The Micro-Immunotherapy Medicine 2LEID Exhibits an Immunostimulant Effect by Boosting Both Innate and Adaptive Immune Responses
Abstract
:1. Introduction
2. Results
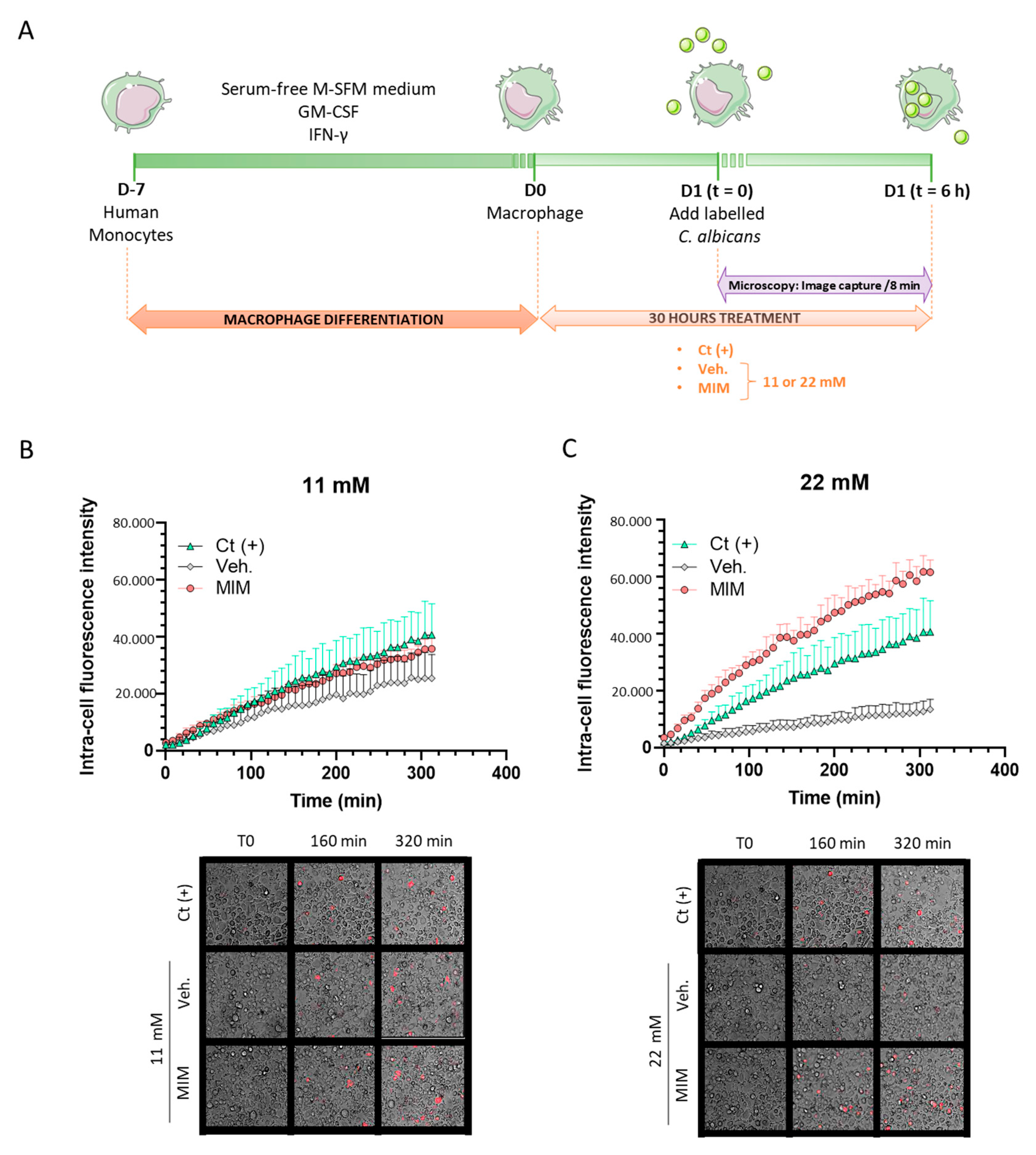
2.1. MIM Stimulates the Phagocytosis Capabilities of Macrophages In Vitro
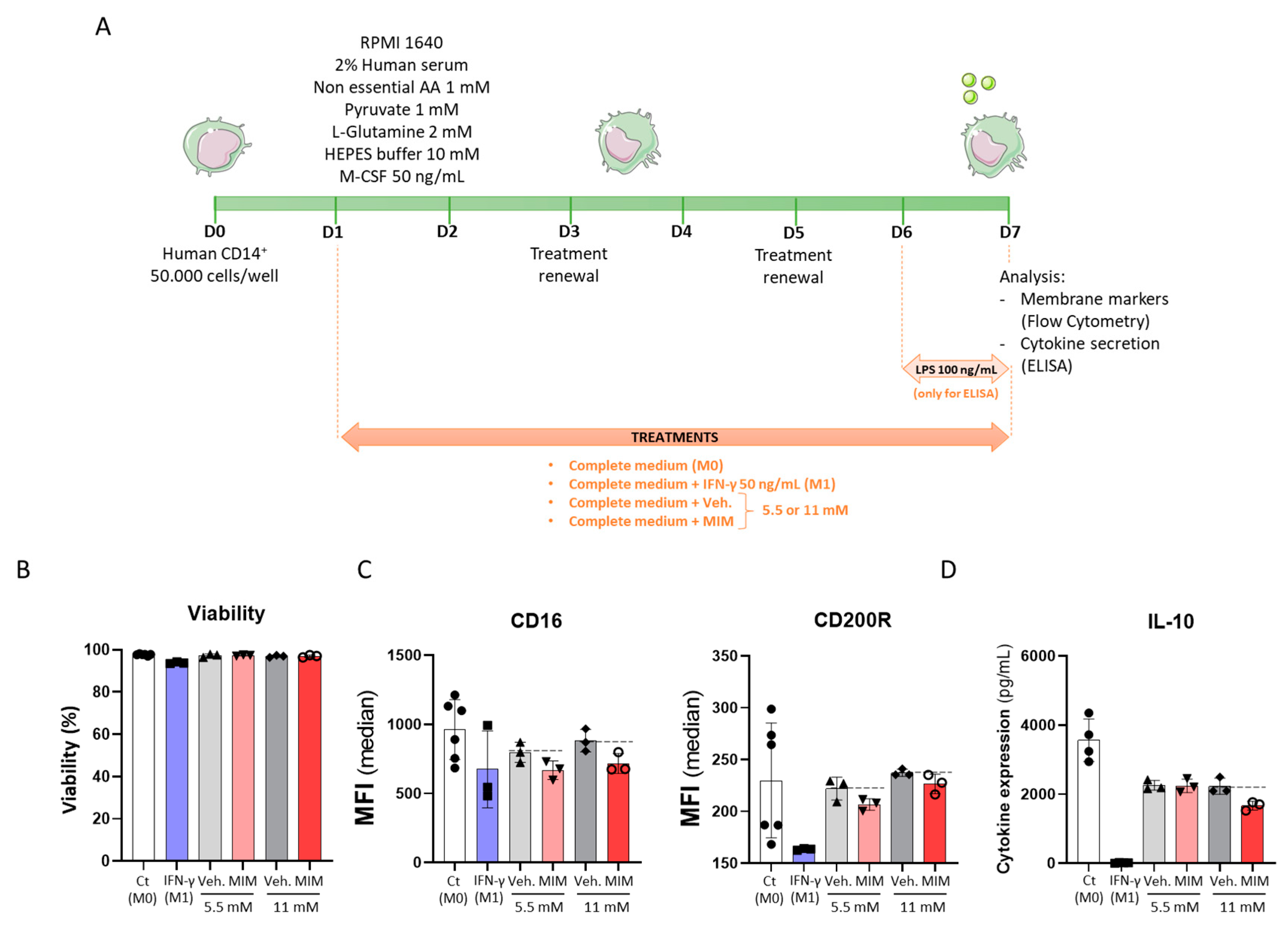
2.2. MIM Reduces the Expression of Cell Surface Markers on CD14+-Derived Macrophages and the Secretion of Anti-Inflammatory Cytokines without Affecting Cell Viability
2.3. Preventive Treatment MIM Improves Antibody Response at Both Local and Systemic Levels in Influenza A Virus-Induced Respiratory Infection
