Transient Focal Cerebral Ischemia Leads to miRNA Alterations in Different Brain Regions, Blood Serum, Liver, and Spleen
Abstract
1. Introduction
2. Results
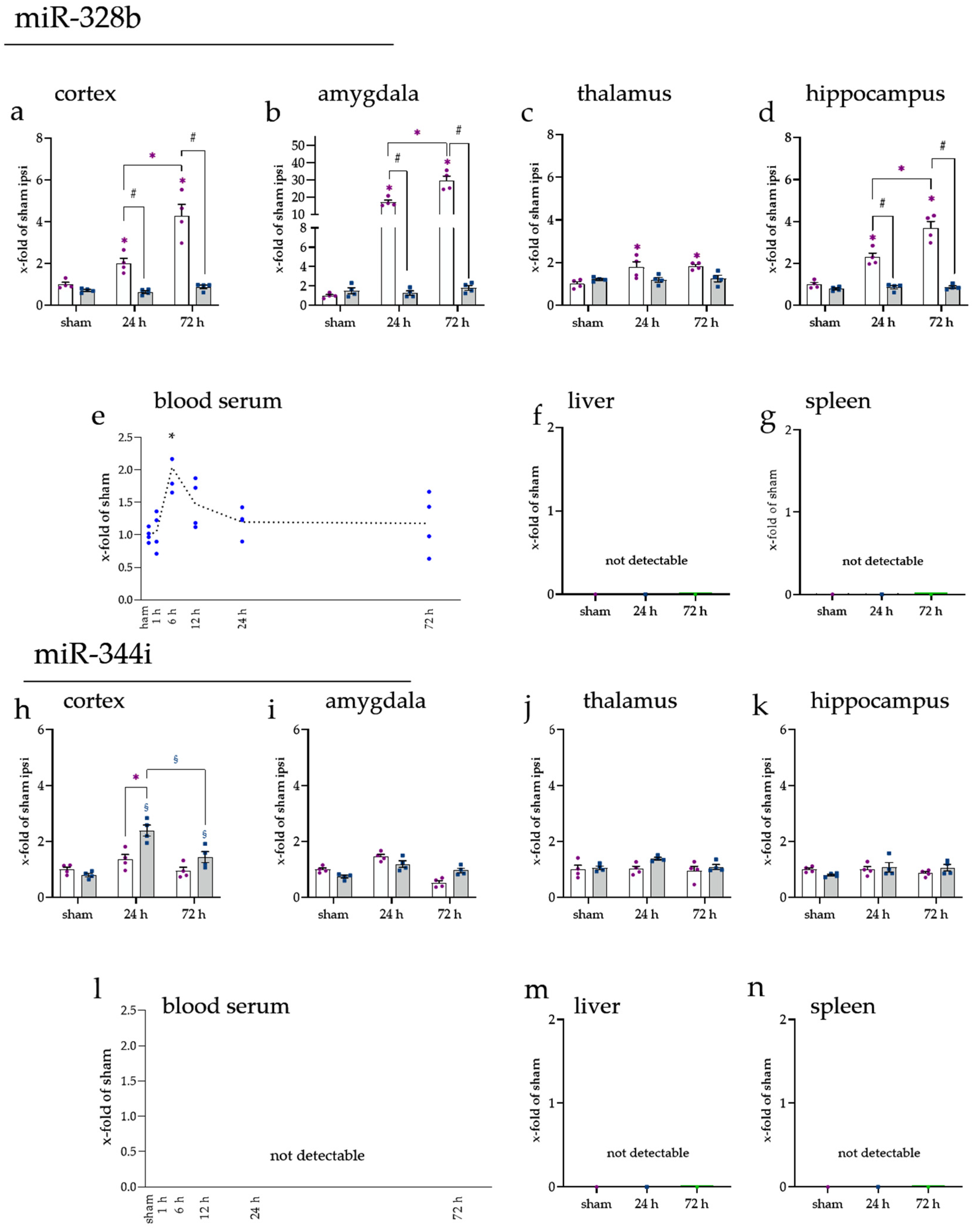
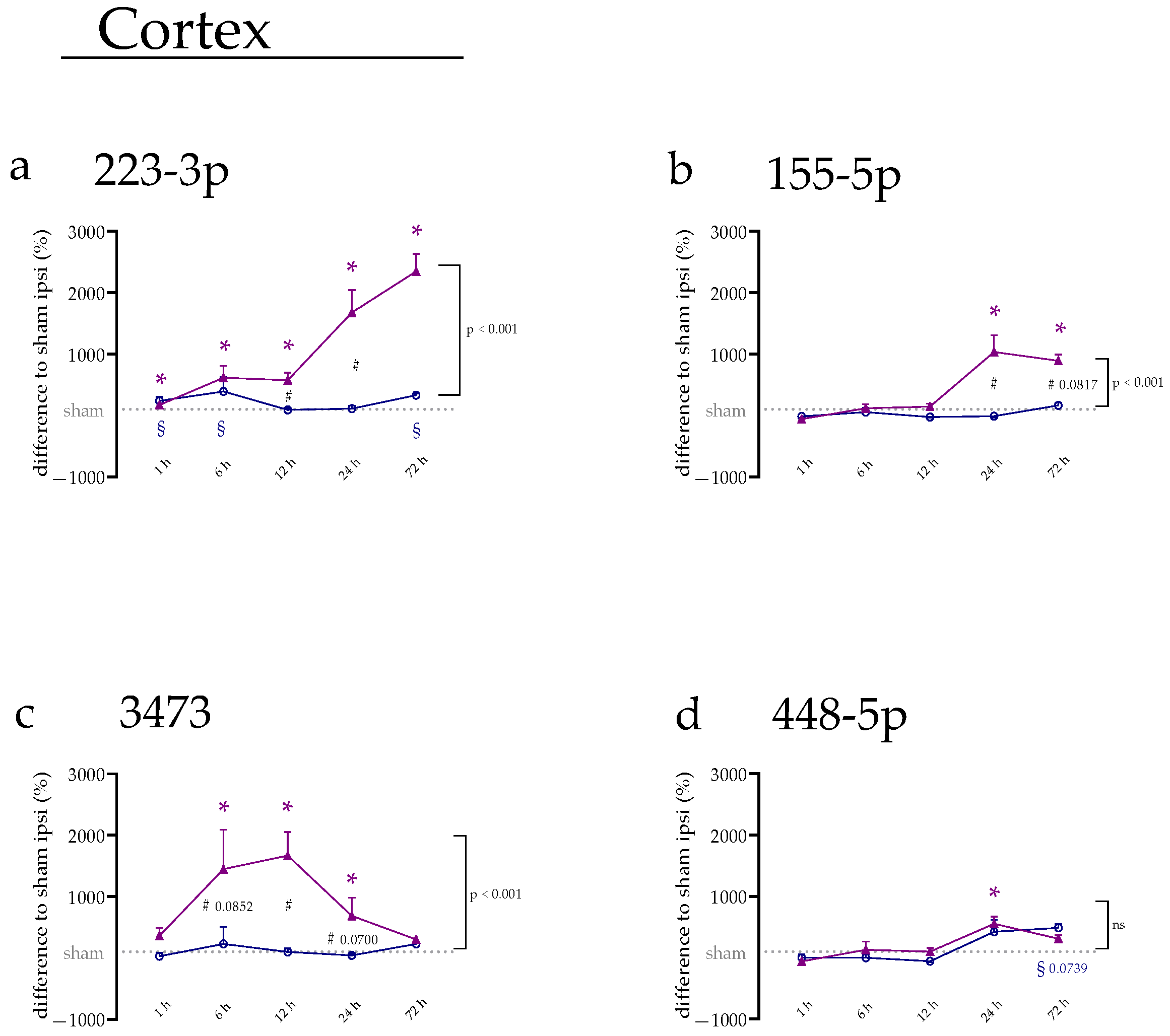
2.1. miRNA Expression Levels Were Mainly Increased on the Ipsilateral Side of the Cerebral Cortex
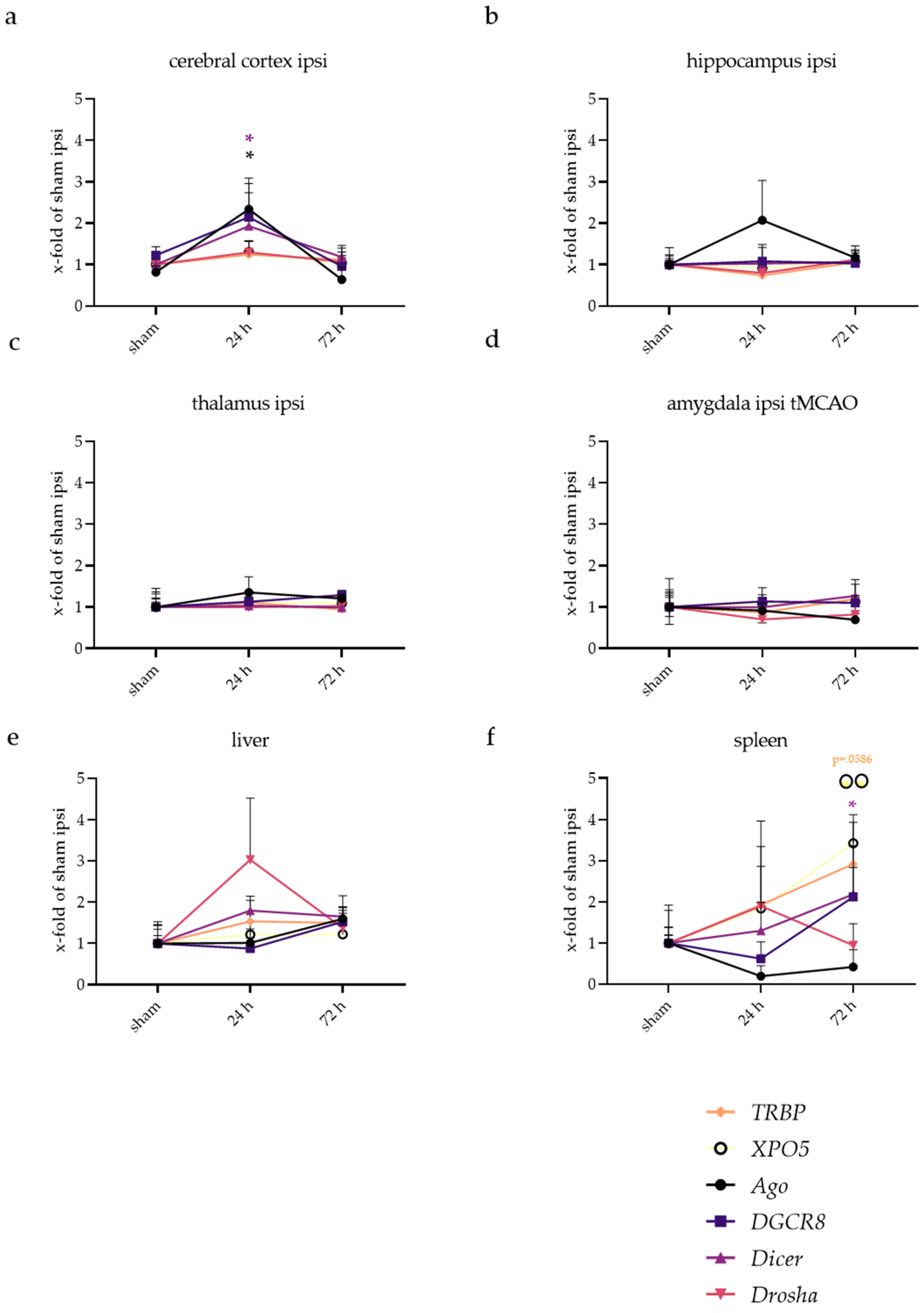
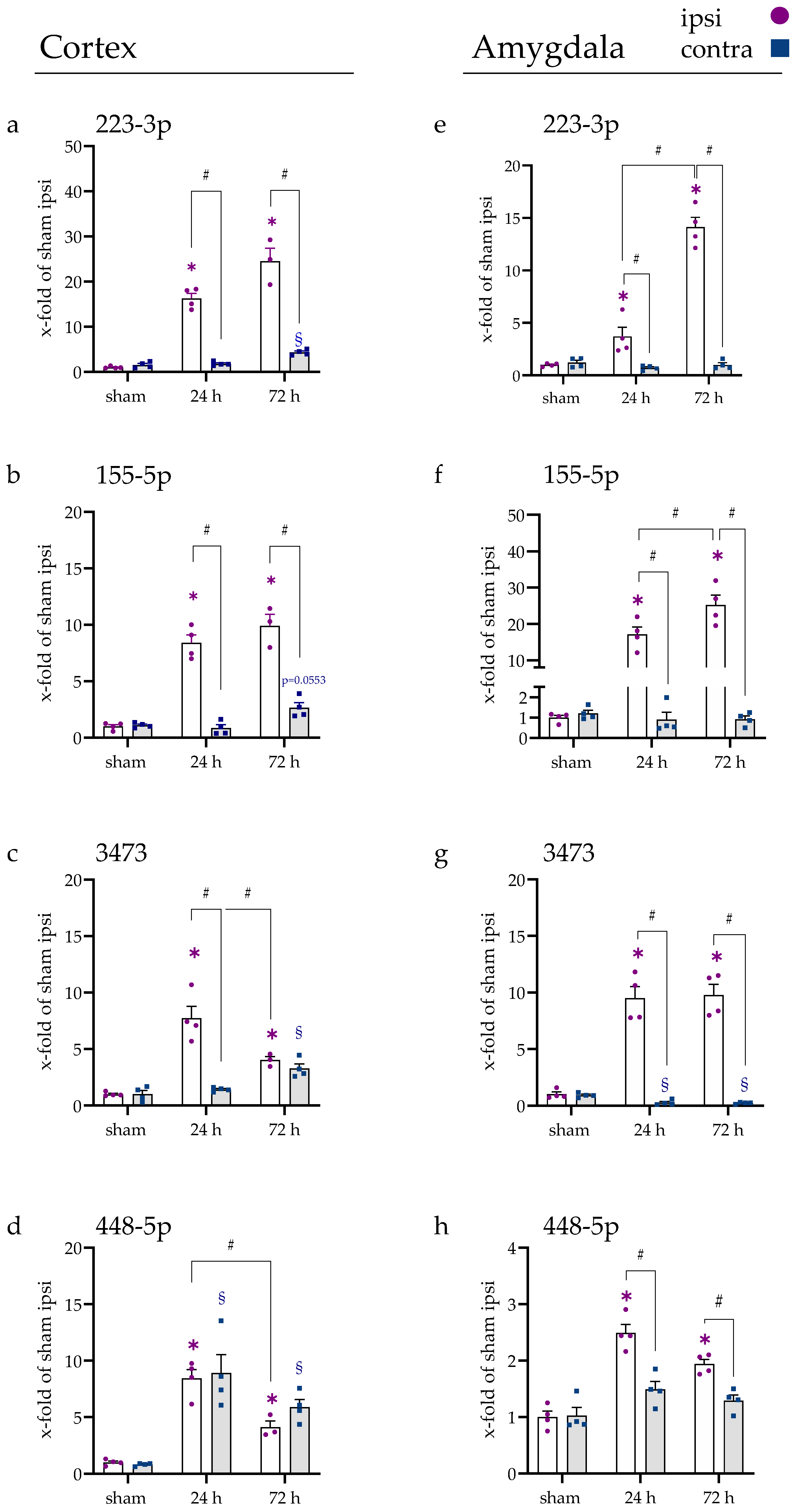
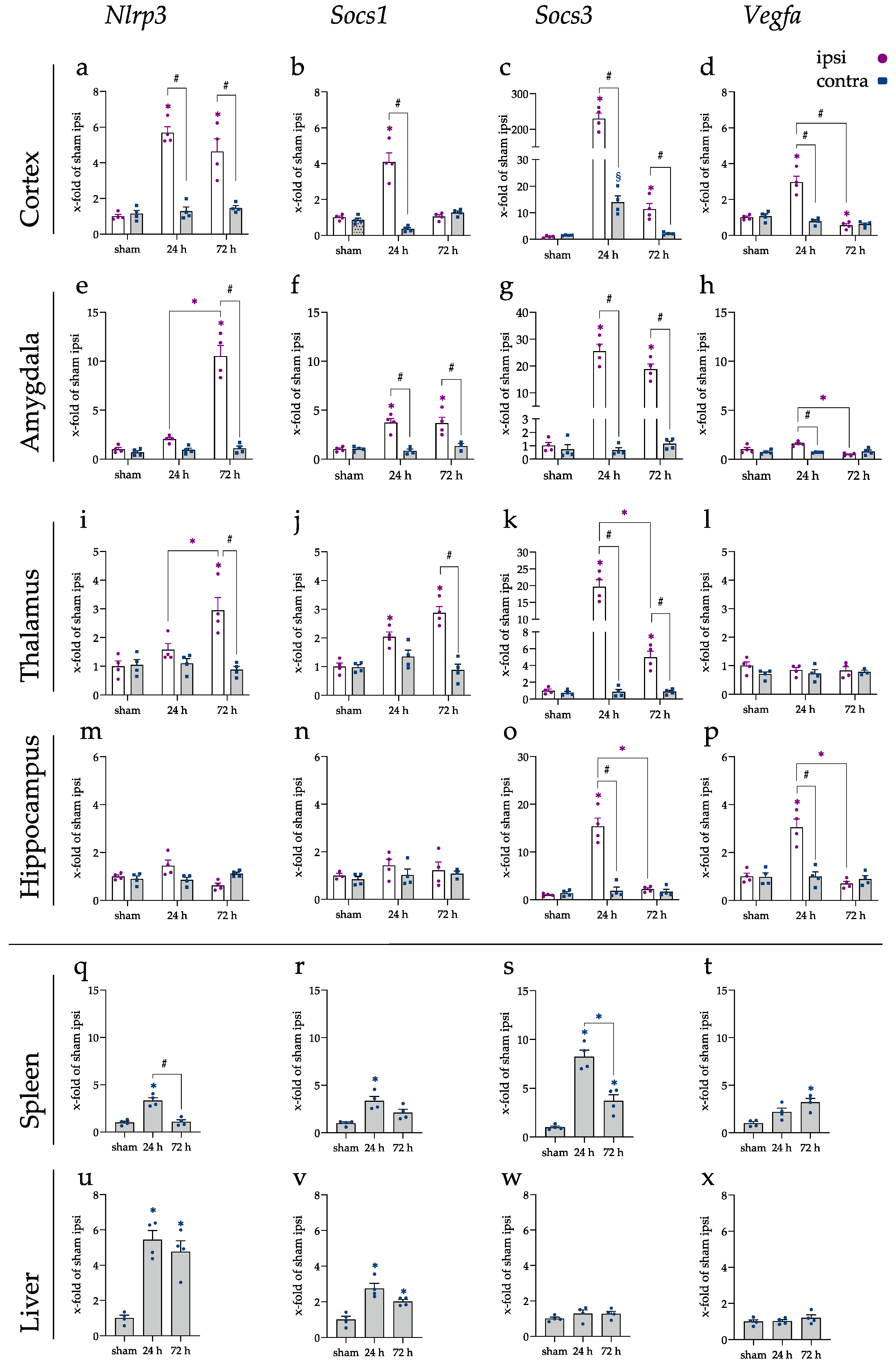
2.2. miRNA Levels Were Increased after 24 and 72 h in the Cerebral Cortex and Amygdala
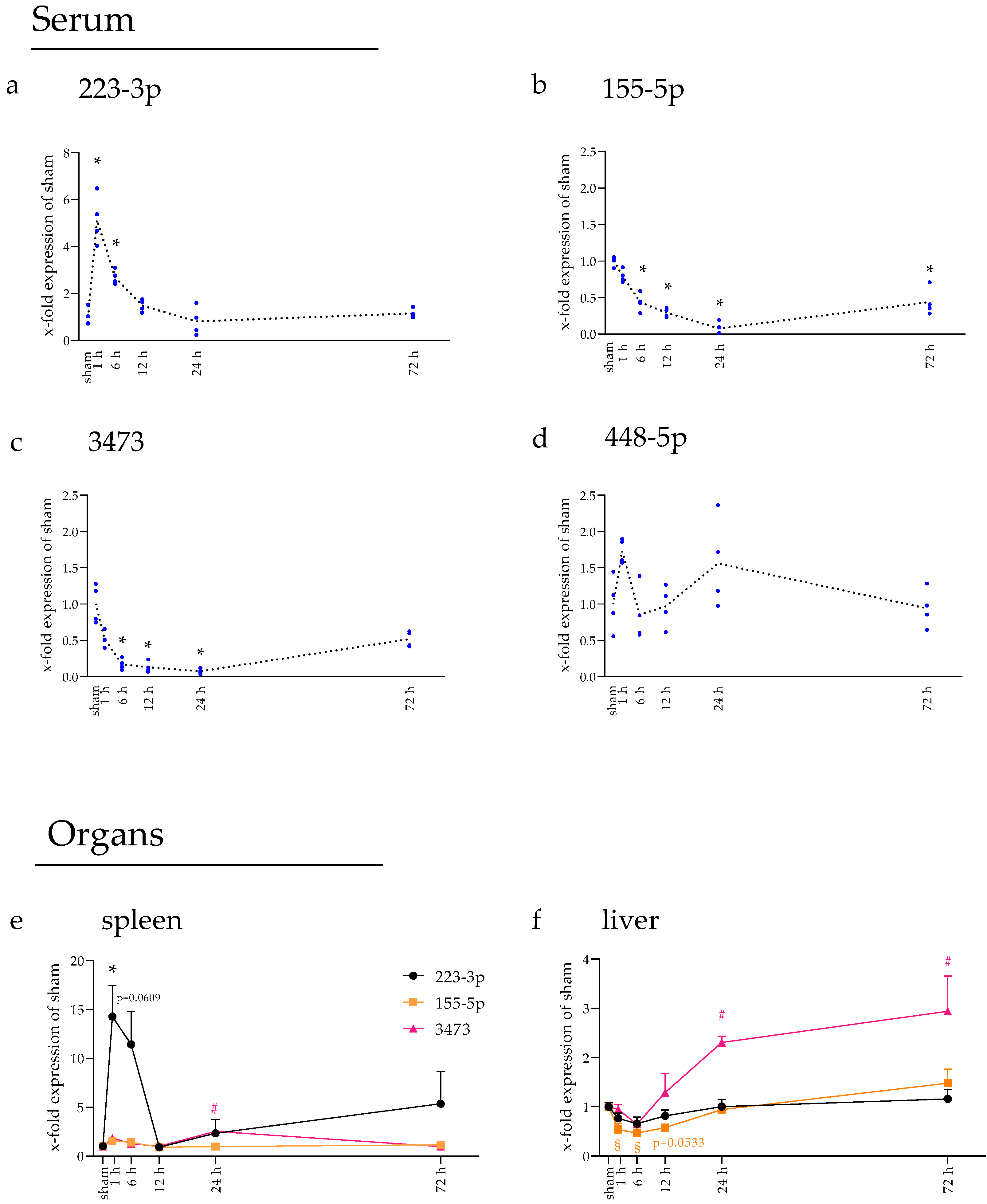
2.3. miRNAs Fluctuated in Blood Serum over Time
2.4. Spleen and Liver Responded to tMCAo with Increased miRNA Expression
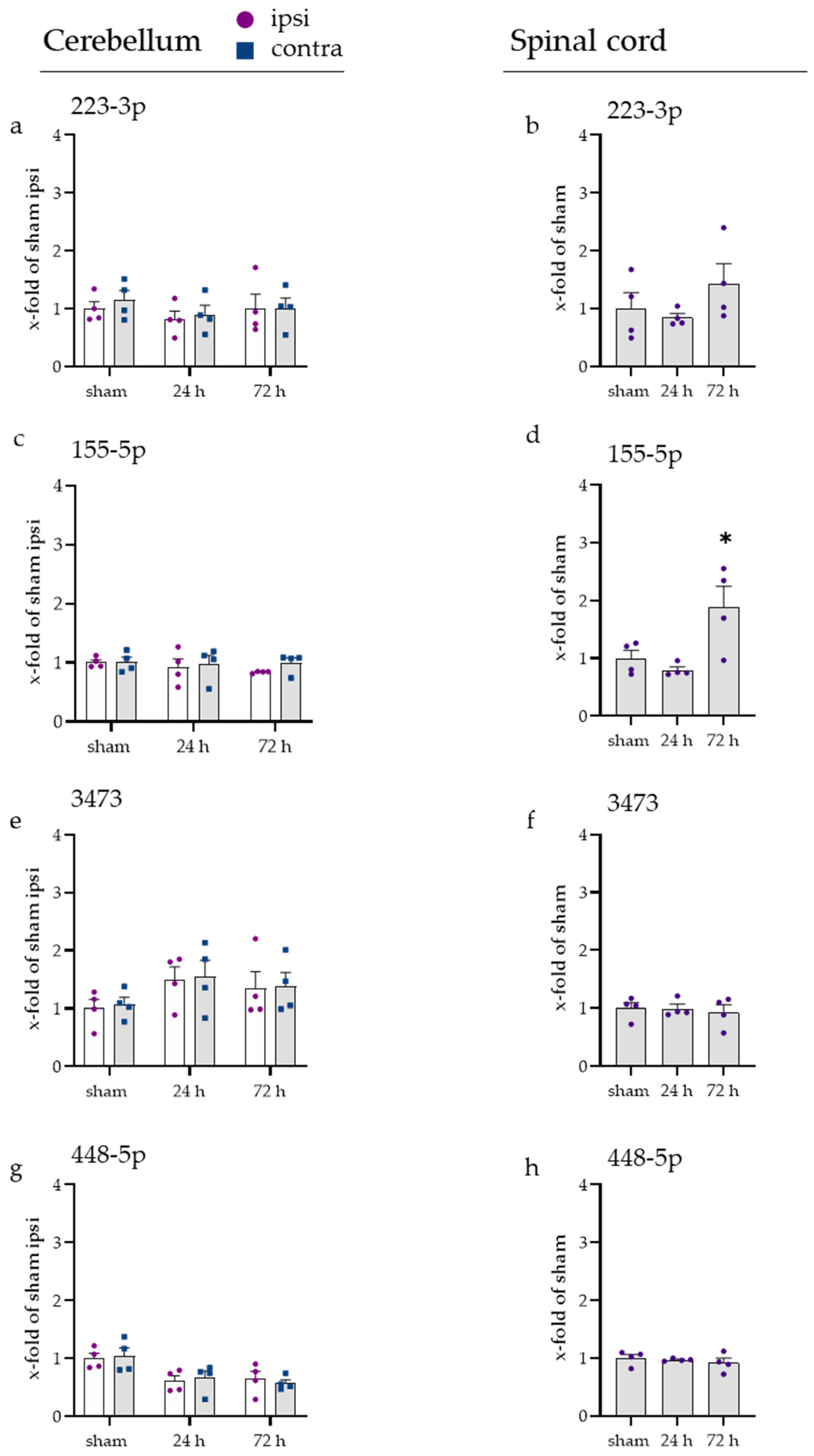
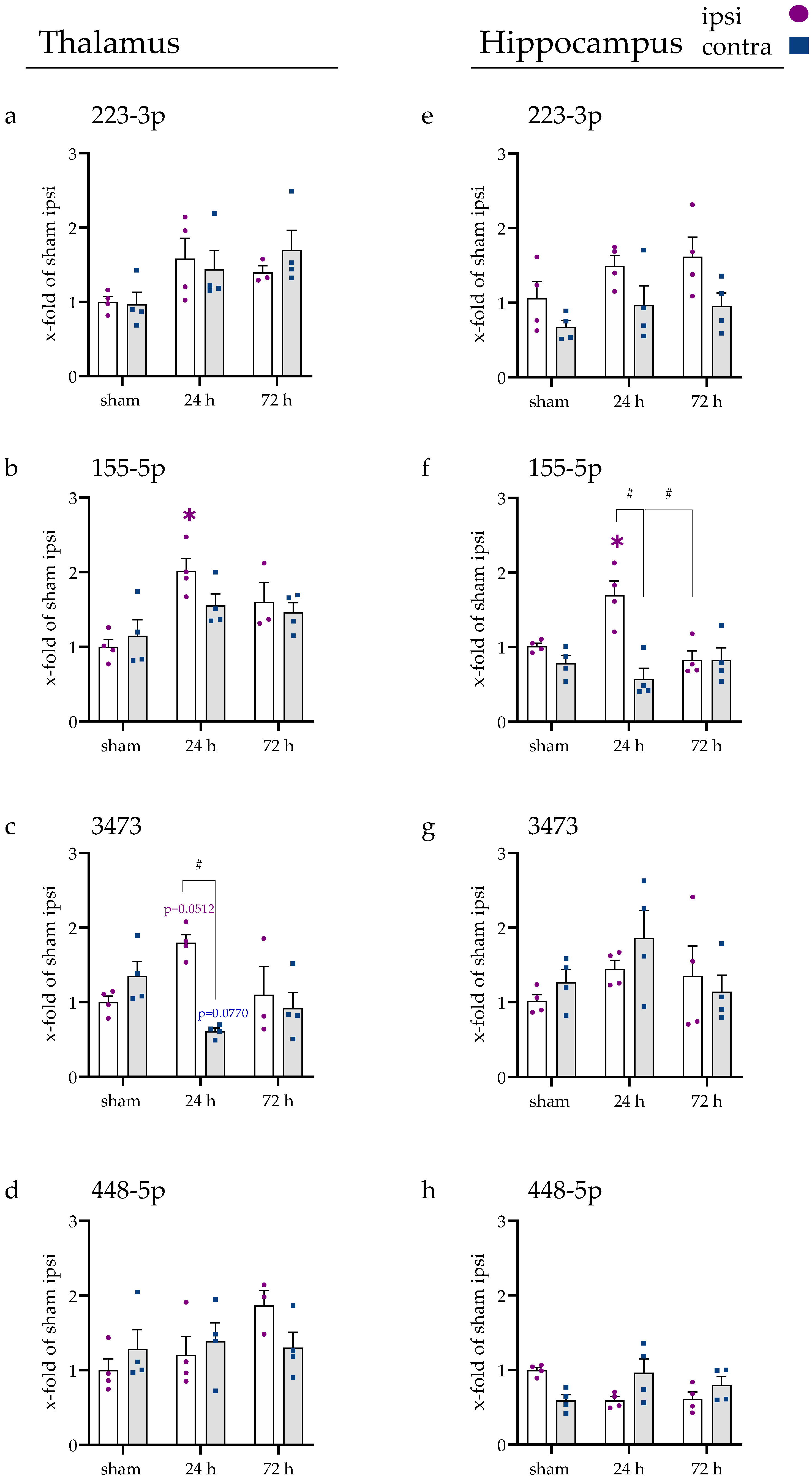
2.5. Inflammatory Responses Also Visible in Injury-Remote Areas
2.6. Brain-Enriched miRNAs miR-328b-3p and miR-344i Were Not Found in the Spleen or Liver
3. Discussion
4. Materials and Methods
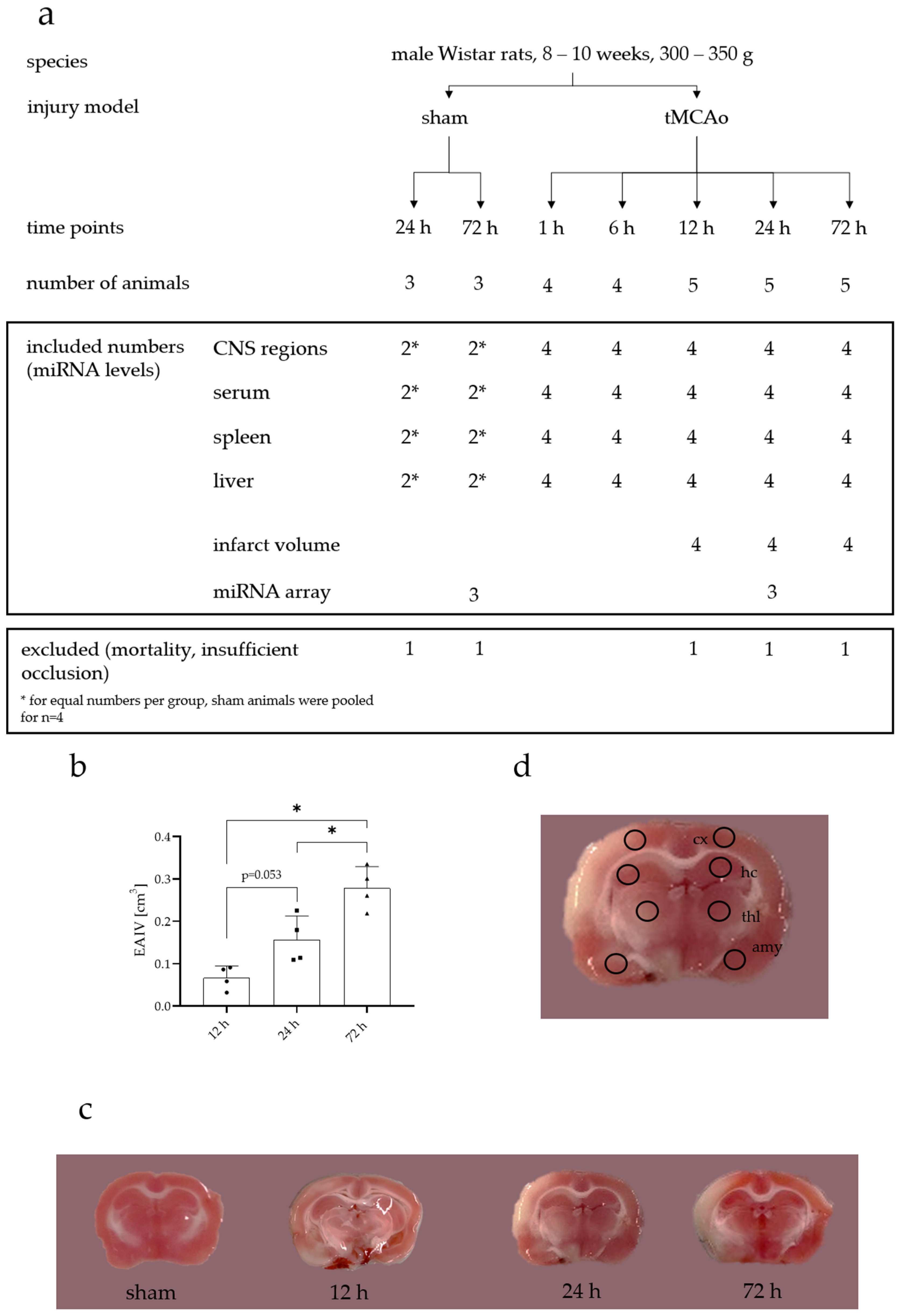
4.1. Animals
4.2. Transient Middle Cerebral Artery Occlusion (tMCAo)
4.3. Neurological Evaluation
4.4. Finalization and Tissue Sampling
4.5. Edema-Adjusted Infarct Volume (EAIV)
4.6. RNA Isolation
4.7. MicroRNA Profiling Using Affymetrix miRNA 4.0 Arrays
4.8. Reverse Transcription (RT) and Quantitative Real-Time PCR (qrtPCR) (cDNA and miRNA cDNA, Normalization Strategy of miRNA)
4.9. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Dirnagl, U.; Iadecola, C.; Moskowitz, M.A. Pathobiology of Ischaemic Stroke: An Integrated View. Trends Neurosci. 1999, 22, 391–397. [Google Scholar] [CrossRef]
- Eltzschig, H.K.; Eckle, T. Ischemia and Reperfusion—From Mechanism to Translation. Nat. Med. 2011, 17, 1391–1401. [Google Scholar] [CrossRef]
- Qian, Y.; Chopp, M.; Chen, J. Emerging Role of MicroRNAs in Ischemic Stroke with Comorbidities. Exp. Neurol. 2020, 331, 113382. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNA Target Recognition and Regulatory Functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef]
- Wang, S.-W.; Liu, Z.; Shi, Z.-S. Non-Coding RNA in Acute Ischemic Stroke: Mechanisms, Biomarkers and Therapeutic Targets. Cell Transplant. 2018, 27, 1763–1777. [Google Scholar] [CrossRef]
- Vasudeva, K.; Munshi, A. MiRNA Dysregulation in Ischaemic Stroke: Focus on Diagnosis, Prognosis, Therapeutic and Protective Biomarkers. Eur. J. Neurosci. 2020, 52, 3610–3627. [Google Scholar] [CrossRef] [PubMed]
- Kim, V.N.; Han, J.; Siomi, M.C. Biogenesis of Small RNAs in Animals. Nat. Rev. Mol. Cell Biol. 2009, 10, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Winter, J.; Jung, S.; Keller, S.; Gregory, R.I.; Diederichs, S. Many Roads to Maturity: MicroRNA Biogenesis Pathways and Their Regulation. Nat. Cell Biol. 2009, 11, 228–234. [Google Scholar] [CrossRef]
- Caby, M.-P.; Lankar, D.; Vincendeau-Scherrer, C.; Raposo, G.; Bonnerot, C. Exosomal-like Vesicles Are Present in Human Blood Plasma. Int. Immunol. 2005, 17, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Witwer, K.W.; Buzás, E.I.; Bemis, L.T.; Bora, A.; Lässer, C.; Lötvall, J.; Nolte-’t Hoen, E.N.; Piper, M.G.; Sivaraman, S.; Skog, J.; et al. Standardization of Sample Collection, Isolation and Analysis Methods in Extracellular Vesicle Research. J. Extracell. Vesicles 2013, 2, 20360. [Google Scholar] [CrossRef]
