chi-miR-487b-3p Inhibits Goat Myoblast Proliferation and Differentiation by Targeting IRS1 through the IRS1/PI3K/Akt Signaling Pathway
Abstract
:1. Introduction
2. Results
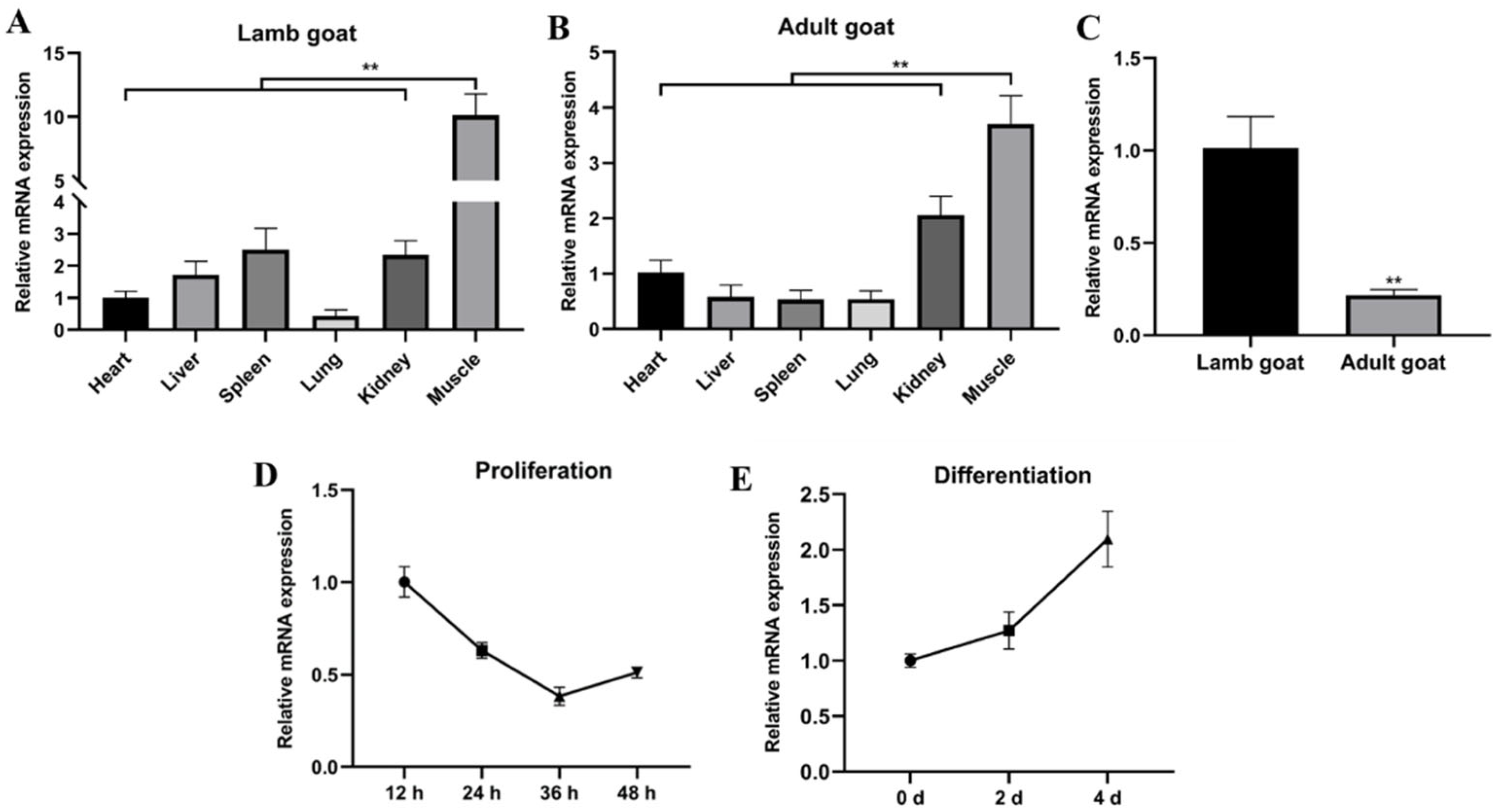
2.1. miR-487b-3p Acts as a Candidate Regulator in Goat Skeletal Myogenesis
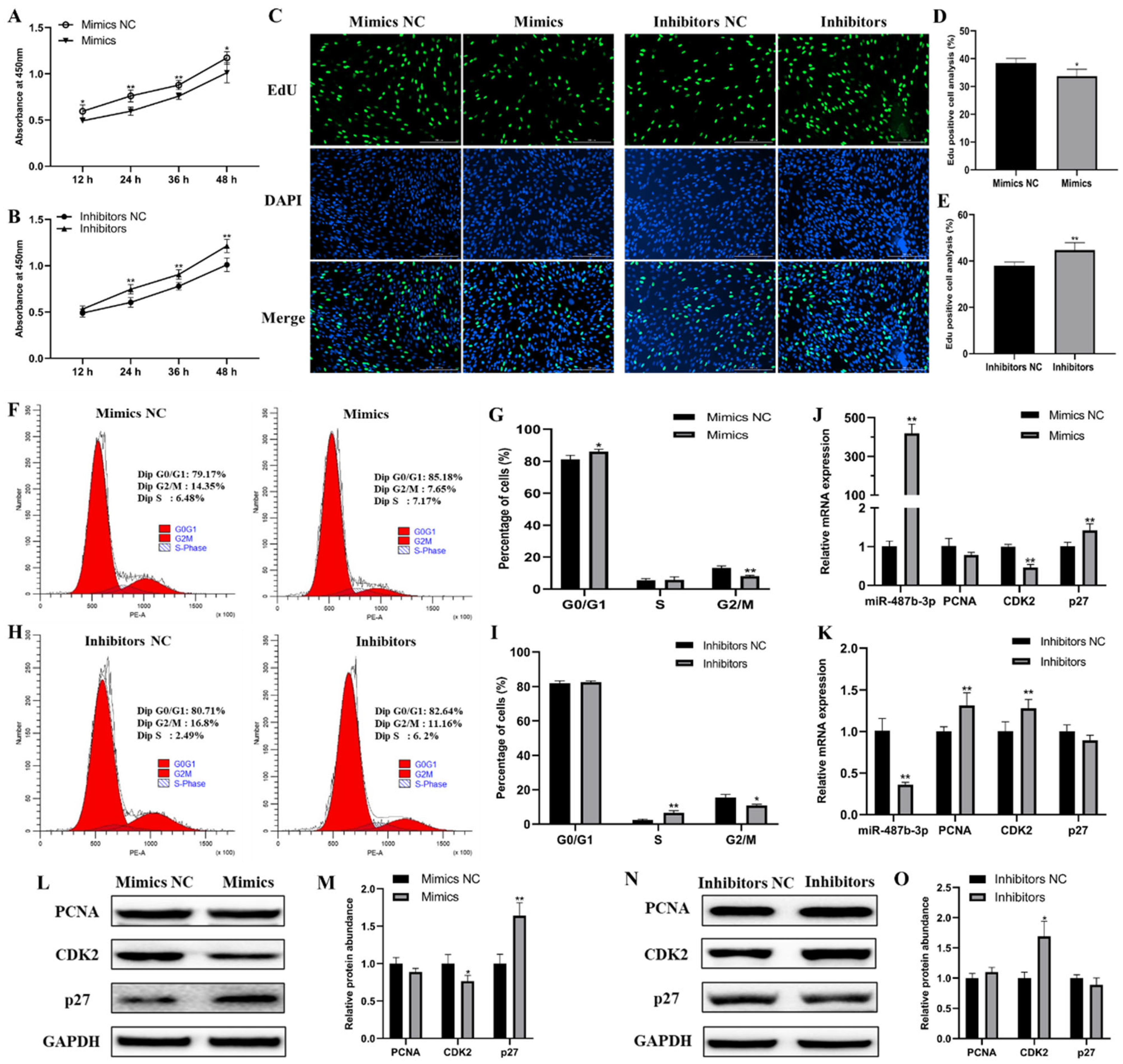
2.2. miR-487b-3p Inhibits Goat Myoblast Proliferation
2.3. miR-487b-3p Inhibits Goat Myoblast Differentiation
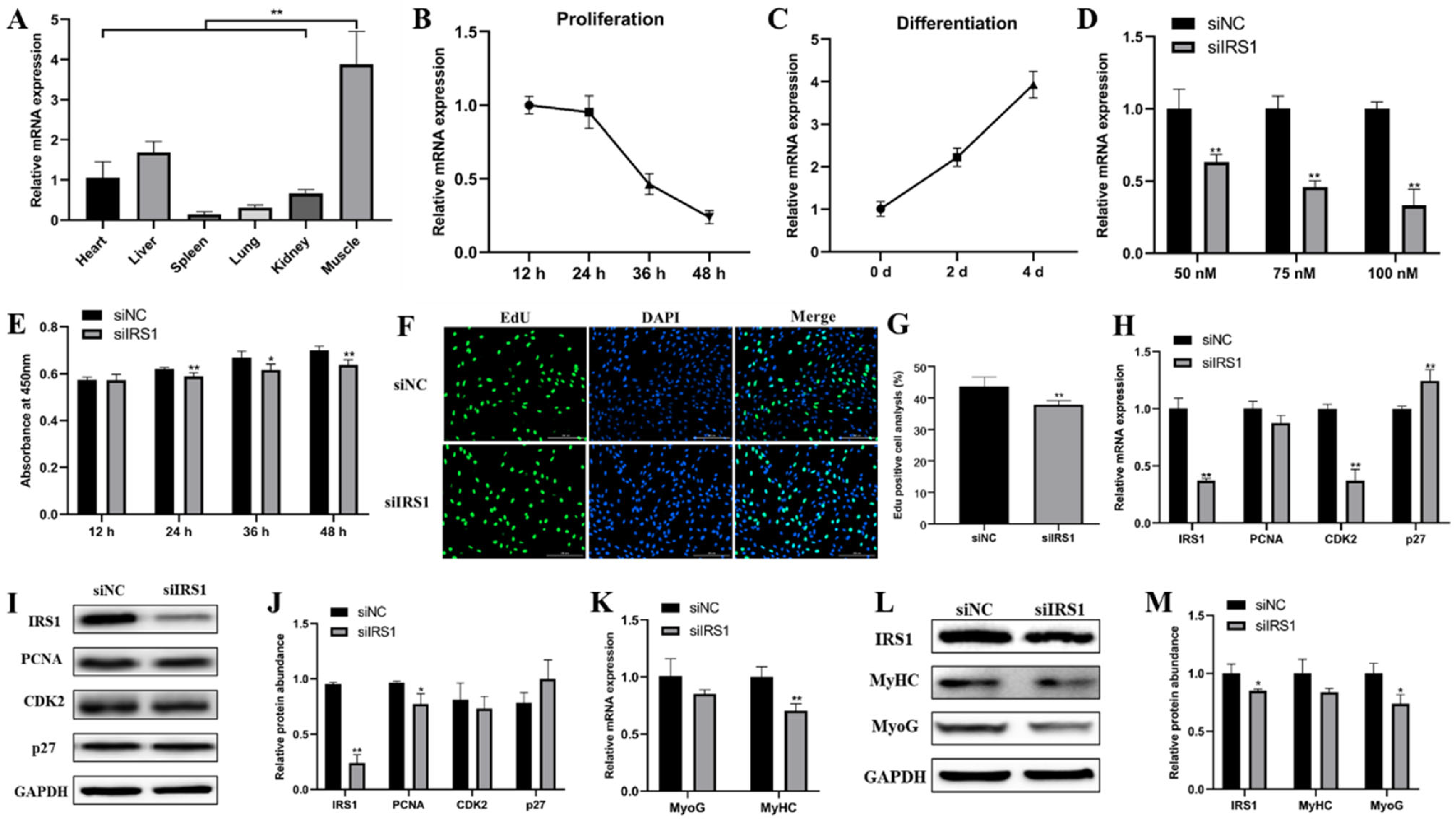
2.4. IRS1 Is a Direct Target of miR-487b-3p
2.5. IRS1 Knockdown Suppresses Goat Myoblast Proliferation and Differentiation
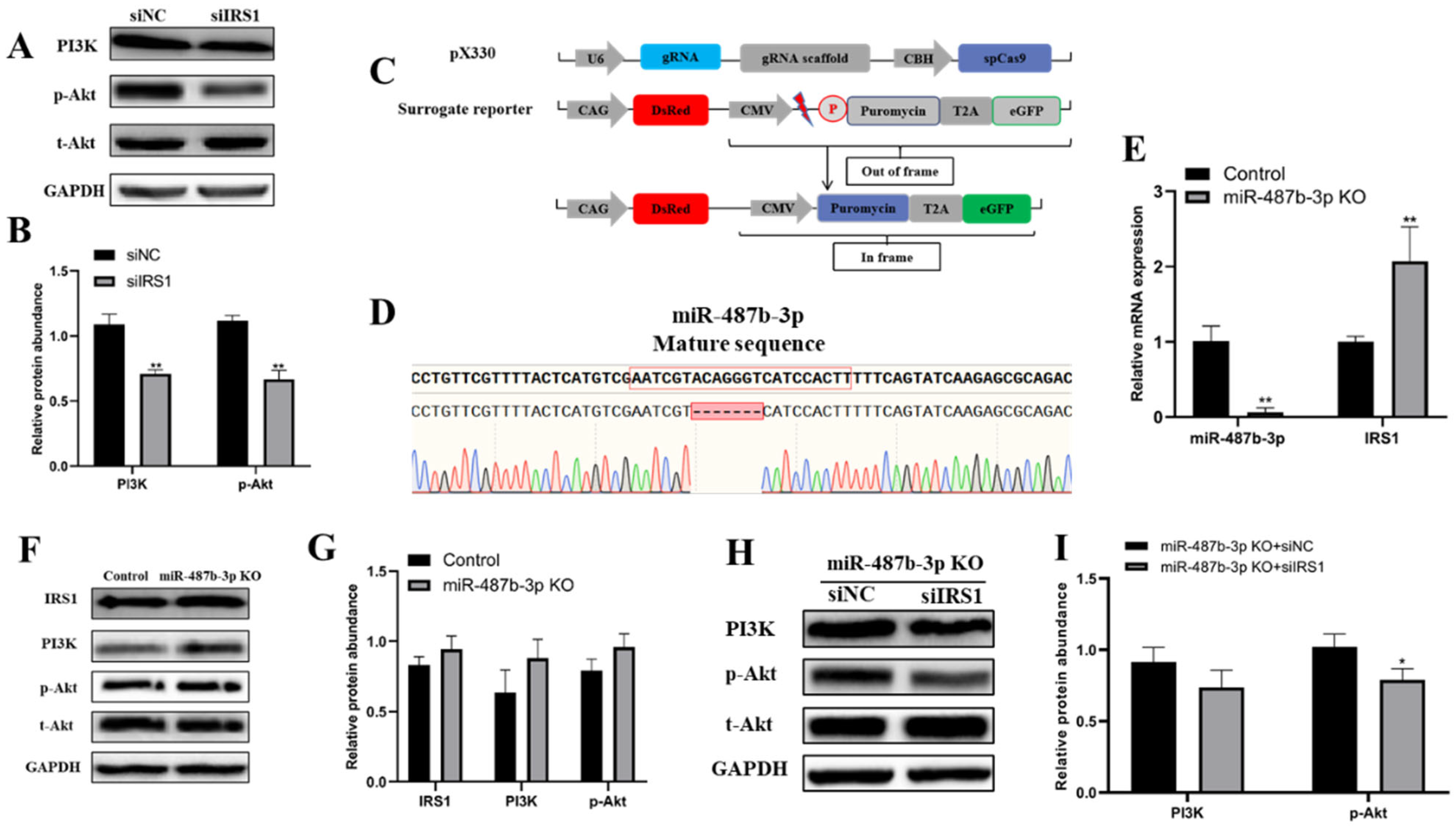
2.6. miR-487b-3p Affects the IRS1/PI3K/Akt Signaling Pathway
3. Discussion
4. Materials and Methods
4.1. Animal Tissue Sample Collection
4.2. Cells Culture
4.3. RNA Isolation and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.4. RNA Oligonucleotides
4.5. Cell Transfection
4.6. Cell Counting Kit-8 (CCK-8) Assays
4.7. 5-Ethynyl-2-deoxyuridine (EdU) Imaging Assays
4.8. Flow Cytometric Analysis
4.9. Bioinformatics Analysis for Target Genes Prediction
4.10. Dual-Luciferase Reporter Assay
4.11. Protein Extraction and Western Blot Analysis
4.12. Immunofluorescence
4.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Verma, M.; Asakura, Y.; Murakonda, B.S.R.; Pengo, T.; Latroche, C.; Chazaud, B.; McLoon, L.K.; Asakura, A. Muscle Satellite Cell Cross-Talk with a Vascular Niche Maintains Quiescence via VEGF and Notch Signaling. Cell Stem Cell 2018, 23, 530–543.e9. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Chu, A.; Chakroun, I.; Islam, U.; Blais, A. Cooperation between myogenic regulatory factors and SIX family transcription factors is important for myoblast differentiation. Nucleic Acids Res. 2010, 38, 6857–6871. [Google Scholar] [CrossRef] [Green Version]
