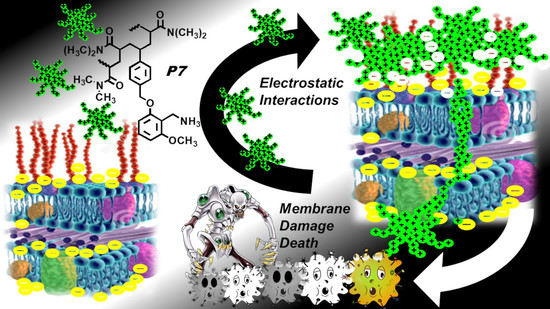
Broad-Spectrum Bactericidal Activity of a Synthetic Random Copolymer Based on 2-Methoxy-6-(4-Vinylbenzyloxy)-Benzylammonium Hydrochloride
Abstract
:1. Introduction
2. Results and Discussion
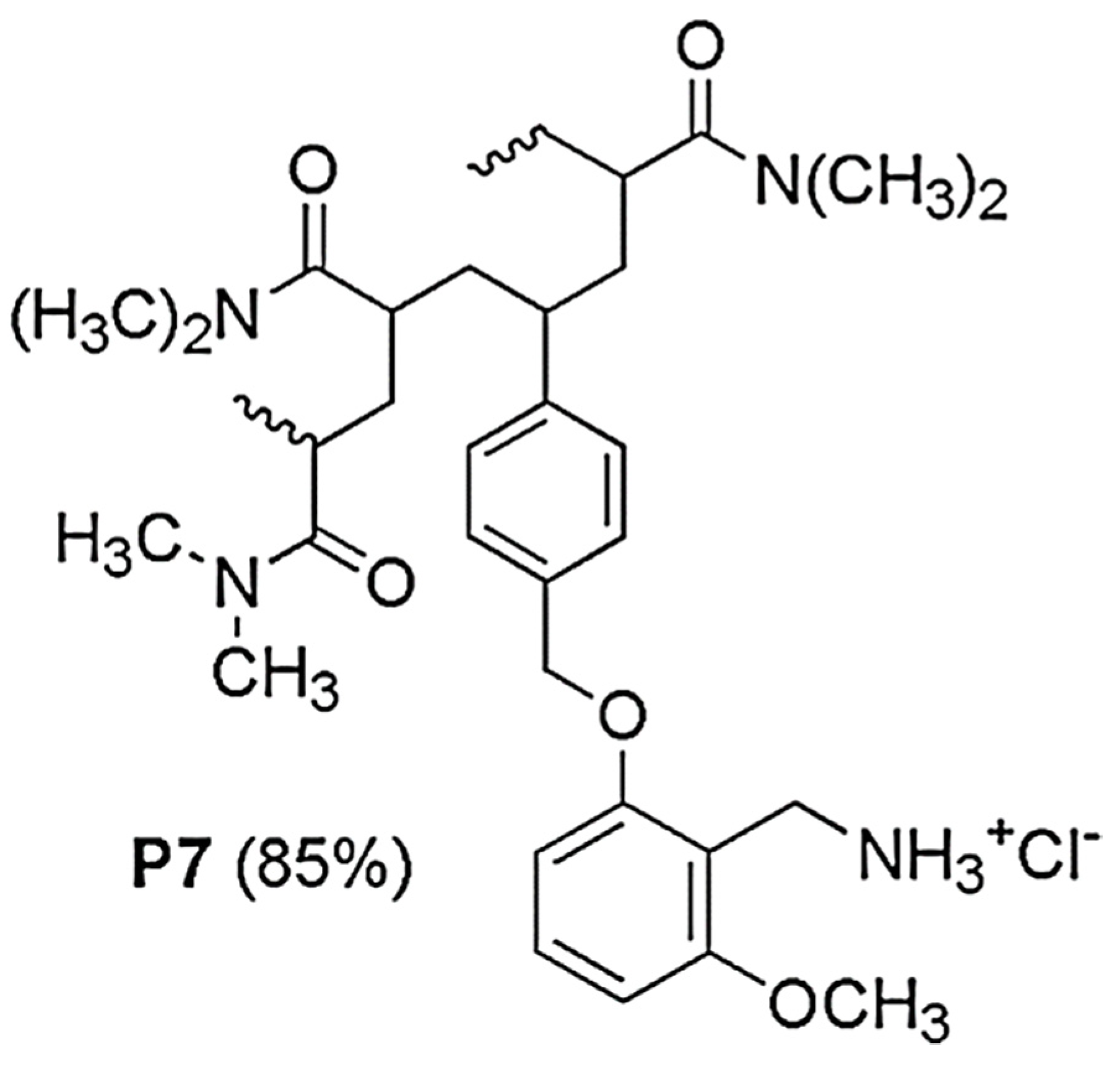
2.1. Synthesis and Spectrophotometric Characterization of 2-Methoxy-6-[(4-Vinyl)Benzyloxy]Benzylammonium Hydrochloride M7 (7)
