A New Protocol of Computer-Assisted Image Analysis Highlights the Presence of Hemocytes in the Regenerating Cephalic Tentacles of Adult Pomacea canaliculata
Abstract
1. Introduction
2. Results
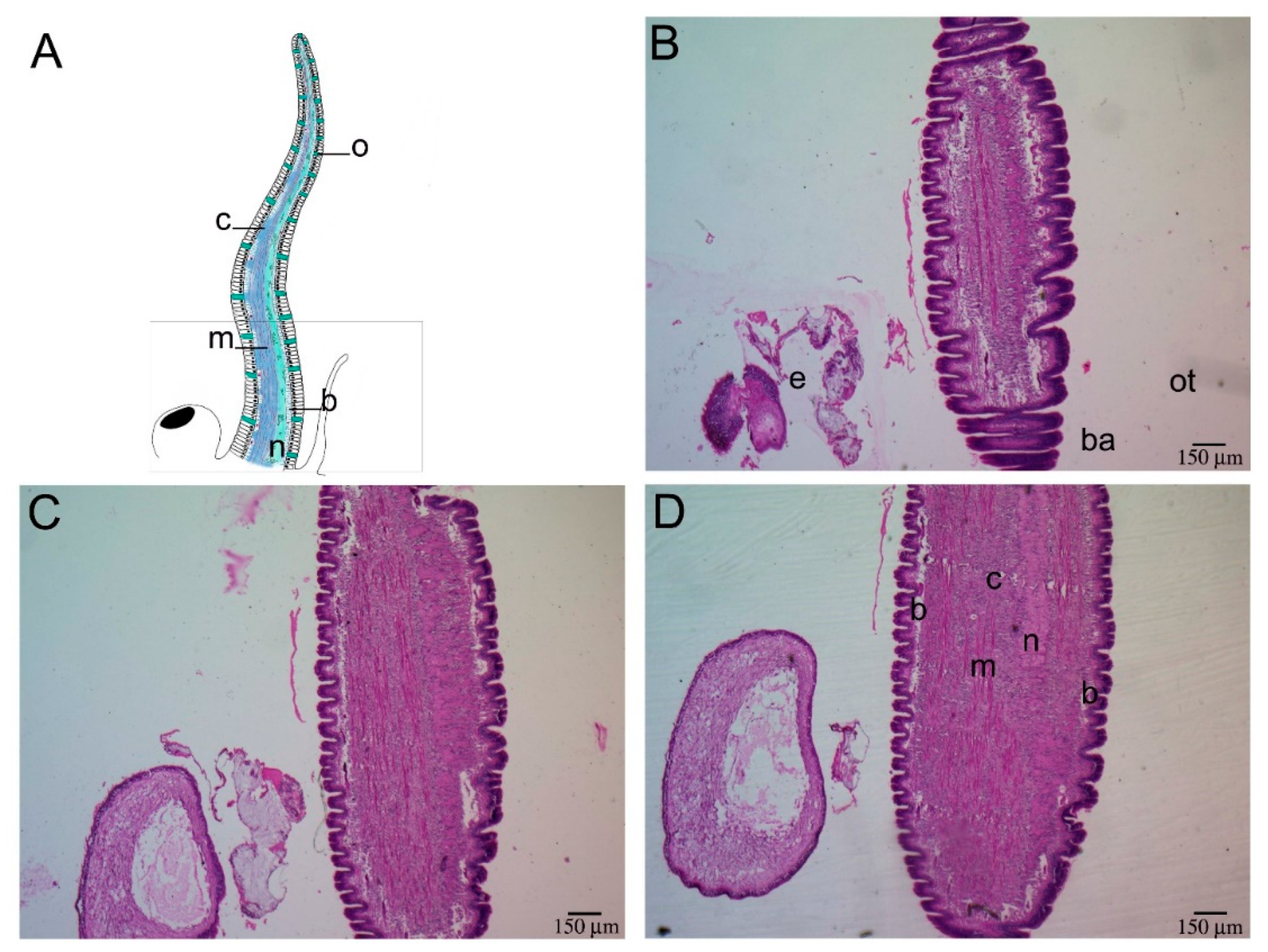
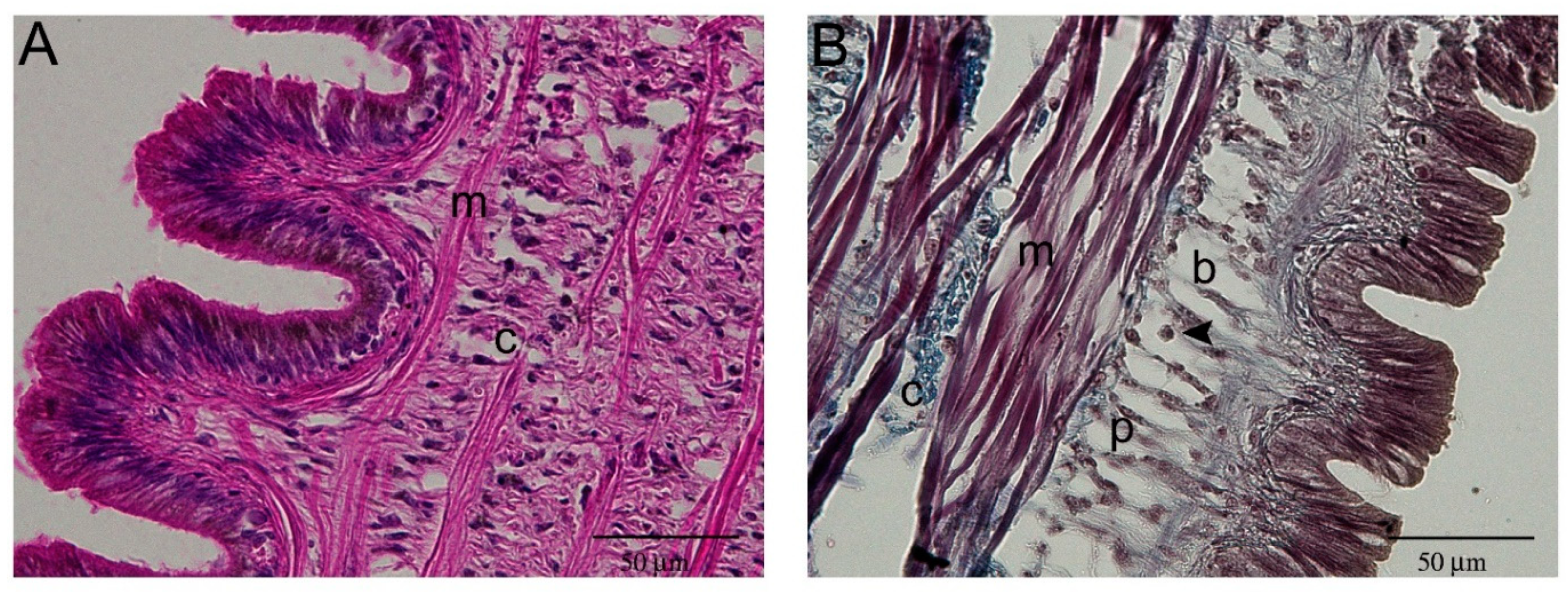
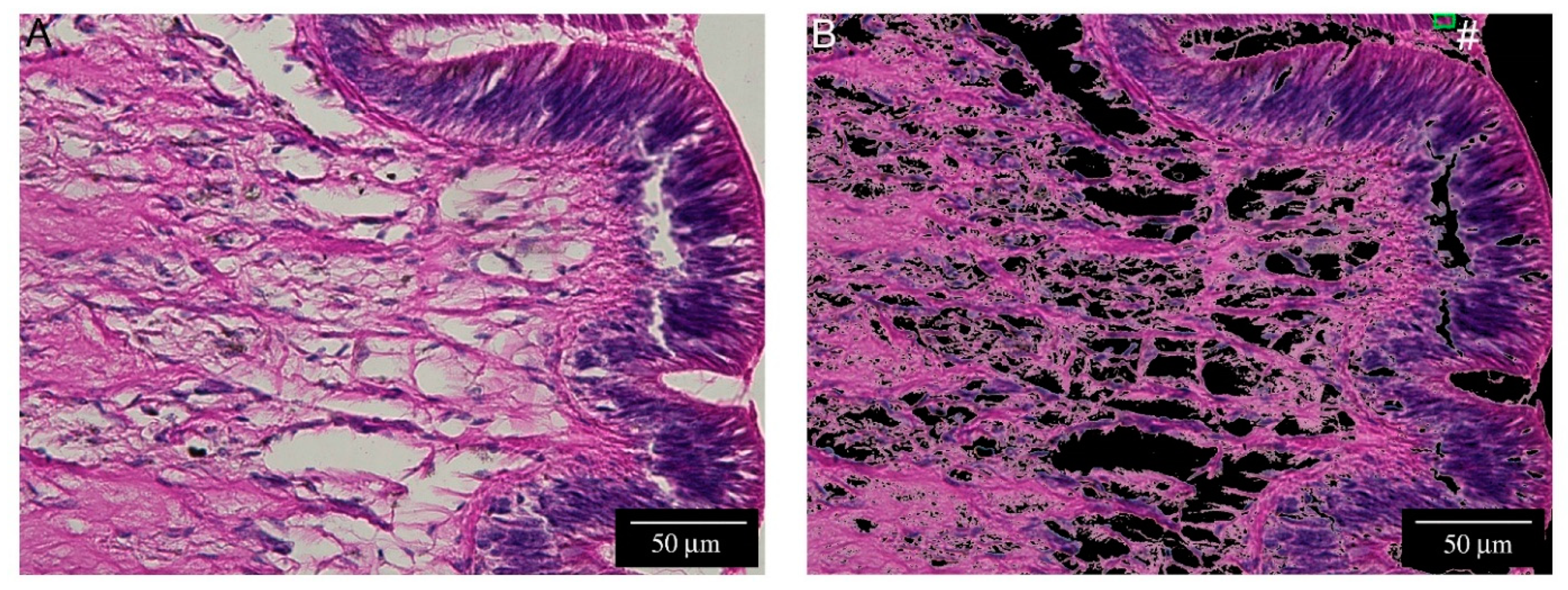
2.1. Histological Description of P. canaliculata Cephalic Tentacle Anatomy
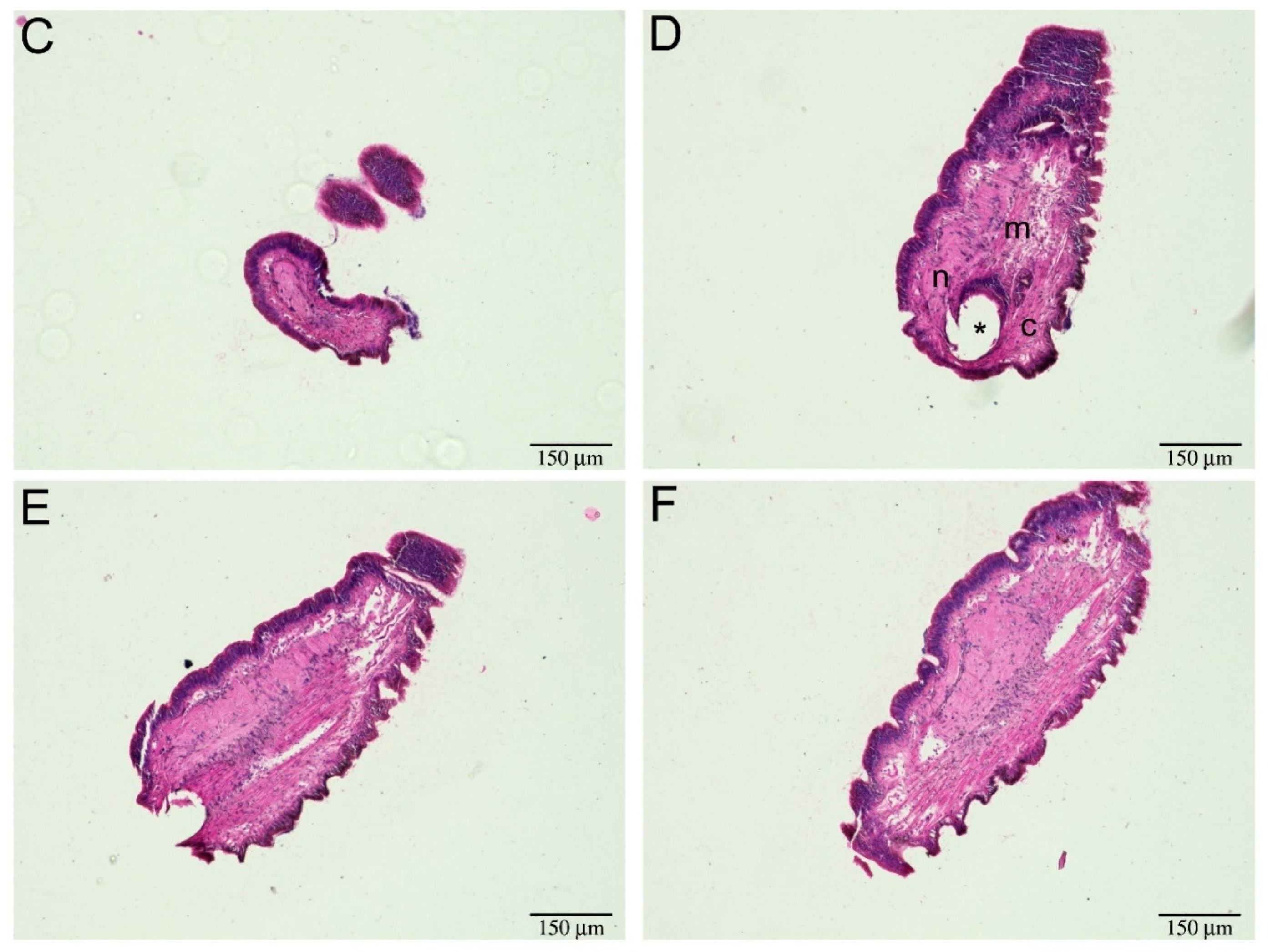
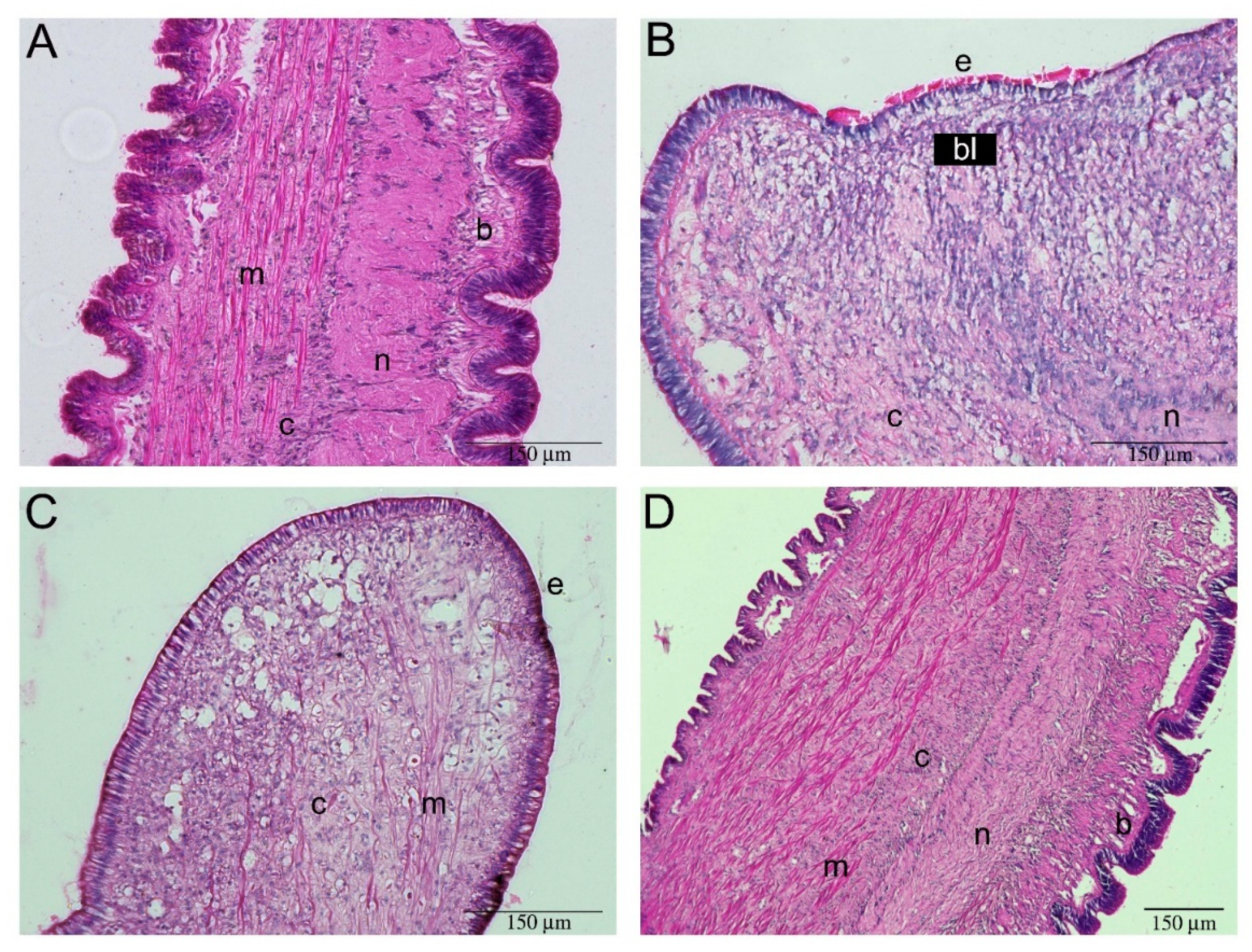
2.2. Tentacle Regeneration
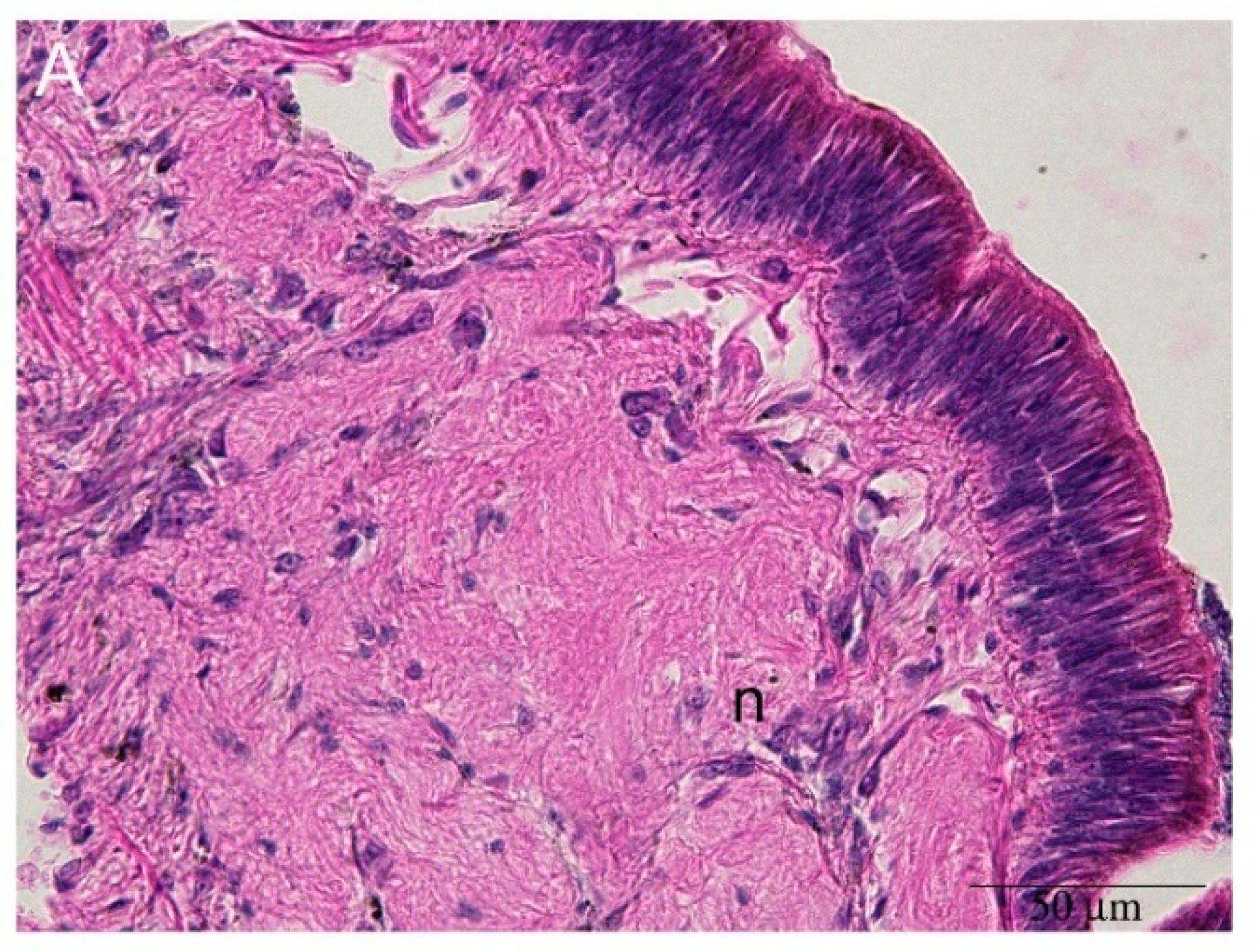
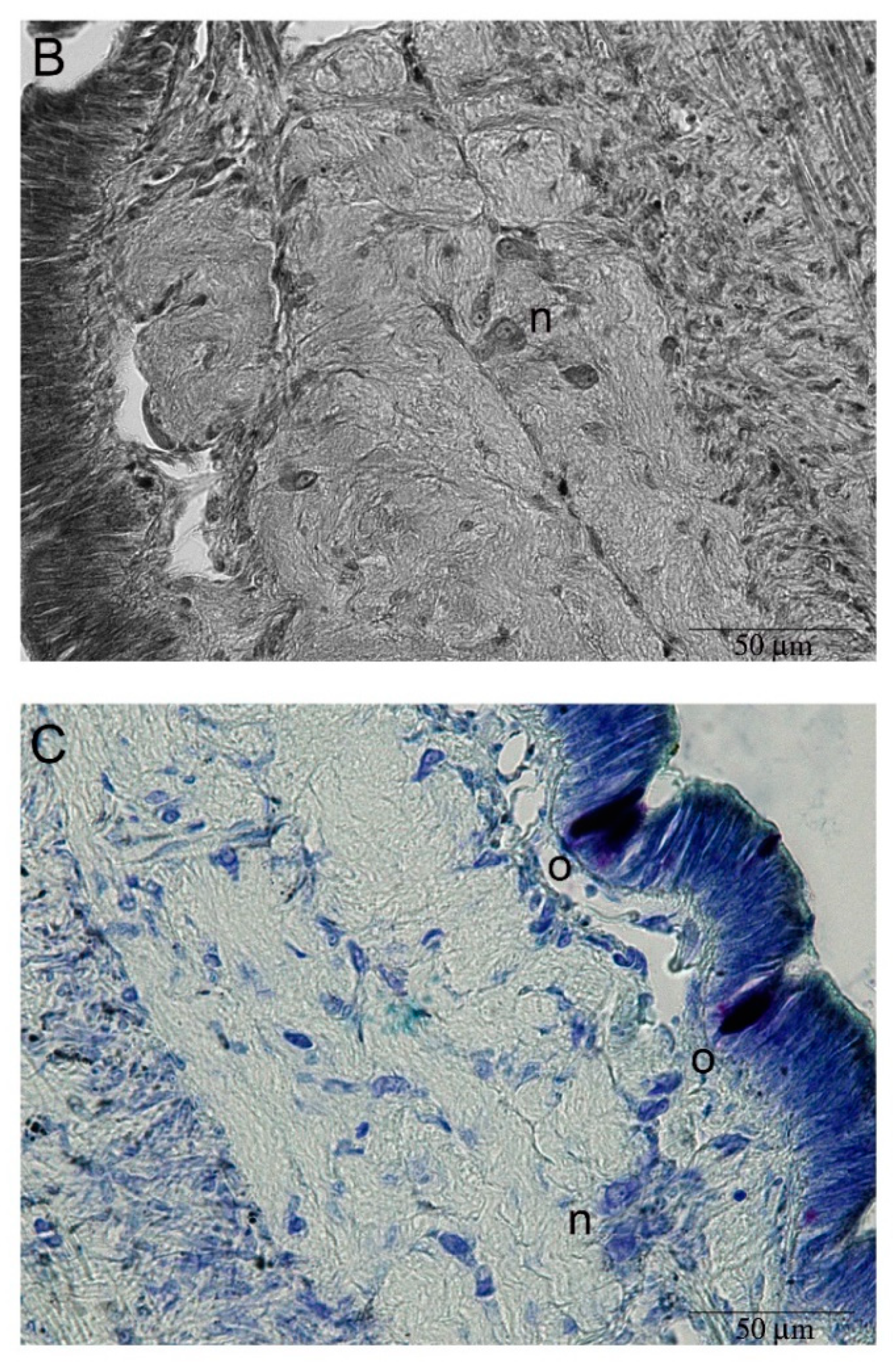
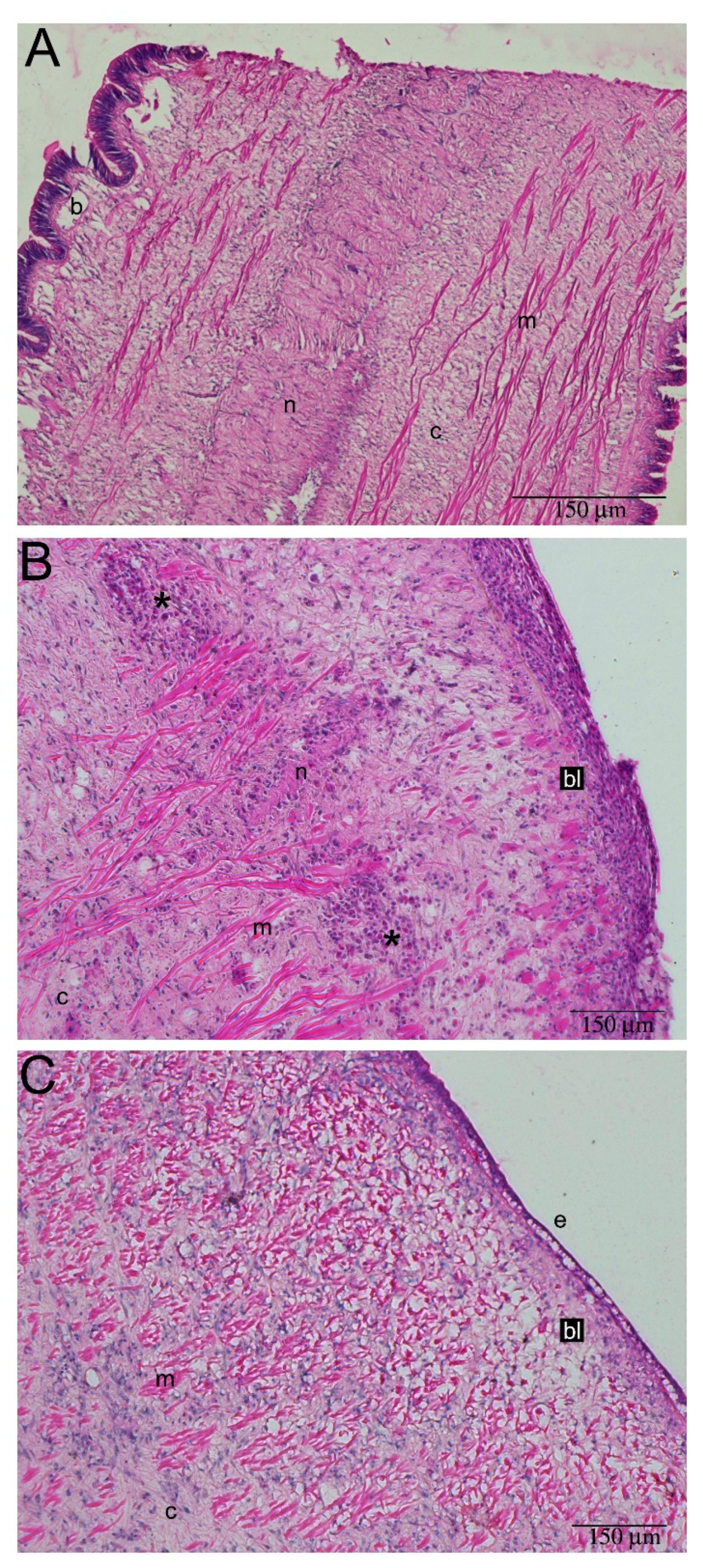
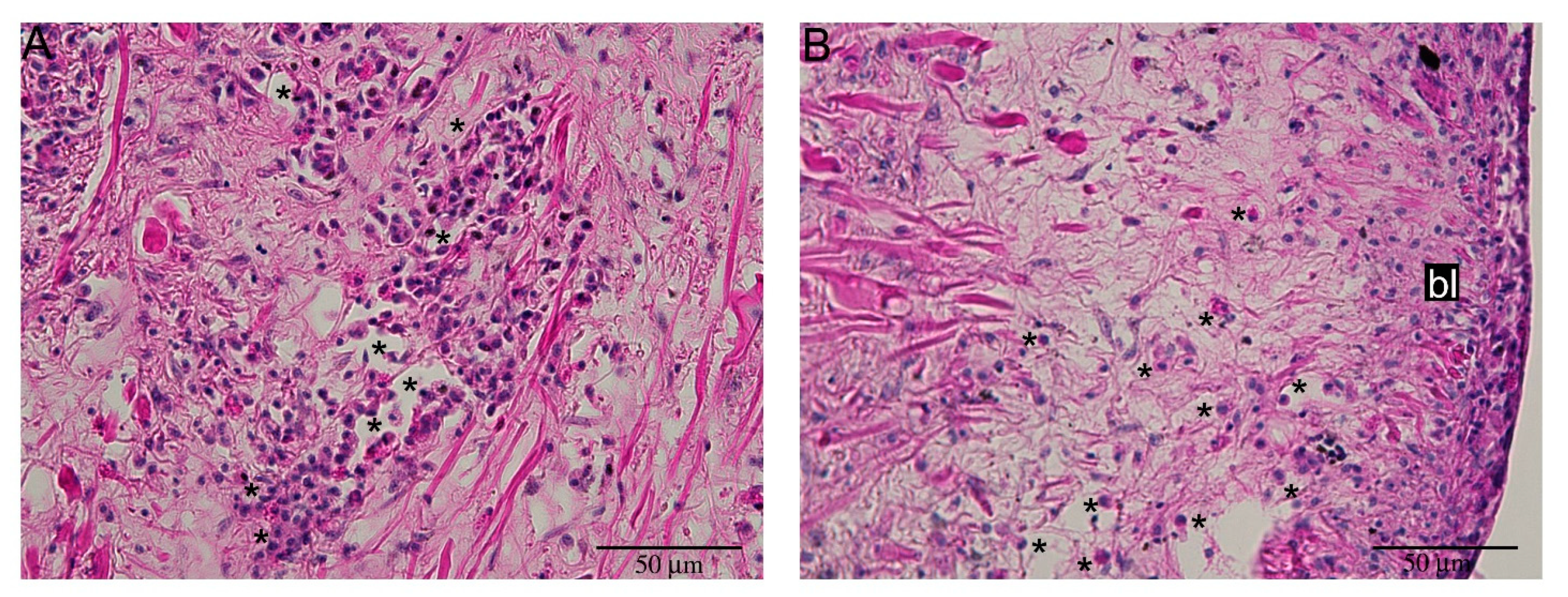
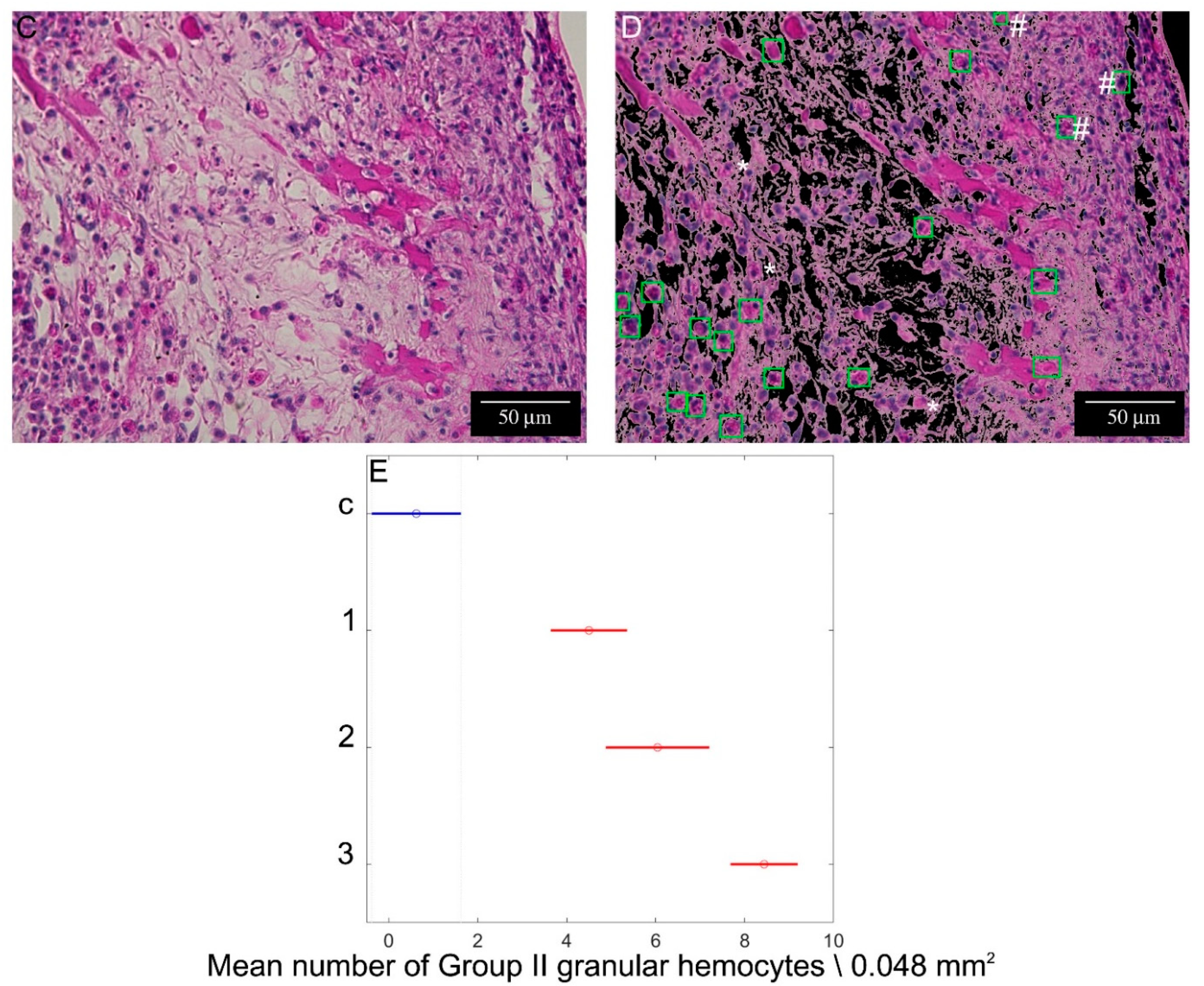
2.3. Hemocyte Identification during Early Blastema Formation
2.4. Automated Hemocyte Count in Control and 12 hpa Slides
3. Discussion
4. Materials and Methods
4.1. Animal Maintenance
4.2. Tentacle Amputation and Processing
4.3. Post-Surgery Maintenance and Blastema Collection
4.4. Histology
4.5. Image Acquisition and Analysis
4.6. Computer-Assisted Image Analysis
- Assembling a training image to model hemocytes.
- Applying MIA on the training image.
- Computer-assisted hemocyte count
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Wilson-Sanders, S.E. Invertebrate models for biomedical research, testing, and education. ILAR J. 2011, 52, 126–152. [Google Scholar] [CrossRef] [PubMed]
- Ferrario, C.; Sugni, M.; Somorjai, I.M.L.; Ballarin, L. Beyond Adult Stem Cells: Dedifferentiation as a Unifying Mechanism Underlying Regeneration in Invertebrate Deuterostomes. Front. Cell Dev. Biol. 2020, 8, 587320. [Google Scholar] [CrossRef] [PubMed]
- Gautam, D.K.; Chimata, A.V.; Gutti, R.K.; Paddibhatla, I. Comparative hematopoiesis and signal transduction in model organisms. J. Cell Physiol. 2021, 1–28. [Google Scholar]