- Hindi, S.M.; Shin, J.; Gallot, Y.S.; Straughn, A.R.; Simionescu-Bankston, A.; Hindi, L.; Xiong, G.; Friedland, R.P.; Kumar, A. MyD88 promotes myoblast fusion in a cell-autonomous manner. Nat. Commun. 2017, 8, 1624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghini, F.; Rubolino, C.; Climent, M.; Simeone, I.; Marzi, M.J.; Nicassio, F. Endogenous transcripts control miRNA levels and activity in mammalian cells by target-directed miRNA degradation. Nat. Commun. 2018, 9, 3119. [Google Scholar] [CrossRef] [PubMed]
- Kooshapur, H.; Choudhury, N.R.; Simon, B.; Mühlbauer, M.; Jussupow, A.; Fernandez, N.; Jones, A.N.; Dallmann, A.; Gabel, F.; Camilloni, C.; et al. Structural basis for terminal loop recognition and stimulation of pri-miRNA-18a processing by hnRNP A1. Nat. Commun. 2018, 9, 2479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.; Kim, J.; Kim, K.; Chang, H.; You, K.; Kim, V.N. Bias-minimized quantification of microRNA reveals widespread alternative processing and 3′ end modification. Nucleic Acids Res. 2019, 47, 2630–2640. [Google Scholar] [CrossRef] [Green Version]
- Siciliano, V.; Garzilli, I.; Fracassi, C.; Criscuolo, S.; Ventre, S.; di Bernardo, D. MiRNAs confer phenotypic robustness to gene networks by suppressing biological noise. Nat. Commun. 2013, 4, 2364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, B.; Lin, H.; Xiao, J.; Lu, Y.; Luo, X.; Li, B.; Zhang, Y.; Xu, C.; Bai, Y.; Wang, H.; et al. The muscle-specific microRNA miR-1 regulates cardiac arrhythmogenic potential by targeting GJA1 and KCNJ2. Nat. Med. 2007, 13, 486–491. [Google Scholar] [CrossRef]
- Yin, H.; Pasut, A.; Soleimani, V.D.; Bentzinger, C.F.; Antoun, G.; Thorn, S.; Seale, P.; Fernando, P.; van Ijcken, W.; Grosveld, F.; et al. MicroRNA-133 controls brown adipose determination in skeletal muscle satellite cells by targeting Prdm16. Cell Metab. 2013, 17, 210–224. [Google Scholar] [CrossRef] [Green Version]
