A Vacuolar Invertase CsVI2 Regulates Sucrose Metabolism and Increases Drought Tolerance in Cucumis sativus L.
Abstract
:1. Introduction
2. Results
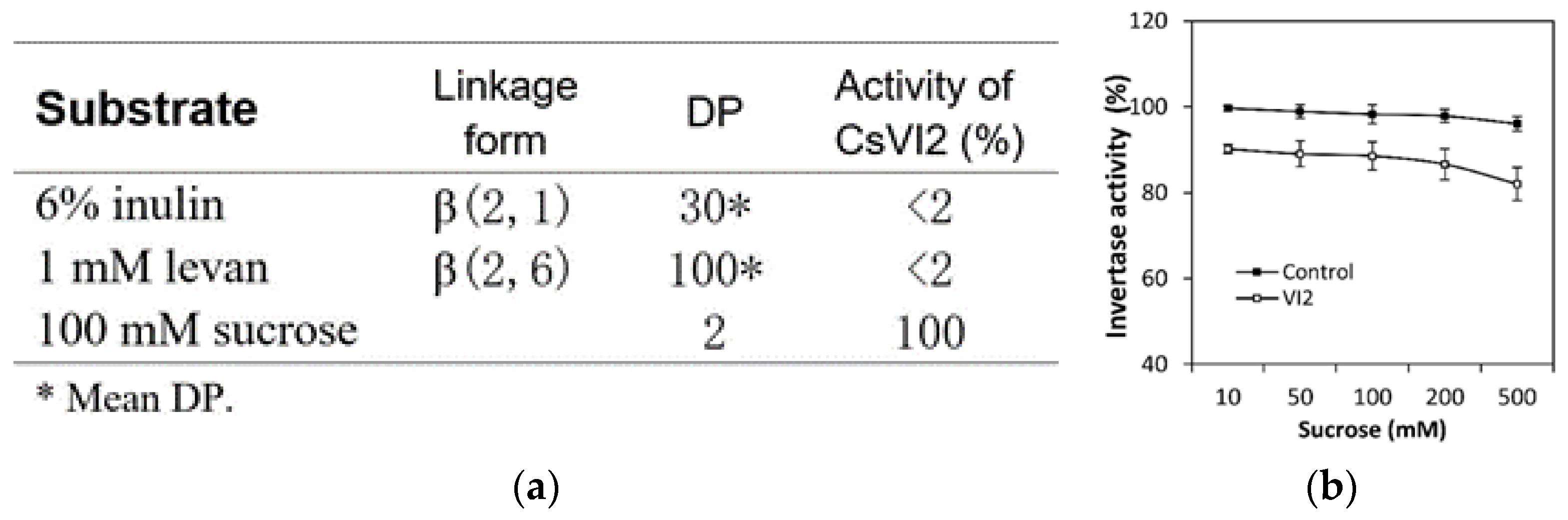
2.1. Cloning and Enzymatic Characterization of CsVI2
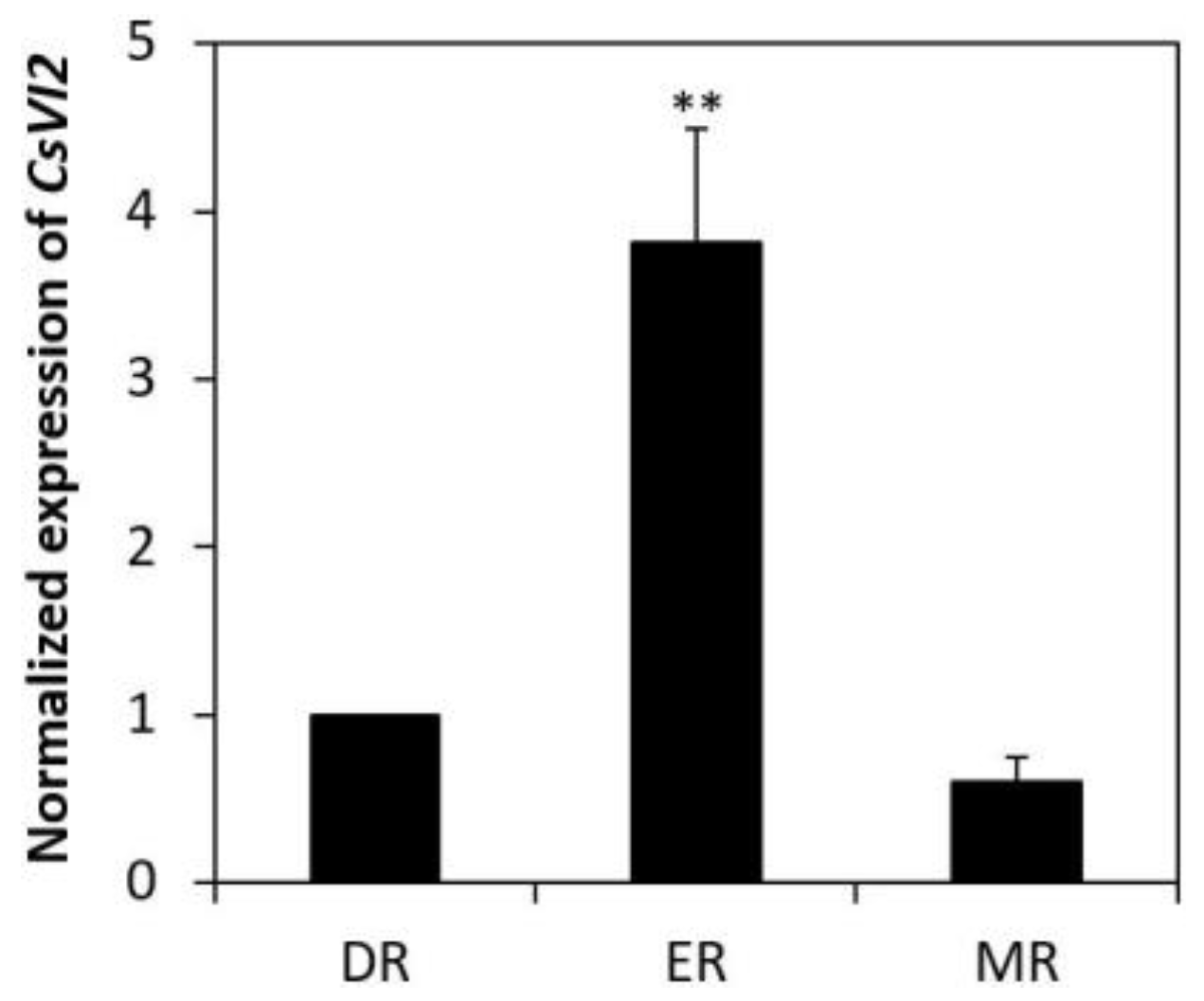
2.2. Expression Profile of CsVI2 in Cucumber Seedlings
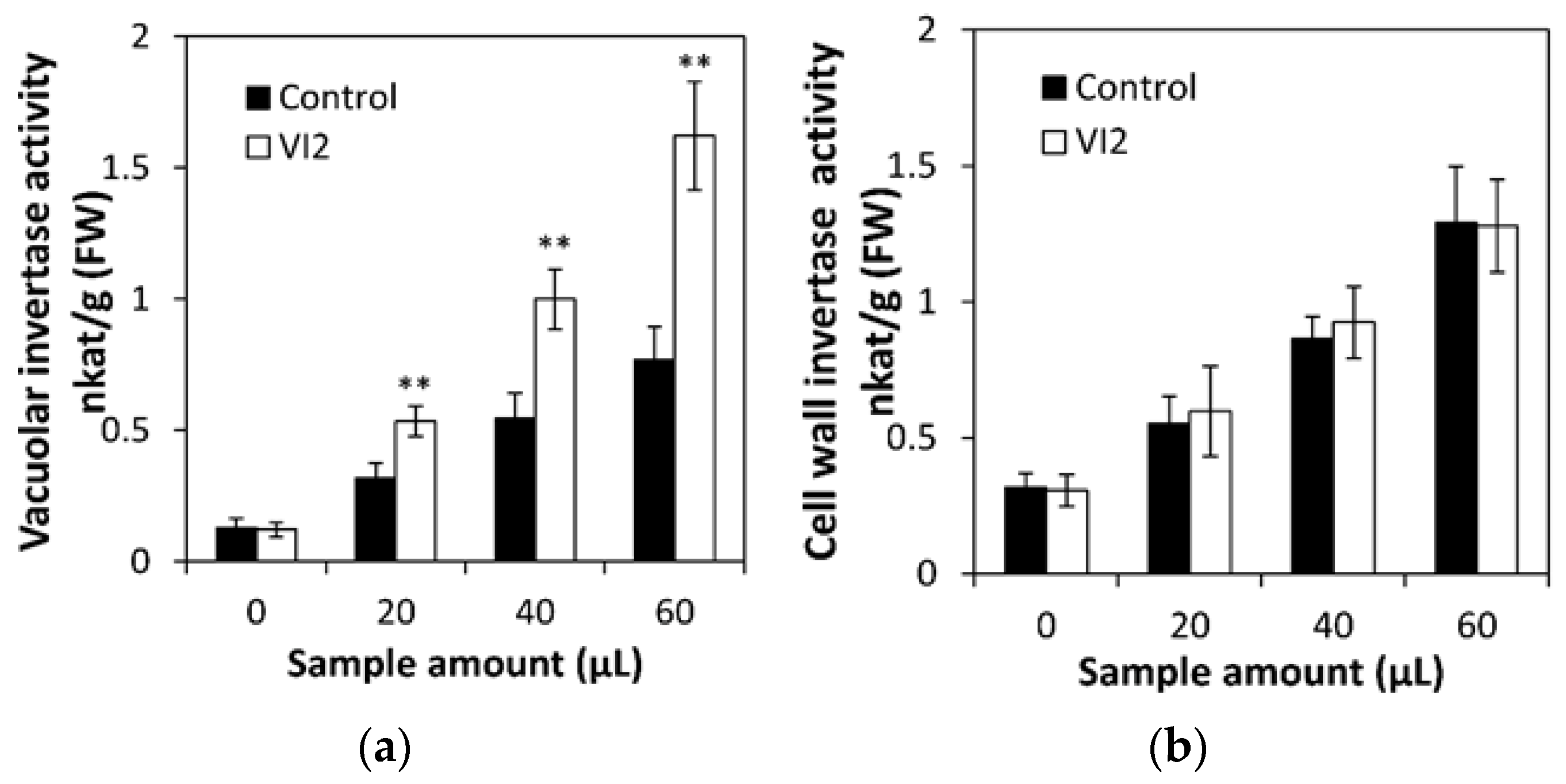
2.3. Drought Stress Induces CsVI2 Expression and Increases Vacuolar Invertase Activity
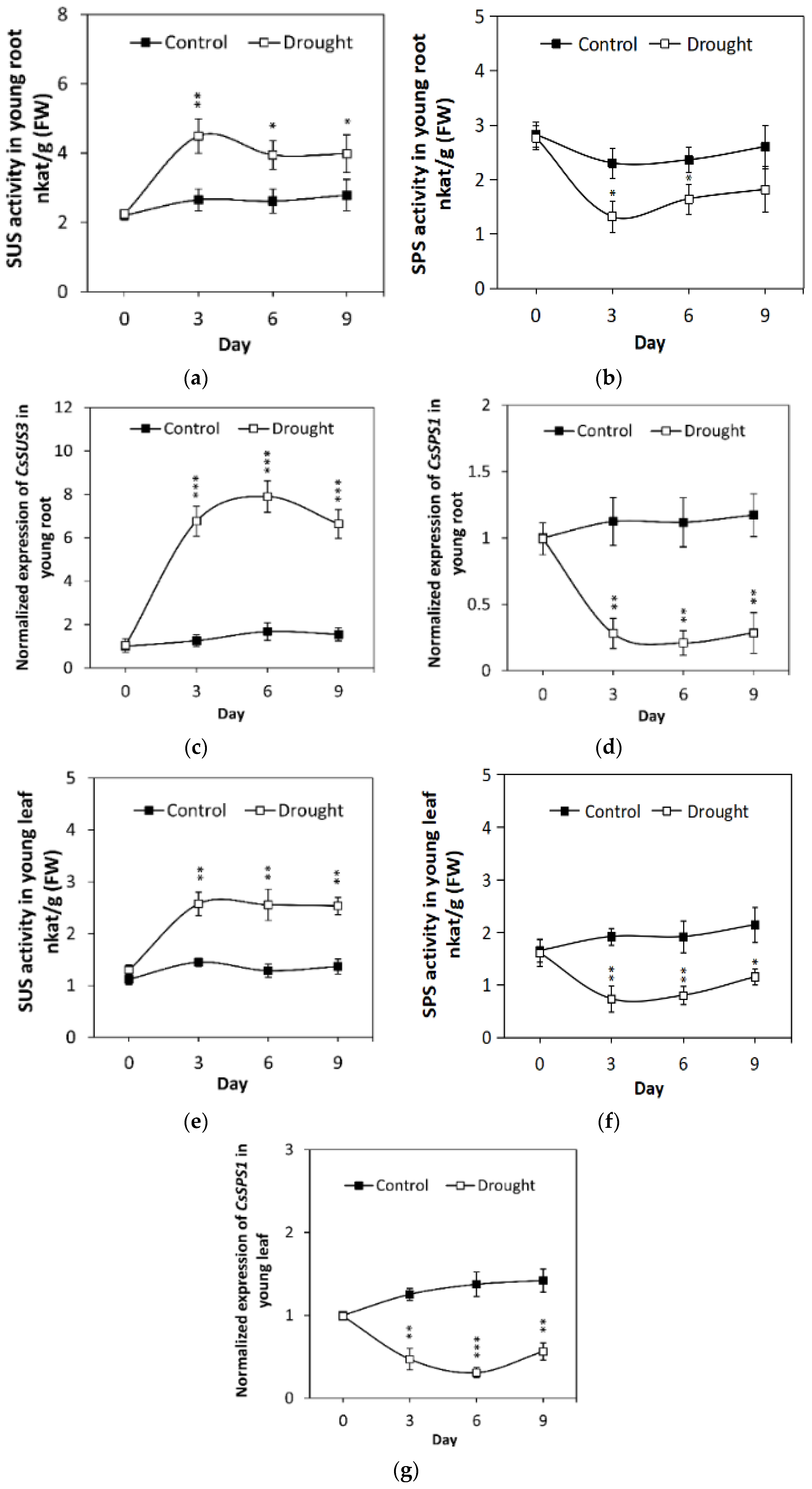
2.4. Drought Stress Effects SUS and SPS Activity and Related Gene Expression
2.5. Overexpressing CsVI2 Enhanced Vacuolar Invertase Activity and Drought Tolerance in Cucumber Seedlings
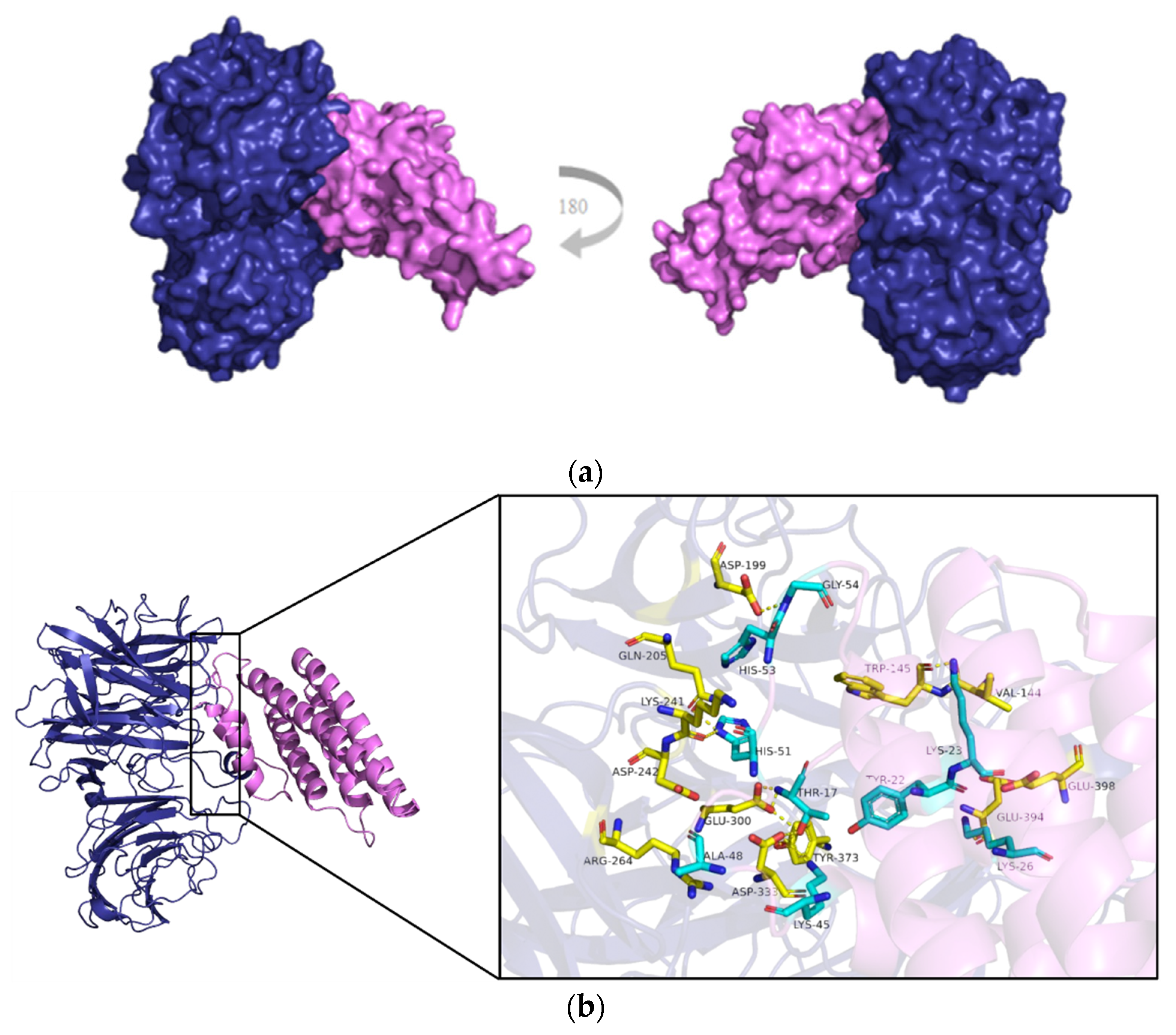
2.6. CsVI2 can Form a Complex with a Putative Cucumber Invertase Inhibitor CsINVINH3
3. Discussion
4. Materials and Methods
4.1. Plant Material and Treatments
4.2. Gene Expression Analysis
4.3. Plant Transformation
4.4. Plant Carbohydrates and Protein Extraction
4.5. Heterologous Expression and Purification of CsVI2
4.6. Enzyme Activity and Carbohydrate Assay
4.7. Protein Complex Modelling
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ruan, Y.L. Sucrose metabolism: Gateway to diverse carbon use and sugar signaling. Annu. Rev. Plant Biol. 2014, 65, 33–67. [Google Scholar] [CrossRef]
- Ruan, Y.L.; Jin, Y.; Yang, Y.J.; Li, G.J.; Boyer, J.S. Sugar input, metabolism, and signaling mediated by invertase: Roles in development, yield potential, and response to drought and heat. Mol. Plant 2010, 3, 942–955. [Google Scholar] [CrossRef]