- Saman, S.; Kim, W.; Raya, M.; Visnick, Y.; Miro, S.; Saman, S.; Jackson, B.; McKee, A.C.; Alvarez, V.E.; Lee, N.C.Y.; et al. Exosome-Associated Tau Is Secreted in Tauopathy Models and Is Selectively Phosphorylated in Cerebrospinal Fluid in Early Alzheimer Disease. J. Biol. Chem. 2012, 287, 3842–3849. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, N.; Iguchi, H.; Yoshioka, Y.; Takeshita, F.; Matsuki, Y.; Ochiya, T. Secretory Mechanisms and Intercellular Transfer of MicroRNAs in Living Cells. J. Biol. Chem. 2010, 285, 17442–17452. [Google Scholar] [CrossRef]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-Mediated Transfer of MRNAs and MicroRNAs Is a Novel Mechanism of Genetic Exchange between Cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Rechavi, O.; Erlich, Y.; Amram, H.; Flomenblit, L.; Karginov, F.V.; Goldstein, I.; Hannon, G.J.; Kloog, Y. Cell Contact-Dependent Acquisition of Cellular and Viral Nonautonomously Encoded Small RNAs. Genes Dev. 2009, 23, 1971–1979. [Google Scholar] [CrossRef] [PubMed]
- Mulcahy, L.A.; Pink, R.C.; Carter, D.R.F. Routes and Mechanisms of Extracellular Vesicle Uptake. J. Extracell. Vesicles 2014, 3, 24641. [Google Scholar] [CrossRef] [PubMed]
- Janas, T.; Janas, M.M.; Sapoń, K.; Janas, T. Mechanisms of RNA Loading into Exosomes. FEBS Lett. 2015, 589, 1391–1398. [Google Scholar] [CrossRef]
- Jeyaseelan, K.; Lim, K.Y.; Armugam, A. MicroRNA Expression in the Blood and Brain of Rats Subjected to Transient Focal Ischemia by Middle Cerebral Artery Occlusion. Stroke 2008, 39, 959–966. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, J.Y.; Xu, L.Y.; Cai, R.; Chen, Z.; Luo, B.Y. MicroRNA Expression Changes in the Hippocampi of Rats Subjected to Global Ischemia. J. Clin. Neurosci. 2010, 17, 774–778. [Google Scholar] [CrossRef]
- Peng, G.; Yuan, Y.; Wu, S.; He, F.; Hu, Y.; Luo, B. MicroRNA Let-7e Is a Potential Circulating Biomarker of Acute Stage Ischemic Stroke. Transl. Stroke Res. 2015, 6, 437–445. [Google Scholar] [CrossRef]
- Liu, D.-Z.; Tian, Y.; Ander, B.P.; Xu, H.; Stamova, B.S.; Zhan, X.; Turner, R.J.; Jickling, G.; Sharp, F.R. Brain and Blood MicroRNA Expression Profiling of Ischemic Stroke, Intracerebral Hemorrhage, and Kainate Seizures. J. Cereb. Blood Flow Metab. 2010, 30, 92–101. [Google Scholar] [CrossRef]
- Haneklaus, M.; Gerlic, M.; Kurowska-Stolarska, M.; Rainey, A.-A.; Pich, D.; McInnes, I.B.; Hammerschmidt, W.; O’Neill, L.A.J.; Masters, S.L. Cutting Edge: MiR-223 and EBV MiR-BART15 Regulate the NLRP3 Inflammasome and IL-1β Production. J. Immunol. 2012, 189, 3795–3799. [Google Scholar] [CrossRef]
- Long, F.-Q.; Kou, C.-X.; Li, K.; Wu, J.; Wang, Q.-Q. MiR-223-3p Inhibits RTp17-Induced Inflammasome Activation and Pyroptosis by Targeting NLRP3. J. Cell. Mol. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Jimenez Calvente, C.; Del Pilar, H.; Tameda, M.; Johnson, C.D.; Feldstein, A.E. MicroRNA 223 3p Negatively Regulates the NLRP3 Inflammasome in Acute and Chronic Liver Injury. Mol. Ther. J. Am. Soc. Gene Ther. 2020, 28, 653–663. [Google Scholar] [CrossRef]
- Jeffries, J.; Zhou, W.; Hsu, A.Y.; Deng, Q. MiRNA-223 at the Crossroads of Inflammation and Cancer. Cancer Lett. 2019, 451, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Qiao, F.; Huang, D.; Wu, Q.; Chen, T.; Badawy, S.; Cheng, G.; Hao, H.; Xie, S.; Wang, X. MiR-155-5p Plays as a “Janus” in the Expression of Inflammatory Cytokines Induced by T-2 Toxin. Food Chem. Toxicol. 2020, 140, 111258. [Google Scholar] [CrossRef]
- Shi, Y.; Li, K.; Xu, K.; Liu, Q.-H. MiR-155-5p Accelerates Cerebral Ischemia-Reperfusion Injury via Targeting DUSP14 by Regulating NF-ΚB and MAPKs Signaling Pathways. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 1408–1419. [Google Scholar] [CrossRef] [PubMed]
