Functions of COP1/SPA E3 Ubiquitin Ligase Mediated by MpCRY in the Liverwort Marchantia polymorpha under Blue Light
Abstract
:1. Introduction
2. Results
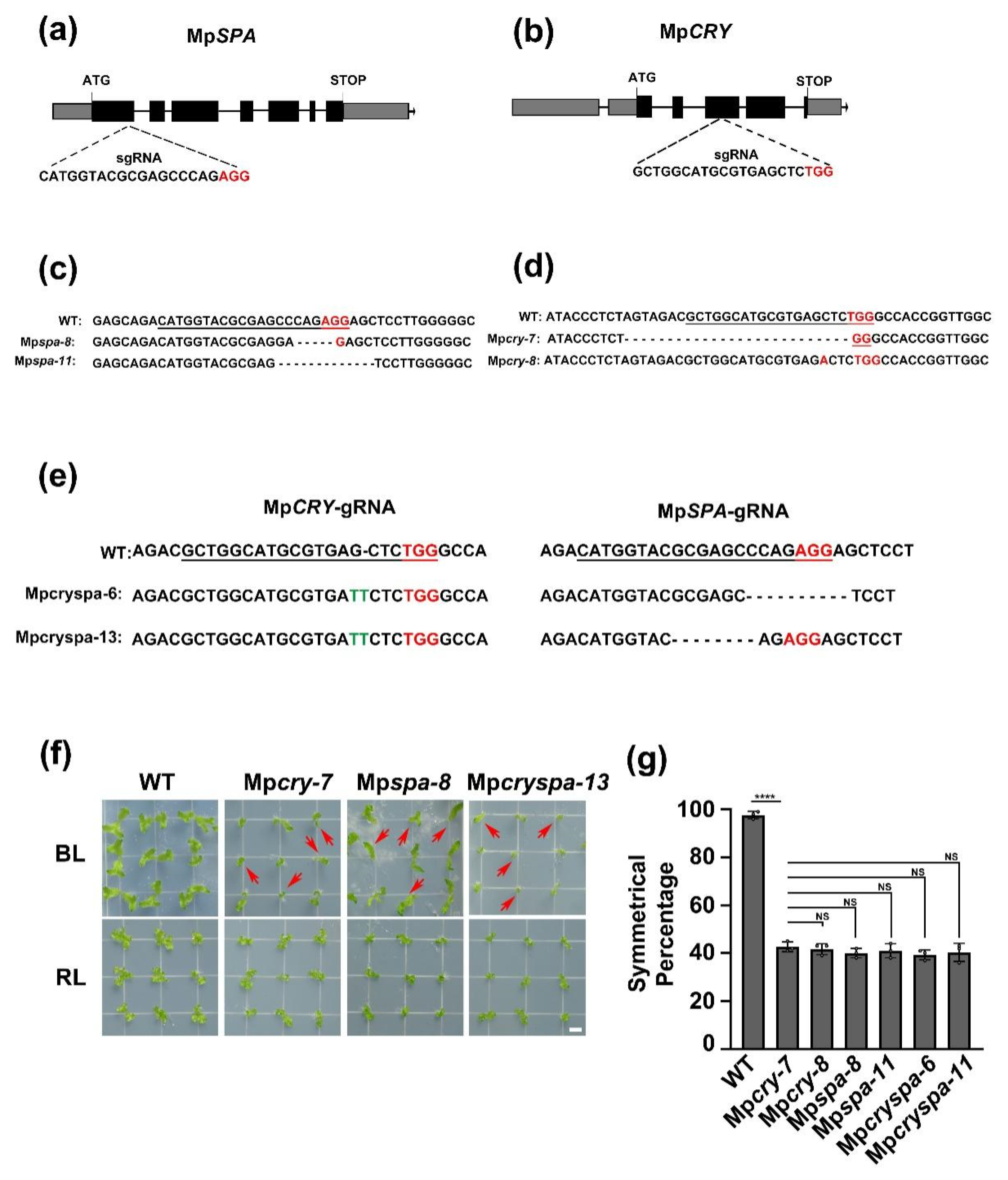
2.1. MpSPA Is Associated with MpCRY to Regulate the Thallus Symmetry of M. polymorpha under Blue Light
2.2. MpSPA Interacts with MpCRY in the Nucleus
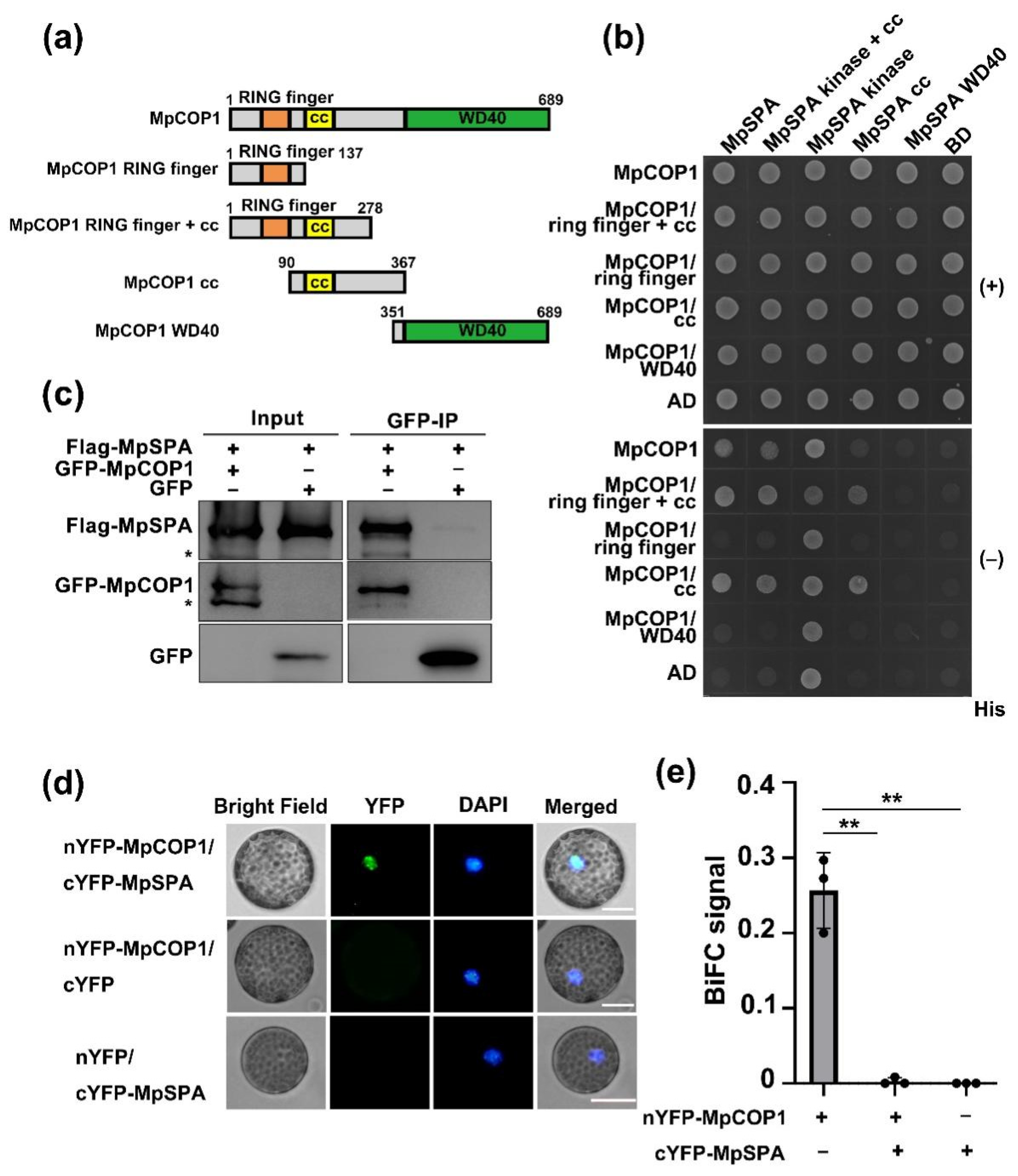
2.3. MpSPA Interacts with MpCOP1 in the Nucleus
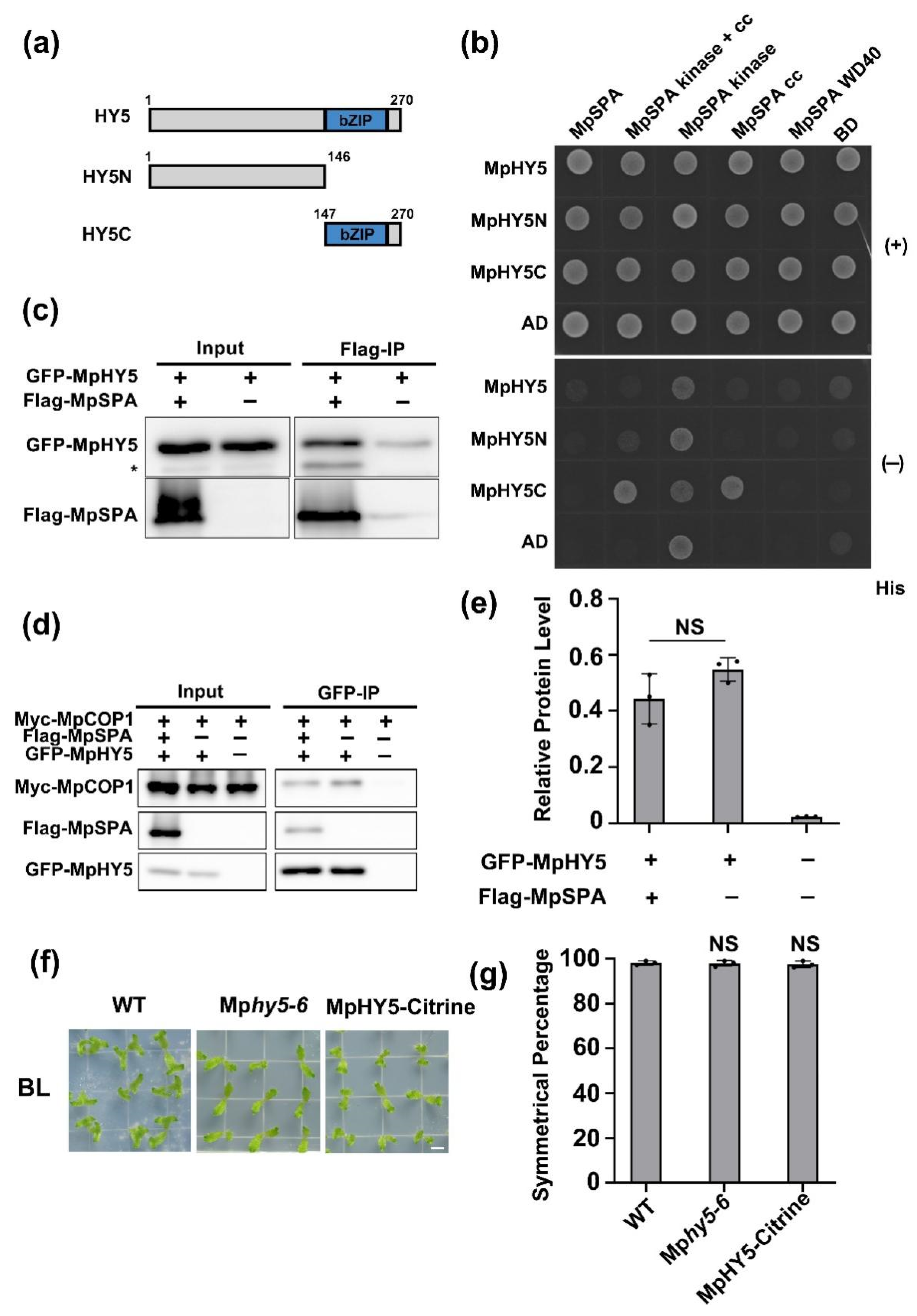
2.4. MpCOP1/MpSPA Complex Interacts with MpHY5
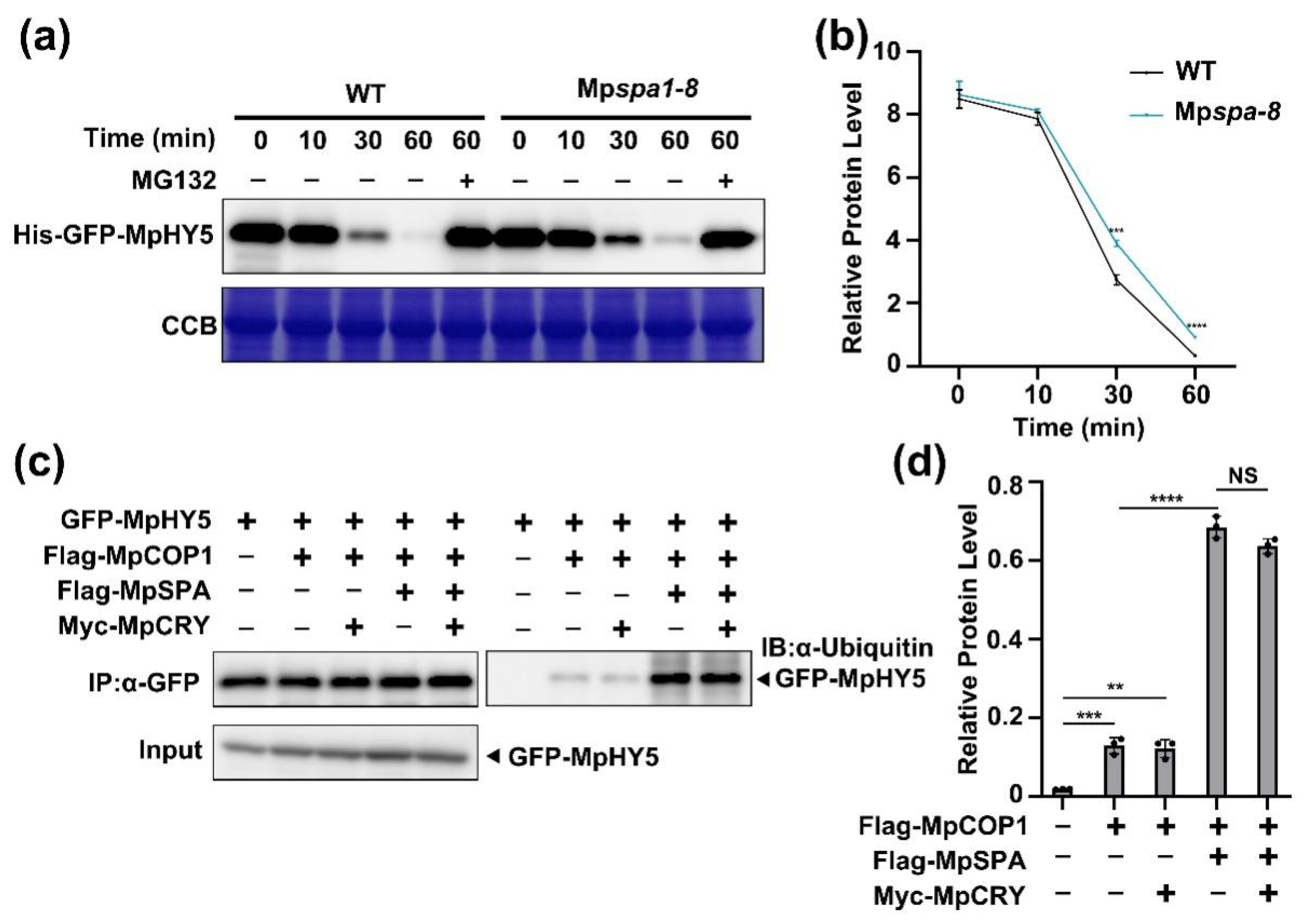
2.5. MpSPA Promotes That MpCOP1 Ubiquitinates MpHY5
2.6. Blue Light Increases MpHY5 Abundance Independently of MpCRY
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. HEK-293T Cell Culture and Transfected
4.3. Generation of Transgenic Lines
4.4. Colocalization and Localization
4.5. Phylogenetic Tree Analysis
4.6. Co-Immunoprecipitation in HEK293T Cells
4.7. Yeast Two-Hybrid Assay
4.8. Bimolecular Fluorescence Complementation (BiFC) Assay
4.9. Ubiquitination Assays in HEK293T Cells
4.10. Antibodies Used for Immunoblotting
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McNellis, T.W.; Deng, X.W. Light control of seedling morphogenetic pattern. Plant Cell 1995, 7, 1749–1761. [Google Scholar] [CrossRef] [Green Version]
- Quail, P.H. Phytochrome photosensory signalling networks. Nat. Reviews. Mol. Cell Biol. 2002, 3, 85–93. [Google Scholar] [CrossRef]
- Liu, Q.; Su, T.; He, W.; Ren, H.; Liu, S.; Chen, Y.; Gao, L.; Hu, X.; Lu, H.; Cao, S.; et al. Photooligomerization Determines Photosensitivity and Photoreactivity of Plant Cryptochromes. Mol. Plant 2020, 13, 398–413. [Google Scholar] [CrossRef]
- Podolec, R.; Ulm, R. Photoreceptor-mediated regulation of the COP1/SPA E3 ubiquitin ligase. Curr. Opin. Plant Biol. 2018, 45, 18–25. [Google Scholar] [CrossRef]
- Ponnu, J. Molecular mechanisms suppressing COP1/SPA E3 ubiquitin ligase activity in blue light. Physiol. Plant. 2020, 169, 418–429. [Google Scholar] [CrossRef]
