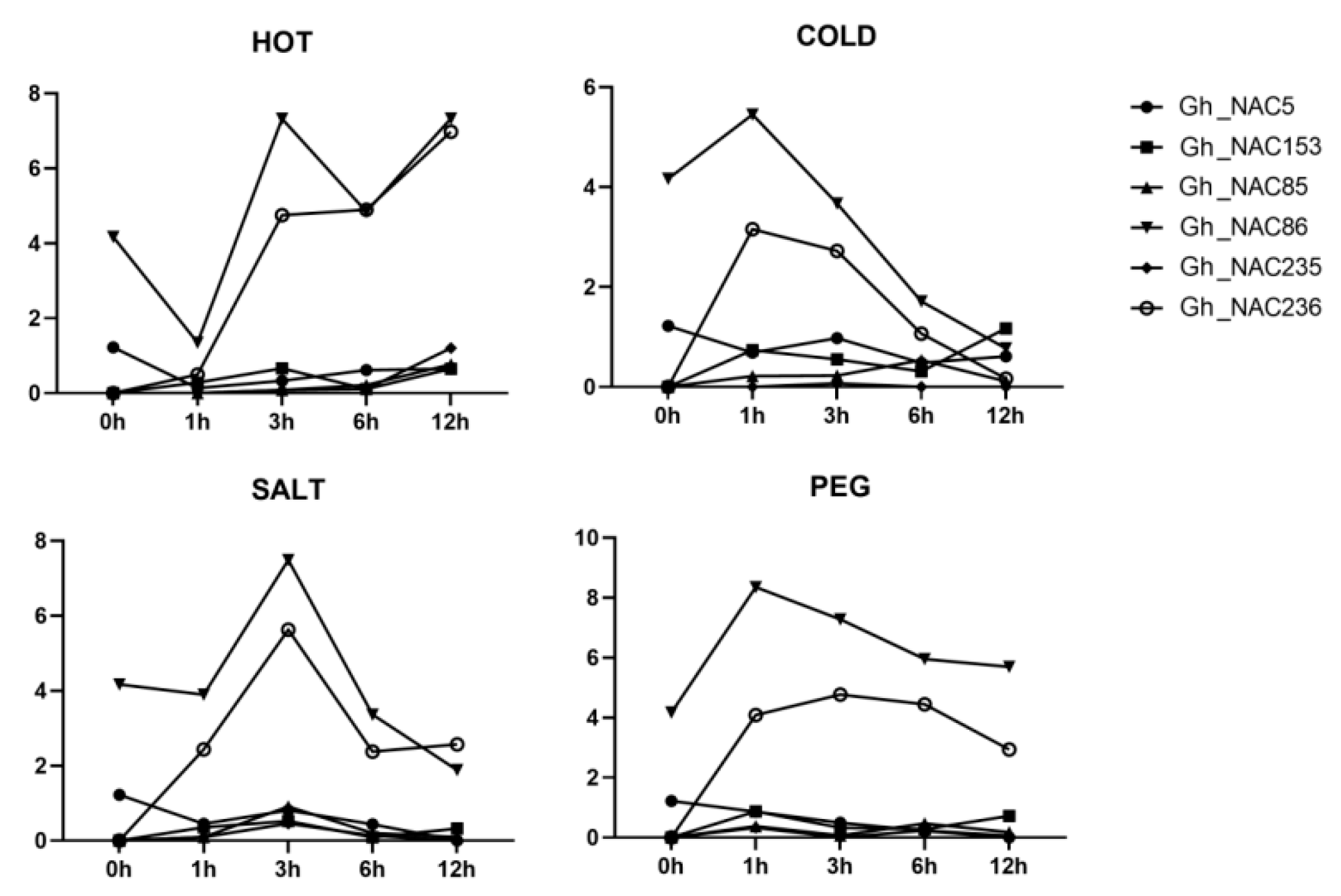
2.7. Expression Profiling of 6 Gland-Related NAC Genes under Various Stresses
2.8. Predicted Regulatory Mechanism of the 6 NAC Genes
3. Discussion
3.1. Identification and Phylogenetic Analysis of NAC Genes in Cotton
3.2. Expression Analysis and Potential Roles of NAC Genes in Pigment Gland Development
3.3. Putative Molecular Regulatory Mechanisms of Gland-Related NAC Genes
4. Materials and Methods
4.1. Identification and Phylogenetic Analysis of NAC Proteins
4.2. Gene Chromosomal Location, Duplication and Synteny Analysis of NACs in Cotton
4.3. Gene Expression and Correlation Analysis of GhMYC2-Like and NAC Genes
4.4. Plant Materials and MeJA Treatment
4.5. Virus Induced Gene Silencing (VIGS)
4.6. RNA Extraction and QRT-PCR Analysis
4.7. Predictions of Cis-Elements, TFBS, miRNA Targets, Interaction Network and Binding Sites
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Palle, S.R.; Campbell, L.M.; Pandeya, D.; Puckhaber, L.; Tollack, L.K.; Marcel, S.; Sundaram, S.; Stipanovic, R.D.; Wedegaertner, T.C.; Hinze, L.; et al. RNAi-mediated Ultra-low gossypol cottonseed trait: Performance of transgenic lines under field conditions. Plant Biotechnol. J. 2013, 11, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.J.; Xu, Z.R.; Pan, X.L.; Yan, X.H.; Wang, Y.B. Advances in gossypol toxicity and processing effects of whole cottonseed in dairy cows feeding. Livest. Sci. 2007, 111, 1–9. [Google Scholar] [CrossRef]
- Gadelha, I.C.; Fonseca, N.B.; Oloris, S.C.; Melo, M.M.; Soto-Blanco, B. Gossypol toxicity from cottonseed products. Sci. World J. 2014, 231635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, J.L.; Christa, E.K.; Orth, R.G.; Gassmann, A.J.; Graham, H.; Tabashnik, B.E.; Yves, C. Fitness cost of resistance to Bt cotton linked with increased gossypol content in pink bollworm larvae. PLoS ONE 2011, 6, e21863. [Google Scholar] [CrossRef] [Green Version]
- Stipanovic, R.D.; Lopez, J.D.; Dowd, M.K.; Puckhaber, L.S.; Duke, S.E. Effect of racemic and (+)- and (−)-gossypol on the survival and development of Helicoverpa zea larvae. J. Chem. Ecol. 2006, 32, 959–968. [Google Scholar] [CrossRef]
- Zhou, M.; Zhang, C.; Wu, Y.; Tang, Y. Metabolic engineering of gossypol in cotton. Appl. Microbiol. Biotechnol. 2013, 97, 6159–6165. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Ruan, J.X.; Huang, J.Q.; Fang, X.; Mao, Y.B.; Wang, L.J.; Chen, X.Y.; Yang, C.Q. Gossypol: Phytoalexin of cotton. Sci. China Life Sci. 2016, 59, 122–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.Y.; Chen, Y.; Heinstein, P.; Davisson, V.J. Cloning, expression, and characterization of (+)-delta-cadinene synthase: A catalyst for cotton phytoalexin biosynthesis. Arch. Biochem. Biophys. 