- Onodera, Y.; Teramura, T.; Takehara, T.; Obora, K.; Mori, T.; Fukuda, K. MiR-155 Induces ROS Generation through Downregulation of Antioxidation-Related Genes in Mesenchymal Stem Cells. Aging Cell 2017, 16, 1369–1380. [Google Scholar] [CrossRef]
- Zheng, X.; Huang, H.; Liu, J.; Li, M.; Liu, M.; Luo, T. Propofol Attenuates Inflammatory Response in LPS-Activated Microglia by Regulating the MiR-155/SOCS1 Pathway. Inflammation 2018, 41, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Chen, H.; Hu, Y.; Li, Q.; Hu, Z.; Ma, T.; Mao, X. Burkholderia Pseudomallei-Derived MiR-3473 Enhances NF-ΚB via Targeting TRAF3 and Is Associated with Different Inflammatory Responses Compared to Burkholderia Thailandensis in Murine Macrophages. BMC Microbiol. 2016, 16, 283. [Google Scholar] [CrossRef]
- Wang, X.; Chen, S.; Ni, J.; Cheng, J.; Jia, J.; Zhen, X. MiRNA-3473b Contributes to Neuroinflammation Following Cerebral Ischemia. Cell Death Dis. 2018, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Zhang, S.; Huang, C.; Li, K.; Zhao, Q.; Li, X. MiR-448-5p/VEGFA Axis Protects Cardiomyocytes from Hypoxia Through Regulating the FAS/FAS-L Signaling Pathway. Int. Heart. J. 2021, 62, 647–657. [Google Scholar] [CrossRef]
- Weis, S.M.; Cheresh, D.A. Pathophysiological Consequences of VEGF-Induced Vascular Permeability. Nature 2005, 437, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Geiseler, S.J.; Morland, C. The Janus Face of VEGF in Stroke. Int. J. Mol. Sci. 2018, 19, 1362. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.; Gröger, L.; Tschernig, T.; Solomon, J.; Laham, O.; Schaum, N.; Wagner, V.; Kern, F.; Schmartz, G.P.; Li, Y.; et al. MiRNATissueAtlas2: An Update to the Human MiRNA Tissue Atlas. Nucleic Acids Res. 2021, gkab808. [Google Scholar] [CrossRef] [PubMed]
- Tabula Muris Consortium. A Single Cell Transcriptomic Atlas Characterizes Aging Tissues in the Mouse. Nature 2020, 583, 590–595. [Google Scholar] [CrossRef]
- Voelz, C.; Habib, P.; Köberlein, S.; Beyer, C.; Slowik, A. Alteration of MiRNA Biogenesis Regulating Proteins in the Human Microglial Cell Line HMC-3 after Ischemic Stress. Mol. Neurobiol. 2021, 58, 1535–1549. [Google Scholar] [CrossRef] [PubMed]
- Herzog, R.; Zendedel, A.; Lammerding, L.; Beyer, C.; Slowik, A. Impact of 17beta-Estradiol and Progesterone on Inflammatory and Apoptotic MicroRNA Expression after Ischemia in a Rat Model. J. Steroid Biochem. Mol. Biol. 2017, 167, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Song, Y.; Huang, J.; Qu, M.; Zhang, Y.; Geng, J.; Zhang, Z.; Liu, J.; Yang, G.-Y. Increased Circulating Exosomal MiRNA-223 Is Associated with Acute Ischemic Stroke. Front. Neurol. 2017, 8, 57. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Huang, J.; Chen, X.; Gu, X.; Wang, Y.; Zeng, L.; Yang, G.-Y. Increase of Circulating MiR-223 and Insulin-like Growth Factor-1 Is Associated with the Pathogenesis of Acute Ischemic Stroke in Patients. BMC Neurol. 2014, 14, 77. [Google Scholar] [CrossRef]
- Duan, X.; Zhan, Q.; Song, B.; Zeng, S.; Zhou, J.; Long, Y.; Lu, J.; Li, Z.; Yuan, M.; Chen, X.; et al. Detection of Platelet MicroRNA Expression in Patients with Diabetes Mellitus with or without Ischemic Stroke. J. Diabetes Complicat. 2014, 28, 705–710. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, C.; Huang, C.; Xu, X.; Teng, J. MiR-155 Knockdown Protects against Cerebral Ischemia and Reperfusion Injury by Targeting MafB. BioMed Res. Int. 2020, 2020, 6458204. [Google Scholar] [CrossRef]