- Saijo, Y.; Sullivan, J.A.; Wang, H.; Yang, J.; Shen, Y.; Rubio, V.; Ma, L.; Hoecker, U.; Deng, X.W. The COP1-SPA1 interaction defines a critical step in phytochrome A-mediated regulation of HY5 activity. Genes Dev. 2003, 17, 2642–2647. [Google Scholar] [CrossRef] [Green Version]
- Laubinger, S.; Marchal, V.; Le Gourrierec, J.; Wenkel, S.; Adrian, J.; Jang, S.; Kulajta, C.; Braun, H.; Coupland, G.; Hoecker, U. Arabidopsis SPA proteins regulate photoperiodic flowering and interact with the floral inducer CONSTANS to regulate its stability. Development 2006, 133, 3213–3222. [Google Scholar] [CrossRef] [Green Version]
- Maier, A.; Schrader, A.; Kokkelink, L.; Falke, C.; Welter, B.; Iniesto, E.; Rubio, V.; Uhrig, J.F.; Hülskamp, M.; Hoecker, U. Light and the E3 ubiquitin ligase COP1/SPA control the protein stability of the MYB transcription factors PAP1 and PAP2 involved in anthocyanin accumulation in Arabidopsis. Plant J. Cell Mol. Biol. 2013, 74, 638–651. [Google Scholar] [CrossRef]
- Yi, C.; Deng, X.W. COP1—From plant photomorphogenesis to mammalian tumorigenesis. Trends Cell Biol. 2005, 15, 618–625. [Google Scholar] [CrossRef]
- Marine, J.C. Spotlight on the role of COP1 in tumorigenesis. Nat. Rev. Cancer 2012, 12, 455–464. [Google Scholar] [CrossRef]
- Hoecker, U. The activities of the E3 ubiquitin ligase COP1/SPA, a key repressor in light signaling. Curr. Opin. Plant Biol. 2017, 37, 63–69. [Google Scholar] [CrossRef]
- Osterlund, M.T.; Ang, L.H.; Deng, X.W. The role of COP1 in repression of Arabidopsis photomorphogenic development. Trends Cell Biol. 1999, 9, 113–118. [Google Scholar] [CrossRef]
- Chen, S.; Lory, N.; Stauber, J.; Hoecker, U. Photoreceptor Specificity in the Light-Induced and COP1-Mediated Rapid Degradation of the Repressor of Photomorphogenesis SPA2 in Arabidopsis. PLoS Genet. 