- Geigenberger, P.; Stitt, M. Sucrose synthase catalyses a readily reversible reaction in vivo in developing potato tubers and other plant tissues. Planta 1993, 189, 329–339. [Google Scholar] [CrossRef]
- Verma, S.; Dubey, R.S. Effect of cadmium on soluble sugars and enzymes of their metabolism in rice. Biol. Plant. 2001, 44, 117–123. [Google Scholar] [CrossRef]
- Kim, J.Y.; Mahe, A.; Brangeon, J.; Prioul, J.L. A maize vacuolar invertase, IVR2, is induced by water stress. Organ/tissue specificity and diurnal modulation of expression. Plant Physiol. 2000, 124, 71–84. [Google Scholar] [CrossRef] [Green Version]
- Sturm, A.; Tang, G.Q. The sucrose-cleaving enzymes of plants are crucial for development, growth and carbon partitioning. Trends Plant Sci. 1999, 4, 401–407. [Google Scholar] [CrossRef]
- Wan, H.; Wu, L.; Yang, Y.; Zhou, G.; Ruan, Y.L. Evolution of sucrose metabolism: The dichotomy of invertases and beyond. Trends Plant Sci. 2018, 23, 163–177. [Google Scholar] [CrossRef]
- Klann, E.M.; Chetelat, R.T.; Bennett, A.B. Expression of acid invertase gene controls sugar composition in tomato (Lycopersicon) fruit. Plant Physiol. 1993, 103, 863–870. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.; Ni, D.A.; Ruan, Y.L. Posttranslational elevation of cell wall invertase activity by silencing its inhibitor in tomato delays leaf senescence and increases seed weight and fruit hexose Level. Plant Cell 2009, 21, 2072–2089. [Google Scholar] [CrossRef] [Green Version]