- Xing, G.; Luo, Z.; Zhong, C.; Pan, X.; Xu, X. Influence of MiR-155 on Cell Apoptosis in Rats with Ischemic Stroke: Role of the Ras Homolog Enriched in Brain (Rheb)/MTOR Pathway. Med. Sci. Monit. 2016, 22, 5141–5153. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zheng, H.-Z.; Jiang, W.; Zhao, X.-F.; Du, J.; Liu, P.-G.; Chang, L.-D.; Li, W.-B.; Hu, H.-T.; Shi, X.-M. Electroacupuncture Induces Acute Changes in Cerebral Cortical MiRNA Profile, Improves Cerebral Blood Flow and Alleviates Neurological Deficits in a Rat Model of Stroke. Neural Regen. Res. 2016, 11, 1940–1950. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pang, Q.-J.; Liu, J.-T.; Wu, H.-H.; Tao, D.-Y. Down-Regulated MiR-448 Relieves Spinal Cord Ischemia/Reperfusion Injury by up-Regulating SIRT1. Braz. J. Med. Biol. Res. 2018, 51, e7319. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.-J.; Xie, A.; Liu, H.; Dudley, S.C. MIR448 Antagomir Reduces Arrhythmic Risk after Myocardial Infarction by Upregulating the Cardiac Sodium Channel. JCI Insight 2020, 5, 140759. [Google Scholar] [CrossRef]
- Harraz, M.M.; Eacker, S.M.; Wang, X.; Dawson, T.M.; Dawson, V.L. MicroRNA-223 Is Neuroprotective by Targeting Glutamate Receptors. Proc. Natl. Acad. Sci. USA 2012, 109, 18962–18967. [Google Scholar] [CrossRef]
- Balakathiresan, N.S.; Chandran, R.; Bhomia, M.; Jia, M.; Li, H.; Maheshwari, R.K. Serum and Amygdala MicroRNA Signatures of Posttraumatic Stress: Fear Correlation and Biomarker Potential. J. Psychiatr. Res. 2014, 57, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-X.; Visavadiya, N.P.; Pandya, J.D.; Nelson, P.T.; Sullivan, P.G.; Springer, J.E. Mitochondria-Associated MicroRNAs in Rat Hippocampus Following Traumatic Brain Injury. Exp. Neurol. 2015, 265, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, L.; Pang, X.; Zhang, J.; Guan, Y. Role of MicroRNA-155 in Modifying Neuroinflammation and Γ-aminobutyric Acid Transporters in Specific Central Regions after Post-ischaemic Seizures. J. Cell. Mol. Med. 2019, 23, 5019–5024. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Dai, Q.; Jiang, L.; Wang, Y.; Yang, T.; Miao, J.; Wang, J.; Han, Y. Downregulation of MicroRNA-448 Improves Isoflurane-Induced Learning and Memory Impairment in Rats. Mol. Med. Rep. 2017, 16, 1578–1583. [Google Scholar] [CrossRef]
- Zhu, H.; Zhou, X.; Ma, C.; Chang, H.; Li, H.; Liu, F.; Lu, J. Low Expression of MiR-448 Induces EMT and Promotes Invasion by Regulating ROCK2 in Hepatocellular Carcinoma. Cell. Physiol. Biochem. 2015, 36, 487–498. [Google Scholar] [CrossRef]
- Guo, J.-C.; Yang, Y.-J.; Zhang, J.-Q.; Guo, M.; Xiang, L.; Yu, S.-F.; Ping, H.; Zhuo, L. MicroRNA-448 Inhibits Stemness Maintenance and Self-Renewal of Hepatocellular Carcinoma Stem Cells through the MAGEA6-Mediated AMPK Signaling Pathway. J. Cell. Physiol. 2019, 234, 23461–23474. [Google Scholar] [CrossRef]
- Katayama, Y.; Maeda, M.; Miyaguchi, K.; Nemoto, S.; Yasen, M.; Tanaka, S.; Mizushima, H.; Fukuoka, Y.; Arii, S.; Tanaka, H. Identification of Pathogenesis-Related MicroRNAs in Hepatocellular Carcinoma by Expression Profiling. Oncol. Lett. 2012, 4, 817–823. [Google Scholar] [CrossRef][Green Version]
- Correia de Sousa, M.; Gjorgjieva, M.; Dolicka, D.; Sobolewski, C.; Foti, M. Deciphering MiRNAs’ Action through MiRNA Editing. Int. J. Mol. Sci. 2019, 20, 6249. [Google Scholar] [CrossRef]
- Ota, H.; Sakurai, M.; Gupta, R.; Valente, L.; Wulff, B.-E.; Ariyoshi, K.; Iizasa, H.; Davuluri, R.V.; Nishikura, K. ADAR1 Forms a Complex with Dicer to Promote MicroRNA Processing and RNA-Induced Gene Silencing. Cell 2013, 153, 575–589. [Google Scholar] [CrossRef]
- Nussbacher, J.K.; Yeo, G.W. Systematic Discovery of RNA Binding Proteins that Regulate MicroRNA Levels. Mol. Cell 2018, 69, 1005–1016.e7. [Google Scholar] [CrossRef]