2.4. MIM Increases the Proliferation and the Activation of PBMCs In Vitro
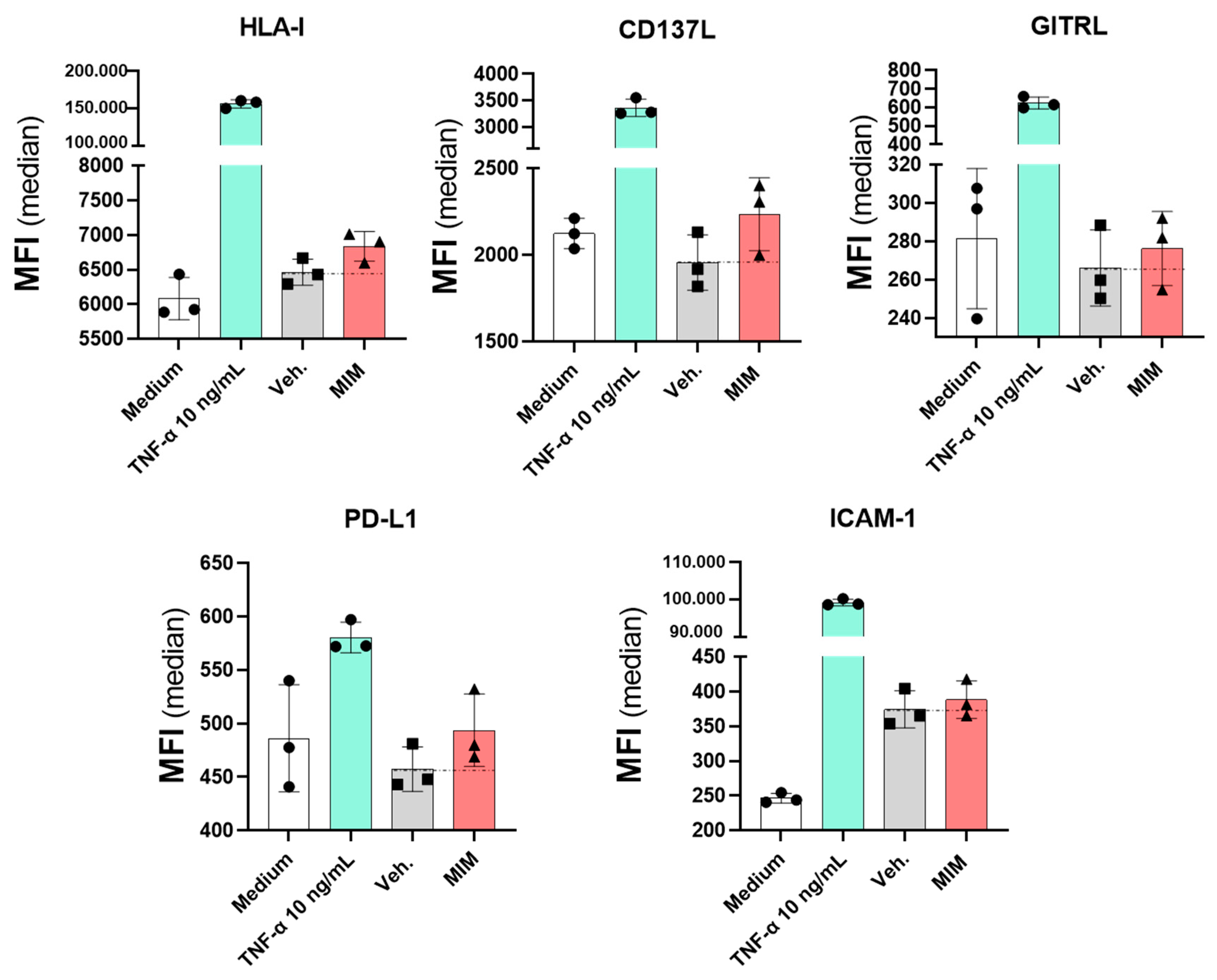
2.5. MIM Modulates the Expression of Endothelial Cell Surface Markers In Vitro
3. Discussion
4. Materials and Methods
4.1. Tested Items
4.2. Phagocytosis Capability Assessment Experiments
4.3. Macrophage Cell Surface Marker Expression and Cytokine Secretion Evaluation
4.4. In Vivo Experiments
4.5. Blood Collection
4.6. Plasmatic Concentrations of Immunoglobulins Assessment
4.7. Bronchoalveolar Lavage Fluid Differential Leukocyte Counts
4.8. Viral Load Determination
4.9. Evaluation of Cell Surface Activation Marker Expression and Proliferation of PBMC Sub-Populations by Flow Cytometry
4.10. HUVEC Immunoprofiling by Flow Cytometry
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Dasaraju, P.V.; Liu, C. Infections of the Respiratory System. In Med. Microbiol., 4th ed.; Baron, S., Ed.; University of Texas, Medical Branch at Galveston: Galveston, TX, USA, 1996. Available online: http://www.ncbi.nlm.nih.gov/books/NBK8142/ (accessed on 10 November 2021).