- Dey, B.K.; Gagan, J.; Dutta, A. miR-206 and -486 induce myoblast differentiation by downregulating Pax7. Mol. Cell Biol. 2011, 31, 203–214. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.; Zhang, Y.; Yang, G.; Chen, X.; Zhang, Y.; Cao, G.; Wang, J.; Sun, Y.; Zhang, P.; Fan, M.; et al. Transforming growth factor-beta-regulated miR-24 promotes skeletal muscle differentiation. Nucleic Acids Res. 2008, 36, 2690–2699. [Google Scholar] [CrossRef]
- Wang, H.; Wang, B.; Zhang, A.; Hassounah, F.; Seow, Y.; Wood, M.; Ma, F.; Klein, J.D.; Price, S.R.; Wang, X.H. Exosome-Mediated miR-29 Transfer Reduces Muscle Atrophy and Kidney Fibrosis in Mice. Mol. Ther. J. Am. Soc. Gene Ther. 2019, 27, 571–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, Y.; Sun, Y.; Chen, J. IGF-II is regulated by microRNA-125b in skeletal myogenesis. J. Cell Biol. 2011, 192, 69–81. [Google Scholar] [CrossRef] [Green Version]
- Naguibneva, I.; Ameyar-Zazoua, M.; Polesskaya, A.; Ait-Si-Ali, S.; Groisman, R.; Souidi, M.; Cuvellier, S.; Harel-Bellan, A. The microRNA miR-181 targets the homeobox protein Hox-A11 during mammalian myoblast differentiation. Nat. Cell Biol. 2006, 8, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Juan, A.H.; Kumar, R.M.; Marx, J.G.; Young, R.A.; Sartorelli, V. Mir-214-dependent regulation of the polycomb protein Ezh2 in skeletal muscle and embryonic stem cells. Mol. Cell 2009, 36, 61–74. [Google Scholar] [CrossRef] [Green Version]
- Mori, M.A.; Ludwig, R.G.; Garcia-Martin, R.; Brandão, B.B.; Kahn, C.R. Extracellular miRNAs: From Biomarkers to Mediators of Physiology and Disease. Cell Metab. 2019, 30, 656–673. [Google Scholar] [CrossRef]