- Gjorgjieva, M.; Sobolewski, C.; Dolicka, D.; Correia de Sousa, M.; Foti, M. MiRNAs and NAFLD: From Pathophysiology to Therapy. Gut 2019, 68, 2065–2079. [Google Scholar] [CrossRef]
- Vasudevan, S.; Tong, Y.; Steitz, J.A. Switching from Repression to Activation: MicroRNAs Can Up-Regulate Translation. Science 2007, 318, 1931–1934. [Google Scholar] [CrossRef]
- Xiao, M.; Li, J.; Li, W.; Wang, Y.; Wu, F.; Xi, Y.; Zhang, L.; Ding, C.; Luo, H.; Li, Y.; et al. MicroRNAs Activate Gene Transcription Epigenetically as an Enhancer Trigger. RNA Biol. 2017, 14, 1326–1334. [Google Scholar] [CrossRef]
- Maes, T.; Cobos, F.A.; Schleich, F.; Sorbello, V.; Henket, M.; De Preter, K.; Bracke, K.R.; Conickx, G.; Mesnil, C.; Vandesompele, J.; et al. Asthma Inflammatory Phenotypes Show Differential MicroRNA Expression in Sputum. J. Allergy Clin. Immunol. 2016, 137, 1433–1446. [Google Scholar] [CrossRef]
- Liu, Y.; Li, C.; Wu, H.; Xie, X.; Sun, Y.; Dai, M. Paeonol Attenuated Inflammatory Response of Endothelial Cells via Stimulating Monocytes-Derived Exosomal MicroRNA-223. Front. Pharmacol. 2018, 9, 1105. [Google Scholar] [CrossRef]
- Merkerova, M.; Belickova, M.; Bruchova, H. Differential Expression of MicroRNAs in Hematopoietic Cell Lineages. Eur. J. Haematol. 2008, 81, 304–310. [Google Scholar] [CrossRef]
- Calvente, C.J.; Tameda, M.; Johnson, C.D.; Del Pilar, H.; Lin, Y.C.; Adronikou, N.; De Mollerat Du Jeu, X.; Llorente, C.; Boyer, J.; Feldstein, A.E. Neutrophils Contribute to Spontaneous Resolution of Liver Inflammation and Fibrosis via MicroRNA-223. J. Clin. Investig. 2019, 129, 4091–4109. [Google Scholar] [CrossRef]
- Izumi, B.; Nakasa, T.; Tanaka, N.; Nakanishi, K.; Kamei, N.; Yamamoto, R.; Nakamae, T.; Ohta, R.; Fujioka, Y.; Yamasaki, K.; et al. MicroRNA-223 Expression in Neutrophils in the Early Phase of Secondary Damage after Spinal Cord Injury. Neurosci. Lett. 2011, 492, 114–118. [Google Scholar] [CrossRef]
- Zeng, Z.; Xia, L.; Fan, X.; Ostriker, A.C.; Yarovinsky, T.; Su, M.; Zhang, Y.; Peng, X.; Xie, Y.; Pi, L.; et al. Platelet-Derived MiR-223 Promotes a Phenotypic Switch in Arterial Injury Repair. J. Clin. Investig. 2019, 129, 1372–1386. [Google Scholar] [CrossRef]
- Nian, K.; Harding, I.C.; Herman, I.M.; Ebong, E.E. Blood-Brain Barrier Damage in Ischemic Stroke and Its Regulation by Endothelial Mechanotransduction. Front. Physiol. 2020, 11, 1681. [Google Scholar] [CrossRef]
- Scheld, M.; Heymann, F.; Zhao, W.; Tohidnezhad, M.; Clarner, T.; Beyer, C.; Zendedel, A. Modulatory Effect of 17β-Estradiol on Myeloid Cell Infiltration into the Male Rat Brain after Ischemic Stroke. J. Steroid Biochem. Mol. Biol. 2020, 202, 105667. [Google Scholar] [CrossRef]
- Ceppi, M.; Pereira, P.M.; Dunand-Sauthier, I.; Barras, E.; Reith, W.; Santos, M.A.; Pierre, P. MicroRNA-155 Modulates the Interleukin-1 Signaling Pathway in Activated Human Monocyte-Derived Dendritic Cells. Proc. Natl. Acad. Sci. USA 2009, 106, 2735–2740. [Google Scholar] [CrossRef]
- Rajasekhar, M.; Olsson, A.M.; Steel, K.J.A.; Georgouli, M.; Ranasinghe, U.; Brender Read, C.; Frederiksen, K.S.; Taams, L.S. MicroRNA-155 Contributes to Enhanced Resistance to Apoptosis in Monocytes from Patients with Rheumatoid Arthritis. J. Autoimmun. 2017, 79, 53–62. [Google Scholar] [CrossRef]
- Marques-Rocha, J.L.; Garcia-Lacarte, M.; Samblas, M.; Bressan, J.; Martínez, J.A.; Milagro, F.I. Regulatory Roles of MiR-155 and Let-7b on the Expression of Inflammation-Related Genes in THP-1 Cells: Effects of Fatty Acids. J. Physiol. Biochem. 2018, 74, 579–589. [Google Scholar] [CrossRef]
