The Role of Genital Tract Microbiome in Fertility: A Systematic Review
Abstract
:1. Introduction
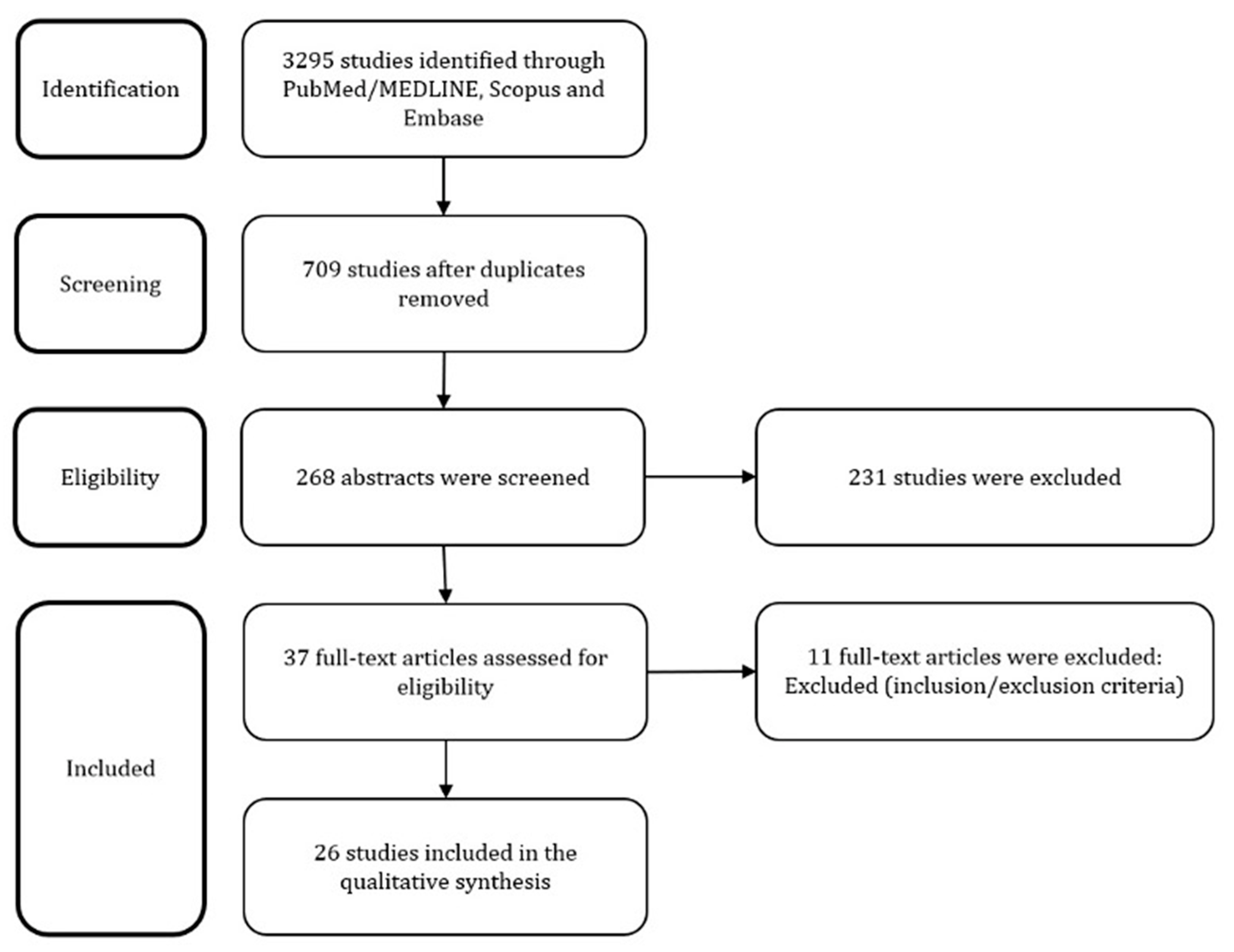
2. Materials and Methods
- -
- Population: women or couples with infertility (any type) or non-pregnant condition.