- Albacete, A.; Cantero-Navarro, E.; Balibrea, M.E.; Großkinsky, D.K.; De La Cruz González, M.; Martínez-Andújar, C.; Smigocki, A.C.; Roitsch, T.; Pérez-Alfocea, F. Hormonal and metabolic regulation of tomato fruit sink activity and yield under salinity. J. Exp. Bot. 2014, 65, 6081–6095. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.F.; Liang, K.; Yin, D.M.; Ni, D.A.; Zhang, Z.G.; Ruan, Y.L. Ectopic expression of a tobacco vacuolar invertase inhibitor in guard cells confers drought tolerance in Arabidopsis. J. Enzyme Inhib. Med. Chem. 2016, 31, 1381–1385. [Google Scholar] [CrossRef] [Green Version]
- Gong, Z.; Xiong, L.; Shi, H.; Yang, S.; Herrera-Estrella, L.R.; Xu, G.; Chao, D.Y.; Li, J.; Wang, P.Y.; Qin, F.; et al. Plant abiotic stress response and nutrient use efficiency. Sci. China Life Sci. 2020, 63, 635–674. [Google Scholar] [CrossRef]
- Liu, Y.H.; Offler, C.E.; Ruan, Y.L. Regulation of fruit and seed response to heat and drought by sugars as nutrients and signals. Front. Plant Sci. 2013, 4, 282. [Google Scholar] [CrossRef] [Green Version]
- Albacete, A.; Cantero-Navarro, E.; Grosskinsky, D.K.; Arias, C.L.; Balibrea, M.E.; Bru, R.; Fragner, L.; Ghanem, M.E.; de la Cruz Gonzalez, M.; Hernandez, J.A.; et al. Ectopic overexpression of the cell wall invertase gene CIN1 leads to dehydration avoidance in tomato. J. Exp. Bot. 2015, 66, 863–878. [Google Scholar] [CrossRef]