- Miao, P.; Tang, Y. Cascade Strand Displacement and Bipedal Walking Based DNA Logic System for miRNA Diagnostics. ACS Cent. Sci. 2021, 7, 1036–1044. [Google Scholar] [CrossRef]
- Wang, J.; Tan, J.; Qi, Q.; Yang, L.; Wang, Y.; Zhang, C.; Hu, L.; Chen, H.; Fang, X. miR-487b-3p Suppresses the Proliferation and Differentiation of Myoblasts by Targeting IRS1 in Skeletal Muscle Myogenesis. Int. J. Biol. Sci. 2018, 14, 760–774. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Geng, L.; Black, A.; Talmon, G.; Berim, L.; Wang, J. The miR-487b-3p/GRM3/TGFβ signaling axis is an important regulator of colon cancer tumorigenesis. Oncogene 2017, 36, 3477–3489. [Google Scholar] [CrossRef] [Green Version]
- Nossent, A.Y.; Eskildsen, T.V.; Andersen, L.B.; Bie, P.; Brønnum, H.; Schneider, M.; Andersen, D.C.; Welten, S.M.J.; Jeppesen, P.L.; Hamming, J.F.; et al. The 14q32 microRNA-487b targets the antiapoptotic insulin receptor substrate 1 in hypertension-induced remodeling of the aorta. Ann. Surg. 2013, 258, 743–751; discussion 752–753. [Google Scholar] [CrossRef] [PubMed]
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.; Barretto, R.; Habib, N.; Hsu, P.D.; Wu, X.; Jiang, W.; Marraffini, L.A.; et al. Multiplex genome engineering using CRISPR/Cas systems. Science 2013, 339, 819–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Posa, D.K.; Baba, S.P. Intracellular pH Regulation of Skeletal Muscle in the Milieu of Insulin Signaling. Nutrients 2020, 12, 2910. [Google Scholar] [CrossRef]
- Ren, C.; Xu, K.; Liu, Z.; Shen, J.; Han, F.; Chen, Z.; Zhang, Z. Dual-Reporter Surrogate Systems for Efficient Enrichment of Genetically Modified Cells. Cell. Mol. Life Sci. 2015, 72, 2763–2772. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Ren, C.; Liu, Z.; Zhang, T.; Zhang, T.; Li, D.; Wang, L.; Yan, Q.; Guo, L.; Shen, J.; et al. Efficient genome engineering in eukaryotes using Cas9 from Streptococcus thermophilus. Cell. Mol. Life Sci. CMLS 2015, 72, 383–399. [Google Scholar] [CrossRef]
