Roles of Oxytocin in Stress Responses, Allostasis and Resilience
Abstract
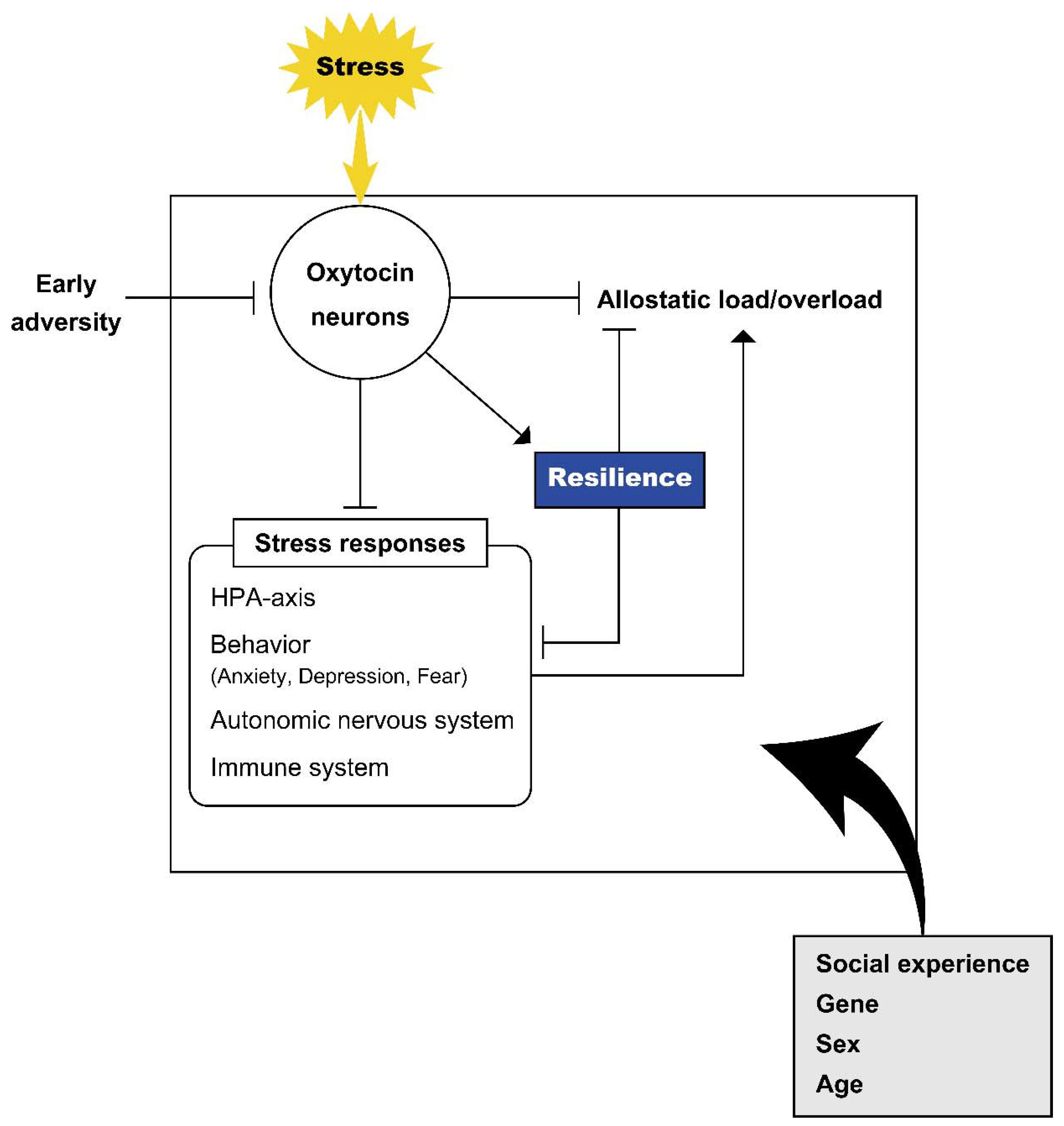
:1. Introduction
2. Stress Responses and Oxytocin
2.1. Activation of Oxytocin Neurons and Facilitation of Oxytocin Release after Various Stressful Stimuli
2.2. Roles of Oxytocin in Stress Responses
2.3. Site of Action of Oxytocin with Respect to Stress Responses
2.3.1. Prefrontal Cortex
2.3.2. Bed Nucleus of the Stria Terminalis
2.3.3. Hypothalamus
2.3.4. Lateral Septum
2.3.5. Amygdala
2.3.6. Raphe Nucleus
2.3.7. Medulla Oblongata
3. Allostasis and Oxytocin
3.1. Oxytocin in the Concept of Allostasis
3.2. Adaptation to Changing Environments in the Oxytocin System
4. Resilience and Oxytocin
4.1. Roles of Oxytocin in Resilience in Animal Models
4.2. Roles of Oxytocin in Humans
5. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Cannon, W.B. Organization for physiological homeostasis. Physiol. Rev. 1929, 9, 399–431. [Google Scholar] [CrossRef]
- Ramsay, D.S.; Woods, S.C. Clarifying the roles of homeostasis and allostasis in physiological regulation. Psychol. Rev. 2014, 121, 225–247. [Google Scholar] [CrossRef] [Green Version]
- Sterling, P.; Eyer, J. Allostasis: A New Paradigm to Explain Arousal Pathology. In Handbook of Life Stress, Cognition and Health; Fisher, S., Reason, J., Eds.; Wiley: Chichester, UK, 1988; pp. 629–649. [Google Scholar]
- McEwen, B.S.; Stellar, E. Stress and the individual. Mechanisms leading to disease. Arch. Intern. Med. 1993, 153, 2093–2101. [Google Scholar] [CrossRef]
- Peters, A.; McEwen, B.S.; Friston, K. Uncertainty and stress: Why it causes diseases and how it is mastered by the brain. Prog. Neurobiol. 2017, 156, 164–188. [Google Scholar] [CrossRef]
- Southwick, S.M.; Bonanno, G.A.; Masten, A.S.; Panter-Brick, C.; Yehuda, R. Resilience definitions, theory, and challenges: Interdisciplinary perspectives. Eur. J. Psychotraumatol. 2014, 5, 25338. [Google Scholar] [CrossRef] [Green Version]
- McEwen, B.S. Protective and damaging effects of stress mediators. N. Engl. J. Med. 1998, 338, 171–179. [Google Scholar] [CrossRef] [Green Version]
- Onaka, T.; Okabe, S.; Takayanagi, Y.; Yoshida, M. Noxious or Non-noxious Inputs to Oxytocin Neurons: Possible Roles in the Control of Behaviors. Interdiscip. Inf. Sci. 2015, 21, 189–195. [Google Scholar] [CrossRef] [Green Version]
- Onaka, T.; Yagi, K. Differential effects of naloxone on neuroendocrine responses to fear-related emotional stress. Exp. Brain Res. 1990, 81, 53–58. [Google Scholar] [CrossRef]
- Duque-Wilckens, N.; Steinman, M.Q.; Busnelli, M.; Chini, B.; Yokoyama, S.; Pham, M.; Laredo, S.A.; Hao, R.; Perkeybile, A.M.; Minie, V.A.; et al. Oxytocin Receptors in the Anteromedial Bed Nucleus of the Stria Terminalis Promote Stress-Induced Social Avoidance in Female California Mice. Biol. Psychiatry 2018, 83, 203–213. [Google Scholar] [CrossRef]
- Nasanbuyan, N.; Yoshida, M.; Takayanagi, Y.; Inutsuka, A.; Nishimori, K.; Yamanaka, A.; Onaka, T. Oxytocin-Oxytocin Receptor Systems Facilitate Social Defeat Posture in Male Mice. Endocrinology 2018, 159, 763–775. [Google Scholar] [CrossRef]
- Bosch, O.J.; Krömer, S.A.; Brunton, P.J.; Neumann, I.D. Release of oxytocin in the hypothalamic paraventricular nucleus, but not central amygdala or lateral septum in lactating residents and virgin intruders during maternal defence. Neuroscience 2004, 124, 439–448. [Google Scholar] [CrossRef]
- Engelmann, M.; Ebner, K.; Landgraf, R.; Holsboer, F.; Wotjak, C.T. Emotional stress triggers intrahypothalamic but not peripheral release of oxytocin in male rats. J. Neuroendocrinol. 1999, 11, 867–872. [Google Scholar] [CrossRef]
- Wotjak, C.T.; Kubota, M.; Liebsch, G.; Montkowski, A.; Holsboer, F.; Neumann, I.; Landgraf, R. Release of vasopressin within the rat paraventricular nucleus in response to emotional stress: A novel mechanism of regulating adrenocorticotropic hormone secretion? J. Neurosci. 1996, 16, 7725–7732. [Google Scholar] [CrossRef] [Green Version]
- Lang, R.E.; Heil, J.W.; Ganten, D.; Hermann, K.; Unger, T.; Rascher, W. Oxytocin unlike vasopressin is a stress hormone in the rat. Neuroendocrinology 1983, 37, 314–316. [Google Scholar] [CrossRef]
- Nishioka, T.; Anselmo-Franci, J.A.; Li, P.; Callahan, M.F.; Morris, M. Stress increases oxytocin release within the hypothalamic paraventricular nucleus. Brain Res. 1998, 781, 57–61. [Google Scholar] [CrossRef]
- Douglas, A.J.; Johnstone, H.A.; Wigger, A.; Landgraf, R.; Russell, J.A.; Neumann, I.D. The role of endogenous opioids in neurohypophysial and hypothalamo-pituitary-adrenal axis hormone secretory responses to stress in pregnant rats. J. Endocrinol. 1998, 158, 285–293. [Google Scholar] [CrossRef] [Green Version]
- Torner, L.; Plotsky, P.M.; Neumann, I.D.; de Jong, T.R. Forced swimming-induced oxytocin release into blood and brain: Effects of adrenalectomy and corticosterone treatment. Psychoneuroendocrinology 2017, 77, 165–174. [Google Scholar] [CrossRef]
- Kasahara, Y.; Takayanagi, Y.; Kawada, T.; Itoi, K.; Nishimori, K. Impaired thermoregulatory ability of oxytocin-deficient mice during cold-exposure. Biosci. Biotechnol. Biochem. 2007, 71, 3122–3126. [Google Scholar] [CrossRef]
- Hada, T.; Onaka, T.; Takahashi, T.; Hiraga, A.; Yagi, K. Effects of novelty stress on neuroendocrine activities and running performance in thoroughbred horses. J. Neuroendocrinol. 2003, 15, 638–648. [Google Scholar] [CrossRef]
- Rivest, S.; Laflamme, N. Neuronal activity and neuropeptide gene transcription in the brains of immune-challenged rats. J. Neuroendocrinol. 1995, 7, 501–525. [Google Scholar] [CrossRef]
- Ericsson, A.; Kovács, K.J.; Sawchenko, P.E. A functional anatomical analysis of central pathways subserving the effects of interleukin-1 on stress-related neuroendocrine neurons. J. Neurosci. 1994, 14, 897–913. [Google Scholar] [CrossRef]
- Onaka, T.; Takayanagi, Y.; Yoshida, M. Roles of oxytocin neurones in the control of stress, energy metabolism, and social behaviour. J. Neuroendocrinol. 2012, 24, 587–598. [Google Scholar] [CrossRef] [PubMed]
- Onaka, T.; Takayanagi, Y. Role of oxytocin in the control of stress and food intake. J. Neuroendocrinol. 2019, 31, e12700. [Google Scholar] [CrossRef]
- Neumann, I.D.; Landgraf, R. Balance of brain oxytocin and vasopressin: Implications for anxiety, depression, and social behaviors. Trends Neurosci. 2012, 35, 649–659. [Google Scholar] [CrossRef]
- Asgari, P.; McKinney, G.; Hodges, T.E.; McCormick, C.M. Social Instability Stress in Adolescence and Social Interaction in Female Rats. Neuroscience 2021, 477, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Jong, T.R.; Menon, R.; Bludau, A.; Grund, T.; Biermeier, V.; Klampfl, S.M.; Jurek, B.; Bosch, O.J.; Hellhammer, J.; Neumann, I.D. Salivary oxytocin concentrations in response to running, sexual self-stimulation, breastfeeding and the TSST: The Regensburg Oxytocin Challenge (ROC) study. Psychoneuroendocrinology 2015, 62, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Landgraf, R.; Häcker, R.; Buhl, H. Plasma vasopressin and oxytocin in response to exercise and during a day-night cycle in man. Endokrinologie 1982, 79, 281–291. [Google Scholar]
- Hew-Butler, T.; Noakes, T.D.; Soldin, S.J.; Verbalis, J.G. Acute changes in endocrine and fluid balance markers during high-intensity, steady-state, and prolonged endurance running: Unexpected increases in oxytocin and brain natriuretic peptide during exercise. Eur. J. Endocrinol. 2008, 159, 729–737. [Google Scholar] [CrossRef] [Green Version]