- -
- Intervention: genital tract microbial assessment
- -
- Comparison: optional comparison with microbiome of fertile women.
- -
- Outcomes: composition of the microbial flora correlated with infertility or ARTs failure
- -
- Study design: only full-text original research articles written in English were considered eligible for analysis, whereas reviews, editorials, opinions or letters, case studies, conference papers, and abstracts were excluded.
3. Results
4. Discussions
4.1. Sampling Methods
4.2. Methods of Analysis
4.3. Vaginal Findings
4.4. Cervical Findings
4.5. Endometrial Findings
4.6. Miscellanea Findings
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Davenport, E.R.; Sanders, J.G.; Song, S.J.; Amato, K.R.; Clark, A.G.; Knight, R. The human microbiome in evolution. BMC Biol. 2017, 15, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Knight, R.; Callewaert, C.; Marotz, C.; Hyde, E.R.; Debelius, J.; McDonald, D.; Sogin, M.L. The Microbiome and Human Biology. Annu. Rev. Genom. Hum. Genet. 2017, 18, 65–86. [Google Scholar] [CrossRef] [PubMed]
- Maruvada, P.; Leone, V.; Kaplan, L.M.; Chang, E.B. The human microbiome and obesity: Moving beyond associations. Cell Host Microbe 2017, 22, 589–599. [Google Scholar] [CrossRef]
- Whipps, J.M.; Lewis, K.; Cooke, R.C. Mycoparasitism and plant disease control 161–187. In Fungi in Biological Control Systems; Burge, N.M., Ed.; Manchester University Press: Manchester, UK, 1988; p. 176. [Google Scholar]
- Prescott, S.L. History of medicine: Origin of the term microbiome and why it matters. Hum. Microbiome J. 2017, 4, 24–25. [Google Scholar] [CrossRef]
- Valenti, P.; Rosa, L.; Capobianco, D.; Lepanto, M.S.; Schiavi, E.; Cutone, A.; Paesano, R.; Mastromarino, P. Role of lactobacilli and lactoferrin in the mucosal cervicovaginal defense. Front. Immunol. 2018, 9, 376. [Google Scholar] [CrossRef] [PubMed]
- Moreno, I.; Simon, C. Relevance of assessing the uterine microbiota in infertility. Fertil. Steril. 2018, 110, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Tagini, F.; Greub, G. Bacterial genome sequencing in clinical microbiology: A pathogen-oriented review. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 2007–2020. [Google Scholar] [CrossRef] [PubMed]
- Proctor, L.M.; Creasy, H.H.; Fettweis, J.M. The integrative human microbiome project. Nature 2019, 569, 641–648. [Google Scholar]
- Buchta, V. Vaginal microbiome. Ces. Gynekol. 2018, 83, 371–379. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [PubMed]
- Hok, T. Comparative endocervical bacteriology of the mucus. Am. J. Obstet. Gynecol. 1967, 98, 781–783. [Google Scholar] [CrossRef]
- Moberg, P.; Eneroth, P.; Harlin, J.; Ljung-wadstrm, A.; Nord, C. Cervical bacterial flora in infertile and pregnant women. Med. Microbiol. Immunol. 1978, 165, 139–145. [Google Scholar] [CrossRef]
- Koskimies, A.; Paavonen, J.; Meyer, B.; Kajanoja, P. Cervicitis and infertility. Am. J. Reprod. Immunol. 1981, 1, 299–302. [Google Scholar] [CrossRef]
- Taylor, P.; Ilesanmi, O.A.; Edozien, L.C. Culture of the endometrium of infertile women culture of the endometrium of infertile women. J. Obstet. Gynaecol. 1995, 15, 50–52. [Google Scholar]
- Fanchin, R.; Harmas, A.; Benaoudia, F.; Lundkvist, U.; Olivennes, F.; Frydman, R. Microbial flora of the cervix assessed at the time of embryo transfer adversely affects in vitro fertilization outcome. Fertil. Steril. 1998, 70, 866–870. [Google Scholar] [CrossRef]
- Salim, R.; Ben-Shlomo, I.; Colodner, R.; Keness, Y.; Shalev, E. Bacterial colonization of the uterine cervix and success rate in assisted reproduction: Results of a prospective survey. Hum. Reprod. 2002, 17, 337–340. [Google Scholar] [CrossRef]