- Fan, H.F.; Ding, L.; Du, C.X.; Wu, X. Effect of short-term water deficit stress on antioxidative systems in cucumber seedling roots. Bot. Stud. 2014, 55, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Parkash, V.; Singh, S.; Deb, S.K.; Ritchie, G.L.; Wallace, R.W. Effect of deficit irrigation on physiology, plant growth, and fruit yield of cucumber cultivars. Plant Stress 2021, 1, 100004. [Google Scholar] [CrossRef]
- Liu, S.; Li, H.; Lv, X.; Ahammed, G.J.; Xia, X.; Zhou, J.; Shi, K.; Asami, T.; Yu, J.; Zhou, Y. Grafting cucumber onto luffa improves drought tolerance by increasing ABA biosynthesis and sensitivity. Sci. Rep. 2016, 6, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Du, Q.; Bai, L.; Sun, M.; Li, Y.; He, C.; Wang, J.; Yu, X.; Yan, Y. Interference of CsGPA1, the α-submit of G protein, reduces drought tolerance in cucumber seedlings. Hortic. Plant J. 2021, 7, 209–220. [Google Scholar] [CrossRef]
- Wang, M.; Jiang, B.; Peng, Q.; Liu, W.; He, X.; Liang, Z.; Lin, Y. Transcriptome analyses in different cucumber cultivars provide novel insights into drought stress responses. Int. J. Mol. Sci. 2018, 19, 2067. [Google Scholar] [CrossRef] [Green Version]
- Ouzounidou, G.; Giannakoula, A.; Ilias, I.; Zamanidis, P. Alleviation of drought and salinity stresses on growth, physiology, biochemistry and quality of two Cucumis sativus L. cultivars by Si application. Rev. Bras. Bot. 2016, 39, 531–539. [Google Scholar] [CrossRef]