2.2. Preparation of Copolymer P7 by Radical Copolymerizations in Solution and Spectroscopic characterizations of P7
2.3. Antibacterial Properties
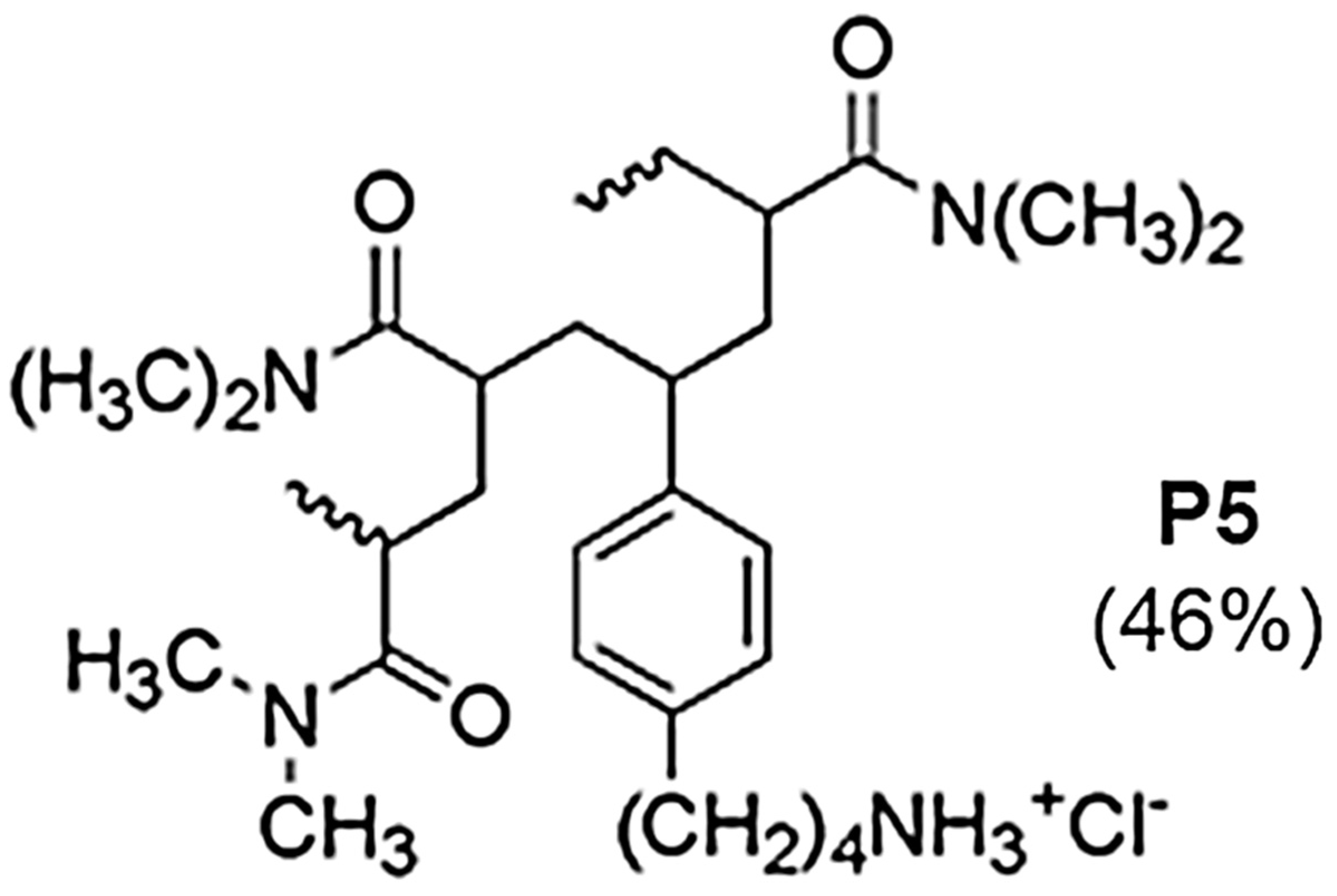
2.3.1. The Reasons for Our Interest in Monomer M7 and Copolymer P7
2.3.2. Antimicrobial Activity of P7 by Determination of MIC and MBC Values
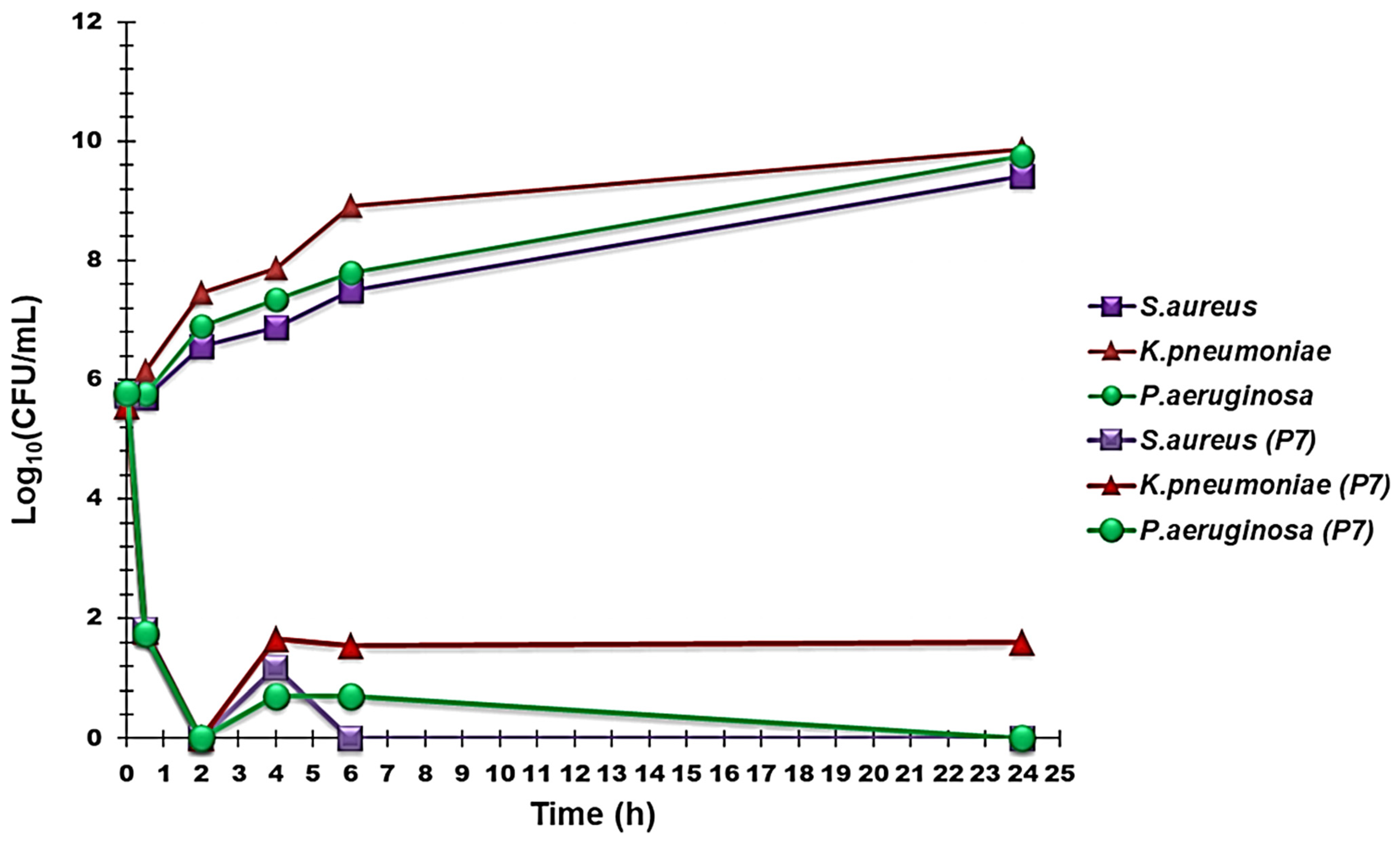
2.3.3. Time–Kill Curves
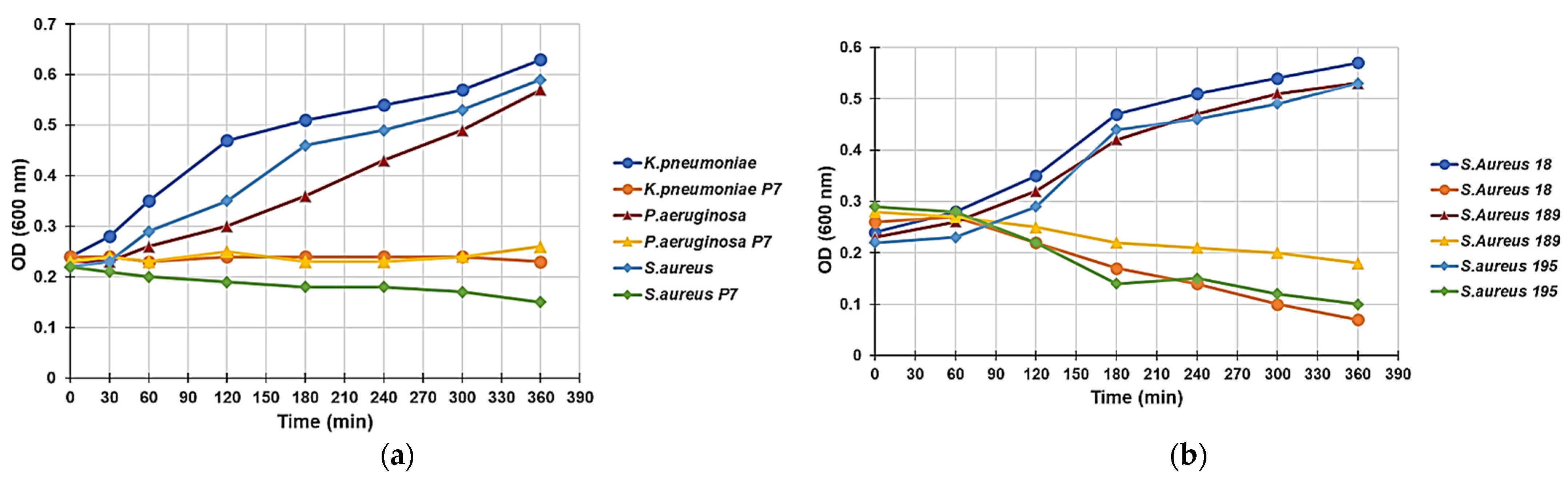
2.3.4. Effect of P5 on the Growth Curve of P. Aeruginosa, K. Pneumoniae, and S. Aureus
3. Materials and Methods
3.1. Chemicals and Instruments
3.2. Microbiology
3.2.1. Microorganisms
3.2.2. Determination of MIC and MBC
3.2.3. Time–Kill Curves
3.2.4. Evaluation of the Antimicrobial Effect of P7 through Turbidimetric Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- My Personal Trailer. Available online: https://www.my-personaltrainer.it/farmaci/citrosil.html (accessed on 15 April 2021).