- Borovkova, N.; Korrovits, P.; Ausmees, K.; Türk, S.; Jõers, K.; Punab, M.; Mändar, R. Anaerobe in fluence of sexual intercourse on genital tract microbiota in infertile couples. Anaerobe 2011, 17, 414–418. [Google Scholar] [CrossRef]
- Mangot-Bertrand, J.; Fenollar, F.; Bretelle, F.; Gamerre, M.; Raoult, D.; Courbiere, B. Molecular diagnosis of bacterial vaginosis: Impact on IVF outcome. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 32, 535–541. [Google Scholar] [CrossRef]
- Pelzer, E.S.; Allan, J.A.; Waterhouse, M.A.; Ross, T.; Beagley, K.W.; Knox, C.L. Microorganisms within human follicular fluid: Effects on IVF. PLoS ONE 2013, 8, e59062. [Google Scholar] [CrossRef]
- Campisciano, G.; Florian, F.; D’Eustacchio, A.; Stanković, D.; Ricci, G.; De Seta, F.; Comar, M. Subclinical alteration of the cervical-vaginal microbiome in women with idiopathic infertility. J. Cell. Physiol. 2017, 232. [Google Scholar] [CrossRef]
- Verstraelen, H.; Vilchez-Vargas, R.; Desimpel, F.; Jauregui, R.; Vankeirsbilck, N.; Weyers, S.; Verhelst, R.; De Sutter, P.; Pieper, D.H.; Van De Wiele, T. Characterisation of the human uterine microbiome in non-pregnant women through deep sequencing of the V1-2 region of the 16S rRNA gene. PeerJ 2016, 4, e1602. [Google Scholar] [CrossRef] [PubMed]
- Moreno, I.; Codoñer, F.M.; Vilella, F.; Valbuena, D.; Martinez-Blanch, J.F.; Jimenez-Almazán, J.; Alonso, R.; Alamá, P.; Remohí, J.; Pellicer, A.; et al. Evidence that the endometrial microbiota has an effect on implantation success or failure. Am. J. Obstet. Gynecol. 2016, 215, 684–703. [Google Scholar] [CrossRef] [PubMed]
- Haahr, T.; Jensen, J.; Thomsen, L.; Duus, L.; Rygaard, K.; Humaidan, P. Abnormal vaginal microbiota may be associated with poor reproductive outcomes: A prospective study in IVF patients. Hum. Reprod. 2016, 31, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Franasiak, J.M.; Werner, M.D.; Juneau, C.R.; Tao, X.; Landis, J.; Zhan, Y.; Treff, N.R.; Scott, R.T. Endometrial microbiome at the time of embryo transfer: Next-generation sequencing of the 16S ribosomal subunit. J. Assist. Reprod. Genet. 2016, 33, 129–136. [Google Scholar] [CrossRef]
- Sahu, M.C.; Mishra, S.P.; Panda, R.; Patnaik, T. Surveillance of microbial flora for infertility couples in an indian tertiary care teaching hospital. Asian J. Pharm. Clin. Res. 2017, 10, 405. [Google Scholar] [CrossRef]
- Graspeuntner, S.; Bohlmann, M.K.; Gillmann, K.; Speer, R.; Kuenzel, S.; Mark, H.; Hoellen, F.; Lettau, R.; Griesinger, G.; König, I.; et al. Microbiota-based analysis reveals specific bacterial traits and a novel strategy for the diagnosis of infectious infertility. PLoS ONE 2018, 13, e0191047. [Google Scholar] [CrossRef]
- Babu, G.; Singaravelu, B.; Srikumar, R.; Reddy, S.V. Comparative study on the vaginal flora and incidence of asymptomatic vaginosis among healthy women and in women with infertility problems of reproductive age. J. Clin. Diagn. Res. 2017, 11, 18–22. [Google Scholar] [CrossRef]
- Tao, X.; Franasiak, J.M.; Zhan, Y.; Scott, R.T.; Rajchel, J.; Bedard, J.; Newby, R.; Treff, N.R.; Chu, T. Characterizing the endometrial microbiome by analyzing the ultra-low bacteria from embryo transfer catheter tips in IVF cycles: Next generation sequencing (NGS) analysis of the 16S ribosomal gene. Hum. Microbiome J. 2017, 3, 15–21. [Google Scholar] [CrossRef]
- Kyono, K.; Hashimoto, T.; Nagai, Y.; Sakuraba, Y. Analysis of endometrial microbiota by 16S ribosomal RNA gene sequencing among infertile patients: A single-center pilot study. Reprod. Med. Biol. 2018, 17, 297–306. [Google Scholar] [CrossRef]