- Fashner, J.; Ericson, K.; Werner, S. Treatment of the Common Cold in Children and Adults. Am. Fam. Phys. 2012, 86, 7. [Google Scholar]
- Heikkinen, T.; Ruuskanen, O. Upper respiratory tract infection. Encycl. Respir. Med. 2006, 385–388. [Google Scholar] [CrossRef]
- Simasek, M. Treatment of the Common Cold. Am. Fam. Phys. 2007, 75, 515–520. [Google Scholar] [PubMed]
- Segerstrom, S.C.; Miller, G.E. Psychological Stress and the Human Immune System: A Meta-Analytic Study of 30 Years of Inquiry. Psychol. Bull. 2004, 130, 601–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prunicki, M.; Cauwenberghs, N.; Lee, J.; Zhou, X.; Movassagh, H.; Noth, E.; Lurmann, F.; Hammond, S.K.; Balmes, J.R.; Desai, M.; et al. Air pollution exposure is linked with methylation of immunoregulatory genes, altered immune cell profiles, and increased blood pressure in children. Sci. Rep. 2021, 11, 4067. [Google Scholar] [CrossRef]
- Zhang, W.; Yu, H.; Shang, J.; Liu, T.; Ma, J.; Gu, X. Association between dietary habits and recurrent respiratory infection in children: A case–control study. J. Tradit. Chin. Med. Sci. 2015, 2, 105–110. [Google Scholar] [CrossRef] [Green Version]
- Haspel, J.A.; Anafi, R.; Brown, M.K.; Cermakian, N.; Depner, C.; Desplats, P.; Gelman, A.E.; Haack, M.; Jelic, S.; Kim, B.S.; et al. Perfect timing: Circadian rhythms, sleep, and immunity—An NIH workshop summary. JCI Insight 2020, 5, 131487. [Google Scholar] [CrossRef] [Green Version]
- Douglas, R.M.; Hemilä, H.; Chalker, E.; Treacy, B. Vitamin C for preventing and treating the common cold. Cochrane Database Syst. Rev. 2007, CD000980. [Google Scholar] [CrossRef] [Green Version]
- Kaufman, D.W.; Kelly, J.P.; Rosenberg, L.; Anderson, T.E.; Mitchell, A.A. Recent patterns of medication use in the ambulatory adult population of the United States: The Slone survey. JAMA 2002, 287, 337–344. [Google Scholar] [CrossRef] [Green Version]
- Thomas, G.; Cluzel, H.; Lafon, J.; Bruhwyler, J.; Lejeune, B. Efficacy of 2LPAPI®, a Micro-Immunotherapy Drug, in Patients with High-Risk Papillomavirus Genital Infection. Adv. Infect. Dis. 2016, 6, 7–14. [Google Scholar] [CrossRef] [Green Version]
- Floris, I.; Chenuet, P.; Togbe, D.; Volteau, C.; Lejeune, B. Potential Role of the Micro-Immunotherapy Medicine 2LALERG in the Treatment of Pollen-Induced Allergic Inflammation. Dose-Response 2020, 18, 1559325820914092. [Google Scholar] [CrossRef] [Green Version]
- Floris, I.; García-González, V.; Palomares, B.; Appel, K.; Lejeune, B. The Micro-Immunotherapy Medicine 2LARTH® Reduces Inflammation and Symptoms of Rheumatoid Arthritis In Vivo. Int. J. Rheumatol. 2020, 2020, 1594573. [Google Scholar] [CrossRef] [Green Version]
- Jacques, C.; Floris, I.; Lejeune, B. Ultra-Low Dose Cytokines in Rheumatoid Arthritis, Three Birds with One Stone as the Rationale of the 2LARTH® Micro-Immunotherapy Treatment. Int. J. Mol. Sci. 2021, 22, 6717. [Google Scholar] [CrossRef] [PubMed]
- Lilli, N.L.; Révy, D.; Robelet, S.; Lejeune, B. Effect of the micro-immunotherapy medicine 2LPARK® on rat primary dopaminergic neurons after 6-OHDA injury: Oxidative stress and survival evaluation in an in vitro model of Parkinson’s disease. Degener. Neurol. Neuromuscul. Dis. 2019, 9, 79–88. [Google Scholar] [CrossRef] [Green Version]