- Lei, Y.; Zhou, S.; Dong, C.; Zhang, A.; Lin, Y. PDMS tri-block copolymers bearing quaternary ammonium salts for epidermal antimicrobial agents: Synthesis, surface adsorption and non-skin-penetration. React. Funct. Polym. 2018, 124, 20–28. [Google Scholar] [CrossRef]
- Jiao, Y.; Niu, L.; Ma, S.; Li, J.; Tay, F.R.; Chen, J. Quaternary ammonium-based biomedical materials: State-of-the-art, toxicological aspects and antimicrobial resistance. Prog. Polym. Sci. 2017, 71, 53–90. [Google Scholar] [CrossRef]
- Zhang, C.; Cui, F.; Zeng, G.; Jiang, M.; Yang, Z.; Yu, Z.; Zhu, M.; Shen, L. Quaternary ammonium compounds (QACs): A review on occurrence, fate and toxicity in the environment. Sci. Total Environ. 2015, 518, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Lam, P.L.; Lee, K.K.H.; Wong, R.S.M.; Cheng, G.Y.M.; Bian, Z.X.; Chui, C.H.; Gambari, R. Recent advances on topical antimicrobials for skin and soft tissue infections and their safety concerns. Crit. Rev. Microbiol. 2017, 44, 40–78. [Google Scholar] [CrossRef] [PubMed]
- Kenawy, E.-R.; Abdel-Hay, F.I.; El-Magd, A.A.; Mahmoud, Y. Biologically active polymers: VII. Synthesis and antimicrobial activity of some crosslinked copolymers with quaternary ammonium and phosphonium groups. React. Funct. Polym. 2006, 66, 419–429. [Google Scholar] [CrossRef]
- Llor, C.; Bjerrum, L. Antimicrobial resistance: Risk associated with antibiotic overuse and initiatives to reduce the problem. Ther. Adv. Drug Saf. 2014, 229–241. [Google Scholar] [CrossRef] [Green Version]
- Alavi, A.; Sibbald, R.G.; Ladizinski, B.; Saraiya, A.; Lee, K.C.; Skotnicki-Grant, S.; Maibach, H. Wound-Related Allergic/Irritant Contact Dermatitis (Article). Adv. Ski. Wound Care 2016, 29, 278–286. [Google Scholar] [CrossRef]
- Licata, A. Adverse drug reactions and organ damage: The liver. Eur. J. Intern. Med. 2016, 28, 9–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siedenbiedel, F.; Tiller, J.C. Antimicrobial Polymers in Solution and on Surfaces: Overview and Functional Principles. Polymers 2012, 4, 46–71. [Google Scholar] [CrossRef] [Green Version]
- Alfei, S.; Schito, A.M. Positively Charged Polymers as Promising Devices against Multidrug Resistant Gram-Negative Bacteria: A Review. Polymers 2020, 12, 1195. [Google Scholar]
- Gelman, M.A.; Weisblum, B.; Lynn, D.M.; Gellman, S.H. Biocidal activity of polystyrenes that are cationic by virtue of protonation. Org. Lett. 2004, 6, 557–560. [Google Scholar] [CrossRef]
- Alfei, S.; Piatti, G.; Caviglia, D.; Schito, A.M. Synthesis, Characterization and Bactericidal Activity of a 4-Ammoniumbuthylstyrene-Based Random Copolymer. Polymers 2021, 13, 1140. [Google Scholar] [CrossRef] [PubMed]
- Palermo, E.; Kuroda, K. Chemical structure of cationic groups in amphiphilic polymethacrylates modulates the antimicrobial and hemolytic activities. Biomacromolecules 2009, 10, 1416–1428. [Google Scholar] [CrossRef] [PubMed]
- Ergene, C.; Yasuharab, K.; Palermo, E.F. Biomimetic antimicrobial polymers: Recent advances in molecular design. Polym. Chem. 2018, 9, 2047. [Google Scholar] [CrossRef] [Green Version]
- Ganewatta, M.S.; Tang, C. Controlling macromolecular structures towards effective antimicrobial polymers. Polymer 2015, 63, A1–A29. [Google Scholar] [CrossRef]
- Gabriel, G.J.; Som, A.; Madkour, A.E.; Eren, T.; Tew, G.N. Infectious disease: Connecting innate immunity to biocidal polymers. Mater. Sci. Eng. R Rep. 2007, 57, 28–64. [Google Scholar] [CrossRef] [Green Version]
- Matsuzaki, K. Control of cell selectivity of antimicrobial peptides. Biochim. Et Biophys. Acta 2009, 1687–1692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuzaki, K.; Sugishita, K.; Fujii, N.; Miyajima, K. Molecular Basis for Membrane Selectivity of an Antimicrobial Peptide, Magainin 2. Biochemistry 1995, 34, 3423–3429. [Google Scholar] [CrossRef]