- Hew-Butler, T.; Jordaan, E.; Stuempfle, K.J.; Speedy, D.B.; Siegel, A.J.; Noakes, T.D.; Soldin, S.J.; Verbalis, J.G. Osmotic and nonosmotic regulation of arginine vasopressin during prolonged endurance exercise. J. Clin. Endocrinol. Metab. 2008, 93, 2072–2078. [Google Scholar] [CrossRef] [Green Version]
- Sanders, G.; Freilicher, J.; Lightman, S.L. Psychological stress of exposure to uncontrollable noise increases plasma oxytocin in high emotionality women. Psychoneuroendocrinology 1990, 15, 47–58. [Google Scholar] [CrossRef]
- Pierrehumbert, B.; Torrisi, R.; Laufer, D.; Halfon, O.; Ansermet, F.; Beck Popovic, M. Oxytocin response to an experimental psychosocial challenge in adults exposed to traumatic experiences during childhood or adolescence. Neuroscience 2010, 166, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Bernhard, A.; van der Merwe, C.; Ackermann, K.; Martinelli, A.; Neumann, I.D.; Freitag, C.M. Adolescent oxytocin response to stress and its behavioral and endocrine correlates. Horm. Behav. 2018, 105, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Tabak, B.A.; McCullough, M.E.; Szeto, A.; Mendez, A.J.; McCabe, P.M. Oxytocin indexes relational distress following interpersonal harms in women. Psychoneuroendocrinology 2011, 36, 115–122. [Google Scholar] [CrossRef] [Green Version]
- Taylor, S.E.; Saphire-Bernstein, S.; Seeman, T.E. Are plasma oxytocin in women and plasma vasopressin in men biomarkers of distressed pair-bond relationships? Psychol. Sci. 2010, 21, 3–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marazziti, D.; Dell’Osso, B.; Baroni, S.; Mungai, F.; Catena, M.; Rucci, P.; Albanese, F.; Giannaccini, G.; Betti, L.; Fabbrini, L.; et al. A relationship between oxytocin and anxiety of romantic attachment. Clin. Pract. Epidemiol. Ment. Health 2006, 2, 28. [Google Scholar] [CrossRef] [Green Version]
- Hoge, E.A.; Pollack, M.H.; Kaufman, R.E.; Zak, P.J.; Simon, N.M. Oxytocin levels in social anxiety disorder. CNS Neurosci. Ther. 2008, 14, 165–170. [Google Scholar] [CrossRef]
- Holt-Lunstad, J.; Birmingham, W.; Light, K.C. The influence of depressive symptomatology and perceived stress on plasma and salivary oxytocin before, during and after a support enhancement intervention. Psychoneuroendocrinology 2011, 36, 1249–1256. [Google Scholar] [CrossRef]
- Purba, J.S.; Hoogendijk, W.J.; Hofman, M.A.; Swaab, D.F. Increased number of vasopressin- and oxytocin-expressing neurons in the paraventricular nucleus of the hypothalamus in depression. Arch. Gen. Psychiatry 1996, 53, 137–143. [Google Scholar] [CrossRef]
- Meynen, G.; Unmehopa, U.A.; Hofman, M.A.; Swaab, D.F.; Hoogendijk, W.J. Hypothalamic oxytocin mRNA expression and melancholic depression. Mol. Psychiatry 2007, 12, 118–119. [Google Scholar] [CrossRef] [Green Version]
- Parker, K.J.; Kenna, H.A.; Zeitzer, J.M.; Keller, J.; Blasey, C.M.; Amico, J.A.; Schatzberg, A.F. Preliminary evidence that plasma oxytocin levels are elevated in major depression. Psychiatry Res. 2010, 178, 359–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altemus, M.; Roca, C.; Galliven, E.; Romanos, C.; Deuster, P. Increased vasopressin and adrenocorticotropin responses to stress in the midluteal phase of the menstrual cycle. J. Clin. Endocrinol. Metab. 2001, 86, 2525–2530. [Google Scholar] [CrossRef] [PubMed]
- Chicharro, J.L.; Hoyos, J.; Bandrés, F.; Gómez Gallego, F.; Pérez, M.; Lucía, A. Plasma oxytocin during intense exercise in professional cyclists. Horm. Res. 2001, 55, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Bakos, J.; Hlavacova, N.; Makatsori, A.; Tybitanclova, K.; Zorad, S.; Hinghofer-Szalkay, H.; Johansson, B.B.; Jezova, D. Oxytocin levels in the posterior pituitary and in the heart are modified by voluntary wheel running. Regul. Pept. 2007, 139, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Seltzer, L.J.; Ziegler, T.E.; Pollak, S.D. Social vocalizations can release oxytocin in humans. Proc. R. Soc. B. 2010, 277, 2661–2666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McQuaid, R.J.; McInnis, O.A.; Paric, A.; Al-Yawer, F.; Matheson, K.; Anisman, H. Relations between plasma oxytocin and cortisol: The stress buffering role of social support. Neurobiol. Stress 2016, 3, 52–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altemus, M.; Redwine, L.S.; Leong, Y.M.; Frye, C.A.; Porges, S.W.; Carter, C.S. Responses to laboratory psychosocial stress in postpartum women. Psychosom. Med. 2001, 63, 814–821. [Google Scholar] [CrossRef]
- Cyranowski, J.M.; Hofkens, T.L.; Frank, E.; Seltman, H.; Cai, H.M.; Amico, J.A. Evidence of dysregulated peripheral oxytocin release among depressed women. Psychosom. Med. 2008, 70, 967–975. [Google Scholar] [CrossRef] [Green Version]
- Heim, C.; Young, L.J.; Newport, D.J.; Mletzko, T.; Miller, A.H.; Nemeroff, C.B. Lower CSF oxytocin concentrations in women with a history of childhood abuse. Mol. Psychiatry 2009, 14, 954–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jokinen, J.; Chatzittofis, A.; Hellström, C.; Nordström, P.; Uvnäs-Moberg, K.; Asberg, M. Low CSF oxytocin reflects high intent in suicide attempters. Psychoneuroendocrinology 2012, 37, 482–490. [Google Scholar] [CrossRef]
- Carmassi, C.; Marazziti, D.; Mucci, F.; Della Vecchia, A.; Barberi, F.M.; Baroni, S.; Giannaccini, G.; Palego, L.; Massimetti, G.; Dell’Osso, L. Decreased Plasma Oxytocin Levels in Patients With PTSD. Front. Psychol. 2021, 12, 612338. [Google Scholar] [CrossRef]
- Ozsoy, S.; Esel, E.; Kula, M. Serum oxytocin levels in patients with depression and the effects of gender and antidepressant treatment. Psychiatry Res. 2009, 169, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Frasch, A.; Zetzsche, T.; Steiger, A.; Jirikowski, G.F. Reduction of plasma oxytocin levels in patients suffering from major depression. Adv. Exp. Med. Biol. 1995, 395, 257–258. [Google Scholar]
- Thul, T.A.; Corwin, E.J.; Carlson, N.S.; Brennan, P.A.; Young, L.J. Oxytocin and postpartum depression: A systematic review. Psychoneuroendocrinology 2020, 120, 104793. [Google Scholar] [CrossRef] [PubMed]
- Windle, R.J.; Shanks, N.; Lightman, S.L.; Ingram, C.D. Central oxytocin administration reduces stress-induced corticosterone release and anxiety behavior in rats. Endocrinology 1997, 138, 2829–2834. [Google Scholar] [CrossRef]
- Windle, R.J.; Kershaw, Y.M.; Shanks, N.; Wood, S.A.; Lightman, S.L.; Ingram, C.D. Oxytocin attenuates stress-induced c-fos mRNA expression in specific forebrain regions associated with modulation of hypothalamo-pituitary-adrenal activity. J. Neurosci. 2004, 24, 2974–2982. [Google Scholar] [CrossRef] [PubMed]
- Neumann, I.D.; Wigger, A.; Torner, L.; Holsboer, F.; Landgraf, R. Brain oxytocin inhibits basal and stress-induced activity of the hypothalamo-pituitary-adrenal axis in male and female rats: Partial action within the paraventricular nucleus. J. Neuroendocrinol. 2000, 12, 235–243. [Google Scholar] [CrossRef]
- Cavanaugh, J.; Carp, S.B.; Rock, C.M.; French, J.A. Oxytocin modulates behavioral and physiological responses to a stressor in marmoset monkeys. Psychoneuroendocrinology 2016, 66, 22–30. [Google Scholar] [CrossRef]
- Parker, K.J.; Buckmaster, C.L.; Schatzberg, A.F.; Lyons, D.M. Intranasal oxytocin administration attenuates the ACTH stress response in monkeys. Psychoneuroendocrinology 2005, 30, 924–929. [Google Scholar] [CrossRef]
- Ditzen, B.; Schaer, M.; Gabriel, B.; Bodenmann, G.; Ehlert, U.; Heinrichs, M. Intranasal oxytocin increases positive communication and reduces cortisol levels during couple conflict. Biol. Psychiatry 2009, 65, 728–731. [Google Scholar] [CrossRef]
- Norman, G.J.; Cacioppo, J.T.; Morris, J.S.; Malarkey, W.B.; Berntson, G.G.; Devries, A.C. Oxytocin increases autonomic cardiac control: Moderation by loneliness. Biol. Psychol. 2011, 86, 174–180. [Google Scholar] [CrossRef]