2015, 11, e1005516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, B.; Zuo, Z.; Liu, H.; Liu, X.; Lin, C. Arabidopsis cryptochrome 1 interacts with SPA1 to suppress COP1 activity in response to blue light. Genes Dev. 2011, 25, 1029–1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, B.D.; Kim, M.R.; Kang, M.Y.; Cha, J.Y.; Han, S.H.; Nawkar, G.M.; Sakuraba, Y.; Lee, S.Y.; Imaizumi, T.; McClung, C.R.; et al. The F-box protein FKF1 inhibits dimerization of COP1 in the control of photoperiodic flowering. Nat. Commun. 2017, 8, 2259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponnu, J.; Riedel, T.; Penner, E.; Schrader, A.; Hoecker, U. Cryptochrome 2 competes with COP1 substrates to repress COP1 ubiquitin ligase activity during Arabidopsis photomorphogenesis. Proc. Natl. Acad. Sci. USA 2019, 116, 27133–27141. [Google Scholar] [CrossRef]
- Balcerowicz, M.; Kerner, K.; Schenkel, C.; Hoecker, U. SPA Proteins Affect the Subcellular Localization of COP1 in the COP1/SPA Ubiquitin Ligase Complex during Photomorphogenesis. Plant Physiol. 2017, 174, 1314–1321. [Google Scholar] [CrossRef] [Green Version]
- Osterlund, M.T.; Deng, X.W. Multiple photoreceptors mediate the light-induced reduction of GUS-COP1 from Arabidopsis hypocotyl nuclei. Plant J. Cell Mol. Biol. 1998, 16, 201–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuo, Z.; Liu, H.; Liu, B.; Liu, X.; Lin, C. Blue light-dependent interaction of CRY2 with SPA1 regulates COP1 activity and floral initiation in Arabidopsis. Curr. Biol. 2011, 21, 841–847. [Google Scholar] [CrossRef] [Green Version]
- Artz, O.; Dickopf, S.; Ranjan, A.; Kreiss, M.; Abraham, E.T.; Boll, V.; Rensing, S.A.; Hoecker, U. Characterization of spa mutants in the moss Physcomitrella provides evidence for functional divergence of SPA genes during the evolution of land plants. New Phytol. 2019, 224, 1613–1626. [Google Scholar] [CrossRef]
- Bowman, J.L.; Kohchi, T.; Yamato, K.T.; Jenkins, J.; Shu, S.; Ishizaki, K.; Yamaoka, S.; Nishihama, R.; Nakamura, Y.; Berger, F.; et al. Insights into Land Plant Evolution Garnered from the Marchantia polymorpha Genome. Cell 2017, 171, 287–304.e215. [Google Scholar] [CrossRef] [Green Version]