- Yan, N.; Sun, Y.; Fang, Y.; Deng, J.; Mu, L.; Xu, K.; Mymryk, J.S.; Zhang, Z. A Universal Surrogate Reporter for Efficient Enrichment of CRISPR/Cas9-Mediated Homology-Directed Repair in Mammalian Cells. Mol. Ther. Nucleic Acids 2020, 19, 775–789. [Google Scholar] [CrossRef]
- Zhu, P.; Zhang, C.; Gao, Y.; Wu, F.; Zhou, Y.; Wu, W. The transcription factor Slug represses p16(Ink4a) and regulates murine muscle stem cell aging. Nat. Commun. 2019, 10, 2568. [Google Scholar] [CrossRef]
- Liu, J.; Gaj, T.; Yang, Y.; Wang, N.; Shui, S.; Kim, S.; Kanchiswamy, C.N.; Kim, J.; Barbas, C.F.R. Efficient delivery of nuclease proteins for genome editing in human stem cells and primary cells. Nat. Protoc. 2015, 10, 1842–1859. [Google Scholar] [CrossRef] [PubMed]
- Diniz, G.P.; Wang, D. Regulation of Skeletal Muscle by microRNAs. Compr. Physiol. 2016, 6, 1279–1294. [Google Scholar]
- Zhang, Z.; Li, J.; Guan, D.; Liang, C.; Zhuo, Z.; Liu, J.; Lu, A.; Zhang, G.; Zhang, B. A newly identified lncRNA MAR1 acts as a miR-487b sponge to promote skeletal muscle differentiation and regeneration. J. Cachexia Sarcopenia Muscle 2018, 9, 613–626. [Google Scholar] [CrossRef] [Green Version]
- Ames, H.M.; Yuan, M.; Vizcaíno, M.A.; Yu, W.; Rodriguez, F.J. MicroRNA profiling of low-grade glial and glioneuronal tumors shows an independent role for cluster 14q32.31 member miR-487b. Mod. Pathol. 2017, 30, 204–216. [Google Scholar] [CrossRef]
- Cheng, M.; Duan, P.; Gao, Z.; Dai, M. MicroRNA-487b-3p inhibits osteosarcoma chemoresistance and metastasis by targeting ALDH1A3. Oncol. Rep. 2020, 44, 2691–2700. [Google Scholar] [CrossRef]