- Wee, B.A.; Thomas, M.; Sweeney, E.L.; Frentiu, F.D.; Samios, M.; Ravel, J.; Gajer, P.; Myers, G.; Timms, P.; Allan, J.A.; et al. A retrospective pilot study to determine whether the reproductive tract microbiota differs between women with a history of infertility and fertile women. Aust. NZ J. Obstet. Gynaecol. 2018, 58, 341–348. [Google Scholar] [CrossRef]
- Koedooder, R.; Singer, M.; Schoenmakers, S. The vaginal microbiome as a predictor for outcome of in vitro fertilization with or without intracytoplasmic sperm injection: A prospective study. Hum. Reprod. 2019, 34, 1042–1054. [Google Scholar] [CrossRef]
- Bernabeu, A.; Lledo, B.; Díaz, M.C.; Lozano, F.M.; Ruiz, V.; Fuentes, A.; Lopez-Pineda, A.; Moliner, B.; Castillo, J.C.; Ortiz, J.A.; et al. Effect of the vaginal microbiome on the pregnancy rate in women receiving assisted reproductive treatment. J. Assist. Reprod. Genet. 2019, 36, 2111–2119. [Google Scholar] [CrossRef]
- Liu, Y.; Ko, E.Y.-L.; Wong, K.K.-W.; Chen, X.; Cheung, W.-C.; Law, T.S.-M.; Chung, J.; Tsui, S.K.-W.; Li, T.C.; Chim, S.S.-C. Endometrial microbiota in infertile women with and without chronic endometritis as diagnosed using a quantitative and reference range-based method. Fertil. Steril. 2019, 112, 707–717.e1. [Google Scholar] [CrossRef]
- Kitaya, K.; Nagai, Y.; Arai, W.; Sakuraba, Y.; Ishikawa, T. Characterization of Microbiota in Endometrial Fluid and Vaginal Secretions in Infertile Women with Repeated Implantation Failure. Mediat. Inflamm. 2019, 2019, 1–10. [Google Scholar] [CrossRef]
- Cheong, H.C.; Yap, P.S.X.; Chong, C.W.; Cheok, Y.Y.; Lee, C.Y.Q.; Tan, G.M.Y.; Sulaiman, S.; Hassan, J.; Sabet, N.S.; Looi, C.Y.; et al. Diversity of endocervical microbiota associated with genital Chlamydia trachomatis infection and infertility among women visiting obstetrics and gynecology clinics in Malaysia. PLoS ONE 2019, 14, e0224658. [Google Scholar] [CrossRef]
- Lynch, T.; Peirano, G.; Lloyd, T.; Read, R.; Carter, J.; Chu, A.; Shaman, J.A.; Jarvis, J.P.; Diamond, E.; Ijaz, U.Z.; et al. Molecular diagnosis of vaginitis: Comparing quantitative pcr and microbiome profiling approaches to current microscopy scoring. J. Clin. Microbiol. 2019, 57. [Google Scholar] [CrossRef]
- Yarza, P.; Yilmaz, P.; Pruesse, E.; Glöckner, F.O.; Ludwig, W.; Schleifer, K.-H.; Whitman, W.; Euzéby, J.; Amann, R.; Rossello-Mora, R. Uniting the classification of cultured and uncultured bacteria and archaea using 16S rRNA gene sequences. Nat. Rev. Microbiol. 2014, 12, 635–645. [Google Scholar] [CrossRef]
- Kralik, P.; Ricchi, M. A basic guide to real time pcr in microbial diagnostics: Definitions, parameters, and everything. Front. Microbiol. 2017, 8, 108. [Google Scholar] [CrossRef]
- Weinstock, G.M. Genomic approaches to studying the human microbiota. Nature 2012, 489, 250–256. [Google Scholar] [CrossRef]
- Budding, A.E.; Grasman, M.E.; Lin, F. IS-pro: High-throughput molecular fingerprinting of the intestinal microbiota. FASEB J. 2010, 24, 4556–4564. [Google Scholar] [CrossRef]
- Lum, D.; Guido, R.; Rodriguez, E.; Lee, T.; Mansuria, S.; D’Ambrosio, L.; Austin, R.M. Brush Cytology of the Fallopian Tube and Implications in Ovarian Cancer Screening. J. Minim. Invasive Gynecol. 2014, 21, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Török, P.; Molnár, S.; Herman, T.; Jashanjeet, S.; Lampé, R.; Riemma, G.; Vitale, S.G. Fallopian tubal obstruction is associated with increased pain experienced during office hysteroscopy: A retrospective study. Updat. Surg. 2020, 72, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Messalli, E.M.; Grauso, F.; Balbi, G.; Napolitano, A.; Seguino, E.; Torella, M. Borderline ovarian tumors: Features and controversial aspects. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 167, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Gizzo, S.; Noventa, M.; Quaranta, M.; Vitagliano, A.; Saccardi, C.; Litta, P.; Antona, D. A Novel Hysteroscopic Approach for Ovarian Cancer Screening/Early Diagnosis [Internet] Oncology Letters; Spandidos Publications: Athens, Greece, 2017; Volume 13, pp. 549–553. [Google Scholar]