- Floris, I.; Rose, T.; Rojas, J.A.C.; Appel, K.; Roesch, C.; Lejeune, B. Pro-Inflammatory Cytokines at Ultra-Low Dose Exert Anti-Inflammatory Effect In Vitro: A Possible Mode of Action Involving Sub-Micron Particles? Dose-Response 2020, 18, 1559325820961723. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.-Q.; Zhang, D.-F.; Tu, E.; Chen, Q.-M.; Chen, W. The mucosal immune system in the oral cavity—an orchestra of T cell diversity. Int. J. Oral. Sci. 2014, 6, 125–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hovav, A.-H. Dendritic cells of the oral mucosa. Mucosal Immunol. 2014, 7, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Pechkovsky, D.V.; Potapnev, M.P.; Zalutskaya, O.M. Different patterns of cytokine regulation of phagocytosis and bacterial killing by human neutrophils. Int. J. Antimicrob. Agents 1996, 7, 33–40. [Google Scholar] [CrossRef]
- Paludan, S.R. Synergistic action of pro-inflammatory agents: Cellular and molecular aspects. J. Leukoc. Biol. 2000, 67, 18–25. [Google Scholar] [CrossRef] [Green Version]
- Wesemann, D.R.; Benveniste, E.N. STAT-1α and IFN-γ as Modulators of TNF-α Signaling in Macrophages: Regulation and Functional Implications of the TNF Receptor 1:STAT-1α Complex. J. Immunol. 2003, 171, 5313–5319. [Google Scholar] [CrossRef] [Green Version]
- Bradley, L.M.; Dalton, D.K.; Croft, M. A direct role for IFN-gamma in regulation of Th1 cell development. J. Immunol. 1996, 157, 1350–1358. [Google Scholar] [PubMed]
- Kak, G.; Raza, M.; Tiwari, B.K. Interferon-gamma (IFN-γ): Exploring its implications in infectious diseases. Biomol. Concepts 2018, 9, 64–79. [Google Scholar] [CrossRef] [PubMed]
- Mily, A.; Kalsum, S.; Loreti, M.G.; Rekha, R.S.; Muvva, J.R.; Lourda, M.; Brighenti, S. Polarization of M1 and M2 Human Monocyte-Derived Cells and Analysis with Flow Cytometry upon Mycobacterium tuberculosis Infection. J. Vis. Exp. 2020, 163, 61807. [Google Scholar] [CrossRef]
- Byrne, A.J.; Mathie, S.A.; Gregory, L.G.; Lloyd, C.M. Pulmonary macrophages: Key players in the innate defence of the airways. Thorax 2015, 70, 1189–1196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chacón-Salinas, R.; Serafín-López, J.; Ramos-Payán, R.; Méndez-Aragón, P.; Hernández-Pando, R.; Soolingen, D.V.; Flores-Romo, L.; Estrada-Parra, S.; Estrada-García, I. Differential pattern of cytokine expression by macrophages infected in vitro with different Mycobacterium tuberculosis genotypes. Clin. Exp. Immunol. 2005, 140, 443–449. [Google Scholar] [CrossRef]
- Riha, P.; Rudd, C.E. CD28 co-signaling in the adaptive immune response. Self Nonself 2010, 1, 231–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsoukas, C.D.; Landgraf, B.; Bentin, J.; Valentine, M.; Lotz, M.; Vaughan, J.H.; Carson, D.A. Activation of resting T lymphocytes by anti-CD3 (T3) antibodies in the absence of monocytes. J. Immunol. 1985, 135, 1719–1723. [Google Scholar] [PubMed]
- Ahout, I.M.L.; Jans, J.; Haroutiounian, L.; Simonetti, E.R.; van der Gaast-de Jongh, C.; Diavatopoulos, D.A.; de Jonge, M.I.; de Groot, R.; Ferwerda, G. Reduced Expression of HLA-DR on Monocytes During Severe Respiratory Syncytial Virus Infections. Pediatr. Infect. Dis. J. 2016, 35, e89. [Google Scholar] [CrossRef]
- Shao, Y.; Saredy, J.; Yang, W.Y.; Sun, Y.; Lu, Y.; Saaoud, F.; Drummer, C.; Johnson, C.; Xu, K.; Jiang, X.; et al. Vascular Endothelial Cells and Innate Immunity. Arterioscler. Thromb. Vasc. Biol. 2020, 40, e138–e152. [Google Scholar] [CrossRef]
- Wedgwood, J.F.; Hatam, L.; Bonagura, V.R. Effect of interferon-gamma and tumor necrosis factor on the expression of class I and class II major histocompatibility molecules by cultured human umbilical vein endothelial cells. Cell. Immunol. 1988, 111, 1–9. [Google Scholar] [CrossRef]