- Chen, X.; Lin, Z.; Xi, W.; Wang, W.; Zhang, D.; Yang, F.; Li, Y.; Huo, Y.; Zhang, T.; Jiang, Y.; et al. DNA methylation-regulated and tumor-suppressive roles of miR-487b in colorectal cancer via targeting MYC, SUZ12, and KRAS. Cancer Med. 2019, 8, 1694–1709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, S.; Jin, L.; Zhang, F.; Sarnow, P.; Kay, M.A. Biological basis for restriction of microRNA targets to the 3’ untranslated region in mammalian mRNAs. Nat. Struct. Mol. Biol. 2009, 16, 144–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef] [Green Version]
- Dietz, C.; Infanger, M.; Romswinkel, A.; Strube, F.; Kraus, A. Apoptosis Induction and Alteration of Cell Adherence in Human Lung Cancer Cells under Simulated Microgravity. Int. J. Mol. Sci. 2019, 20, 3601. [Google Scholar] [CrossRef] [Green Version]
- Madhala-Levy, D.; Williams, V.C.; Hughes, S.M.; Reshef, R.; Halevy, O. Cooperation between Shh and IGF-I in promoting myogenic proliferation and differentiation via the MAPK/ERK and PI3K/Akt pathways requires Smo activity. J. Cell Physiol. 2012, 227, 1455–1464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, M.; Wang, X.; Chen, X.; Cai, R.; Chen, F.; Dong, W.; Yang, G.; Pang, W. MicroRNA-432 targeting E2F3 and P55PIK inhibits myogenesis through PI3K/AKT/mTOR signaling pathway. RNA Biol. 2017, 14, 347–360. [Google Scholar] [CrossRef] [Green Version]
- Bai, L.; Liang, R.; Yang, Y.; Hou, X.; Wang, Z.; Zhu, S.; Wang, C.; Tang, Z.; Li, K. MicroRNA-21 Regulates PI3K/Akt/mTOR Signaling by Targeting TGFβI during Skeletal Muscle Development in Pigs. PLoS ONE 2015, 10, e0119396. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Pan, J.; Zhang, L.; Wei, Y.; Wang, C. Long non-coding RNA CRNDE sponges miR-384 to promote proliferation and metastasis of pancreatic cancer cells through upregulating IRS1. Cell Proliferat. 2017, 50, e12389. [Google Scholar] [CrossRef] [Green Version]