- Alfei, S.; Marengo, B.; Valenti, G.E.; Domenicotti, C. Synthesis of Polystyrene-Based Cationic Nanomaterials with Pro-Oxidant Cytotoxic Activity on Etoposide-Resistant Neuroblastoma Cells. Nanomaterials 2021, 11, 977. [Google Scholar] [CrossRef] [PubMed]
- Eliassen, L.T.; Berge, G.; Leknessund, A.; Wikman, M.; Lindin, I.; Løkke, C.; Ponthan, F.; Johnsen, J.I.; Sveinbjørnsson, B.; Kogner, P.; et al. The antimicrobial peptide, Lactoferricin B, is cytotoxic to neuroblastoma cells in vitro and inhibits xenograft growth in vivo. Int. J. Cancer 2006, 119, 493–500. [Google Scholar] [CrossRef] [Green Version]
- Alfei, S.; Schito, A.M. From Nanobiotechnology, Positively Charged Biomimetic Dendrimers as Novel Antibacterial Agents: A Review. Nanomaterials 2020, 10, 2022. [Google Scholar] [CrossRef]
- Schito, A.M.; Schito, G.C.; Alfei, S. Synthesis and Antibacterial Activity of Cationic Amino Acid-Conjugated Dendrimers Loaded with a Mixture of Two Triterpenoid Acids. Polymers 2021, 13, 521. [Google Scholar] [CrossRef]
- Schito, A.M.; Alfei, S. Antibacterial Activity of Non-Cytotoxic, Amino Acid-Modified Polycationic Dendrimers against Pseudomonas aeruginosa and Other Non-Fermenting Gram-Negative Bacteria. Polymers 2020, 12, 1818. [Google Scholar] [CrossRef]
- Tan, J.; Tay, J.; Hedrick, J.; Yang, Y.Y. Synthetic macromolecules as therapeutics that overcome resistance in cancer and microbial infection. Biomaterials 2020, 252, 120078. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Xu, L.; Xiao, X.; Wang, Z. Preparation, characterization, and antibacterial activity of cationic nanopolystyrenes. J. Appl. Polym. Sci. 2019, 137, 48405. [Google Scholar] [CrossRef]
- Ikeda, T.; Tazuke, S.; Suzuki, Y. Biologically active polycations 4: Synthesis and antimicrobial activity of poly(trialkylvinylbenzylammonium chloride)s. Makromol. Chem. 1984, 185, 869–876. [Google Scholar] [CrossRef]
- Timofeeva, L.; Kleshcheva, N. Antimicrobial polymers: Mechanism of action, factors of activity, and applications. Appl. Microbiol. Biotechnol. 2011, 89, 475–492. [Google Scholar] [CrossRef]
- Kougia, E.; Tselepi, M.; Vasilopoulos, G.; Lainioti, G.C.; Koromilas, N.D.; Druvari, D.; Bokias, G.; Vantarakis, A.; Kallitsis, J.K. Evaluation of Antimicrobial E_ciency of New Polymers Comprised by Covalently Attached and/or Electrostatically Bound Bacteriostatic Species, Based on Quaternary Ammonium Compounds. Molecules 2015, 20, 21313–21327. [Google Scholar] [CrossRef] [PubMed]
- Vancomycin. Available online: https://en.wikipedia.org/wiki/Vancomycin (accessed on 15 April 2021).
- Eloff, J.N.; Famakin, J.O.; Katerere, D.P.R. Isolation of an antibacterial stilbene from Combretum woodii (Combretaceae) leaves. Afr. J. Biotechnol. 2005, 4, 1167–1171. [Google Scholar]
- World Health Organization. Critically Important Antimicrobials for Human Medicine, 6th ed.; World Health Organ: Geneva, Switzerland, 2019; hdl: 10665/312266. [Google Scholar]
- World Health Organization. World Health Organization Model List of Essential Medicines: 21st List 2019; World Health Organ: Geneva, Switzerland, 2019; hdl: 10665/325771. [Google Scholar]
- Kanazawa, A.; Ikeda, T.; Endo, T. Novel polycationic biocides: Synthesis and antibacterial activity of polymeric phosphonium salts. J. Polym. Sci. A Polym. Chem. 1993, 31, 335–343. [Google Scholar] [CrossRef]
- Santos, M.R.E.; Mendonça, P.V.; Almeida, M.C.; Branco, R.; Serra, A.C.; Morais, P.V.; Coelho, J.F.J. Increasing the Antimicrobial Activity of Amphiphilic Cationic Copolymers by the Facile Synthesis of High Molecular Weight Stars by Supplemental Activator and Reducing Agent Atom Transfer Radical Polymerization. Biomacromolecules 2019, 20, 1146–1156. [Google Scholar] [CrossRef]