- Wang, X.; Yang, L.; Huang, F.; Zhang, Q.; Liu, S.; Ma, L.; You, Z. Inflammatory cytokines IL-17 and TNF-α up-regulate PD-L1 expression in human prostate and colon cancer cells. Immunol. Lett. 2017, 184, 7–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.-W.; Sung, H.-C.; Lin, S.-R.; Wu, C.-W.; Lee, C.-W.; Lee, I.-T.; Yang, Y.-F.; Yu, I.-S.; Lin, S.-W.; Chiang, M.-H.; et al. Resveratrol attenuates ICAM-1 expression and monocyte adhesiveness to TNF-α-treated endothelial cells: Evidence for an anti-inflammatory cascade mediated by the miR-221/222/AMPK/p38/NF-κB pathway. Sci. Rep. 2017, 7, 44689. [Google Scholar] [CrossRef] [PubMed]
- Byrne, A.M.; Goleva, E.; Chouiali, F.; Kaplan, M.H.; Hamid, Q.A.; Leung, D.Y.M. Induction of GITRL expression in human keratinocytes by Th2 cytokines and TNF-α: Implications for atopic dermatitis. Clin. Exp. Allergy 2012, 42, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Small, A.G.; King, J.R.; Rathjen, D.A.; Ferrante, A. The Role of Phagocytes in Immunity to Candida albicans. In Candida Albicans; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef] [Green Version]
- Calabrese, E.J.; Giordano, J. Ultra low doses and biological amplification: Approaching Avogadro’s number. Pharmacol. Res. 2021, 170, 105738. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhou, S.; Sun, C.; Lei, T.; Peng, J.; Li, W.; Ding, P.; Lu, J.; Zhao, Y. Interferon-γ inhibits nonopsonized phagocytosis of macrophages via an mTORC1-c/EBPβ pathway. J. Innate Immun. 2015, 7, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity 2014, 41, 14–20. [Google Scholar] [CrossRef] [Green Version]
- Koning, N.; van Eijk, M.; Pouwels, W.; Brouwer, M.S.M.; Voehringer, D.; Huitinga, I.; Hoek, R.M.; Raes, G.; Hamann, J. Expression of the inhibitory CD200 receptor is associated with alternative macrophage activation. J. Innate Immun. 2010, 2, 195–200. [Google Scholar] [CrossRef] [Green Version]
- Goulding, J.; Godlee, A.; Vekaria, S.; Hilty, M.; Snelgrove, R.; Hussell, T. Lowering the threshold of lung innate immune cell activation alters susceptibility to secondary bacterial superinfection. J. Infect. Dis. 2011, 204, 1086–1094. [Google Scholar] [CrossRef] [Green Version]
- Zhong, H.; Bao, W.; Liu, Y.; Yazdanbakhsh, K. Inflammation Response Cytokines IFN-γ and IL-10 Regulate Monocyte Subset Differentiation. Blood 2019, 134, 3586. [Google Scholar] [CrossRef]
- Herrero, C.; Hu, X.; Li, W.P.; Samuels, S.; Sharif, M.N.; Kotenko, S.; Ivashkiv, L.B. Reprogramming of IL-10 activity and signaling by IFN-gamma. J. Immunol. 2003, 171, 5034–5041. [Google Scholar] [CrossRef] [Green Version]
- Geng, P.; Zhu, H.; Zhou, W.; Su, C.; Chen, M.; Huang, C.; Xia, C.; Huang, H.; Cao, Y.; Shi, X. Baicalin Inhibits Influenza A Virus Infection via Promotion of M1 Macrophage Polarization. Front. Pharmacol. 2020, 11, 1298. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, M.I.; Catalan-Dibene, J.; Zlotnik, A. B cells responses and cytokine production are regulated by their immune microenvironment. Cytokine 2015, 74, 318–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, S.; Ruprecht, R.M. Immunoglobulin M: An Ancient Antiviral Weapon—Rediscovered. Front. Immunol. 2020, 11, 1943. [Google Scholar] [CrossRef] [PubMed]
- Jayasekera, J.P.; Moseman, E.A.; Carroll, M.C. Natural antibody and complement mediate neutralization of influenza virus in the absence of prior immunity. J. Virol. 2007, 81, 3487–3494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tagliacarne, S.C.; Valsecchi, C.; Benazzo, M.; Nichelatti, M.; Marseglia, A.; Ciprandi, G.; Bernasconi, S. Low-dose multicomponent medication modulates humoral and cellular immune response in an ex-vivo study on children subjected to adenoid surgery. Immunol. Lett. 2018, 203, 95–101. [Google Scholar] [CrossRef]