- Giampaolino, P.; Foreste, V.; Di Filippo, C.; Gallo, A.; Mercorio, A.; Serafino, P.; Improda, F.; Verrazzo, P.; Zara, G.; Buonfantino, C.; et al. Microbiome and PCOS: State-Of-Art and Future Aspects. Int. J. Mol. Sci. 2021, 22, 2048. [Google Scholar] [CrossRef]
- Chiofalo, B.; Palmara, V.; Vilos, G.A.; Pacheco, L.A.; Lasmar, R.B.; Shawki, O.; Giacobbe, V.; Alibrandi, A.; Di Guardo, F.; Vitale, S.G. Reproductive outcomes of infertile women undergoing “see and treat” office hysteroscopy: A retrospective observational study. Minim. Invasive Ther. Allied Technol. 2021, 30, 147–153. [Google Scholar] [CrossRef]
- Vitale, S.G.; Haimovich, S.; Riemma, G.; Ludwin, A.; Zizolfi, B.; De Angelis, M.C.; Carugno, J. Innovations in hysteroscopic surgery: Expanding the meaning of “in-office". Minim. Invasive Ther. Allied Technol. 2021, 30, 125–132. [Google Scholar] [CrossRef]
- Vitale, S.G. The Biopsy Snake Grasper Sec. VITALE: A New Tool for Office Hysteroscopy. J. Minim. Invasive Gynecol. 2020, 27, 1414–1416. [Google Scholar] [CrossRef]
- Shi, Y.; Zhu, Y.; Fan, S.; Vitagliano, A.; Liu, X.; Liao, Y.; Liang, Y.; Vitale, S.G. Clinical Characteristics and Antifungal Susceptibility of Candida nivariensis from Vulvovaginal Candidiasis. Gynecol. Obstet. Investig. 2020, 85, 88–93. [Google Scholar] [CrossRef] [PubMed]
| Database | Number of Retrieved Studies | Search Strategy |
|---|---|---|
| PubMed/MEDLINE | 650 | (“Microbiota” [Mesh] OR microbiot * OR microbiom * OR microfilm * OR flora OR microflor *) AND (“Infertility” [Mesh] OR infertility OR fertility OR sterility) |
| Embase | 963 | (microbiot * OR microbiom * OR microfilm * OR ‘flora’/exp OR flora OR microflor *) AND (infertility OR fertility OR sterility) |
| Scopus | 1682 | TITLE-ABS-KEY ((microbiot * OR microbiom * OR microfilm * OR flora OR microflor *) AND (infertility OR fertility OR sterility)) |
| Authors | Year | Country | Aim | Basic Data on Studied Infertile Women/Couples | Specimen Type | Diagnostic Method | Data on Cultures Growth | Impact on Fertility or Infertility Treatment Outcomes | Additional Information |
|---|---|---|---|---|---|---|---|---|---|
| Hok et al. [12] | 1967 | Indonesia | To examine the incidence of various bacteria in endocervical mucus obtained from the internal os of women with gynecological issues. | 112 infertile woman qualified for tubal insufflation | Endocervical mucus | Bacteria culturing | No differences between groups except higher occurrence of Mycobacterium tuberculosis in infertile patients; the growth pattern in infertile patients was as Streptococcus—29%; Gaffkya tetragena—20%; Bacillus—15%; Pseudomonas aeruginosa—15%; Proteus—13%; Alcaligenes faecalis—6%; Diphtheroid rods—5%: | In 3% cases of infertility, Mycobacterium tuberculosis was found and was expected to be the reason of infertility. | |
| Moberg et al. [13] | 1978 | Sweden | To describe the predominant cervical bacterial flora in infertile, early-pregnancy and labour patients | 47 infertile women | Cervical swabs | Bacteria culturing | Alpha-hemolytic streptococci—23% Streptococci group B—0% Streptococci group C—5% Streptococci group D—18% Staphylococcus aureus—4% Staphylococcus epidermidis—41% Enterobacteriaceae—9% | The largest proportion of patients with both aerobic and anaerobic bacteria was found in the labour group, followed by the early pregnancy and the infertile groups. The same relation between the groups was noted for aerobic bacteria only; only anaerobic bacteria was found in 51% of infertile patients, 26% of early pregnancy and 0% in labour group | - |