- Komatsu, A.; Terai, M.; Ishizaki, K.; Suetsugu, N.; Tsuboi, H.; Nishihama, R.; Yamato, K.T.; Wada, M.; Kohchi, T. Phototropin encoded by a single-copy gene mediates chloroplast photorelocation movements in the liverwort Marchantia polymorpha. Plant Physiol. 2014, 166, 411–427. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, T.; Takio, S.; Yamamoto, I.; Satoh, T. Characterization of cDNA of the liverwort phytochrome gene, and phytochrome involvement in the light-dependent and light-independent protochlorophyllide oxidoreductase gene expression in Marchantia paleacea var. diptera. Plant Cell Physiol. 2001, 42, 576–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inoue, K.; Nishihama, R.; Kataoka, H.; Hosaka, M.; Manabe, R.; Nomoto, M.; Tada, Y.; Ishizaki, K.; Kohchi, T. Phytochrome signaling is mediated by PHYTOCHROME INTERACTING FACTOR in the liverwort Marchantia polymorpha. Plant Cell 2016, 28, 1406–1421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soriano, G.; Cloix, C.; Heilmann, M.; Nunez-Olivera, E.; Martinez-Abaigar, J.; Jenkins, G.I. Evolutionary conservation of structure and function of the UVR8 photoreceptor from the liverwort Marchantia polymorpha and the moss Physcomitrella patens. New Phytol. 2018, 217, 151–162. [Google Scholar] [CrossRef] [Green Version]
- Kubota, A.; Kita, S.; Ishizaki, K.; Nishihama, R.; Yamato, K.T.; Kohchi, T. Co-option of a photoperiodic growth-phase transition system during land plant evolution. Nat. Commun. 2014, 5, 3668. [Google Scholar] [CrossRef] [PubMed]
- Hoecker, U.; Tepperman, J.M.; Quail, P.H. SPA1, a WD-repeat protein specific to phytochrome A signal transduction. Science 1999, 284, 496–499. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.; Yang, H.; Guo, H.; Mockler, T.; Chen, J.; Cashmore, A.R. Enhancement of blue-light sensitivity of Arabidopsis seedlings by a blue light receptor cryptochrome 2. Proc. Natl. Acad. Sci. USA 1998, 95, 2686–2690. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Sayegh, R.; Maymon, M.; Warpeha, K.; Klejnot, J.; Yang, H.; Huang, J.; Lee, J.; Kaufman, L.; Lin, C. Formation of nuclear bodies of Arabidopsis CRY2 in response to blue light is associated with its blue light-dependent degradation. Plant Cell 2009, 21, 118–130. [Google Scholar] [CrossRef] [Green Version]