- Andrews, P.L.R. Introduction: Laboratory invertebrates: Only spineless, or spineless and painless? ILAR J. 2011, 52, 121–125. [Google Scholar] [CrossRef]
- Bosch, T.C. Why polyps regenerate and we don’t: Towards a cellular and molecular framework for Hydra regeneration. Dev. Biol. 2007, 303, 421–433. [Google Scholar] [CrossRef]
- Duncan, E.M.; Sánchez Alvarado, A. Regulation of Genomic Output and (Pluri)potency in Regeneration. Annu. Rev. Genet. 2019, 53, 327–346. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, E.M.; Reddien, P.W. The cellular basis for animal regeneration. Dev. Cell 2011, 21, 172–185. [Google Scholar] [CrossRef]
- Giangrande, A.; Licciano, M. Regeneration and clonality in Metazoa. The price to pay for evolving complexity. Inv. Reprod. Dev. 2014, 58, 1–8. [Google Scholar] [CrossRef]
- Boidin-Wichlacz, C.; Vergote, D.; Slomianny, C.; Jouy, N.; Salzet, M.; Tasiemski, A. Morphological and functional characterization of leech circulating blood cells: Role in immunity and neural repair. Cell Mol. Life Sci. 2012, 69, 1717–1731. [Google Scholar] [CrossRef]
- Molnar, L.; Pollak, E.; Skopek, Z.; Gutt, E.; Kruk, J.; Morgan, A.J.; Plytycz, B. Immune system participates in brain regeneration and restoration of reproduction in the earthworm Dendrobaena veneta. Dev. Comp. Immunol. 2015, 52, 269–279. [Google Scholar] [CrossRef]
- Franchini, A.; Ottaviani, E. Repair of molluscan tissue injury: Role of PDGF and TGF-beta1. Tissue Cell 2000, 32, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Farr, M.; Zhu, D.F.; Povelones, M.; Valcich, D.; Ambron, R.T. Direct interactions between immunocytes and neurons after axotomy in Aplysia. J. Neurobiol. 2001, 46, 89–96. [Google Scholar] [CrossRef]