- Elton, T.S.; Selemon, H.; Elton, S.M.; Parinandi, N.L. Regulation of the MIR155 Host Gene in Physiological and Pathological Processes. Gene 2013, 532, 1–12. [Google Scholar] [CrossRef]
- Gomez, I.; Ward, B.; Souilhol, C.; Recarti, C.; Ariaans, M.; Johnston, J.; Burnett, A.; Mahmoud, M.; Luong, L.A.; West, L.; et al. Neutrophil Microvesicles Drive Atherosclerosis by Delivering MiR-155 to Atheroprone Endothelium. Nat. Commun. 2020, 11, 214. [Google Scholar] [CrossRef] [PubMed]
- Butin-Israeli, V.; Bui, T.M.; Wiesolek, H.L.; Mascarenhas, L.; Lee, J.J.; Mehl, L.C.; Knutson, K.R.; Adam, S.A.; Goldman, R.D.; Beyder, A.; et al. Neutrophil-Induced Genomic Instability Impedes Resolution of Inflammation and Wound Healing. J. Clin. Investig. 2019, 129, 712–726. [Google Scholar] [CrossRef]
- Wu, R.; He, Q.; Chen, H.; Xu, M.; Zhao, N.; Xiao, Y.; Tu, Q.-Q.; Zhang, W.; Bi, X. MicroRNA-448 Promotes Multiple Sclerosis Development through Induction of Th17 Response through Targeting Protein Tyrosine Phosphatase Non-Receptor Type 2 (PTPN2). Biochem. Biophys. Res. Commun. 2017, 486, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Lammerding, L.; Slowik, A.; Johann, S.; Beyer, C.; Zendedel, A. Poststroke Inflammasome Expression and Regulation in the Peri-Infarct Area by Gonadal Steroids after Transient Focal Ischemia in the Rat Brain. Neuroendocrinology 2016, 103, 460–475. [Google Scholar] [CrossRef]
- Merksz, M.; Ambach, G.; Palkovits, M. Blood Supply of the Rat Amygdala. Acta Morphol. Acad. Sci. Hung. 1978, 26, 139–171. [Google Scholar]
- Gubskiy, I.L.; Namestnikova, D.D.; Cherkashova, E.A.; Chekhonin, V.P.; Baklaushev, V.P.; Gubsky, L.V.; Yarygin, K.N. MRI Guiding of the Middle Cerebral Artery Occlusion in Rats Aimed to Improve Stroke Modeling. Transl. Stroke Res. 2018, 9, 417–425. [Google Scholar] [CrossRef]
- Shah, F.A.; Li, T.; Kury, L.T.A.; Zeb, A.; Khatoon, S.; Liu, G.; Yang, X.; Liu, F.; Yao, H.; Khan, A.-U.; et al. Pathological Comparisons of the Hippocampal Changes in the Transient and Permanent Middle Cerebral Artery Occlusion Rat Models. Front. Neurol. 2019, 10, 1178. [Google Scholar] [CrossRef]
- Ozdemir, Y.G.; Bolay, H.; Erdem, E.; Dalkara, T. Occlusion of the MCA by an Intraluminal Filament May Cause Disturbances in the Hippocampal Blood Flow Due to Anomalies of Circle of Willis and Filament Thickness. Brain Res. 1999, 822, 260–264. [Google Scholar] [CrossRef]
- Block, F.; Dihné, M.; Loos, M. Inflammation in Areas of Remote Changes Following Focal Brain Lesion. Prog. Neurobiol. 2005, 75, 342–365. [Google Scholar] [CrossRef]
- Garcia, J.H.; Liu, K.F.; Ye, Z.R.; Gutierrez, J.A. Incomplete Infarct and Delayed Neuronal Death after Transient Middle Cerebral Artery Occlusion in Rats. Stroke 1997, 28, 2303–2309; discussion 2310. [Google Scholar] [CrossRef]
- Xiong, B.; Li, A.; Lou, Y.; Chen, S.; Long, B.; Peng, J.; Yang, Z.; Xu, T.; Yang, X.; Li, X.; et al. Precise Cerebral Vascular Atlas in Stereotaxic Coordinates of Whole Mouse Brain. Front. Neuroanat. 2017, 11, 128. [Google Scholar] [CrossRef]
- Franke, M.; Bieber, M.; Kraft, P.; Weber, A.N.R.; Stoll, G.; Schuhmann, M.K. The NLRP3 Inflammasome Drives Inflammation in Ischemia/Reperfusion Injury after Transient Middle Cerebral Artery Occlusion in Mice. Brain. Behav. Immun. 2021, 92, 223–233. [Google Scholar] [CrossRef]
- Ismael, S.; Zhao, L.; Nasoohi, S.; Ishrat, T. Inhibition of the NLRP3-Inflammasome as a Potential Approach for Neuroprotection after Stroke. Sci. Rep. 2018, 8, 5971. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Ma, S.; Yi, Y.; Yu, H.; Pei, L.; Feng, D. The Role of NLRP3 Inflammasome in Stroke and Central Poststroke Pain. Medicine 2018, 97, e11861. [Google Scholar] [CrossRef]