1995, 324, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Y.; Wang, M.; Chen, Y.; Davisson, V.J.; Heinstein, P. Cloning and heterologous expression of a second (+)-delta-cadinene synthase from Gossypium arboreum. J. Nat. Prod. 1996, 59, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Sunilkumar, G.; Campbell, L.M.; Puckhaber, L.; Stipanovic, R.D.; Rathore, K.S. Engineering cottonseed for use in human nutrition by tissue-specific reduction of toxic gossypol. Proc. Natl. Acad. Sci. USA 2006, 103, 18054–18059. [Google Scholar] [CrossRef] [Green Version]
- Tian, X.; Ruan, J.X.; Huang, J.Q.; Yang, C.Q.; Fang, X.; Chen, Z.W.; Hong, H.; Wang, L.J.; Mao, Y.B.; Lu, S.; et al. Characterization of gossypol biosynthetic pathway. Proc. Natl. Acad. Sci. USA 2018, 115, E5410–E5418. [Google Scholar] [CrossRef] [Green Version]
- Rathore, K.S.; Sundaram, S.; Sunilkumar, G.; Campbell, L.M.; Puckhaber, L.; Marcel, S.; Palle, S.R.; Stipanovic, R.D.; Wedegaertner, T.C. Ultra-low gossypol cottonseed: Generational stability of the seed-specific, RNAi-mediated phenotype and resumption of terpenoid profile following seed germination. Plant Biotechnol. J. 2012, 10, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.Z.; Zhou, Y.F.; Wang, X.; Jiao, Z.J. Programmed cell death during pigment gland formation in Gossypium hirsutum leaves. Plant Biol. 2010, 12, 895–902. [Google Scholar] [CrossRef]
- Stanford, E.E.; Viehoever, A. Chemistry and histology of the glands of the cotton plant, with notes on the occurrence of similar glands in related plants. J. Agric. Res. 1918, 13, 419–435. [Google Scholar]
- Bell, A.A. Phytoalexin production and Verticillium wilt resistance in cotton. Tech. Rep. Arch. Image Libr. 1969, 59, 1119–1127. [Google Scholar]
- Singh, I.; Weaver, J. Growth and infestation of boll weevils on normal-glanded, glandless, and high-gossypol strains of cotton. J. Econ. Entomol. 1972, 65, 821–824. [Google Scholar] [CrossRef]
- Wilson, F.; Smith, J. Some genetic relationships between gland density and gossypol content in Gossypium hirsutum L. Crop Sci. 1976, 16, 830–832. [Google Scholar] [CrossRef]
- Mohan, P.; Singh, P.; Dongre, A.; Narayanan, S. Gossypol-gland density and free gossypol content in seed and cotyledonary leaf of upland cotton (Gossypium hirsutum). Indian J. Agric. Sci. 1995, 65, 66–68. [Google Scholar]
- McMichael, S. Glandless boll in upland cotton and its use in the study of natural crossing. Agron. J. 1954, 46, 527–528. [Google Scholar] [CrossRef] [Green Version]
- McMichael, S.C. Combined effects of glandless genes gl2 and gl3 on pigment glands in the cotton plant. Agron. J. 1960, 52, 385–386. [Google Scholar] [CrossRef]
- Miravalle, R.J. Action of the genes controlling the character glandless seed in cotton. Crop Sci. 1962, 2, 447. [Google Scholar] [CrossRef]
- Lee, J.A. Genetical studies concerning the distribution of pigment glands in the cotyledons and leaves of upland cotton. Genetics 1962, 47, 131–142. [Google Scholar] [CrossRef]