- Lawlor, N.; Nehar-Belaid, D.; Grassmann, J.D.S.; Stoeckius, M.; Smibert, P.; Stitzel, M.L.; Pascual, V.; Banchereau, J.; Williams, A.; Ucar, D. Single Cell Analysis of Blood Mononuclear Cells Stimulated Through Either LPS or Anti-CD3 and Anti-CD28. Front. Immunol. 2021, 12, 636720. [Google Scholar] [CrossRef] [PubMed]
- Samstag, Y.; Emmrich, F.; Staehelin, T. Activation of human T lymphocytes: Differential effects of CD3- and CD8-mediated signals. Proc. Natl. Acad. Sci. USA 1988, 85, 9689–9693. [Google Scholar] [CrossRef] [Green Version]
- Borrego, F.; Peña, J.; Solana, R. Regulation of CD69 expression on human natural killer cells: Differential involvement of protein kinase C and protein tyrosine kinases. Eur. J. Immunol. 1993, 23, 1039–1043. [Google Scholar] [CrossRef]
- Ochiai, K.; Kagami, M.; Nakazawa, T.; Sugiyama, T.; Sueishi, M.; Ito, M.; Tomioka, H. Regulation of CD69 Expression on Eosinophil Precursors by Interferon-γ. Int. Arch. Allergy Immunol. 2000, 122, 28–32. [Google Scholar] [CrossRef]
- López-Cabrera, M.; Muñoz, E.; Blázquez, M.V.; Ursa, M.A.; Santis, A.G.; Sánchez-Madrid, F. Transcriptional regulation of the gene encoding the human C-type lectin leukocyte receptor AIM/CD69 and functional characterization of its tumor necrosis factor-alpha-responsive elements. J. Biol. Chem. 1995, 270, 21545–21551. [Google Scholar] [CrossRef] [Green Version]
- Voigt, J.; Hünniger, K.; Bouzani, M.; Jacobsen, I.D.; Barz, D.; Hube, B.; Löffler, J.; Kurzai, O. Human natural killer cells acting as phagocytes against Candida albicans and mounting an inflammatory response that modulates neutrophil antifungal activity. J. Infect. Dis. 2014, 209, 616–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hershman, M.J.; Appel, S.H.; Wellhausen, S.R.; Sonnenfeld, G.; Polk, H.C. Interferon-gamma treatment increases HLA-DR expression on monocytes in severely injured patients. Clin. Exp. Immunol. 1989, 77, 67–70. [Google Scholar] [PubMed]
- Kinter, A.L.; Godbout, E.J.; McNally, J.P.; Sereti, I.; Roby, G.A.; O’Shea, M.A.; Fauci, A.S. The common gamma-chain cytokines IL-2, IL-7, IL-15, and IL-21 induce the expression of programmed death-1 and its ligands. J. Immunol. 2008, 181, 6738–6746. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.; Song, B.; Liu, F.; Sun, D.; Wang, K.; Qu, H. TGF-β induces HLA-G expression through inhibiting miR-152 in gastric cancer cells. J. Biomed. Sci. 2015, 22, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, Y.; Tanigaki, T.; Heimer, D.; Wang, W.; Ross, W.G.; Murphy, G.A.; Sakai, A.; Sussman, H.H.; Vu, T.H.; Raffin, T.A. TGF-beta 1 causes increased endothelial ICAM-1 expression and lung injury. J. Appl. Physiol. 1994, 77, 1281–1287. [Google Scholar] [CrossRef]
- Romano, M.; Sironi, M.; Toniatti, C.; Polentarutti, N.; Fruscella, P.; Ghezzi, P.; Faggioni, R.; Luini, W.; van Hinsbergh, V.; Sozzani, S.; et al. Role of IL-6 and its soluble receptor in induction of chemokines and leukocyte recruitment. Immunity 1997, 6, 315–325. [Google Scholar] [CrossRef] [Green Version]
- Valenzuela, N.M.; Reed, E.F. Antibodies to HLA Molecules Mimic Agonistic Stimulation to Trigger Vascular Cell Changes and Induce Allograft Injury. Curr. Transplant. Rep. 2015, 2, 222–232. [Google Scholar] [CrossRef] [Green Version]
- Kwon, B. Regulation of Inflammation by Bidirectional Signaling through CD137 and Its Ligand. Immune Netw. 2012, 12, 176–180. [Google Scholar] [CrossRef] [Green Version]
- Croft, M. The role of TNF superfamily members in T-cell function and diseases. Nat. Rev. Immunol. 2009, 9, 271–285. [Google Scholar] [CrossRef] [Green Version]
- Tian, J.; Zhang, B.; Rui, K.; Wang, S. The Role of GITR/GITRL Interaction in Autoimmune Diseases. Front. Immunol. 2020, 11, 588682. [Google Scholar] [CrossRef]