- Feng, Z.; Zheng, F.; Wu, S.; Li, R.; Li, Y.; Zhong, J.; Zhao, H. Functional characterization of a cucumber (Cucumis sativus L.) vacuolar invertase, CsVI1, involved in hexose accumulation and response to low temperature stress. Int. J. Mol. Sci. 2021, 22, 9365. [Google Scholar] [CrossRef]
- Li, X.; Du, J.; Guo, J.; Wang, H.; Ma, S.; Lü, J.; Sui, X.; Zhang, Z. The functions of cucumber sucrose phosphate synthases 4 (CsSPS4) in carbon metabolism and transport in sucrose-and stachyose-transporting plants. J. Plant Physiol. 2018, 228, 150–157. [Google Scholar] [CrossRef]
- Wang, H.; Sui, X.; Guo, J.; Wang, Z.; Cheng, J.; Ma, S.; Li, X.; Zhang, Z. Antisense suppression of cucumber (Cucumis sativus L.) sucrose synthase 3 (CsSUS3) reduces hypoxic stress tolerance. Plant Cell Environ. 2014, 37, 795–810. [Google Scholar] [CrossRef]
- Rausch, T.; Greiner, S. Plant protein inhibitors of invertases. Biochim. Biophys. Acta-Proteins Proteom. 2004, 1696, 253–261. [Google Scholar] [CrossRef]
- Hothorn, M.; Van Den Endec, W.; Lammens, W.; Rybin, V.; Scheffzek, K. Structural insights into the pH-controlled targeting of plant cell-wall invertase by a specific inhibitor protein. Proc. Natl. Acad. Sci. USA 2010, 107, 17427–17432. [Google Scholar] [CrossRef] [Green Version]
- Ji, X.M.; Raveendran, M.; Oane, R.; Ismail, A.; Lafitte, R.; Bruskiewich, R.; Cheng, S.H.; Bennett, J. Tissue-specific expression and drought responsiveness of cell-wall invertase genes of rice at flowering. Plant Mol. Biol. 2005, 59, 945–964. [Google Scholar] [CrossRef]