- Hermann, P.M.; Nicol, J.J.; Bulloch, A.G.M.; Wildering, W.C. RGD-dependent mechanisms in the endoneurial phagocyte response and axonal regeneration in the nervous system of the snail Lymnaea stagnalis. J. Exp. Biol. 2008, 211, 491–501. [Google Scholar] [CrossRef][Green Version]
- Moffett, S.B. Neural regeneration in gastropod molluscs. Prog. Neurobiol. 1995, 46, 289–330. [Google Scholar] [CrossRef]
- Matsuo, R.; Ito, E. Spontaneous regeneration of the central nervous system in gastropods. Biol. Bull. 2011, 221, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Hermann, P.M.; Nicol, J.J.; Nagle, G.T.; Bulloch, A.G.M.; Wildering, W.C. Epidermal growth factor-dependent enhancement of axonal regeneration in the pond snail Lymnaea stagnalis: Role of phagocyte survival. J. Comp. Neurol. 2005, 492, 383–400. [Google Scholar] [CrossRef]
- Malagoli, D. Going beyond a static picture: The apple snail Pomacea canaliculata can tell us the life history of molluscan hemocytes. Invertbr. Surv. J. 2018, 15, 61–65. [Google Scholar]
- Liu, Q.; Zhao, L.L.; Yang, S.; Zhang, J.E.; Zhao, N.Q.; Wu, H.; He, T.; Yan, T.M.; Guo, J. Regeneration of excised shell by the invasive apple snail Pomacea canaliculata. Mar. Freshw. Behav. Physiol. 2017, 50, 17–29. [Google Scholar] [CrossRef]
- Bever, M.M.; Borgens, R.B. Eye regeneration in the Mystery snail. J. Exp. Zool. 1988, 245, 33–42. [Google Scholar] [CrossRef]
- Accorsi, A.; Ross, E.; Ottaviani, E.; Sanchez-Alvarado, A. Pomacea canaliculata: A new model system for studying development and regeneration of complex eyes. J. Histochem. 2017, 61, 11. [Google Scholar]
- Accorsi, A.; Ross, E.; McClain, M.; McKinney, S.; Sánchez Alvarado, A. Eur J of Histochemistry Complete Regeneration of a Camera-type Eye in the Research Organism Pomacea canaliculata. FASEB J. 2018, 32, 232.4. [Google Scholar]