- Murray, J.C. A new locus for glanded stem in tetraploid cotton. J. Hered. 1965, 1, 42–44. [Google Scholar] [CrossRef]
- Afifi, A.; Bary, A.; Kamel, S.; Heikal, I. Bahtim 110, a new strain of Egyptian cotton free from gossypol. Emp. Cotton Grow. Rev. 1966, 43, 112–120. [Google Scholar]
- Kohel, R.; Lee, J. Genetic analysis of Egyptian glandless cotton. Crop Sci. 1984, 24, 1119–1121. [Google Scholar] [CrossRef]
- Cheng, H.L.; Lu, C.R.; Yu, J.Z.; Zou, C.S.; Zhang, Y.P.; Wang, Q.L.; Huang, J.; Feng, X.X.; Jiang, P.F.; Yang, W.C.; et al. Fine mapping and candidate gene analysis of the dominant glandless gene Gl2e in cotton (Gossypium spp.). Theor. Appl. Genet. 2016, 129, 1347–1355. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Hu, Y.; Yang, C.Q.; Liu, B.L.; Fang, L.; Wan, Q.; Liang, W.H.; Mei, G.F.; Wang, L.J.; Wang, H.P.; et al. Genetic basis for glandular trichome formation in cotton. Nat. Commun. 2016, 7, 10456. [Google Scholar] [CrossRef] [PubMed]
- Janga, M.R.; Pandeya, D.; Campbell, L.M.; Konganti, K.; Villafuerte, S.T.; Puckhaber, L.; Pepper, A.; Stipanovic, R.D.; Scheffler, J.A.; Rathore, K.S. Genes regulating gland development in the cotton plant. Plant Biotechnol. J. 2019, 17, 1142–1153. [Google Scholar] [CrossRef]
- Ali, M.; Cheng, H.L.; Soomro, M.; Li, S.Y.; Bilal, T.M.; Nazir, M.F.; Feng, X.X.; Zhang, Y.P.; Zuo, D.Y.; Lv, L.M.; et al. Comparative transcriptomic analysis to identify the genes related to delayed gland morphogenesis in Gossypium bickii. Genes 2020, 11, 472. [Google Scholar] [CrossRef]
- Cai, Y.F.; Cai, X.Y.; Wang, Q.L.; Wang, P.; Zhang, Y.; Cai, C.W.; Xu, Y.C.; Wang, K.B.; Zhou, Z.L.; Wang, C.X.; et al. Genome sequencing of the Australian wild diploid species Gossypium australe highlights disease resistance and delayed gland morphogenesis. Plant Biotechnol. J. 2020, 18, 814–828. [Google Scholar] [CrossRef] [Green Version]
- Le, D.T.; Nishiyama, R.; Watanabe, Y.; Mochida, K.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Tran, L.S. Genome-wide survey and expression analysis of the plant-specific NAC transcription factor family in soybean during development and dehydration stress. DNA Res. 2011, 18, 263–276. [Google Scholar] [CrossRef] [Green Version]
- Olsen, A.N.; Ernst, H.A.; Leggio, L.L.; Skriver, K. NAC transcription factors: Structurally distinct, functionally diverse. Trends Plant Sci. 2005, 10, 79–87. [Google Scholar] [CrossRef]
- Souer, E.; Van, H.A.; Kloos, D.; Mol, J.; Koes, R. The no apical meristem gene of Petunia is required for pattern formation in embryos and flowers and is expressed at meristem and primordia boundaries. Cell 1996, 85, 159–170. [Google Scholar] [CrossRef] [Green Version]