- Quintana, D.S.; Kemp, A.H.; Alvares, G.A.; Guastella, A.J. A role for autonomic cardiac control in the effects of oxytocin on social behavior and psychiatric illness. Front. Neurosci. 2013, 7, 48. [Google Scholar] [CrossRef] [Green Version]
- Kasahara, Y.; Sato, K.; Takayanagi, Y.; Mizukami, H.; Ozawa, K.; Hidema, S.; So, K.H.; Kawada, T.; Inoue, N.; Ikeda, I.; et al. Oxytocin receptor in the hypothalamus is sufficient to rescue normal thermoregulatory function in male oxytocin receptor knockout mice. Endocrinology 2013, 154, 4305–4315. [Google Scholar] [CrossRef] [Green Version]
- Takayanagi, Y.; Kasahara, Y.; Onaka, T.; Takahashi, N.; Kawada, T.; Nishimori, K. Oxytocin receptor-deficient mice developed late-onset obesity. Neuroreport 2008, 19, 951–955. [Google Scholar] [CrossRef] [PubMed]
- Imami, A.S.; O’Donovan, S.M.; Creeden, J.F.; Wu, X.; Eby, H.; McCullumsmith, C.B.; Uvnäs-Moberg, K.; McCullumsmith, R.E.; Andari, E. Oxytocin’s anti-inflammatory and proimmune functions in COVID-19: A transcriptomic signature-based approach. Physiol. Genom. 2020, 52, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Buemann, B.; Marazziti, D.; Uvnäs-Moberg, K. Can intravenous oxytocin infusion counteract hyperinflammation in COVID-19 infected patients? World J. Biol. Psychiatry 2021, 22, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Friuli, M.; Eramo, B.; Valenza, M.; Scuderi, C.; Provensi, G.; Romano, A. Targeting the Oxytocinergic System: A Possible Pharmacological Strategy for the Treatment of Inflammation Occurring in Different Chronic Diseases. Int. J. Mol. Sci. 2021, 22, 10250. [Google Scholar] [CrossRef]
- Neumann, I.D.; Slattery, D.A. Oxytocin in General Anxiety and Social Fear: A Translational Approach. Biol. Psychiatry 2016, 79, 213–221. [Google Scholar] [CrossRef] [Green Version]
- Baldi, E.; Costa, A.; Rani, B.; Passani, M.B.; Blandina, P.; Romano, A.; Provensi, G. Oxytocin and Fear Memory Extinction: Possible Implications for the Therapy of Fear Disorders? Int. J. Mol. Sci. 2021, 22, 10000. [Google Scholar] [CrossRef]
- Janeček, M.; Dabrowska, J. Oxytocin facilitates adaptive fear and attenuates anxiety responses in animal models and human studies-potential interaction with the corticotropin-releasing factor (CRF) system in the bed nucleus of the stria terminalis (BNST). Cell Tissue Res. 2019, 375, 143–172. [Google Scholar] [CrossRef]
- Amico, J.A.; Mantella, R.C.; Vollmer, R.R.; Li, X. Anxiety and stress responses in female oxytocin deficient mice. J. Neuroendocrinol. 2004, 16, 319–324. [Google Scholar] [CrossRef]
- Yoshida, M.; Takayanagi, Y.; Inoue, K.; Kimura, T.; Young, L.J.; Onaka, T.; Nishimori, K. Evidence that oxytocin exerts anxiolytic effects via oxytocin receptor expressed in serotonergic neurons in mice. J. Neurosci. 2009, 29, 2259–2271. [Google Scholar] [CrossRef]
- Heinrichs, M.; Baumgartner, T.; Kirschbaum, C.; Ehlert, U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol. Psychiatry 2003, 54, 1389–1398. [Google Scholar] [CrossRef]
- Domes, G.; Heinrichs, M.; Gläscher, J.; Büchel, C.; Braus, D.F.; Herpertz, S.C. Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biol. Psychiatry 2007, 62, 1187–1190. [Google Scholar] [CrossRef]
- Kanat, M.; Heinrichs, M.; Mader, I.; van Elst, L.T.; Domes, G. Oxytocin Modulates Amygdala Reactivity to Masked Fearful Eyes. Neuropsychopharmacology 2015, 40, 2632–2638. [Google Scholar] [CrossRef] [Green Version]
- Kanat, M.; Heinrichs, M.; Schwarzwald, R.; Domes, G. Oxytocin attenuates neural reactivity to masked threat cues from the eyes. Neuropsychopharmacology 2015, 40, 287–295. [Google Scholar] [CrossRef] [Green Version]
- Kirsch, P.; Esslinger, C.; Chen, Q.; Mier, D.; Lis, S.; Siddhanti, S.; Gruppe, H.; Mattay, V.S.; Gallhofer, B.; Meyer-Lindenberg, A. Oxytocin modulates neural circuitry for social cognition and fear in humans. J. Neurosci. 2005, 25, 11489–11493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quintana, D.S.; Westlye, L.T.; Alnæs, D.; Rustan, Ø.G.; Kaufmann, T.; Smerud, K.T.; Mahmoud, R.A.; Djupesland, P.G.; Andreassen, O.A. Low dose intranasal oxytocin delivered with Breath Powered device dampens amygdala response to emotional stimuli: A peripheral effect-controlled within-subjects randomized dose-response fMRI trial. Psychoneuroendocrinology 2016, 69, 180–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kreuder, A.K.; Scheele, D.; Schultz, J.; Hennig, J.; Marsh, N.; Dellert, T.; Ettinger, U.; Philipsen, A.; Babasiz, M.; Herscheid, A.; et al. Common and dissociable effects of oxytocin and lorazepam on the neurocircuitry of fear. Proc. Natl. Acad. Sci. USA 2020, 117, 11781–11787. [Google Scholar] [CrossRef] [PubMed]
- Labuschagne, I.; Phan, K.L.; Wood, A.; Angstadt, M.; Chua, P.; Heinrichs, M.; Stout, J.C.; Nathan, P.J. Oxytocin attenuates amygdala reactivity to fear in generalized social anxiety disorder. Neuropsychopharmacology 2010, 35, 2403–2413. [Google Scholar] [CrossRef] [Green Version]
- Holt-Lunstad, J.; Smith, T.B.; Layton, J.B. Social relationships and mortality risk: A meta-analytic review. PLoS Med. 2010, 7, e1000316. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Lindenberg, A.; Tost, H. Neural mechanisms of social risk for psychiatric disorders. Nat. Neurosci. 2012, 15, 663–668. [Google Scholar] [CrossRef]
- Hostinar, C.E.; Sullivan, R.M.; Gunnar, M.R. Psychobiological mechanisms underlying the social buffering of the hypothalamic-pituitary-adrenocortical axis: A review of animal models and human studies across development. Psychol. Bull. 2014, 140, 256–282. [Google Scholar] [CrossRef]
- Smith, A.S.; Wang, Z. Hypothalamic oxytocin mediates social buffering of the stress response. Biol. Psychiatry 2014, 76, 281–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sicorello, M.; Dieckmann, L.; Moser, D.; Lux, V.; Luhmann, M.; Schlotz, W.; Kumsta, R. Oxytocin and the stress buffering effect of social company: A genetic study in daily life. Soc. Cogn. Affect. Neurosci. 2020, 15, 293–301. [Google Scholar] [CrossRef]
- Burkett, J.P.; Andari, E.; Johnson, Z.V.; Curry, D.C.; de Waal, F.B.; Young, L.J. Oxytocin-dependent consolation behavior in rodents. Science 2016, 351, 375–378. [Google Scholar] [CrossRef] [Green Version]
- Li, L.F.; Yuan, W.; He, Z.X.; Wang, L.M.; Jing, X.Y.; Zhang, J.; Yang, Y.; Guo, Q.Q.; Zhang, X.N.; Cai, W.Q.; et al. Involvement of oxytocin and GABA in consolation behavior elicited by socially defeated individuals in mandarin voles. Psychoneuroendocrinology 2019, 103, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Yoshida, M.; Jayathilake, B.W.; Inutsuka, A.; Nishimori, K.; Takayanagi, Y.; Onaka, T. Indispensable role of the oxytocin receptor for allogrooming toward socially distressed cage mates in female mice. J. Neuroendocrinol. 2021, 33, e12980. [Google Scholar] [CrossRef] [PubMed]
- Scatliffe, N.; Casavant, S.; Vittner, D.; Cong, X. Oxytocin and early parent-infant interactions: A systematic review. Int. J. Nurs. Sci. 2019, 6, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Waldherr, M.; Neumann, I.D. Centrally released oxytocin mediates mating-induced anxiolysis in male rats. Proc. Natl. Acad. Sci. USA 2007, 104, 16681–16684. [Google Scholar] [CrossRef] [Green Version]
- Phan, J.; Alhassen, L.; Argelagos, A.; Alhassen, W.; Vachirakorntong, B.; Lin, Z.; Sanathara, N.; Alachkar, A. Mating and parenting experiences sculpture mood-modulating effects of oxytocin-MCH signaling. Sci. Rep. 2020, 10, 13611. [Google Scholar] [CrossRef]