- Locock, K.E.S.; Michl, T.D.; Stevens, N.; Hayball, J.D.; Vasilev, K.; Postma, A.; Griesser, H.J.; Meagher, L.; Haeussler, M. Antimicrobial Polymethacrylates Synthesized as Mimics of Tryptophan-Rich Cationic Peptides. ACS Macro Lett. 2014, 3, 319–323. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Park, Y.K.; Chung, E.S.; Na, I.Y.; Ko, K.S. Evolved Resistance to Colistin and Its Loss Due to Genetic Reversion in Pseudomonas Aeruginosa. Sci. Rep. 2016, 6, 25543. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, T.; Bustos, R.-H.; González, D.; Garzón, V.; García, J.-C.; Ramírez, D. An Approach to Measuring Colistin Plasma Levels Regarding the Treatment of Multidrug-Resistant Bacterial Infection. Antibiotics 2019, 8, 100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Zhu, J.; Hu, Q.; Rao, X. Morganella morganii, a non-negligent opportunistic pathogen. Int. J. Infect. Dis. 2016, 50, 10–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Band, V.I.; Weiss, D.S. Mechanisms of Antimicrobial Peptide Resistance in Gram-Negative Bacteria. Antibiotics 2015, 4, 18–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostrowska, K.; Kamysz, W.; Dawgul, M.; Różalski, A. Synthetic amphibian peptides and short amino-acids derivatives against planktonic cells and mature biofilm of Providencia stuartii clinical strains. Pol. J. Microbiol. 2014, 63, 423–431. [Google Scholar] [CrossRef]
- Mizutani, M.; Palermo, E.F.; Thoma, L.M.; Satoh, K.; Kamigaito, M.; Kuroda, K. Design and Synthesis of Self-Degradable Antibacterial Polymers by Simultaneous Chain- and Step-Growth Radical Copolymerization. Biomacromolecules 2012, 13, 1554–1563. [Google Scholar] [CrossRef] [PubMed]
- Barman, S.; Konai, M.M.; Samaddar, S.; Haldar, J. Amino Acid Conjugated Polymers: Antibacterial Agents Effective against Drug-Resistant Acinetobacter baumannii with No Detectable Resistance. Acs Appl. Mater. Interfaces 2019, 11, 33559–33572. [Google Scholar] [CrossRef]
- Weiyang, L.; Shrinivas, V.; Guansheng, Z.; Bisha, D.; Tan, P.K.J.; Xu, L.; Weimin, F.; Yi, Y.Y. Antimicrobial polymers as therapeutics for treatment of multidrug-resistant Klebsiella pneumoniae lung infection. Acta Biomater. 2018, 78, 78–88. [Google Scholar] [CrossRef]
- Sudip, M.; Swagatam, B.; Riya, M.; Jayanta, H. Amphiphilic Cationic Macromolecules Highly Effective Against Multi-Drug Resistant Gram-Positive Bacteria and Fungi With No Detectable Resistance. Front. Bioeng. Biotechnol. 2020, 8, e55. [Google Scholar] [CrossRef]
- Landman, D.; Georgescu, C.; Martin, D.A.; Quale, J. Polymyxins revisited. Clin. Microbiol. Rev. 2008, 21, 449–465. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, I.; Ghosh, A.; Bhadury, P.; De, P. Side-Chain Amino Acid-Based Cationic Antibacterial Polymers: Investigating the Morphological Switching of a Polymer-Treated Bacterial Cell. ACS Omega 2017, 2, 1633–1644. [Google Scholar] [CrossRef] [PubMed]
- Gough, N.R. Stressing bacteria to death. Sci. Signal. 2011, 4, e164. [Google Scholar] [CrossRef]
- EUCAST. European Committee on Antimicrobial Susceptibility Testing. Available online: https://www.eucast.org/ast_of_bacteria/ (accessed on 15 April 2021).
- Pearson, R.D.; Steigbigel, R.T.; Davis, H.T.; Chapman, S.W. Method for reliable determination of minimal lethal antibiotic concentrations. Antimicrob. Agents Chemother. 1980, 18, 699–708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schito, A.M.; Piatti, G.; Stauder, M.; Bisio, A.; Giacomelli, E.; Romussi, G.; Pruzzo, C. Effects of demethylfruticuline A and fruticuline A from Salvia corrugata Vahl. on biofilm production in vitro by multiresistant strains of Staphylococcus aureus, Staphylococcus epidermidis and Enterococcus Faecalis. Int. J. Antimicrob. Agents 2011, 37, 129–134. [Google Scholar] [CrossRef] [Green Version]