- Accorsi, A. Soluble Factors in the Immune-Neuroendocrine System of Invertebrate Models. Ph.D. Thesis, University of Modena and Reggio Emilia, Modena, Italy, 2015. Available online: https://morethesis.unimore.it/theses/available/etd-03122015-083844/ (accessed on 26 April 2021).
- Zaitseva, O.V. Structural organization of receptor elements and organs of the land mollusk Pomatia elegans. Neurosci. Behav. Physiol. 1997, 27, 533–540. [Google Scholar] [CrossRef]
- Zaitseva, O.V. Structure of sensory organs and skin innervation in the mollusc Pomacea paludosa (Prosobranchia). J. Evol. Biochem. Physiol. 1998, 34, 233–242. [Google Scholar]
- Accorsi, A.; Bucci, L.; de Eguileor, M.; Ottaviani, E.; Malagoli, D. Comparative analysis of circulating hemocytes of the freshwater snail Pomacea canaliculata. Fish. Shellfish Immunol. 2013, 34, 1260–1268. [Google Scholar] [CrossRef]
- Scharrer, B.; Paemen, L.; Smith, E.M.; Hughes, T.K.; Lui, Y.; Pope, M.; Stefano, G.B. The presence and effects of mammalian signal molecules in immunocytes of the insect Leucophaea maderae. Cell Tissue Res. 1996, 283, 93–97. [Google Scholar] [CrossRef]
- Malagoli, D.; Franchini, A.; Ottaviani, E. Synergistic role of cAMP and IP(3) in corticotropin-releasing hormone-induced cell shape changes in invertebrate immunocytes. Peptides 2000, 21, 175–182. [Google Scholar] [CrossRef]
- Radtke, A.J.; Kandov, E.; Lowekamp, B.; Speranza, E.; Chu, C.J.; Gola, A.; Thakur, N.; Shih, R.; Yao, L.; Yaniv, Z.R.; et al. IBEX: A versatile multiplex optical imaging approach for deep phenotyping and spatial analysis of cells in complex tissues. Proc. Natl. Acad. Sci. USA 2020, 117, 33455–33465. [Google Scholar] [CrossRef]