- Detillion, C.E.; Craft, T.K.; Glasper, E.R.; Prendergast, B.J.; DeVries, A.C. Social facilitation of wound healing. Psychoneuroendocrinology 2004, 29, 1004–1011. [Google Scholar] [CrossRef]
- Ditzen, B.; Heinrichs, M. Psychobiology of social support: The social dimension of stress buffering. Restor. Neurol. Neurosci. 2014, 32, 149–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, F.S.; Kumsta, R.; von Dawans, B.; Monakhov, M.; Ebstein, R.P.; Heinrichs, M. Common oxytocin receptor gene (OXTR) polymorphism and social support interact to reduce stress in humans. Proc. Natl. Acad. Sci. USA 2011, 108, 19937–19942. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.S.; Sherman, D.K.; Sasaki, J.Y.; Xu, J.; Chu, T.Q.; Ryu, C.; Suh, E.M.; Graham, K.; Taylor, S.E. Culture, distress, and oxytocin receptor polymorphism (OXTR) interact to influence emotional support seeking. Proc. Natl. Acad. Sci. USA 2010, 107, 15717–15721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, S.; Slattery, D.A.; Uschold-Schmidt, N.; Reber, S.O.; Neumann, I.D. Dose-dependent effects of chronic central infusion of oxytocin on anxiety, oxytocin receptor binding and stress-related parameters in mice. Psychoneuroendocrinology 2014, 42, 225–236. [Google Scholar] [CrossRef]
- Slattery, D.A.; Neumann, I.D. Chronic icv oxytocin attenuates the pathological high anxiety state of selectively bred Wistar rats. Neuropharmacology 2010, 58, 56–61. [Google Scholar] [CrossRef]
- Bosch, O.J.; Neumann, I.D. Brain vasopressin is an important regulator of maternal behavior independent of dams’ trait anxiety. Proc. Natl. Acad. Sci. USA 2008, 105, 17139–17144. [Google Scholar] [CrossRef] [Green Version]
- Guzmán, Y.F.; Tronson, N.C.; Jovasevic, V.; Sato, K.; Guedea, A.L.; Mizukami, H.; Nishimori, K.; Radulovic, J. Fear-enhancing effects of septal oxytocin receptors. Nat. Neurosci. 2013, 16, 1185–1187. [Google Scholar] [CrossRef]
- Winter, J.; Meyer, M.; Berger, I.; Royer, M.; Bianchi, M.; Kuffner, K.; Peters, S.; Stang, S.; Langgartner, D.; Hartmann, F.; et al. Chronic oxytocin-driven alternative splicing of Crfr2alpha induces anxiety (published online ahead of print, 25 May 2021). Mol. Psychiatry 2021. [Google Scholar] [CrossRef]
- Fischer-Shofty, M.; Shamay-Tsoory, S.G.; Harari, H.; Levkovitz, Y. The effect of intranasal administration of oxytocin on fear recognition. Neuropsychologia 2010, 48, 179–184. [Google Scholar] [CrossRef]
- Striepens, N.; Scheele, D.; Kendrick, K.M.; Becker, B.; Schäfer, L.; Schwalba, K.; Reul, J.; Maier, W.; Hurlemann, R. Oxytocin facilitates protective responses to aversive social stimuli in males. Proc. Natl. Acad. Sci. USA 2012, 109, 18144–18149. [Google Scholar] [CrossRef] [Green Version]
- Domes, G.; Lischke, A.; Berger, C.; Grossmann, A.; Hauenstein, K.; Heinrichs, M.; Herpertz, S.C. Effects of intranasal oxytocin on emotional face processing in women. Psychoneuroendocrinology 2010, 35, 83–93. [Google Scholar] [CrossRef]
- De Cagna, F.; Fusar-Poli, L.; Damiani, S.; Rocchetti, M.; Giovanna, G.; Mori, A.; Politi, P.; Brondino, N. The Role of Intranasal Oxytocin in Anxiety and Depressive Disorders: A Systematic Review of Randomized Controlled Trials. Clin. Psychopharmacol. Neurosci. 2019, 17, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Onaka, T.; Takayanagi, Y. The oxytocin system and early-life experience-dependent plastic changes. J. Neuroendocrinol. 2021, 33, e13049. [Google Scholar] [CrossRef] [PubMed]
- Grinevich, V.; Neumann, I.D. Brain oxytocin: How puzzle stones from animal studies translate into psychiatry. Mol. Psychiatry 2021, 26, 265–279. [Google Scholar] [CrossRef]
- Sabihi, S.; Dong, S.M.; Maurer, S.D.; Post, C.; Leuner, B. Oxytocin in the medial prefrontal cortex attenuates anxiety: Anatomical and receptor specificity and mechanism of action. Neuropharmacology 2017, 125, 1–12. [Google Scholar] [CrossRef]
- Li, K.; Nakajima, M.; Ibañez-Tallon, I.; Heintz, N. A Cortical Circuit for Sexually Dimorphic Oxytocin-Dependent Anxiety Behaviors. Cell 2016, 167, 60–72.e11. [Google Scholar] [CrossRef] [Green Version]
- He, Z.; Young, L.; Ma, X.M.; Guo, Q.; Wang, L.; Yang, Y.; Luo, L.; Yuan, W.; Li, L.; Zhang, J.; et al. Increased anxiety and decreased sociability induced by paternal deprivation involve the PVN-PrL OTergic pathway. Elife 2019, 8, e44026. [Google Scholar] [CrossRef]
- Lahoud, N.; Maroun, M. Oxytocinergic manipulations in corticolimbic circuit differentially affect fear acquisition and extinction. Psychoneuroendocrinology 2013, 38, 2184–2195. [Google Scholar] [CrossRef]
- Brill-Maoz, N.; Maroun, M. Extinction of fear is facilitated by social presence: Synergism with prefrontal oxytocin. Psychoneuroendocrinology 2016, 66, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Li, X.H.; Matsuura, T.; Xue, M.; Chen, Q.Y.; Liu, R.H.; Lu, J.S.; Shi, W.; Fan, K.; Zhou, Z.; Miao, Z.; et al. Oxytocin in the anterior cingulate cortex attenuates neuropathic pain and emotional anxiety by inhibiting presynaptic long-term potentiation. Cell Rep. 2021, 36, 109411. [Google Scholar] [CrossRef]
- Luo, L.; Becker, B.; Geng, Y.; Zhao, Z.; Gao, S.; Zhao, W.; Yao, S.; Zheng, X.; Ma, X.; Gao, Z.; et al. Sex-dependent neural effect of oxytocin during subliminal processing of negative emotion faces. Neuroimage 2017, 162, 127–137. [Google Scholar] [CrossRef]
- Martinon, D.; Lis, P.; Roman, A.N.; Tornesi, P.; Applebey, S.V.; Buechner, G.; Olivera, V.; Dabrowska, J. Oxytocin receptors in the dorsolateral bed nucleus of the stria terminalis (BNST) bias fear learning toward temporally predictable cued fear. Transl. Psychiatry 2019, 9, 140. [Google Scholar] [CrossRef]
- Moaddab, M.; Dabrowska, J. Oxytocin receptor neurotransmission in the dorsolateral bed nucleus of the stria terminalis facilitates the acquisition of cued fear in the fear-potentiated startle paradigm in rats. Neuropharmacology 2017, 121, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Duque-Wilckens, N.; Torres, L.Y.; Yokoyama, S.; Minie, V.A.; Tran, A.M.; Petkova, S.P.; Hao, R.; Ramos-Maciel, S.; Rios, R.A.; Jackson, K.; et al. Extrahypothalamic oxytocin neurons drive stress-induced social vigilance and avoidance. Proc. Natl. Acad. Sci. USA 2020, 117, 26406–26413. [Google Scholar] [CrossRef]
- Blume, A.; Bosch, O.J.; Miklos, S.; Torner, L.; Wales, L.; Waldherr, M.; Neumann, I.D. Oxytocin reduces anxiety via ERK1/2 activation: Local effect within the rat hypothalamic paraventricular nucleus. Eur. J. Neurosci. 2008, 27, 1947–1956. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.S.; Tabbaa, M.; Lei, K.; Eastham, P.; Butler, M.J.; Linton, L.; Altshuler, R.; Liu, Y.; Wang, Z. Local oxytocin tempers anxiety by activating GABAA receptors in the hypothalamic paraventricular nucleus. Psychoneuroendocrinology 2016, 63, 50–58. [Google Scholar] [CrossRef] [Green Version]
- Pati, D.; Harden, S.W.; Sheng, W.; Kelly, K.B.; de Kloet, A.D.; Krause, E.G.; Frazier, C.J. Endogenous oxytocin inhibits hypothalamic corticotrophin-releasing hormone neurones following acute hypernatraemia. J. Neuroendocrinol. 2020, 32, e12839. [Google Scholar] [CrossRef] [PubMed]
- Grillon, C.; Krimsky, M.; Charney, D.R.; Vytal, K.; Ernst, M.; Cornwell, B. Oxytocin increases anxiety to unpredictable threat. Mol. Psychiatry 2013, 18, 958–960. [Google Scholar] [CrossRef]
- Huang, T.; Guan, F.; Licinio, J.; Wong, M.L.; Yang, Y. Activation of septal OXTr neurons induces anxiety- but not depressive-like behaviors (published online ahead of print, 6 September 2021). Mol. Psychiatry 2021. [Google Scholar] [CrossRef]
- Zoicas, I.; Slattery, D.A.; Neumann, I.D. Brain oxytocin in social fear conditioning and its extinction: Involvement of the lateral septum. Neuropsychopharmacology 2014, 39, 3027–3035. [Google Scholar] [CrossRef] [Green Version]