- Motohashi, N.; Alexander, M.S.; Shimizu-Motohashi, Y.; Myers, J.A.; Kawahara, G.; Kunkel, L.M. Regulation of IRS1/Akt insulin signaling by microRNA-128a during myogenesis. J. Cell Sci. 2013, 126, 2678–2691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Cai, B.; Abdalla, B.A.; Zhu, X.; Zheng, M.; Han, P.; Nie, Q.; Zhang, X. LncIRS1 controls muscle atrophy via sponging miR-15 family to activate IGF1-PI3K/AKT pathway. J. Cachexia Sarcopenia Muscle 2019, 10, 391–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyu, M.; Shalitana, A.; Luo, J.; He, H.; Sun, S.; Wang, P. Overexpression of the Tuberous sclerosis complex 2 (TSC2) gene inhibits goat myoblasts proliferation and differentiation in understanding the underlying mechanism of muscle development. Gene 2020, 757, 144943. [Google Scholar] [CrossRef] [PubMed]


| Gene | Primer Name | Primer Sequence (5′ to 3′) | Reference |
|---|---|---|---|
| miR-487b-3p | Stem-loop | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAAGTGG | [18] |
| miR-487b-3p-F | CGGGCAATCGTACAGGGT | This manuscript | |
| miR-487b-3p-R | CAGTGCAGGGTCCGAGGTAT | ||
| 18S-rRNA | 18S-rRNA-F | GTGGTGTTGAGGAAAGCAGACA | [18] |
| 18S-rRNA-R | TGATCACACGTTCCACCTCATC | ||
| IRS1 | IRS1-F | GTAGTGGCAAACTCCTGTCTTGT | This manuscript |
| IRS1-R | GAGTAGTAGGAGAGGACGGGCT | ||
| PCNA | PCNA-F | CGCTTAAGGATCTCATCAATGAG | This manuscript |
| PCNA-R | GTTACGGTCGCAGCGGTAAG | ||
| CDK2 | CDK2-F | TCATGGATGCCTCTGCACTC | [42] |
| CDK2-R | CTCTGGCTAGTCCGAAGTCTG | ||
| p27 | p27-F | CGGCGGTGCCTTTACTT | [42] |
| p27-R | GCAGGTCGCTTCCTTATCC | ||
| MyoG | MyoG-F | GGACCCTACAGATGCCCACAA | This manuscript |