- García-Tejero, I.; Jiménez-Bocanegra, J.A.; Martínez, G.; Romero, R.; Durán-Zuazo, V.H.; Muriel-Fernández, J.L. Positive impact of regulated deficit irrigation on yield and fruit quality in a commercial citrus orchard [Citrus sinensis (L.) Osbeck, cv. salustiano]. Agric. Water Manag. 2010, 97, 614–622. [Google Scholar] [CrossRef]
- Chaves, M.M.; Maroco, J.P.; Pereira, J.S. Understanding plant responses to drought—From genes to the whole plant. Funct. Plant Biol. 2003, 30, 239–264. [Google Scholar] [CrossRef]
- Sinkevich, M.S.; Naraykina, N.V.; Trunova, T.I. Involvement of sugars in the antioxidant defense against paraquat-induced oxidative stress in potato transformed with yeast invertase gene. In Doklady Biological Sciences; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar] [CrossRef]
- Xu, W.; Cui, K.; Xu, A.; Nie, L.; Huang, J.; Peng, S. Drought stress condition increases root to shoot ratio via alteration of carbohydrate partitioning and enzymatic activity in rice seedlings. Acta Physiol. Plant. 2015, 37, 9. [Google Scholar] [CrossRef]
- Zhang, C.; Li, X.; He, Y.; Zhang, J.; Yan, T.; Liu, X. Physiological investigation of C4-phosphoenolpyruvate-carboxylase-introduced rice line shows that sucrose metabolism is involved in the improved drought tolerance. Plant Physiol. Biochem. 2017, 115, 328–342. [Google Scholar] [CrossRef]