- Menon, R.; Grund, T.; Zoicas, I.; Althammer, F.; Fiedler, D.; Biermeier, V.; Bosch, O.J.; Hiraoka, Y.; Nishimori, K.; Eliava, M.; et al. Oxytocin Signaling in the Lateral Septum Prevents Social Fear during Lactation. Curr. Biol. 2018, 28, 1066–1078.e6. [Google Scholar] [CrossRef] [Green Version]
- Han, R.T.; Kim, Y.B.; Park, E.H.; Kim, J.Y.; Ryu, C.; Kim, H.Y.; Lee, J.; Pahk, K.; Shanyu, C.; Kim, H.; et al. Long-Term Isolation Elicits Depression and Anxiety-Related Behaviors by Reducing Oxytocin-Induced GABAergic Transmission in Central Amygdala. Front. Mol. Neurosci. 2018, 11, 246. [Google Scholar] [CrossRef] [Green Version]
- Bale, T.L.; Davis, A.M.; Auger, A.P.; Dorsa, D.M.; McCarthy, M.M. CNS region-specific oxytocin receptor expression: Importance in regulation of anxiety and sex behavior. J. Neurosci. 2001, 21, 2546–2552. [Google Scholar] [CrossRef] [PubMed]
- Knobloch, H.S.; Charlet, A.; Hoffmann, L.C.; Eliava, M.; Khrulev, S.; Cetin, A.H.; Osten, P.; Schwarz, M.K.; Seeburg, P.H.; Stoop, R.; et al. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron 2012, 73, 553–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viviani, D.; Charlet, A.; van den Burg, E.; Robinet, C.; Hurni, N.; Abatis, M.; Magara, F.; Stoop, R. Oxytocin selectively gates fear responses through distinct outputs from the central amygdala. Science 2011, 333, 104–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rickenbacher, E.; Perry, R.E.; Sullivan, R.M.; Moita, M.A. Freezing suppression by oxytocin in central amygdala allows alternate defensive behaviours and mother-pup interactions. Elife 2017, 6, e24080. [Google Scholar] [CrossRef]
- Ferretti, V.; Maltese, F.; Contarini, G.; Nigro, M.; Bonavia, A.; Huang, H.; Gigliucci, V.; Morelli, G.; Scheggia, D.; Managò, F.; et al. Oxytocin Signaling in the Central Amygdala Modulates Emotion Discrimination in Mice. Curr. Biol. 2019, 29, 1938–1953.e6. [Google Scholar] [CrossRef]
- Pagani, J.H.; Williams Avram, S.K.; Cui, Z.; Song, J.; Mezey, É.; Senerth, J.M.; Baumann, M.H.; Young, W.S. Raphe serotonin neuron-specific oxytocin receptor knockout reduces aggression without affecting anxiety-like behavior in male mice only. Genes Brain Behav. 2015, 14, 167–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.; Travagli, R.A. Hypothalamic-vagal oxytocinergic neurocircuitry modulates gastric emptying and motility following stress. J. Physiol. 2020, 598, 4941–4955. [Google Scholar] [CrossRef] [PubMed]
- Schulkin, J.; Sterling, P. Allostasis: A Brain-Centered, Predictive Mode of Physiological Regulation. Trends Neurosci. 2019, 42, 740–752. [Google Scholar] [CrossRef]
- Ferris, C.F.; Foote, K.B.; Meltser, H.M.; Plenby, M.G.; Smith, K.L.; Insel, T.R. Oxytocin in the amygdala facilitates maternal aggression. Ann. N. Y. Acad. Sci. 1992, 652, 456–457. [Google Scholar] [CrossRef]
- De Dreu, C.K. Oxytocin modulates cooperation within and competition between groups: An integrative review and research agenda. Horm. Behav. 2012, 61, 419–428. [Google Scholar] [CrossRef]
- Stallen, M.; De Dreu, C.K.; Shalvi, S.; Smidts, A.; Sanfey, A.G. The herding hormone: Oxytocin stimulates in-group conformity. Psychol. Sci. 2012, 23, 1288–1292. [Google Scholar] [CrossRef]
- Zhang, H.; Gross, J.; De Dreu, C.; Ma, Y. Oxytocin promotes coordinated out-group attack during intergroup conflict in humans. Elife 2019, 8, e40698. [Google Scholar] [CrossRef]
- Schaller, F.; Watrin, F.; Sturny, R.; Massacrier, A.; Szepetowski, P.; Muscatelli, F. A single postnatal injection of oxytocin rescues the lethal feeding behaviour in mouse newborns deficient for the imprinted Magel2 gene. Hum. Mol. Genet. 2010, 19, 4895–4905. [Google Scholar] [CrossRef] [PubMed]
- Quintana, D.S.; Guastella, A.J. An Allostatic Theory of Oxytocin. Trends Cogn. Sci. 2020, 24, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Grinevich, V.; Stoop, R. Interplay between Oxytocin and Sensory Systems in the Orchestration of Socio-Emotional Behaviors. Neuron 2018, 99, 887–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oettl, L.L.; Ravi, N.; Schneider, M.; Scheller, M.F.; Schneider, P.; Mitre, M.; da Silva Gouveia, M.; Froemke, R.C.; Chao, M.V.; Young, W.S.; et al. Oxytocin Enhances Social Recognition by Modulating Cortical Control of Early Olfactory Processing. Neuron 2016, 90, 609–621. [Google Scholar] [CrossRef] [Green Version]
- Marlin, B.J.; Mitre, M.; D’amour J, A.; Chao, M.V.; Froemke, R.C. Oxytocin enables maternal behaviour by balancing cortical inhibition. Nature 2015, 520, 499–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kapetaniou, G.E.; Reinhard, M.A.; Christian, P.; Jobst, A.; Tobler, P.N.; Padberg, F.; Soutschek, A. The role of oxytocin in delay of gratification and flexibility in non-social decision making. Elife 2021, 10, e61844. [Google Scholar] [CrossRef] [PubMed]
- Roberts, B.Z.; Young, J.W.; He, Y.V.; Cope, Z.A.; Shilling, P.D.; Feifel, D. Oxytocin improves probabilistic reversal learning but not effortful motivation in Brown Norway rats. Neuropharmacology 2019, 150, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Sala, M.; Braida, D.; Lentini, D.; Busnelli, M.; Bulgheroni, E.; Capurro, V.; Finardi, A.; Donzelli, A.; Pattini, L.; Rubino, T.; et al. Pharmacologic rescue of impaired cognitive flexibility, social deficits, increased aggression, and seizure susceptibility in oxytocin receptor null mice: A neurobehavioral model of autism. Biol. Psychiatry 2011, 69, 875–882. [Google Scholar] [CrossRef]
- Eckstein, M.; Scheele, D.; Patin, A.; Preckel, K.; Becker, B.; Walter, A.; Domschke, K.; Grinevich, V.; Maier, W.; Hurlemann, R. Oxytocin Facilitates Pavlovian Fear Learning in Males. Neuropsychopharmacology 2016, 41, 932–939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terburg, D.; Scheggia, D.; Triana Del Rio, R.; Klumpers, F.; Ciobanu, A.C.; Morgan, B.; Montoya, E.R.; Bos, P.A.; Giobellina, G.; van den Burg, E.H.; et al. The Basolateral Amygdala Is Essential for Rapid Escape: A Human and Rodent Study. Cell 2018, 175, 723–735.e16. [Google Scholar] [CrossRef] [Green Version]
- Robinson, D.A.; Wei, F.; Wang, G.D.; Li, P.; Kim, S.J.; Vogt, S.K.; Muglia, L.J.; Zhuo, M. Oxytocin mediates stress-induced analgesia in adult mice. J. Physiol. 2002, 540, 593–606. [Google Scholar] [CrossRef]
- Li, Y.X.; An, H.; Wen, Z.; Tao, Z.Y.; Cao, D.Y. Can oxytocin inhibit stress-induced hyperalgesia? Neuropeptides 2020, 79, 101996. [Google Scholar] [CrossRef]
- Rash, J.A.; Aguirre-Camacho, A.; Campbell, T.S. Oxytocin and pain: A systematic review and synthesis of findings. Clin. J. Pain 2014, 30, 453–462. [Google Scholar] [CrossRef]
- Mazzuca, M.; Minlebaev, M.; Shakirzyanova, A.; Tyzio, R.; Taccola, G.; Janackova, S.; Gataullina, S.; Ben-Ari, Y.; Giniatullin, R.; Khazipov, R. Newborn Analgesia Mediated by Oxytocin during Delivery. Front. Cell. Neurosci. 2011, 5, 3. [Google Scholar] [CrossRef] [Green Version]
- Tyzio, R.; Cossart, R.; Khalilov, I.; Minlebaev, M.; Hubner, C.A.; Represa, A.; Ben-Ari, Y.; Khazipov, R. Maternal oxytocin triggers a transient inhibitory switch in GABA signaling in the fetal brain during delivery. Science 2006, 314, 1788–1792. [Google Scholar] [CrossRef] [Green Version]
- Bosch, O.J. Maternal aggression in rodents: Brain oxytocin and vasopressin mediate pup defence. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20130085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srisawat, R.; Ludwig, M.; Bull, P.M.; Douglas, A.J.; Russell, J.A.; Leng, G. Nitric oxide and the oxytocin system in pregnancy. J. Neurosci. 2000, 20, 6721–6727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunton, P.J.; Bales, J.; Russell, J.A. Allopregnanolone and induction of endogenous opioid inhibition of oxytocin responses to immune stress in pregnant rats. J. Neuroendocrinol. 2012, 24, 690–700. [Google Scholar] [CrossRef]