- Tokmakova, A.S.; Serebryakova, M.K.; Prokhorova, E.E.; GL Ataev, G.L. Study of the proliferative activity of hemolymph cells in pulmonate molluscs. ISJ Invertebr. Surv. J. 2020, 17, 63–74. [Google Scholar]
- Rodriguez, C.; Prieto, G.I.; Vega, I.A.; Castro-Vazquez, A. Assessment of the kidney and lung as immune barriers and hematopoietic sites in the invasive apple snail Pomacea canaliculata. PeerJ 2018, 6, e5789. [Google Scholar] [CrossRef]
- Boraldi, F.; Lofaro, F.D.; Accorsi, A.; Ross, E.; Malagoli, D. Toward the Molecular Deciphering of Pomacea canaliculata Immunity: First Proteomic Analysis of Circulating Hemocytes. Proteomics 2019, 19, 1800314. [Google Scholar] [CrossRef]
- Carpenter, A.E.; Jones, T.R.; Lamprecht, M.R.; Clarke, C.; Kang, I.H.; Friman, O.; Guertin, D.A.; Chang, J.H.; Lindquist, R.A.; Moffat, J.; et al. CellProfiler: Image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 2006, 7, 100. [Google Scholar] [CrossRef]
- Wilson, J.M.; Laurent, P. Fish Gill Morphology: Inside Out. J. Exp. Zool. 2002, 293, 192–213. [Google Scholar] [CrossRef]
- De Luca, M.; Aiuti, A.; Cossu, G.; Parmar, M.; Pellegrini, G.; Robey, P.G. Advances in stem cell research and therapeutic development. Nat. Cell Biol. 2019, 21, 801–811. [Google Scholar] [CrossRef]
- Zullo, L.; Bozzo, M.; Daya, A.; Di Clemente, A.; Mancini, F.P.; Megighian, A.; Nesher, N.; Röttinger, E.; Shomrat, T.; Tiozzo, S.; et al. The Diversity of Muscles and Their Regenerative Potential across Animals. Cells 2020, 19, 1925. [Google Scholar] [CrossRef]
- Montanari, A.; Bergamini, G.; Ferrari, A.; Ferri, A.; Nasi, M.; Roberto Simonini, R.; Malagoli, D. The Immune Response of the Invasive Golden Apple Snail to a Nematode-Based Molluscicide Involves Different Organs. Biology 2020, 9, 371. [Google Scholar] [CrossRef]