- Weidler, G.; Zur Oven-Krockhaus, S.; Heunemann, M.; Orth, C.; Schleifenbaum, F.; Harter, K.; Hoecker, U.; Batschauer, A. Degradation of Arabidopsis CRY2 is regulated by SPA proteins and phytochrome A. Plant Cell 2012, 24, 2610–2623. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Wang, X.; Deng, W.; Mo, W.; Gao, J.; Liu, Q.; Zhang, C.; Wang, Q.; Lin, C.; Zuo, Z. Using HEK293T Expression System to Study Photoactive Plant Cryptochromes. Front. Plant Sci. 2016, 7, 940. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.; Spalding, E.P. Separate functions for nuclear and cytoplasmic cryptochrome 1 during photomorphogenesis of Arabidopsis seedlings. Proc. Natl. Acad. Sci. USA 2007, 104, 18813–18818. [Google Scholar] [CrossRef] [Green Version]
- Van Buskirk, E.K.; Decker, P.V.; Chen, M. Photobodies in light signaling. Plant Physiol. 2012, 158, 52–60. [Google Scholar] [CrossRef] [Green Version]
- Ranjan, A.; Dickopf, S.; Ullrich, K.K.; Rensing, S.A.; Hoecker, U. Functional analysis of COP1 and SPA orthologs from Physcomitrella and rice during photomorphogenesis of transgenic Arabidopsis reveals distinct evolutionary conservation. BMC Plant Biol. 2014, 14, 178. [Google Scholar] [CrossRef] [Green Version]
- Ruan, W.; Guo, M.; Wang, X.; Guo, Z.; Xu, Z.; Xu, L.; Zhao, H.; Sun, H.; Yan, C.; Yi, K. Two RING-Finger Ubiquitin E3 Ligases Regulate the Degradation of SPX4, An Internal Phosphate Sensor, for Phosphate Homeostasis and Signaling in Rice. Mol. Plant 2019, 12, 1060–1074. [Google Scholar] [CrossRef]
- Seo, H.S.; Yang, J.Y.; Ishikawa, M.; Bolle, C.; Ballesteros, M.L.; Chua, N.H. LAF1 ubiquitination by COP1 controls photomorphogenesis and is stimulated by SPA1. Nature 2003, 423, 995–999. [Google Scholar] [CrossRef]