- Ooka, H.; Satoh, K.; Doi, K.; Nagata, T.; Otomo, Y.; Murakami, K.; Matsubara, K.; Osato, N.; Kawai, J.; Carninci, P.; et al. Comprehensive analysis of NAC family genes in Oryza sativa and Arabidopsis thaliana. DNA Res. 2003, 10, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Nuruzzaman, M.; Manimekalai, R.; Sharoni, A.M.; Satoh, K.; Kondoh, H.; Ooka, H.; Kikuchi, S. Genome-wide analysis of NAC transcription factor family in rice. Gene 2010, 465, 30–44. [Google Scholar] [CrossRef]
- Wang, N.; Zheng, Y.; Xin, H.P.; Fang, L.C.; Li, S.H. Comprehensive analysis of NAC domain transcription factor gene family in Vitis vinifera. Plant Cell Rep. 2013, 32, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Shang, H.H.; Wang, Z.N.; Zou, C.S.; Zhang, Z.; Li, W.J.; Li, J.W.; Shi, Y.Z.; Gong, W.K.; Chen, T.T.; Liu, A.Y.; et al. Comprehensive analysis of NAC transcription factors in diploid Gossypium: Sequence conservation and expression analysis uncover their roles during fiber development. Sci. China Life Sci. 2016, 59, 142–153. [Google Scholar] [CrossRef] [Green Version]
- Fan, K.; Li, F.; Chen, J.H.; Li, Z.W.; Lin, W.W.; Cai, S.Z.; Liu, J.P.; Lin, W.X. Asymmetric evolution and expansion of the NAC transcription factor in polyploidized cotton. Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Hu, M.L.; Li, J.Y.; Chen, L.; Li, M.; Zhang, S.Q.; Zhang, X.L.; Yang, X.Y. Comprehensive analysis of NAC transcription factors uncovers their roles during fiber development and stress response in cotton. BMC Plant Biol. 2018, 18, 150. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.J.; Fu, M.C.; Li, H.; Chen, Y.Z.; Wang, L.G.; Liu, R.Z. Systematic analysis of NAC transcription factors in Gossypium barbadense uncovers their roles in response to Verticillium wilt. Peer J. 2019, 7, e7995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shang, H.H.; Li, W.; Zou, C.S.; Yuan, Y.Y. Analyses of the NAC transcription factor gene family in Gossypium raimondii Ulbr.: Chromosomal location, structure, phylogeny, and expression patterns. J. Integr. Plant biol. 2013, 55, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Nuruzzaman, M.; Sharoni, A.M.; Kikuchi, S. Roles of NAC transcription factors in the regulation of biotic and abiotic stress responses in plants. Front. Microbiol. 2013, 4, 248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, X.; Wang, H.; Cai, J.T.; Li, D.Y.; Song, F.M. NAC transcription factors in plant immunity. Phytopathol. Res. 2019, 1, 1–13. [Google Scholar] [CrossRef]
- Ma, X.M.; Zhang, Y.J.; Tureckova, V.; Xue, G.P.; Fernie, A.R.; Mueller-Roeber, B.; Balazadeh, B. The NAC transcription factor SlNAP2 regulates leaf senescence and fruit yield in tomato. Plant Physiol. 2018, 177, 1286–1302. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, B.; Hasson, A.; Belcram, K.; Cortizo, M.; Morin, H.; Nikovics, K.; Vialette-Guiraud, A.; Takeda, S.; Aida, M.; Laufs, P.; et al. A conserved role for Cup-Shaped Cotyledon genes during ovule development. Plant J. 2015, 83, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, N.; Ji, D.D.; Zhang, W.X.; Wang, Y.; Yu, Y.C.; Zhao, S.Z.; Lyu, M.H.; You, J.J.; Zhang, Y.Y.; et al. A GmSIN1/GmNCED3s/GmRbohBs feed-forward loop acts as a signal amplifier that regulates root growth in soybean exposed to salt stress. Plant Cell 2019, 31, 2107–2130. [Google Scholar] [CrossRef] [Green Version]