- Dalgaard, P.; Ross, T.; Kamperman, L.; Neumeyer, K.; McMeekin, T.A. Estimation of bacterial growth rates from turbidimetric and viable count data. Int. J. Food Microbiol. 1994, 23, 391–404. [Google Scholar] [CrossRef]
| Analysis | Feature | Determinations | |
|---|---|---|---|
| FTIR | NH3+ | 3500 cm−1 | |
| C-H alkyl | 2800–2900 cm−1 | ||
| Overtones * | 2000–1700 cm−1 | ||
| C=ONH | 1649 cm−1 | ||
| -C=C- * | 1575, 1510 cm−1 | ||
| o-disubstituted § | 754 cm−1 | ||
| VPO 1 analysis (MeOH, 45 °C) | Mn | 13719 | |
| Volumetric Titration | µequivNH2/gP7 | 305 | |
| DLS 2 Analysis | Z-Ave 3 (nm) | 220 ± 18 | |
| PDI 4 | 0.809 ± 0.004 | ||
| Z-potential 5 (ζ-p) | +49.8 ± 5.8 | ||
| Potentiometric Titration | Max dpH/dV 6 | 10.75 | 4 |
| HCl 0.1N (mL) 7 | 0.6 | 1.2 | |
| pH 8 | 6.85 | 4.80 | |
| P7 (13719) 2 | M7 (274) 2 | |||
|---|---|---|---|---|
| Strains | MIC µM (µg/mL) | MBC µM (µg/mL) | MIC µM (µg/mL) | MBC µM (µg/mL) |
| Enterococcus Genus | ||||
| E. faecalis 1 * | 2.3 (32) | 2.3 (32) | 233.6 (64) | 467.1 (128) |
| E. faecalis 18 * | 2.3 (32) | 2.3 (32) | 233.6 (64) | 467.1 (128) |
| E. faecalis 51 * | 2.3 (32) | 4.6 (64) | 233.6 (64) | 467.1 (128) |
| E. faecalis 365 * | 2.3 (32) | 4.6 (64) | 467.1 (128) | 467.1 (128) |
| E. faecium 325 * | 1.15 (16) | 2.3 (32) | 116.8 (32) | 233.6 (64) |
| E. faecium 341 * | 1.15 (16) | 2.3 (32) | 233.6 (64) | 467.1 (128) |
| E. faecium 364 * | 0.6 (8) | 1.15 (16) | 233.6 (64) | 467.1 (128) |
| Minor Strains | ||||
| E. casseliflavus 184 ° | 1.15 (16) | 2.3 (32) | 233.6 (64) | 467.1 (128) |
| E. durans 103 ° | 1.15 (16) | 2.3 (32) | 467.1 (128) | 934.3 (256) |
| E. gallinarum 150 * | 1.15 (16) | 2.3 (32) | 467.1 (128) | 467.1 (128) |
| Staphylococcus Genus | ||||
| S. aureus 18 ** | 4.6 (64) | 4.6 (64) | 233.6 (64) | 467.1 (128) |
| S. aureus 195 ** | 4.6 (64) | 4.6 (64) | 233.6 (64) | 467.1 (128) |
| S. aureus 189 | 4.6 (64) | 4.6 (64) | 233.6 (64) | 467.1 (128) |
| S. epidermidis 22 ** | 1.15 (16) | 2.3 (32) | 233.6 (64) | 467.1 (128) |
| S. epidermidis 180 *** | 1.15 (16) | 2.3 (32) | 116.8 (32) | 233.6 (64) |
| S. epidermidis 181 *** | 1.15 (16) | 2.3 (32) | 233.6 (64) | 467.1 (128) |
| S. haemolyticus 193 ** | 3.15 (16) | 3.15 (16) | 233.6 (64) | 233.6 (64) |
| S. hominis 125 ** | 0.6 (8) | 0.6 (8) | 116.8 (32) | 116.8 (32) |
| S. lugdunensis 129 | 1.15 (16) | 1.15 (16) | 116.8 (32) | 233.6 (64) |
| S. warneri 74 | 1.15 (16) | 1.15 (16) | 233.6 (64) | 233.6 (64) |
| S. saprophyticus 41 | 1.15 (16) | 2.3 (32) | 116.8 (32) | 233.6 (64) |
| S. simulans 163 ** | 1.15 (16) | 1.15 (16) | 467.1 (128) | 467.1 (128) |
| Sporogenic Isolate | ||||
| B. subtilis | 1.15 (16) | 1.15 (16) | 233.6 (64) | 467.1 (128) |
| P7 (13719) 2 | M7 (274) 2 | |||
|---|---|---|---|---|
| Strains | MIC µM (µg/mL) | MBC µM (µg/mL) | MIC µM (µg/mL) | MBC µM (µg/mL) |
| Enterobacteriaceae Family | ||||
| E. coli 224 S | 2.3 (32) | 2.3 (32) | 467.1 (128) | 467.1 (128) |
| E. coli 238 # | 4.6 (64) | 4.6 (64) | 934.3 (256) | 934.3 (256) |
| E. coli 246 § | 2.3 (32) | 2.3 (32) | 467.1 (128) | 467.1 (128) |
| Y. enterocolitica 342 | 9.3 (128) | 18.6 (256) | 467.1 (128) | 934.3 (256) |
| S. marcescens 228 | >18.6 (>256) | nt 3 | 1868.6 (512) | 1868.6 (512) |
| P. mirabilis 254 | 18.6 (256) | 18.6 (256) | 1868.6 (512) | 1868.6 (512) |
| M. morganii 372 | 18.6 (256) | >18.6 (>256) | 1868.6 (512) | 1868.6 (512) |
| K. oxytoca 252 | 9.3 (128) | 9.3 (128) | 467.1 (128) | 467.1 (128) |
| K. pneumoniae 231 # | 4.6 (64) | 4.6 (64) | 467.1 (128) | 467.1 (128) |
| K. pneumoniae 232 # | 9.3 (128) | 18.6 (256) | 467.1 (128) | 467.1 (128) |
| K. pneumoniae 233 # | 4.6 (64) | 4.6 (64) | 467.1 (128) | 467.1 (128) |
| K. pneumoniae 260 # | 9.3 (128) | 9.3 (128) | 467.1 (128) | 467.1 (128) |
| K. pneumoniae 366 # | 4.6 (64) | 4.6 (64) | 467.1 (128) | 467.1 (128) |