- Leng, G.; Russell, J.A. The osmoresponsiveness of oxytocin and vasopressin neurones: Mechanisms, allostasis and evolution. J. Neuroendocrinol. 2019, 31, e12662. [Google Scholar] [CrossRef]
- Armstrong, W.E.; Hatton, G.I. The puzzle of pulsatile oxytocin secretion during lactation: Some new pieces. Am. J. Physiol. Integr. Comp. Physiol. 2006, 291, R26–R28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uvnäs-Moberg, K.; Prime, D.K. Oxytocin effects in mothers and infants during breastfeeding. Infant 2013, 9, 201–206. [Google Scholar]
- Zhang, G.; Cai, D. Circadian intervention of obesity development via resting-stage feeding manipulation or oxytocin treatment. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E1004–E1012. [Google Scholar] [CrossRef]
- Yamashita, M.; Takayanagi, Y.; Yoshida, M.; Nishimori, K.; Kusama, M.; Onaka, T. Involvement of prolactin-releasing peptide in the activation of oxytocin neurones in response to food intake. J. Neuroendocrinol. 2013, 25, 455–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.; Coleman, F.H.; Kopenhaver Doheny, K.; Travagli, R.A. Stress Adaptation Upregulates Oxytocin within Hypothalamo-Vagal Neurocircuits. Neuroscience 2018, 390, 198–205. [Google Scholar] [CrossRef]
- Babić, R.; Babić, M.; Rastović, P.; Ćurlin, M.; Šimić, J.; Mandić, K.; Pavlović, K. Resilience in Health and Illness. Psychiatr. Danub. 2020, 32, 226–232. [Google Scholar]
- Sharma, S.R.; Gonda, X.; Dome, P.; Tarazi, F.I. What’s Love Got to do with it: Role of oxytocin in trauma, attachment and resilience. Pharmacol. Ther. 2020, 214, 107602. [Google Scholar] [CrossRef] [PubMed]
- Cathomas, F.; Murrough, J.W.; Nestler, E.J.; Han, M.H.; Russo, S.J. Neurobiology of Resilience: Interface Between Mind and Body. Biol. Psychiatry 2019, 86, 410–420. [Google Scholar] [CrossRef]
- Feldman, R. What is resilience: An affiliative neuroscience approach. World Psychiatry 2020, 19, 132–150. [Google Scholar] [CrossRef]
- Walker, S.C.; Cavieres, A.; Peñaloza-Sancho, V.; El-Deredy, W.; McGlone, F.P.; Dagnino-Subiabre, A. C-low threshold mechanoafferent targeted dynamic touch modulates stress resilience in rats exposed to chronic mild stress (published online ahead of print, 27 August 2020). Eur. J. Neurosci. 2020. [Google Scholar] [CrossRef]
- Okabe, S.; Yoshida, M.; Takayanagi, Y.; Onaka, T. Activation of hypothalamic oxytocin neurons following tactile stimuli in rats. Neurosci. Lett. 2015, 600, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Okabe, S.; Takayanagi, Y.; Yoshida, M.; Onaka, T. Gentle stroking stimuli induce affiliative responsiveness to humans in male rats. Sci. Rep. 2020, 10, 9135. [Google Scholar] [CrossRef]
- Okabe, S.; Takayanagi, Y.; Yoshida, M.; Onaka, T. Post-weaning stroking stimuli induce affiliative behavior toward humans and influence brain activity in female rats. Sci. Rep. 2021, 11, 3805. [Google Scholar] [CrossRef]
- Faraji, J.; Karimi, M.; Soltanpour, N.; Moharrerie, A.; Rouhzadeh, Z.; Lotfi, H.; Hosseini, S.A.; Jafari, S.Y.; Roudaki, S.; Moeeini, R.; et al. Oxytocin-mediated social enrichment promotes longer telomeres and novelty seeking. Elife 2018, 7, e40262. [Google Scholar] [CrossRef]
- Wood, S.K. Individual differences in the neurobiology of social stress: Implications for depression-cardiovascular disease comorbidity. Curr. Neuropharmacol. 2014, 12, 205–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calpe-López, C.; García-Pardo, M.P.; Martínez-Caballero, M.A.; Santos-Ortíz, A.; Aguilar, M.A. Behavioral Traits Associated With Resilience to the Effects of Repeated Social Defeat on Cocaine-Induced Conditioned Place Preference in Mice. Front. Behav. Neurosci. 2019, 13, 278. [Google Scholar] [CrossRef]
- Shi, D.D.; Zhang, Y.D.; Ren, Y.Y.; Peng, S.Y.; Yuan, T.F.; Wang, Z. Predictable maternal separation confers adult stress resilience via the medial prefrontal cortex oxytocin signaling pathway in rats (published online ahead of print, 24 September 2021). Mol. Psychiatry 2021. [Google Scholar] [CrossRef]
- Bardo, M.T.; Hammerslag, L.R.; Malone, S.G. Effect of early life social adversity on drug abuse vulnerability: Focus on corticotropin-releasing factor and oxytocin. Neuropharmacology 2021, 191, 108567. [Google Scholar] [CrossRef] [PubMed]
- Barrett, C.E.; Arambula, S.E.; Young, L.J. The oxytocin system promotes resilience to the effects of neonatal isolation on adult social attachment in female prairie voles. Transl. Psychiatry 2015, 5, e606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mairesse, J.; Zinni, M.; Pansiot, J.; Hassan-Abdi, R.; Demene, C.; Colella, M.; Charriaut-Marlangue, C.; Rideau Batista Novais, A.; Tanter, M.; Maccari, S.; et al. Oxytocin receptor agonist reduces perinatal brain damage by targeting microglia. Glia 2019, 67, 345–359. [Google Scholar] [CrossRef]
- Amini-Khoei, H.; Mohammadi-Asl, A.; Amiri, S.; Hosseini, M.J.; Momeny, M.; Hassanipour, M.; Rastegar, M.; Haj-Mirzaian, A.; Mirzaian, A.H.; Sanjarimoghaddam, H.; et al. Oxytocin mitigated the depressive-like behaviors of maternal separation stress through modulating mitochondrial function and neuroinflammation. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 76, 169–178. [Google Scholar] [CrossRef]
- Ji, H.; Su, W.; Zhou, R.; Feng, J.; Lin, Y.; Zhang, Y.; Wang, X.; Chen, X.; Li, J. Intranasal oxytocin administration improves depression-like behaviors in adult rats that experienced neonatal maternal deprivation. Behav. Pharmacol. 2016, 27, 689–696. [Google Scholar] [CrossRef]
- Mansouri, M.; Pouretemad, H.; Roghani, M.; Wegener, G.; Ardalan, M. Autistic-like behaviours and associated brain structural plasticity are modulated by oxytocin in maternally separated rats. Behav. Brain Res. 2020, 393, 112756. [Google Scholar] [CrossRef] [PubMed]
- Joushi, S.; Esmaeilpour, K.; Masoumi-Ardakani, Y.; Esmaeili-Mahani, S.; Sheibani, V. Intranasal oxytocin administration facilitates the induction of long-term potentiation and promotes cognitive performance of maternally separated rats. Psychoneuroendocrinology 2021, 123, 105044. [Google Scholar] [CrossRef]
- Reguilón, M.D.; Ferrer-Pérez, C.; Miñarro, J.; Rodríguez-Arias, M. Oxytocin reverses ethanol consumption and neuroinflammation induced by social defeat in male mice. Horm. Behav. 2021, 127, 104875. [Google Scholar] [CrossRef]
- Lestanova, Z.; Bacova, Z.; Kiss, A.; Havranek, T.; Strbak, V.; Bakos, J. Oxytocin Increases Neurite Length and Expression of Cytoskeletal Proteins Associated with Neuronal Growth. J. Mol. Neurosci. 2016, 59, 184–192. [Google Scholar] [CrossRef]
- Filova, B.; Reichova, A.; Zatkova, M.; Srancikova, A.; Bukatova, S.; Bacova, Z.; Bakos, J. Expression of synaptic proteins in the hippocampus is modulated by neonatal oxytocin treatment. Neurosci. Lett. 2020, 725, 134912. [Google Scholar] [CrossRef]
- Lin, Y.T.; Chen, C.C.; Huang, C.C.; Nishimori, K.; Hsu, K.S. Oxytocin stimulates hippocampal neurogenesis via oxytocin receptor expressed in CA3 pyramidal neurons. Nat. Commun. 2017, 8, 537. [Google Scholar] [CrossRef]
- Bertoni, A.; Schaller, F.; Tyzio, R.; Gaillard, S.; Santini, F.; Xolin, M.; Diabira, D.; Vaidyanathan, R.; Matarazzo, V.; Medina, I.; et al. Oxytocin administration in neonates shapes hippocampal circuitry and restores social behavior in a mouse model of autism (published online ahead of print, 21 July 2021). Mol. Psychiatry 2021. [Google Scholar] [CrossRef]