- Daneva, A.; Gao, Z.; Van Durme, M.; Nowack, M.K. Functions and regulation of programmed cell death in plant development. Annu. Rev. Cell Dev. Biol. 2016, 32, 441–468. [Google Scholar] [CrossRef]
- Zhao, C.; Avci, U.; Grant, E.H.; Haigler, C.H.; Beers, E.P. XND1, a member of the NAC domain family in Arabidopsis thaliana, negatively regulates lignocellulose synthesis and programmed cell death in xylem. Plant J. 2008, 53, 425–436. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Goué, N.; Igarashi, H.; Ohtani, M.; Nakano, Y.; Mortimer, J.C.; Nishikubo, N.; Kubo, M.; Katayama, Y.; Kakegawa, K.; et al. Vascular-Related Nac-Domain6 and Vascular-Related Nac-Domain7 effectively induce transdifferentiation into xylem vessel elements under control of an induction system. Plant Physiol. 2010, 153, 906–914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, R.Q.; Lee, C.H.; Ye, Z.H. Global analysis of direct targets of secondary wall NAC master switches in Arabidopsis. Mol. Plant 2010, 3, 1087–1103. [Google Scholar] [CrossRef]
- Matallana-Ramirez, L.P.; Rauf, M.; Farage-Barhom, S.; Dortay, H.; Xue, G.; Dröge-Laser, W.; Lers, A.; Balazadeh, S.; Mueller-Roeber, B. NAC transcription factor ORE1 and senescence-induced BIFUNCTIONAL NUCLEASE1 (BFN1) constitute a regulatory cascade in Arabidopsis. Mol. Plant 2013, 6, 1438–1452. [Google Scholar] [CrossRef] [Green Version]
- Fendrych, M.; Van Hautegem, T.; Van Durme, M.; Olvera-Carrillo, Y.; Huysmans, M.; Karimi, M.; Lippens, S.; Guérin, C.J.; Krebs, M.; Schumacher, K.; et al. Programmed cell death controlled by ANAC033/SOMBRERO determines root cap organ size in Arabidopsis. Curr. Biol. 2014, 24, 931–940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huysmans, M.; Buono, R.A.; Skorzinski, N.; Radio, M.C.; De Winter, F.; Parizot, B.; Mertens, J.; Karimi, M.; Fendrych, M.; Nowack, M.K. NAC transcription factors ANAC087 and ANAC046 control distinct aspects of programmed cell death in the Arabidopsis columella and lateral root Cap. Plant Cell 2018, 30, 2197–2213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, F.G.; Fan, G.Y.; Lu, C.R.; Xiao, G.H.; Zou, C.S.; Kohel, R.J.; Ma, Z.Y.; Shang, H.H.; Ma, X.F.; Wu, J.Y.; et al. Genome sequence of cultivated Upland cotton (Gossypium hirsutum TM-1) provides insights into genome evolution. Nat. Biotechnol. 2015, 33, 524–530. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, P.; Soria, M.; Blesa, D.; DiRienzo, J.; Moschen, S.; Rivarola, M.; Clavijo, B.J.; Gonzalez, S.; Peluffo, L.; Principi, D.; et al. Development, characterization and experimental validation of a cultivated sunflower (Helianthus annuus L.) gene expression oligonucleotide microarray. PLoS ONE 2012, 7, e45899. [Google Scholar] [CrossRef]