- Althoff, F.; Kopischke, S.; Zobell, O.; Ide, K.; Ishizaki, K.; Kohchi, T.; Zachgo, S. Comparison of the MpEF1α and CaMV35 promoters for application in Marchantia polymorpha overexpression studies. Transgenic Res. 2014, 23, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Somers, D.E.; Devlin, P.F.; Kay, S.A. Phytochromes and cryptochromes in the entrainment of the Arabidopsis circadian clock. Science 1998, 282, 1488–1490. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, M.; Cashmore, A.R. HY4 gene of A. thaliana encodes a protein with characteristics of a blue-light photoreceptor. Nature 1993, 366, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Barrero, J.M.; Downie, A.B.; Xu, Q.; Gubler, F. A role for barley CRYPTOCHROME1 in light regulation of grain dormancy and germination. Plant Cell 2014, 26, 1094–1104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Zuo, Z.; Wang, X.; Gu, L.; Yoshizumi, T.; Yang, Z.; Yang, L.; Liu, Q.; Liu, W.; Han, Y.J.; et al. Photoactivation and inactivation of Arabidopsis cryptochrome 2. Science 2016, 354, 343–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Yu, X.; Li, K.; Klejnot, J.; Yang, H.; Lisiero, D.; Lin, C. Photoexcited CRY2 interacts with CIB1 to regulate transcription and floral initiation in Arabidopsis. Science 2008, 322, 1535–1539. [Google Scholar] [CrossRef] [Green Version]
- Gu, N.N.; Zhang, Y.C.; Yang, H.Q. Substitution of a conserved glycine in the PHR domain of Arabidopsis cryptochrome 1 confers a constitutive light response. Mol. Plant 2012, 5, 85–97. [Google Scholar] [CrossRef] [Green Version]
- Zhou, T.; Meng, L.; Ma, Y.; Liu, Q.; Zhang, Y.; Yang, Z.; Yang, D.; Bian, M. Overexpression of sweet sorghum cryptochrome 1a confers hypersensitivity to blue light, abscisic acid and salinity in Arabidopsis. Plant Cell Rep. 2018, 37, 251–264. [Google Scholar] [CrossRef]
- Laubinger, S.; Hoecker, U. The SPA1-like proteins SPA3 and SPA4 repress photomorphogenesis in the light. Plant J. Cell Mol. Biol. 2003, 35, 373–385. [Google Scholar] [CrossRef]