| K. pneumoniae 367 # | 9.3 (128) | 9.3 (128) | 467.1 (128) | 467.1 (128) |
| K. pneumoniae 369 | 9.3 (128) | 18.6 (256) | 467.1 (128) | 467.1 (128) |
| K. pneumoniae 377 S | 4.6 (64) | 4.6 (64) | 467.1 (128) | 467.1 (128) |
| Salmonella gr. B 227 | 4.6 (64) | 4.6 (64) | 467.1 (128) | 467.1 (128) |
| P. stuartii 374 | 9.3 (128) | 9.3 (128) | 1868.6 (512) | 1868.6 (512) |
| Non-Fermenting Species | ||||
| A. baumannii 257 | 2.3 (32) | 4.6 (64) | 467.1 (128) | 934.3 (256) |
| A. baumannii 279 | 2.3 (32) | 2.3 (32) | 467.1 (128) | 467.1 (128) |
| A. baumannii 236 | 9.3 (128) | 9.3 (128) | 233.6 (64) | 467.1 (128) |
| A. baumannii 245 | 9.3 (128) | 4.6 (64) | 467.1 (128) | 934.3 (256) |
| A. baumannii 383 | 4.6 (64) | 4.6 (64) | 467.1 (128) | 467.1 (128) |
| A. pittii 272 | 2.3 (32) | 2.3 (32) | 233.6 (64) | 467.1 (128) |
| P. aeruginosa 229 | 4.6 (64) | 4.6 (64) | >1868.6 (>512) | nt 3 |
| P. aeruginosa 247 | 4.6 (64) | 9.3 (128) | >1868.6 (>512) | nt 3 |
| P. aeruginosa 256 | 4.6 (64) | 4.6 (64) | >1868.6 (>512) | nt 3 |
| P. aeruginosa 259 | 9.3 (128) | 18.6 (256) | >1868.6 (>512) | nt 3 |
| P. aeruginosa 268 | 4.6 (64) | 4.6 (64) | >1868.6 (>512) | nt 3 |
| P. aeruginosa 269 | 2.3 (32) | 2.3 (32) | >1868.6 (>512) | nt 3 |
| P. fluorescens 278 | 2.3 (32) | 2.3 (32) | 934.3 (256) | 934.3 (256) |
| P. putida 262 | 4.6 (64) | 4.6 (64) | 934.3 (256) | 934.3 (256) |
| S. maltophylia 255 | 9.3 (128) | 18.6 (256) | 467.1 (128) | 934.3 (256) |
| S. maltophylia 280 | 4.6 (64) | 9.3 (128) | 467.1 (128) | 934.3 (256) |
| S. maltophylia 2 | 2.3 (32) | 2.3 (32) | 467.1 (128) | 467.1 (128) |
| S. maltophylia 3 | 4.6 (64) | 9.3 (128) | 467.1 (128) | 467.1 (128) |
| S. maltophylia 4 | 2.3 (32) | 4.6 (64) | 233.6 (64) | 233.6 (64) |
| S. maltophylia 5 | 9.3 (128) | 18.6 (256) | 467.1 (128) | 467.1 (128) |
| P7 (13719) 2 | Commercial Antibiotics | |
|---|---|---|
| Strains | MIC (µM) | MIC (µM) |
| Enterococcus Genus | ||
| E. faecalis * | 2.3 | 22.1–88.3 3 |
| E. faecium * | 1.15 | 88.3–176.6 3 |
| Staphylococcus Genus | ||
| S. aureus ** | 4.6 | 637.7–1275 4 |
| S. epidermidis *** | 1.15 | 637.7 4 |
| Sporogenic Isolate | ||
| B. subtilis | 1.15 | 212.4 5 |
| Enterobacteriaceae Family | ||
| E. coli # | 4.6 | 16.8 6 96.6 7 13.3–26.5 5 |
| K. pneumoniae # | 4.6–9.3 | 4.4–16.8 6 96.6–193.2 7 6.7–13.3 5 |
| Salmonella gr. B | 4.6 | 235.5 8 |
| M. morganii | 18.6 | 212.4 5 |
| Non-Fermenting Species | ||
| A. baumannii | 2.3–9.3 | 96.6–193.2 7 |
| P. aeruginosa | 2.3–9.3 | 19.6–156.5 9 |
| S. maltophylia | 2.3–9.3 | 58.8–117.7 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schito, A.M.; Piatti, G.; Caviglia, D.; Zuccari, G.; Alfei, S. Broad-Spectrum Bactericidal Activity of a Synthetic Random Copolymer Based on 2-Methoxy-6-(4-Vinylbenzyloxy)-Benzylammonium Hydrochloride. Int. J. Mol. Sci. 2021, 22, 5021. https://doi.org/10.3390/ijms22095021
Schito AM, Piatti G, Caviglia D, Zuccari G, Alfei S. Broad-Spectrum Bactericidal Activity of a Synthetic Random Copolymer Based on 2-Methoxy-6-(4-Vinylbenzyloxy)-Benzylammonium Hydrochloride. International Journal of Molecular Sciences. 2021; 22(9):5021. https://doi.org/10.3390/ijms22095021
Chicago/Turabian StyleSchito, Anna Maria, Gabriela Piatti, Debora Caviglia, Guendalina Zuccari, and Silvana Alfei. 2021. "Broad-Spectrum Bactericidal Activity of a Synthetic Random Copolymer Based on 2-Methoxy-6-(4-Vinylbenzyloxy)-Benzylammonium Hydrochloride" International Journal of Molecular Sciences 22, no. 9: 5021. https://doi.org/10.3390/ijms22095021
APA StyleSchito, A. M., Piatti, G., Caviglia, D., Zuccari, G., & Alfei, S. (2021). Broad-Spectrum Bactericidal Activity of a Synthetic Random Copolymer Based on 2-Methoxy-6-(4-Vinylbenzyloxy)-Benzylammonium Hydrochloride. International Journal of Molecular Sciences, 22(9), 5021. https://doi.org/10.3390/ijms22095021