- Nocentini, G.; Riccardi, C. GITR: A multifaceted regulator of immunity belonging to the tumor necrosis factor receptor superfamily. Eur. J. Immunol. 2005, 35, 1016–1022. [Google Scholar] [CrossRef]
- Dittmer, U.; He, H.; Messer, R.J.; Schimmer, S.; Olbrich, A.R.M.; Ohlen, C.; Greenberg, P.D.; Stromnes, I.M.; Iwashiro, M.; Sakaguchi, S.; et al. Functional Impairment of CD8+ T Cells by Regulatory T Cells during Persistent Retroviral Infection. Immunity 2004, 20, 293–303. [Google Scholar] [CrossRef] [Green Version]
- Yamazaki, T.; Akiba, H.; Iwai, H.; Matsuda, H.; Aoki, M.; Tanno, Y.; Shin, T.; Tsuchiya, H.; Pardoll, D.M.; Okumura, K.; et al. Expression of programmed death 1 ligands by murine T cells and APC. J. Immunol. 2002, 169, 5538–5545. [Google Scholar] [CrossRef] [Green Version]
- Eppihimer, M.J.; Gunn, J.; Freeman, G.J.; Greenfield, E.A.; Chernova, T.; Erickson, J.; Leonard, J.P. Expression and regulation of the PD-L1 immunoinhibitory molecule on microvascular endothelial cells. Microcirculation 2002, 9, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.C.U.; Jablonski-Westrich, D.; Haubold, U.; Gutierrez-Ramos, J.-C.; Springer, T.; Hamann, A. Overlapping and selective roles of endothelial intercellular adhesion molecule-1 (ICAM-1) and ICAM-2 in lymphocyte trafficking. J. Immunol. 2003, 171, 2588–2593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dustin, M.L.; Rothlein, R.; Bhan, A.K.; Dinarello, C.A.; Springer, T.A. Induction by IL 1 and interferon-gamma: Tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1). J. Immunol. 1986, 137, 245–254. [Google Scholar] [PubMed]
- Wung, B.S.; Ni, C.W.; Wang, D.L. ICAM-1 induction by TNFα and IL-6 is mediated by distinct pathways via Rac in endothelial cells. J. Biomed. Sci. 2005, 12, 91–101. [Google Scholar] [CrossRef]
- Othumpangat, S.; Noti, J.D.; McMillen, C.M.; Beezhold, D.H. ICAM-1 regulates the survival of influenza virus in lung epithelial cells during the early stages of infection. Virology 2016, 487, 85–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaux, D.L. Research methods: Know when your numbers are significant. Nature 2012, 492, 180–181. [Google Scholar] [CrossRef]


| Ingredients (CH) | MIM Entire Sequence | MIM Tested Capsule |
|---|---|---|
| hr-IL-1β | 5–10 | 10 |
| hr-IL-2 | 5–10 | 10 |
| hr-IL-5 | 6–10 | 10 |
| hr-IL-6 | 6–10 | 10 |
| hr-IFN-γ | 6–10 | 6 |
| hr-TGF-β | 30–10 | 10 |
| hr-TNF-α | 5–10 | 5 |
| DNA | 8–10 | 10 |
| RNA | 8–10 | 10 |
| SNA-HLA I | 10 | 10 |
| SNA-HLA II | 16–10 | 10 |
| SNA-EID | 16–10 | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacques, C.; Chatelais, M.; Fekir, K.; Fauconnier, L.; Mellier, M.; Togbe, D.; Floris, I. The Micro-Immunotherapy Medicine 2LEID Exhibits an Immunostimulant Effect by Boosting Both Innate and Adaptive Immune Responses. Int. J. Mol. Sci. 2022, 23, 110. https://doi.org/10.3390/ijms23010110
Jacques C, Chatelais M, Fekir K, Fauconnier L, Mellier M, Togbe D, Floris I. The Micro-Immunotherapy Medicine 2LEID Exhibits an Immunostimulant Effect by Boosting Both Innate and Adaptive Immune Responses. International Journal of Molecular Sciences. 2022; 23(1):110. https://doi.org/10.3390/ijms23010110
Chicago/Turabian StyleJacques, Camille, Mathias Chatelais, Karim Fekir, Louis Fauconnier, Manon Mellier, Dieudonnée Togbe, and Ilaria Floris. 2022. "The Micro-Immunotherapy Medicine 2LEID Exhibits an Immunostimulant Effect by Boosting Both Innate and Adaptive Immune Responses" International Journal of Molecular Sciences 23, no. 1: 110. https://doi.org/10.3390/ijms23010110