- Anur, R.M.; Mufithah, N.; Sawitri, W.D.; Sakakibara, H.; Sugiharto, B. Overexpression of sucrose phosphate synthase enhanced sucrose content and biomass production in transgenic sugarcane. Plants 2020, 9, 200. [Google Scholar] [CrossRef] [Green Version]
- Du, Y.; Zhao, Q.; Chen, L.; Yao, X.; Zhang, W.; Zhang, B.; Xie, F. Effect of drought stress on sugar metabolism in leaves and roots of soybean seedlings. Plant Physiol. Biochem. 2020, 146, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Huang, Y.; Loka, D.A.; Bai, H.; Liu, Y.; Wang, S.; Zhou, Z. Drought-induced disturbance of carbohydrate metabolism in anthers and male abortion of two Gossypium hirsutum cultivars differing in drought tolerance. Plant Cell Rep. 2020, 39, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Roitsch, T.; González, M.C. Function and regulation of plant invertases: Sweet sensations. Trends Plant Sci. 2004, 9, 606–613. [Google Scholar] [CrossRef]
- Cséke, C.; Buchanan, B.B. Regulation of the formation and utilization of photosynthate in leaves. BBA Rev. Bioenerg. 1986, 853, 43–63. [Google Scholar] [CrossRef]
- Kusch, U.; Harms, K.; Rausch, T.; Greiner, S. Inhibitors of plant invertases do not affect the structurally related enzymes of fructan metabolism. New Phytol. 2009, 181, 601–612. [Google Scholar] [CrossRef]
- Mohiuddin, A.K.M.; Chowdhury, M.K.U.; Abdullah, Z.C.; Napis, S. Influence of silver nitrate (ethylene inhibitor) on cucumber in vitro shoot regeneration. Plant Cell. Tissue Organ Cult. 1997, 51, 75–78. [Google Scholar] [CrossRef]