- Baker, B.J.; Akhtar, L.N.; Benveniste, E.N. SOCS1 and SOCS3 in the Control of CNS Immunity. Trends Immunol. 2009, 30, 392–400. [Google Scholar] [CrossRef]
- Hu, J.; Wang, W.; Hao, Q.; Zhang, T.; Yin, H.; Wang, M.; Zhang, C.; Zhang, C.; Zhang, L.; Zhang, X.; et al. Suppressors of Cytokine Signalling (SOCS)-1 Inhibits Neuroinflammation by Regulating ROS and TLR4 in BV2 Cells. Inflamm. Res. 2020, 69, 27–39. [Google Scholar] [CrossRef]
- Wen, Y.; Zhang, X.; Dong, L.; Zhao, J.; Zhang, C.; Zhu, C. Acetylbritannilactone Modulates MicroRNA-155-Mediated Inflammatory Response in Ischemic Cerebral Tissues. Mol. Med. 2015, 21, 197–209. [Google Scholar] [CrossRef]
- Dinapoli, V.A.; Benkovic, S.A.; Li, X.; Kelly, K.A.; Miller, D.B.; Rosen, C.L.; Huber, J.D.; O’Callaghan, J.P. Age Exaggerates Proinflammatory Cytokine Signaling and Truncates Signal Transducers and Activators of Transcription 3 Signaling Following Ischemic Stroke in the Rat. Neuroscience 2010, 170, 633–644. [Google Scholar] [CrossRef]
- Raghavendra Rao, V.L.; Bowen, K.K.; Dhodda, V.K.; Song, G.; Franklin, J.L.; Gavva, N.R.; Dempsey, R.J. Gene Expression Analysis of Spontaneously Hypertensive Rat Cerebral Cortex Following Transient Focal Cerebral Ischemia: GeneChip® Analysis after Stroke. J. Neurochem. 2002, 83, 1072–1086. [Google Scholar] [CrossRef]
- Aliena-Valero, A.; Rius-Pérez, S.; Baixauli-Martín, J.; Torregrosa, G.; Chamorro, Á.; Pérez, S.; Salom, J.B. Uric Acid Neuroprotection Associated to IL-6/STAT3 Signaling Pathway Activation in Rat Ischemic Stroke. Mol. Neurobiol. 2021, 58, 408–423. [Google Scholar] [CrossRef]
- Modo, M.; Mellodew, K.; Cash, D.; Fraser, S.E.; Meade, T.J.; Price, J.; Williams, S.C.R. Mapping Transplanted Stem Cell Migration after a Stroke: A Serial, In Vivo Magnetic Resonance Imaging Study. NeuroImage 2004, 21, 311–317. [Google Scholar] [CrossRef]
- Habib, P.; Harms, J.; Zendedel, A.; Beyer, C.; Slowik, A. Gonadal Hormones E2 and P Mitigate Cerebral Ischemia-Induced Upregulation of the AIM2 and NLRC4 Inflammasomes in Rats. Int. J. Mol. Sci. 2020, 21, 4795. [Google Scholar] [CrossRef]
- Garcia, J.H.; Wagner, S.; Liu, K.F.; Hu, X.J. Neurological Deficit and Extent of Neuronal Necrosis Attributable to Middle Cerebral Artery Occlusion in Rats. Statistical Validation. Stroke 1995, 26, 627–634; discussion 635. [Google Scholar] [CrossRef]
- Hatfield, R.H.; Mendelow, A.D.; Perry, R.H.; Alvarez, L.M.; Modha, P. Triphenyltetrazolium Chloride (TTC) as a Marker for Ischaemic Changes in Rat Brain Following Permanent Middle Cerebral Artery Occlusion. Neuropathol. Appl. Neurobiol. 1991, 17, 61–67. [Google Scholar] [CrossRef]
- Dora, R.; Aniko, S.; Sandor, V.; Tamas, K.; Akira, A. Delayed Systemic Administration of PACAP38 Is Neuroprotective in Transient Middle Cerebral Artery Occlusion in the Rat. Stroke 2000, 31, 1411–1417. [Google Scholar] [CrossRef][Green Version]
- Nouraee, C.; Fisher, M.; Di Napoli, M.; Salazar, P.; Farr, T.D.; Jafarli, A.; Divani, A.A. A Brief Review of Edema-Adjusted Infarct Volume Measurement Techniques for Rodent Focal Cerebral Ischemia Models with Practical Recommendations. J. Vasc. Interv. Neurol. 2019, 10, 38–45. [Google Scholar]
- Irizarry, R.A.; Hobbs, B.; Collin, F.; Beazer-Barclay, Y.D.; Antonellis, K.J.; Scherf, U.; Speed, T.P. Exploration, Normalization, and Summaries of High Density Oligonucleotide Array Probe Level Data. Biostat. Oxf. Engl. 2003, 4, 249–264. [Google Scholar] [CrossRef]