| Koskimies et al. [14] | 1981 | Finland | To examine the cervical factor using microbiologic, cytologic, colposcopic, and histologic investigations in a group of women with reproductive failure and clinically “inflame cervixes” | 52 infertile women | Cervical swabs and serum | Bacteria and yeast culturing and anti-chlamydial antibodies immunofluorescence | Chlamydia trachomatis—8% Escherichia coli—10% Staphylococcus epidermidis—21% Lactobacillus—8% Enterococcus—6% Staphylococcus aureus—4% Bacteroidea sp.—4% Bacteroides fragilis—2% Group B streptococcus—2% Peptostreptococcus—2% “Normal mixed flora”—13% No growth—21% Candida albicans—23% Trichomonas vaginalis—2% Neisseria gonorrhoeae—0 | Women examined for infertility have been shown to have significantly higher levels of anti-chlamydial antibodies than the controls | No testing for Ureaplasma urealyticum was performed |
| Taylor & Ilesanmi et al. [15] | 1995 | Nigeria | To examine the microbial flora, if any, of the endometrium of infertile women and re-appraise the value of endometrial and vaginal bacterial cultures in the assessment of the infertile woman | 73 infertile women 12 (16.4%) primary 61 (83.6%) secondary | Endometrial biopsy | Histological examination and bacteria culturing | None of the cultures yielded any growth and the Gram stains were all negative, both for organisms and pus cells. | - | No testing for Chlamydia was performed. |
| Fanchini et al. [16] | 1998 | France | To analyze bacteriologically the catheters used for determining cervical obstruction before ET for the presence of microorganisms and to investigate the possible consequences on the outcome of IVF-ET | 279 infertile women undergoing controlled ovarian hyperstimulation and IVF-ET | Samples from ET catherer | Bacteria culturing | 51% cultures were positive—Mostly Escherichia coli (64%) and Streptococcus species (8%) Only aerobic—86% Only anaerobic—5% Both aerobic and anaerobic—9% Gram-negative—62% Gram-positive—25% Both gram-negative and gram-positive—13% | Clinical, ongoing pregnancy and implantation rates were lower in the positive than in the negative culture group (24% vs. 37%; 17% vs. 28%; and 9% vs. 16%, respectively) | No testing for Chlamydia or Mycoplasma hominis was performed |
| Salim et al. [17] | 2002 | Israel | To examine whether the nature of bacterial flora, found in the uterine cervical canal at embryo transfer, is associated with the rate of conception in assisted reproductive techniques | 204 patients who underwent embryo transfer 68% were of fresh embryos, following recent vaginal oocyte retrieval and prophylactic antibiotic therapy, and 32% of frozen–thawed embryos | Cervical canal swab | Bacteria culturing | In 75 patients (37%) sterile cervical cultures or lactobacillus were recorded; No difference in colonization was found between women who underwent frozen–thawed versus fresh embryo transfer (57 and 67% respectively); Escherichia coli was found in 8.5% of the positive cultures | Any Gram-negative colonization was associated with no conception; From 75 sterile culture patients 31% conceived; among the 129 in whom any pathogenic micro-organism was recovered only 16% conceived | |