- Ferrer-Pérez, C.; Reguilón, M.D.; Manzanedo, C.; Miñarro, J.; Rodríguez-Arias, M. Social Housing Conditions Modulate the Long-Lasting Increase in Cocaine Reward Induced by Intermittent Social Defeat. Front. Behav. Neurosci. 2019, 13, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrer-Pérez, C.; Reguilón, M.D.; Miñarro, J.; Rodríguez-Arias, M. Endogenous oxytocin is essential for the buffering effects of pair housing against the increase in cocaine reward induced by social stress. Physiol. Behav. 2020, 221, 112913. [Google Scholar] [CrossRef]
- Hansson, A.C.; Koopmann, A.; Uhrig, S.; Bühler, S.; Domi, E.; Kiessling, E.; Ciccocioppo, R.; Froemke, R.C.; Grinevich, V.; Kiefer, F.; et al. Oxytocin Reduces Alcohol Cue-Reactivity in Alcohol-Dependent Rats and Humans. Neuropsychopharmacology 2018, 43, 1235–1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tunstall, B.J.; Kirson, D.; Zallar, L.J.; McConnell, S.A.; Vendruscolo, J.C.M.; Ho, C.P.; Oleata, C.S.; Khom, S.; Manning, M.; Lee, M.R.; et al. Oxytocin blocks enhanced motivation for alcohol in alcohol dependence and blocks alcohol effects on GABAergic transmission in the central amygdala. PLoS Biol. 2019, 17, e2006421. [Google Scholar] [CrossRef]
- Le Dorze, C.; Borreca, A.; Pignataro, A.; Ammassari-Teule, M.; Gisquet-Verrier, P. Emotional remodeling with oxytocin durably rescues trauma-induced behavioral and neuro-morphological changes in rats: A promising treatment for PTSD. Transl. Psychiatry 2020, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Nair, H.P.; Gutman, A.R.; Davis, M.; Young, L.J. Central oxytocin, vasopressin, and corticotropin-releasing factor receptor densities in the basal forebrain predict isolation potentiated startle in rats. J. Neurosci. 2005, 25, 11479–11488. [Google Scholar] [CrossRef] [Green Version]
- Opacka-Juffry, J.; Mohiyeddini, C. Experience of stress in childhood negatively correlates with plasma oxytocin concentration in adult men. Stress 2012, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bertsch, K.; Schmidinger, I.; Neumann, I.D.; Herpertz, S.C. Reduced plasma oxytocin levels in female patients with borderline personality disorder. Horm. Behav. 2013, 63, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.E.; Bertsch, K.; Bülau, K.; Herpertz, S.C.; Buchheim, A. Emotional neglect in childhood shapes social dysfunctioning in adults by influencing the oxytocin and the attachment system: Results from a population-based study. Int. J. Psychophysiol. 2019, 136, 73–80. [Google Scholar] [CrossRef]
- Young Kuchenbecker, S.; Pressman, S.D.; Celniker, J.; Grewen, K.M.; Sumida, K.D.; Jonathan, N.; Everett, B.; Slavich, G.M. Oxytocin, cortisol, and cognitive control during acute and naturalistic stress. Stress 2021, 24, 370–383. [Google Scholar] [CrossRef] [PubMed]
- Priel, A.; Djalovski, A.; Zagoory-Sharon, O.; Feldman, R. Maternal depression impacts child psychopathology across the first decade of life: Oxytocin and synchrony as markers of resilience. J. Child Psychol. Psychiatry 2019, 60, 30–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feldman, R.; Vengrober, A.; Ebstein, R.P. Affiliation buffers stress: Cumulative genetic risk in oxytocin-vasopressin genes combines with early caregiving to predict PTSD in war-exposed young children. Transl. Psychiatry 2014, 4, e370. [Google Scholar] [CrossRef] [Green Version]
- Brüne, M. Does the oxytocin receptor (OXTR) polymorphism (rs2254298) confer ‘vulnerability’ for psychopathology or ‘differential susceptibility’? Insights from evolution. BMC Med. 2012, 10, 38. [Google Scholar] [CrossRef] [Green Version]
- Thompson, R.J.; Parker, K.J.; Hallmayer, J.F.; Waugh, C.E.; Gotlib, I.H. Oxytocin receptor gene polymorphism (rs2254298) interacts with familial risk for psychopathology to predict symptoms of depression and anxiety in adolescent girls. Psychoneuroendocrinology 2011, 36, 144–147. [Google Scholar] [CrossRef] [Green Version]
- Womersley, J.S.; Hemmings, S.M.J.; Ziegler, C.; Gutridge, A.; Ahmed-Leitao, F.; Rosenstein, D.; Domschke, K.; Seedat, S. Childhood emotional neglect and oxytocin receptor variants: Association with limbic brain volumes. World J. Biol. Psychiatry 2020, 21, 513–528. [Google Scholar] [CrossRef] [PubMed]
- McQuaid, R.J.; McInnis, O.A.; Stead, J.D.; Matheson, K.; Anisman, H. A paradoxical association of an oxytocin receptor gene polymorphism: Early-life adversity and vulnerability to depression. Front. Neurosci. 2013, 7, 128. [Google Scholar] [CrossRef] [Green Version]
- Bradley, B.; Westen, D.; Mercer, K.B.; Binder, E.B.; Jovanovic, T.; Crain, D.; Wingo, A.; Heim, C. Association between childhood maltreatment and adult emotional dysregulation in a low-income, urban, African American sample: Moderation by oxytocin receptor gene. Dev. Psychopathol. 2011, 23, 439–452. [Google Scholar] [CrossRef] [Green Version]
- Hostinar, C.E.; Cicchetti, D.; Rogosch, F.A. Oxytocin receptor gene polymorphism, perceived social support, and psychological symptoms in maltreated adolescents. Dev. Psychopathol. 2014, 26, 465–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradley, B.; Davis, T.A.; Wingo, A.P.; Mercer, K.B.; Ressler, K.J. Family environment and adult resilience: Contributions of positive parenting and the oxytocin receptor gene. Eur. J. Psychotraumatol. 2013, 4, 21659. [Google Scholar] [CrossRef]
- Sippel, L.M.; Han, S.; Watkins, L.E.; Harpaz-Rotem, I.; Southwick, S.M.; Krystal, J.H.; Olff, M.; Sherva, R.; Farrer, L.A.; Kranzler, H.R.; et al. Oxytocin receptor gene polymorphisms, attachment, and PTSD: Results from the National Health and Resilience in Veterans Study. J. Psychiatr. Res. 2017, 94, 139–147. [Google Scholar] [CrossRef]
- Malhi, G.S.; Das, P.; Outhred, T.; Dobson-Stone, C.; Bell, E.; Gessler, D.; Bryant, R.; Mannie, Z. Interactions of OXTR rs53576 and emotional trauma on hippocampal volumes and perceived social support in adolescent girls. Psychoneuroendocrinology 2020, 115, 104635. [Google Scholar] [CrossRef]
- Milaniak, I.; Cecil, C.A.M.; Barker, E.D.; Relton, C.L.; Gaunt, T.R.; McArdle, W.; Jaffee, S.R. Variation in DNA methylation of the oxytocin receptor gene predicts children’s resilience to prenatal stress. Dev. Psychopathol. 2017, 29, 1663–1674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowen, M.T.; Neumann, I.D. Rebalancing the Addicted Brain: Oxytocin Interference with the Neural Substrates of Addiction. Trends Neurosci. 2017, 40, 691–708. [Google Scholar] [CrossRef] [PubMed]
- King, C.E.; Gano, A.; Becker, H.C. The role of oxytocin in alcohol and drug abuse. Brain Res. 2020, 1736, 146761. [Google Scholar] [CrossRef]
- Joseph, J.E.; McRae-Clark, A.; Sherman, B.J.; Baker, N.L.; Moran-Santa Maria, M.; Brady, K.T. Neural correlates of oxytocin and cue reactivity in cocaine-dependent men and women with and without childhood trauma. Psychopharmacology 2020, 237, 249–261. [Google Scholar] [CrossRef]
- Eidelman-Rothman, M.; Goldstein, A.; Levy, J.; Weisman, O.; Schneiderman, I.; Mankuta, D.; Zagoory-Sharon, O.; Feldman, R. Oxytocin affects spontaneous neural oscillations in trauma-exposed war veterans. Front. Behav. Neurosci. 2015, 9, 165. [Google Scholar] [CrossRef] [Green Version]
- van Zuiden, M.; Frijling, J.L.; Nawijn, L.; Koch, S.B.J.; Goslings, J.C.; Luitse, J.S.; Biesheuvel, T.H.; Honig, A.; Veltman, D.J.; Olff, M. Intranasal Oxytocin to Prevent Posttraumatic Stress Disorder Symptoms: A Randomized Controlled Trial in Emergency Department Patients. Biol. Psychiatry 2017, 81, 1030–1040. [Google Scholar] [CrossRef] [Green Version]