- Accorsi, A.; Benatti, S.; Ross, E.; Nasi, M.; Malagoli, D. A prokineticin-like protein responds to immune challenges in the gastropod pest Pomacea canaliculata. Dev. Comp. Immunol. 2017, 72, 37–43. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Y.; Ren, Y.; Wang, H.; Li, S.; Jiang, F.; Yin, L.; Qiao, X.; Zhang, G.; Qian, W.; et al. The genome of the golden apple snail Pomacea canaliculata provides insight into stress tolerance and invasive adaptation. Gigascience 2018, 7, 101. [Google Scholar] [CrossRef]
- Rodriguez, C.; Simon, V.; Conget, P.; Vega, I.A. Both quiescent and proliferating cells circulate in the blood of the invasive apple snail Pomacea canaliculata. Fish. Shellfish Immunol. 2020, 107, 95–103. [Google Scholar] [CrossRef]
- Accorsi, A.; Ottaviani, E.; Malagoli, D. Effects of repeated hemolymph withdrawals on the hemocyte populations and hematopoiesis in Pomacea canaliculata. Fish. Shellfish Immunol. 2014, 38, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Grimm-Jørgensen, Y. Somatostatin and calcitonin stimulate neurite regeneration of molluscan neurons in vitro. Brain Res. 1987, 403, 121–126. [Google Scholar] [CrossRef]
- Mount, A.S.; Wheeler, A.P.; Paradkar, R.P.; Snider, D. Hemocyte-mediated shell mineralization in the eastern oyster. Science 2004, 304, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, S.; Liu, Y.; Liu, C.; Xie, L.; Zhang, R. Hemocytes in the extrapallial space of Pinctada fucata are involved in immunity and biomineralization. Sci. Rep. 2018, 8, 4657. [Google Scholar] [CrossRef] [PubMed]