| Borovkova et al. [18] | 2011 | Estonia | To clarify the influence of sexual intercourse on partner’s genital tract microbiota in infertile couples | 17 infertile couples | Self-collected vaginal samples taken 3–5 days later before intercourse and 8–12 h after intercourse and semen samples collected during menstruation of the partner | PCR analysis | Lactobacilli—88% Coagulase (−) staphylococci—88% Ureaplasma parvum—59%; its prevalence was higher in these women whose partner had IP (80% vs. 50%) Corynebacteria—76% Anaerobic Gram (+) bacteria—76% Streptococci—65% Trichomonas vaginalis—6% | - | After the intercourse, median 4 new species emerge in one woman and median 2 species disappear; this tendency was more prominent in the partners of IP patients |
| Mangot-Bertrand et al. [19] | 2012 | France | To assess BV prevalence for infertile patients treated by IVF and its impact on the pregnancy rate | 307 women undergoing IVF | Vaginal sampling performed with sterile cytobrush and two sterile cotton swabs | Nugent score and RT-PCR | The prevalence of BV in the whole study group was 9%; among women who performed vaginal douching—22%; whereas among patients who did not douche—8% | The embryo implantation rate does not decrease significantly between the two groups (36% in group 1 BV− vs. 28% in group 2 BV+) nor does the clinical pregnancies rate (33% vs. 28% (both p > 0.05) | Regarding ongoing clinical pregnancies, authors did not observe any significant difference between the two groups concerning early miscarriage rate, PROM, preterm labour, gestational age at delivery, mode of delivery or birthweight |
| Pelzer et al. [20] | 2013 | United States | To test human follicular fluid for the presence of microorganisms and to correlate these findings with the IVF outcomes | 263 couples 202 infertile women during IVF procedures | Follicular fluid samples and vaginal swabs | Culturing and PCR analysis | Microorganisms were detected within 100% of cultured vaginal swabs; Lactobacillus spp., Bifidobacterium spp. And Staphylococcus spp. were the most prevalent species detected in the lower genital tract specimens of all women. The culture analyses revealed that cultivable bacterial species (1–5 species) were present in 99% of follicular fluids tested. The rates of colonization in infertile women ranged from 24% to 37% with no evidence of a difference in colonization rates across cohorts based on the causes of infertility | Adverse IVF outcomes were associated with microbial colonization of follicular fluid. No single species was associated with decreased fertilization rates; however, the presence of Lactobacillus spp. in both right and left follicles was associated with higher rates of embryo transfer; the presence of Propionibacterium spp. and Streptococcus spp. in right follicles was associated with poor embryo transfer rates | Microorganisms isolated from follicular fluids were classified as: (1) ‘colonizers’ if microorganisms were detected within the follicular fluid, but not in vaginal swab; or (2) ‘contaminants’ if microorganisms detected in the vagina were also detected within the follicular fluid; The authors found significant differences in the microbiome of both (left and right) ovaries |