| MyoG-R | TTGGTATGGTTTCATCTGGG | ||
| MyHC | MyHC-F | GTGAAGGAGGACCAGGTGTTG G | [42] |
| MyHC-R | GTTGATGGTGACGCAGAAGAG | ||
| GAPDH | GAPDH-F | CCACGCCATCACTGCCACCC | [42] |
| GAPDH-R | CAGCCTTGGCAGCGCCAGTA |
| Name | Sense Sequence (5′-3′) |
|---|---|
| Mimics | AAUCGUACAGGGUCAUCCACUU |
| Mimics NC | UUCUCCGAACGUGUCACGUTT |
| Inhibitors | AAGUGGAUGACCCUGUACGAUU |
| Inhibitors NC | CAGUACUUUUGUGUAGUACAA |
| siIRS1 | GCAUGCACAAGCGAUUCUUTT |
| AAGAAUCGCUUGUGCAUGCTT | |
| siNC | UUCUCCGAACGUGUCACGUTT |
| ACGUGACACGUUCGGAGAATT |
| Name | Primer Name | Primer Sequence (5′ to 3′) |
|---|---|---|
| Wild-IRS1-3′UTR | Wild-IRS1-F | cggCTCGAGCAGCAAATCCTCCTTTAACTC |
| Wild-IRS1-R | aatGCGGCCGCGCACGATATACAACGTGCAG | |
| Mutant-IRS1-3′UTR | Mutant-IRS1-F | CTCAGTAGATGGGCTAATGCACCC |
| Mutant-IRS1-R | GAAATGGGTGCCGATTACCATCTAC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyu, M.; Wang, X.; Meng, X.; Qian, H.; Li, Q.; Ma, B.; Zhang, Z.; Xu, K. chi-miR-487b-3p Inhibits Goat Myoblast Proliferation and Differentiation by Targeting IRS1 through the IRS1/PI3K/Akt Signaling Pathway. Int. J. Mol. Sci. 2022, 23, 115. https://doi.org/10.3390/ijms23010115
Lyu M, Wang X, Meng X, Qian H, Li Q, Ma B, Zhang Z, Xu K. chi-miR-487b-3p Inhibits Goat Myoblast Proliferation and Differentiation by Targeting IRS1 through the IRS1/PI3K/Akt Signaling Pathway. International Journal of Molecular Sciences. 2022; 23(1):115. https://doi.org/10.3390/ijms23010115
Chicago/Turabian StyleLyu, Ming, Xu Wang, Xiangyu Meng, Hongrun Qian, Qian Li, Baoxia Ma, Zhiying Zhang, and Kun Xu. 2022. "chi-miR-487b-3p Inhibits Goat Myoblast Proliferation and Differentiation by Targeting IRS1 through the IRS1/PI3K/Akt Signaling Pathway" International Journal of Molecular Sciences 23, no. 1: 115. https://doi.org/10.3390/ijms23010115