- Sack, M.; Spieler, D.; Wizelman, L.; Epple, G.; Stich, J.; Zaba, M.; Schmidt, U. Intranasal oxytocin reduces provoked symptoms in female patients with posttraumatic stress disorder despite exerting sympathomimetic and positive chronotropic effects in a randomized controlled trial. BMC Med. 2017, 15, 40. [Google Scholar] [CrossRef] [Green Version]
- Grimm, S.; Pestke, K.; Feeser, M.; Aust, S.; Weigand, A.; Wang, J.; Wingenfeld, K.; Pruessner, J.C.; La Marca, R.; Böker, H.; et al. Early life stress modulates oxytocin effects on limbic system during acute psychosocial stress. Soc. Cogn. Affect. Neurosci. 2014, 9, 1828–1835. [Google Scholar] [CrossRef] [Green Version]
- MacDonald, E.; Dadds, M.R.; Brennan, J.L.; Williams, K.; Levy, F.; Cauchi, A.J. A review of safety, side-effects and subjective reactions to intranasal oxytocin in human research. Psychoneuroendocrinology 2011, 36, 1114–1126. [Google Scholar] [CrossRef]
- Verhees, M.; Houben, J.; Ceulemans, E.; Bakermans-Kranenburg, M.J.; van, I.M.H.; Bosmans, G. No side-effects of single intranasal oxytocin administration in middle childhood. Psychopharmacology 2018, 235, 2471–2477. [Google Scholar] [CrossRef] [Green Version]
- Cai, Q.; Feng, L.; Yap, K.Z. Systematic review and meta-analysis of reported adverse events of long-term intranasal oxytocin treatment for autism spectrum disorder. Psychiatry Clin. Neurosci. 2018, 72, 140–151. [Google Scholar] [CrossRef]
- Bowen, M.T.; McGregor, I.S. Oxytocin and vasopressin modulate the social response to threat: A preclinical study. Int. J. Neuropsychopharmacol. 2014, 17, 1621–1633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hicks, C.; Ramos, L.; Reekie, T.; Misagh, G.H.; Narlawar, R.; Kassiou, M.; McGregor, I.S. Body temperature and cardiac changes induced by peripherally administered oxytocin, vasopressin and the non-peptide oxytocin receptor agonist WAY 267,464: A biotelemetry study in rats. Br. J. Pharmacol. 2014, 171, 2868–2887. [Google Scholar] [CrossRef] [Green Version]
- Ramos, L.; Hicks, C.; Kevin, R.; Caminer, A.; Narlawar, R.; Kassiou, M.; McGregor, I.S. Acute prosocial effects of oxytocin and vasopressin when given alone or in combination with 3,4-methylenedioxymethamphetamine in rats: Involvement of the V1A receptor. Neuropsychopharmacology 2013, 38, 2249–2259. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; McCann, K.E.; McNeill, J.K. 4th.; Larkin, T.E., 2nd; Huhman, K.L.; Albers, H.E. Oxytocin induces social communication by activating arginine-vasopressin V1a receptors and not oxytocin receptors. Psychoneuroendocrinology 2014, 50, 14–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.R.; Scheidweiler, K.B.; Diao, X.X.; Akhlaghi, F.; Cummins, A.; Huestis, M.A.; Leggio, L.; Averbeck, B.B. Oxytocin by intranasal and intravenous routes reaches the cerebrospinal fluid in rhesus macaques: Determination using a novel oxytocin assay. Mol. Psychiatry 2018, 23, 115–122. [Google Scholar] [CrossRef]
- Neumann, I.D.; Maloumby, R.; Beiderbeck, D.I.; Lukas, M.; Landgraf, R. Increased brain and plasma oxytocin after nasal and peripheral administration in rats and mice. Psychoneuroendocrinology 2013, 38, 1985–1993. [Google Scholar] [CrossRef] [PubMed]
- Striepens, N.; Kendrick, K.M.; Hanking, V.; Landgraf, R.; Wüllner, U.; Maier, W.; Hurlemann, R. Elevated cerebrospinal fluid and blood concentrations of oxytocin following its intranasal administration in humans. Sci. Rep. 2013, 3, 3440. [Google Scholar] [CrossRef]
- Tzabazis, A.; Kori, S.; Mechanic, J.; Miller, J.; Pascual, C.; Manering, N.; Carson, D.; Klukinov, M.; Spierings, E.; Jacobs, D.; et al. Oxytocin and Migraine Headache. Headache 2017, 57 (Suppl. 2), 64–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quintana, D.S.; Lischke, A.; Grace, S.; Scheele, D.; Ma, Y.; Becker, B. Advances in the field of intranasal oxytocin research: Lessons learned and future directions for clinical research. Mol. Psychiatry 2021, 26, 80–91. [Google Scholar] [CrossRef]
- Leng, G.; Ludwig, M. Intranasal Oxytocin: Myths and Delusions. Biol. Psychiatry 2016, 79, 243–250. [Google Scholar] [CrossRef] [Green Version]
- Ichinose, W.; Cherepanov, S.M.; Shabalova, A.A.; Yokoyama, S.; Yuhi, T.; Yamaguchi, H.; Watanabe, A.; Yamamoto, Y.; Okamoto, H.; Horike, S.; et al. Development of a Highly Potent Analogue and a Long-Acting Analogue of Oxytocin for the Treatment of Social Impairment-Like Behaviors. J. Med. Chem. 2019, 62, 3297–3310. [Google Scholar] [CrossRef]
- Neumann, I.; Landgraf, R.; Takahashi, Y.; Pittman, Q.J.; Russell, J.A. Stimulation of oxytocin release within the supraoptic nucleus and into blood by CCK-8. Am. J. Physiol. 1994, 267, R1626–R1631. [Google Scholar] [CrossRef]
- Sabatier, N.; Caquineau, C.; Dayanithi, G.; Bull, P.; Douglas, A.J.; Guan, X.M.; Jiang, M.; Van der Ploeg, L.; Leng, G. Alpha-melanocyte-stimulating hormone stimulates oxytocin release from the dendrites of hypothalamic neurons while inhibiting oxytocin release from their terminals in the neurohypophysis. J. Neurosci. 2003, 23, 10351–10358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takayanagi, Y.; Yoshida, M.; Takashima, A.; Takanami, K.; Yoshida, S.; Nishimori, K.; Nishijima, I.; Sakamoto, H.; Yamagata, T.; Onaka, T. Activation of Supraoptic Oxytocin Neurons by Secretin Facilitates Social Recognition. Biol. Psychiatry 2017, 81, 243–251. [Google Scholar] [CrossRef] [Green Version]
- Nagasawa, M.; Mitsui, S.; En, S.; Ohtani, N.; Ohta, M.; Sakuma, Y.; Onaka, T.; Mogi, K.; Kikusui, T. Social evolution. Oxytocin-gaze positive loop and the coevolution of human-dog bonds. Science 2015, 348, 333–336. [Google Scholar] [CrossRef]
- Li, Q.; Becker, B.; Wernicke, J.; Chen, Y.; Zhang, Y.; Li, R.; Le, J.; Kou, J.; Zhao, W.; Kendrick, K.M. Foot massage evokes oxytocin release and activation of orbitofrontal cortex and superior temporal sulcus. Psychoneuroendocrinology 2019, 101, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Morhenn, V.; Beavin, L.E.; Zak, P.J. Massage increases oxytocin and reduces adrenocorticotropin hormone in humans. Altern. Ther. Health Med. 2012, 18, 11–18. [Google Scholar] [PubMed]
- Portnova, G.V.; Proskurnina, E.V.; Sokolova, S.V.; Skorokhodov, I.V.; Varlamov, A.A. Perceived pleasantness of gentle touch in healthy individuals is related to salivary oxytocin response and EEG markers of arousal. Exp. Brain Res. 2020, 238, 2257–2268. [Google Scholar] [CrossRef] [PubMed]
- Dukíc, H. Music, Brain Plasticity and the Resilience: The Pillars of New Receptive Therapy. Psychiatr. Danub. 2018, 30, 141–147. [Google Scholar] [PubMed]
- Russo, S.J.; Murrough, J.W.; Han, M.H.; Charney, D.S.; Nestler, E.J. Neurobiology of resilience. Nat. Neurosci. 2012, 15, 1475–1484. [Google Scholar] [CrossRef] [Green Version]
- Ungar, M. Resilience after maltreatment: The importance of social services as facilitators of positive adaptation. Child Abus. Negl. 2013, 37, 110–115. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takayanagi, Y.; Onaka, T. Roles of Oxytocin in Stress Responses, Allostasis and Resilience. Int. J. Mol. Sci. 2022, 23, 150. https://doi.org/10.3390/ijms23010150
Takayanagi Y, Onaka T. Roles of Oxytocin in Stress Responses, Allostasis and Resilience. International Journal of Molecular Sciences. 2022; 23(1):150. https://doi.org/10.3390/ijms23010150
Chicago/Turabian StyleTakayanagi, Yuki, and Tatsushi Onaka. 2022. "Roles of Oxytocin in Stress Responses, Allostasis and Resilience" International Journal of Molecular Sciences 23, no. 1: 150. https://doi.org/10.3390/ijms23010150
APA StyleTakayanagi, Y., & Onaka, T. (2022). Roles of Oxytocin in Stress Responses, Allostasis and Resilience. International Journal of Molecular Sciences, 23(1), 150. https://doi.org/10.3390/ijms23010150