- Moschen, S.; Bengoa Luoni, S.; Paniego, N.B.; Hopp, H.E.; Dosio, G.A.; Fernandez, P.; Heinz, R.A. Identification of candidate genes associated with leaf senescence in cultivated sunflower (Helianthus annuus L.). PLoS ONE 2014, 9, e104379. [Google Scholar] [CrossRef] [PubMed]
- Kazan, K.; Manners, J.M. MYC2: The master in action. Mol. Plant 2013, 6, 686–703. [Google Scholar] [CrossRef] [Green Version]
- D’Ario, M.; Griffiths-Jones, S.; Kim, M. Small RNAs: Big impact on plant development. Trends Plant Sci. 2017, 22, 1056–1068. [Google Scholar] [CrossRef] [Green Version]
- Kozomara, A.; Birgaoanu, M.; Griffiths-Jones, S. miRBase: From microRNA sequences to function. Nucleic Acids Res. 2019, 47, D155–D162. [Google Scholar] [CrossRef]
- Ashraf, J.; Zuo, D.Y.; Wang, Q.L.; Malik, W.; Zhang, Y.P.; Abid, M.A.; Cheng, H.L.; Yang, Q.H.; Song, G.L. Recent insights into cotton functional genomics: Progress and future perspectives. Plant Biotechnol. J. 2018, 16, 699–713. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.C.; Yu, M.; Zou, C.S.; Lu, C.R.; Yu, D.Q.; Cheng, H.L.; Jiang, P.F.; Feng, X.X.; Zhang, Y.P.; Wang, Q.L.; et al. Genome-wide comparative analysis of RNA-binding Glycine-rich protein family genes between Gossypium arboreum and Gossypium raimondii. PLoS ONE 2019, 14, e0218938. [Google Scholar] [CrossRef]
- Cannon, S.B.; Mitra, A.; Baumgarten, A.; Young, N.D.; May, G. The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biol. 2004, 4, 10. [Google Scholar] [CrossRef] [Green Version]
- Hickman, R.; Van Verk, M.C.; Van Dijken, A.; Mendes, M.P.; Vroegop-Vos, I.A.; Caarls, L.; Steenbergen, M.; Van der Nagel, I.; Wesselink, G.J.; Jironkin, A.; et al. Architecture and dynamics of the jasmonic acid gene regulatory network. Plant Cell 2017, 29, 2086–2105. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Chen, J.D.; Fang, L.; Zhang, Z.Y.; Ma, W.; Niu, Y.C.; Ju, L.Z.; Deng, J.Q.; Zhao, T.; Lian, J.M.; et al. Gossypium barbadense and Gossypium hirsutum genomes provide insights into the origin and evolution of allotetraploid cotton. Nat. Genet. 2019, 51, 739–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, X.M.; Huang, G.; He, S.P.; Yang, Z.E.; Sun, G.F.; Ma, X.F.; Liu, N.; Zhang, X.Y.; Sun, J.L.; Liu, M.; et al. Resequencing of 243 diploid cotton accessions based on an updated A genome identifies the genetic basis of key agronomic traits. Nat. Genet. 2018, 50, 796–802. [Google Scholar] [CrossRef]