- Croq, F.; Vizioli, J.; Tuzova, M.; Tahtouh, M.; Sautiere, P.E.; Van Camp, C.; Salzet, M.; Cruikshank, W.W.; Pestel, J.; Lefebvre, C. A homologous form of human interleukin 16 is implicated in microglia recruitment following nervous system injury in leech Hirudo medicinalis. Glia 2010, 58, 1649–1962. [Google Scholar] [CrossRef] [PubMed]
- Rodet, F.; Tasiemski, A.; Boidin-Wichlacz, C.; Van Camp, C.; Vuillaume, C.; Slomianny, C.; Salzet, M. Hm-MyD88 and Hm-SARM: Two key regulators of the neuroimmune system and neural repair in the medicinal leech. Sci. Rep. 2015, 5, 9624. [Google Scholar] [CrossRef] [PubMed]
- Benton, J.L.; Kery, R.; Li, J.; Noonin, C.; Söderhäll, I.; Beltz, B.S. Cells from the immune system generate adult-born neurons in crayfish. Dev. Cell 2014, 30, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Brenneis, G.; Beltz, B.S. Adult neurogenesis in crayfish: Origin, expansion, and migration of neural progenitor lineages in a pseudostratified neuroepithelium. J. Comp. Neurol. 2020, 528, 1459–1485. [Google Scholar] [CrossRef] [PubMed]
- Ottaviani, E.; Accorsi, A.; Rigillo, G.; Malagoli, D.; Blom, J.M.; Tascedda, F. Epigenetic modification in neurons of the mollusc Pomacea canaliculata after immune challenge. Brain Res. 2013, 1537, 18–26. [Google Scholar] [CrossRef]
- Prats-Montalbána, J.M.; de Juanb, A.; Ferrera, A. Multivariate image analysis: A review with applications. Chemom. Intell. Lab. Syst. 2011, 107, 1–2. [Google Scholar] [CrossRef]
- Nur, B.A.M.; Nurashikin, A.F.; Syed, K.A.; Aidil, A.Z.A.; Zaipatimah, A.; Wong, B.Y.; Zainul, A.M.S. Image processing of an agriculture produce: Determination of size and ripeness of a banana. In Proceedings of the 2008 International Symposium on Information Technology, Kuala Lumpur, Malaysia, 26–28 August 2008; pp. 1–7. [Google Scholar]