- Busk, P.K. A Tool for Design of Primers for MicroRNA-Specific Quantitative RT-QPCR. BMC Bioinform. 2014, 15, 29. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- de Ronde, M.W.J.; Ruijter, J.M.; Moerland, P.D.; Creemers, E.E.; Pinto-Sietsma, S.-J. Study Design and QPCR Data Analysis Guidelines for Reliable Circulating MiRNA Biomarker Experiments: A Review. Clin. Chem. 2018, 64, 1308–1318. [Google Scholar] [CrossRef] [PubMed]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate Normalization of Real-Time Quantitative RT-PCR Data by Geometric Averaging of Multiple Internal Control Genes. Genome Biol. 2002, 3, RESEARCH0034. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenbach, H.; da Silva, A.M.; Calin, G.; Pantel, K. Data Normalization Strategies for MicroRNA Quantification. Clin. Chem. 2015, 61, 1333–1342. [Google Scholar] [CrossRef] [PubMed]
| Primer Name | Sense | Anti-Sense |
|---|---|---|
| U6 | CCCTGCGCAAGGATGA | AGGTCCAGTTTTTTTTTTTTTTTAATTTG |
| cel-miR-39 | GTCACCGGGTGTAAATCAG | GGTCCAGTTTTTTTTTTTTTTTCAAG |
| miR-103 | GCAGAGCAGCATTGTACAG | GGTCCAGTTTTTTTTTTTTTTTCATAG |
| miR-107 | GCAGAGCAGCATTGTACAG | GGTCCAGTTTTTTTTTTTTTTTGATAG |
| miR-223-3p | CGCAGTGTCAGTTTGTCA | CCAGTTTTTTTTTTTTTTTGGGGTA |
| miR-155-5p | CGCAGTTAATGCTAATTGTGATAG | AGGTCCAGTTTTTTTTTTTTTTTACC |
| miR-3473 | CAGTCTAGGGCTGGAGAG | CCAGTTTTTTTTTTTTTTTAGCCATC |
| miR-448-5p | CGCAGAACATCCTGCATAG | GTTTTTTTTTTTTTTTGGCAGCAC |
| miR-328b-3p | GCTGGCCCTCTCTGC | AGGTCCAGTTTTTTTTTTTTTTTAGG |
| miR-344i | CTCTAGCCAGGGCTTGA | TCCAGTTTTTTTTTTTTTTTGCAGT |
| cyclophilin A | GGCAAATGCTGGACCAAACAC | TTAGAGTTGTCCACAGTCGGAGATG |
| Gapdh | AACCCATCACCATCTTCCAG | GTGGTTCACACCCATCACAA |
| Ago | CCCCCACCTCCCATGTTTAC | GACCTGGCAGTTGCTCTGAT |
| Dicer | GAAGAGGAGACCAGCGTTCC | CGGGTTTGGGGTAACTCTCC |
| Drosha | CTGGGACGAAACCAAGCTCT | CATAACTCAACTGTGCAGGGC |
| Trbp | GGATCATGGCCGGTAGCAAA | CTAGGCAGACGATAGACCC |
| Dgcr8 | GCGAAGAATAAAGCTGCCCG | TTGTCAGCTCGTAGACTCGC |
| Xpo5 | AGAATCTGGTCGCTTGGTGG | CCGTCAGAAGGGCAAGATGT |
| Nrlp3 | TCTGTTCATTGGCTGCGGAT | GCCTTTTTCGAACTTGCCGT |
| Socs1 | CCGCTCCCACTCTGATTACC | CTCAGGGGTCCCCAGTAGAA |
| Socs3 | CGACGGAACCTTCCTTTGAGG | AGAGGTCGGCTCAGTACCAG |
| Vegfa | ATCTTCCAGGAGTACCCCGAT | CGCATGATCTGCATAGTGACG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voelz, C.; Ebrahimy, N.; Zhao, W.; Habib, P.; Zendedel, A.; Pufe, T.; Beyer, C.; Slowik, A. Transient Focal Cerebral Ischemia Leads to miRNA Alterations in Different Brain Regions, Blood Serum, Liver, and Spleen. Int. J. Mol. Sci. 2022, 23, 161. https://doi.org/10.3390/ijms23010161
Voelz C, Ebrahimy N, Zhao W, Habib P, Zendedel A, Pufe T, Beyer C, Slowik A. Transient Focal Cerebral Ischemia Leads to miRNA Alterations in Different Brain Regions, Blood Serum, Liver, and Spleen. International Journal of Molecular Sciences. 2022; 23(1):161. https://doi.org/10.3390/ijms23010161
Chicago/Turabian StyleVoelz, Clara, Nahal Ebrahimy, Weiyi Zhao, Pardes Habib, Adib Zendedel, Thomas Pufe, Cordian Beyer, and Alexander Slowik. 2022. "Transient Focal Cerebral Ischemia Leads to miRNA Alterations in Different Brain Regions, Blood Serum, Liver, and Spleen" International Journal of Molecular Sciences 23, no. 1: 161. https://doi.org/10.3390/ijms23010161
APA StyleVoelz, C., Ebrahimy, N., Zhao, W., Habib, P., Zendedel, A., Pufe, T., Beyer, C., & Slowik, A. (2022). Transient Focal Cerebral Ischemia Leads to miRNA Alterations in Different Brain Regions, Blood Serum, Liver, and Spleen. International Journal of Molecular Sciences, 23(1), 161. https://doi.org/10.3390/ijms23010161