- Paterson, A.H.; Wendel, J.F.; Gundlach, H.; Guo, H.; Jenkins, J.; Jin, D.; Llewellyn, D.; Showmaker, K.C.; Shu, S.; Udall, J.; et al. Repeated polyploidization of Gossypium genomes and the evolution of spinnable cotton fibres. Nature 2012, 492, 423–427. [Google Scholar] [CrossRef] [Green Version]
- Madeira, F.; Park, Y.m.; Lee, J.; Buso, N.; Gur, T.; Madhusoodanan, N.; Basutkar, P.; Tivey, A.R.N.; Potter, S.C.; Finn, R.D.; et al. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 2019, 47, W636–W641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, T.; Liang, C.Z.; Meng, Z.G.; Sun, G.Q.; Meng, Z.H.; Guo, S.D.; Zhang, R. CottonFGD: An integrated functional genomics database for cotton. BMC Plant Biol. 2017, 17, 101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subramanian, B.; Gao, S.; Lercher, M.J.; Hu, S.; Chen, W.H. Evolview v3: A webserver for visualization, annotation, and management of phylogenetic trees. Nucleic Acids Res. 2019, 47, W270–W275. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.H.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Cui, Y.P.; Su, Y.; Wang, J.J.; Jia, B.; Wu, M.; Pei, W.F.; Zhang, J.F.; Yu, J.W. Genome-wide characterization and analysis of CIPK gene family in two cultivated allopolyploid cotton species: Sequence variation, association with seed oil content, and the role of GhCIPK6. Int. J. Mol. Sci. 2020, 21, 863. [Google Scholar] [CrossRef] [Green Version]
- Tang, H.; Bowers, J.E.; Wang, X.; Ming, R.; Alam, M.; Paterson, A.H. Synteny and collinearity in plant genomes. Science 2008, 320, 486–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patro, R.; Duggal, G.; Love, M.I.; Irizarry, R.A.; Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 2017, 14, 417–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, X.Q.; Shan, L.B. Functional genomic analysis of cotton genes with agrobacterium-mediated virus-induced gene silencing. Methods Mol. Biol. 2013, 975, 157–165. [Google Scholar] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, H.; Feng, X.; Zuo, D.; Zhang, Y.; Wang, Q.; Lv, L.; Wu, C.; Li, S.; Dai, Y.; Qu, D.; et al. Gene Expression Correlation Analysis Reveals MYC-NAC Regulatory Network in Cotton Pigment Gland Development. Int. J. Mol. Sci. 2021, 22, 5007. https://doi.org/10.3390/ijms22095007
Cheng H, Feng X, Zuo D, Zhang Y, Wang Q, Lv L, Wu C, Li S, Dai Y, Qu D, et al. Gene Expression Correlation Analysis Reveals MYC-NAC Regulatory Network in Cotton Pigment Gland Development. International Journal of Molecular Sciences. 2021; 22(9):5007. https://doi.org/10.3390/ijms22095007
Chicago/Turabian StyleCheng, Hailiang, Xiaoxu Feng, Dongyun Zuo, Youping Zhang, Qiaolian Wang, Limin Lv, Chaofeng Wu, Shuyan Li, Yuanli Dai, Da Qu, and et al. 2021. "Gene Expression Correlation Analysis Reveals MYC-NAC Regulatory Network in Cotton Pigment Gland Development" International Journal of Molecular Sciences 22, no. 9: 5007. https://doi.org/10.3390/ijms22095007