- MATLAB© Image Processing Toolbox Release 2020a–User’s Guide: Chapter 10 Page 41:45. Available online: https://www.mathworks.com/help/pdf_doc/images/images_ug.pdf (accessed on 26 April 2021).
- Li Vigni, M.; Prats-Montalban, J.M.; Ferrer, A.; Cocchi, M. Coupling 2D-wavelet decomposition and multivariate image analysis (2D WT-MIA). J. Chemom. 2017, 32, 2970. [Google Scholar] [CrossRef]
- Driscoll, W.C. Robustness of the ANOVA and Tukey-Kramer Statistical Tests. Comput. Ind. Eng. 1996, 31, 265–268. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bergamini, G.; Ahmad, M.; Cocchi, M.; Malagoli, D. A New Protocol of Computer-Assisted Image Analysis Highlights the Presence of Hemocytes in the Regenerating Cephalic Tentacles of Adult Pomacea canaliculata. Int. J. Mol. Sci. 2021, 22, 5023. https://doi.org/10.3390/ijms22095023
Bergamini G, Ahmad M, Cocchi M, Malagoli D. A New Protocol of Computer-Assisted Image Analysis Highlights the Presence of Hemocytes in the Regenerating Cephalic Tentacles of Adult Pomacea canaliculata. International Journal of Molecular Sciences. 2021; 22(9):5023. https://doi.org/10.3390/ijms22095023
Chicago/Turabian StyleBergamini, Giulia, Mohamad Ahmad, Marina Cocchi, and Davide Malagoli. 2021. "A New Protocol of Computer-Assisted Image Analysis Highlights the Presence of Hemocytes in the Regenerating Cephalic Tentacles of Adult Pomacea canaliculata" International Journal of Molecular Sciences 22, no. 9: 5023. https://doi.org/10.3390/ijms22095023
APA StyleBergamini, G., Ahmad, M., Cocchi, M., & Malagoli, D. (2021). A New Protocol of Computer-Assisted Image Analysis Highlights the Presence of Hemocytes in the Regenerating Cephalic Tentacles of Adult Pomacea canaliculata. International Journal of Molecular Sciences, 22(9), 5023. https://doi.org/10.3390/ijms22095023