- Su, T.; Han, M.; Min, J.; Zhou, H.; Zhang, Q.; Zhao, J.; Fang, Y. Functional characterization of invertase inhibitors PtC/VIF1 and 2 revealed their involvements in the defense response to fungal pathogen in populus trichocarpa. Front. Plant Sci. 2020, 10, 1654. [Google Scholar] [CrossRef]
- Hothorn, M.; D’Angelo, I.; Márquez, J.A.; Greiner, S.; Scheffzek, K. The Invertase Inhibitor Nt-CIF from Tobacco: A Highly Thermostable Four-helix Bundle with an Unusual N-terminal Extension. J. Mol. Biol. 2004, 335, 987–995. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Zheng, F.; Feng, Z.; Li, Y.; Ma, M.; Wang, G.; Zhao, H. A Vacuolar Invertase CsVI2 Regulates Sucrose Metabolism and Increases Drought Tolerance in Cucumis sativus L. Int. J. Mol. Sci. 2022, 23, 176. https://doi.org/10.3390/ijms23010176
Chen L, Zheng F, Feng Z, Li Y, Ma M, Wang G, Zhao H. A Vacuolar Invertase CsVI2 Regulates Sucrose Metabolism and Increases Drought Tolerance in Cucumis sativus L. International Journal of Molecular Sciences. 2022; 23(1):176. https://doi.org/10.3390/ijms23010176
Chicago/Turabian StyleChen, Lin, Fenghua Zheng, Zili Feng, Yue Li, Muxuan Ma, Guoping Wang, and Hongbo Zhao. 2022. "A Vacuolar Invertase CsVI2 Regulates Sucrose Metabolism and Increases Drought Tolerance in Cucumis sativus L." International Journal of Molecular Sciences 23, no. 1: 176. https://doi.org/10.3390/ijms23010176