- Ishizaki, K.; Chiyoda, S.; Yamato, K.T.; Kohchi, T. Agrobacterium-mediated transformation of the haploid liverwort Marchantia polymorpha L., an emerging model for plant biology. Plant Cell Physiol. 2008, 49, 1084–1091. [Google Scholar] [CrossRef] [Green Version]
- Gamborg, O.L.; Miller, R.A.; Ojima, K. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 1968, 50, 151–158. [Google Scholar] [CrossRef]
- Yang, L.; Mo, W.; Yu, X.; Yao, N.; Zhou, Z.; Fan, X.; Zhang, L.; Piao, M.; Li, S.; Yang, D.; et al. Reconstituting Arabidopsis CRY2 Signaling Pathway in Mammalian Cells Reveals Regulation of Transcription by Direct Binding of CRY2 to DNA. Cell Rep. 2018, 24, 585–593.e584. [Google Scholar] [CrossRef] [Green Version]
- Sugano, S.S.; Nishihama, R. CRISPR/Cas9-Based Genome Editing of Transcription Factor Genes in Marchantia polymorpha. Methods Mol. Biol. 2018, 1830, 109–126. [Google Scholar] [CrossRef] [PubMed]
- Sugano, S.S.; Nishihama, R.; Shirakawa, M.; Takagi, J.; Matsuda, Y.; Ishida, S.; Shimada, T.; Hara-Nishimura, I.; Osakabe, K.; Kohchi, T. Efficient CRISPR/Cas9-based genome editing and its application to conditional genetic analysis in Marchantia polymorpha. PLoS ONE 2018, 13, e0205117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishizaki, K.; Nishihama, R.; Ueda, M.; Inoue, K.; Ishida, S.; Nishimura, Y.; Shikanai, T.; Kohchi, T. Development of Gateway Binary Vector Series with Four Different Selection Markers for the Liverwort Marchantia polymorpha. PLoS ONE 2015, 10, e0138876. [Google Scholar] [CrossRef] [Green Version]
- Kubota, A.; Ishizaki, K.; Hosaka, M.; Kohchi, T. Efficient Agrobacterium-mediated transformation of the liverwort Marchantia polymorpha using regenerating thalli. Biosci. Biotechnol. Biochem. 2013, 77, 167–172. [Google Scholar] [CrossRef] [Green Version]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [Green Version]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, F.H.; Shen, S.C.; Lee, L.Y.; Lee, S.H.; Chan, M.T.; Lin, C.S. Tape-Arabidopsis Sandwich—A simpler Arabidopsis protoplast isolation method. Plant Methods 2009, 5, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, H.; Han, J.; Yang, P.; Mao, W.; Liu, X.; Qiu, L.; Qian, C.; Liu, Y.; Chen, Z.; Ouyang, X.; et al. Two E3 ligases antagonistically regulate the UV-B response in Arabidopsis. Proc. Natl. Acad. Sci. USA 2019, 116, 4722–4731. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.E.; Manteiga, J.C.; Henninger, J.E.; Sabari, B.R.; Dall’Agnese, A.; Hannett, N.M.; Spille, J.-H.; Afeyan, L.K.; Zamudio, A.V.; Shrinivas, K.; et al. Pol II phosphorylation regulates a switch between transcriptional and splicing condensates. Nature 2019, 572, 543–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Li, T.; Su, S.; Peng, H.; Li, S.; Li, K.; Ji, L.; Xing, Y.; Zhang, J.; Du, X.; et al. Functions of COP1/SPA E3 Ubiquitin Ligase Mediated by MpCRY in the Liverwort Marchantia polymorpha under Blue Light. Int. J. Mol. Sci. 2022, 23, 158. https://doi.org/10.3390/ijms23010158
Zhang L, Li T, Su S, Peng H, Li S, Li K, Ji L, Xing Y, Zhang J, Du X, et al. Functions of COP1/SPA E3 Ubiquitin Ligase Mediated by MpCRY in the Liverwort Marchantia polymorpha under Blue Light. International Journal of Molecular Sciences. 2022; 23(1):158. https://doi.org/10.3390/ijms23010158
Chicago/Turabian StyleZhang, Li, Tianhong Li, Shengzhong Su, Hao Peng, Sudi Li, Ke Li, Luyao Ji, Yaoyun Xing, Junchuan Zhang, Xinglin Du, and et al. 2022. "Functions of COP1/SPA E3 Ubiquitin Ligase Mediated by MpCRY in the Liverwort Marchantia polymorpha under Blue Light" International Journal of Molecular Sciences 23, no. 1: 158. https://doi.org/10.3390/ijms23010158
APA StyleZhang, L., Li, T., Su, S., Peng, H., Li, S., Li, K., Ji, L., Xing, Y., Zhang, J., Du, X., Bian, M., Liao, Y., Yang, Z., & Zuo, Z. (2022). Functions of COP1/SPA E3 Ubiquitin Ligase Mediated by MpCRY in the Liverwort Marchantia polymorpha under Blue Light. International Journal of Molecular Sciences, 23(1), 158. https://doi.org/10.3390/ijms23010158