| Campisciano et al. [21] | 2016 | Italy | To compare the vaginal microbiome of idiopathic and non-idiopathic infertile as well as fertile women to identify bacterial species suitable as biomarkers | 27 infertile women attending the ART | Cervical–vaginal fluids | V3-16S rDNA sequencing | Lactobacillus iners, Lactobacillus crispatus, and Lactobacillus gasseri distinguished idiopathic infertile women from the other groups. In these group, a microbial profile similar to that observed in BVwomen has been detected; in Idiopathic we observe a lack of Fusobacteria, which are present only in Vaginosis, and the presence of Clostridia, missing only in Control. Infertile has not Bacteroidia and Tenericutes, which are present in the other three groups, mainly in Idiopathic and Vaginosis. At the lowest taxonomic level, among species belonging to Lactobacilli (Firmicutes), Lactobacillus gasseri, Lactobacillus iners, and Lactobacillus crispatus are the most abundant in Idiopathic. Lactobacillus gasseri is overrepresented in Idiopathic compared to the other three groups; Lactobacillus iners is underrepresented in Idiopathic compared to Control, and its abundance decreases in Vaginosis and Infertile. Lactobacillus crispatus is more abundant in Control than Idiopathic, while Vaginosis shows the lowest abundance. Veillonella (Firmicutes) and Staphylococcus pasteuri/warneri (Firmicutes) are common to Idiopathic and Vaginosis. Among Actinobacteria, Gardnerella vaginalis is underrepresented only in Control. Atopobium vaginae (Actinobacteria) and Prevotellabivia (Bacteroidetes) are shared between Idiopathic and Vaginosis. | ||
| Verstraelen et al. [22] | 2016 | Belgium | To investigate the putative presence of a uterine microbiome in a selected series of non-pregnant women through deep sequencing of the V1-2 hypervariable region of the 16S ribosomal RNA (rRNA) gene | 19 patients (various reproductive conditions, including subfertility) | Endometrial sample | PCR analysis RNA sequencing | In 90% of subjects, the community was fairly similar, with different species of Bacteroides and Pelomonas taxa. In six cases, additional high abundance of Lactobacillus crispatus and Lactobacillus iners was found in the presence of Bacteroides; 15 phylotypes were present in all samples; in 90% of the women community architecture was similar in as much Bacteroides xylanisolvens, Bacteroides thetaiotaomicron, Bacteroides fragilis and an undetermined Pelomonas taxon constituted over one third of the endometrial bacterial community | ||
| Moreno et al. [23] | 2016 | Spain | To test the existence of an endometrial microbiota that differs from that in the vagina and analyze the impact of the endometrial microbial community on reproductive outcome in infertile patients undergoing IVF | 35 infertile patients | Endometrial fluid | RNA sequencing | The most represented genus was Lactobacillus (72% of identified bacteria); Gardnerella (12.6%), Bifidobacterium (3.7%), Streptococcus (3.2%), and Prevotella (0.866%) were the other most common genera | The presence of a non-Lactobacillus-dominated microbiota in a receptive endometrium was associated with significant decreases in implantation (61% vs. 23%) pregnancy (71% vs. 33%) ongoing pregnancy (59% vs. 13%) and live birth (59% vs. 7%) rates | If bacterial communities from paired endometrial fluid and vaginal aspirate samples within the same subjects were interrogated, different bacterial communities were detected between the uterine cavity and the vagina of subjects |
| Haahret al. [24] | 2016 | Denmark | To characterize what is the diagnostic performance of qPCR assays compared with Nugent scoring for abnormal vaginal microbiota and for predicting the success rate of IVF treatment | 130 infertile women | Vaginal swabs | Nugent’s criteria and PCR analysis | The prevalence of BV defined by Nugent score was 21%,whereas the prevalence of an abnormal vaginal microbiota was 28% defined by qPCR; there were high concentrations of Gardnerella vaginalis and/or Atopobium vaginae | Abnormal vaginal microbiota may negatively affect the clinical pregnancy rate in IVF patients; only 9% with qPCR defined abnormal vaginal microbiota obtained a clinical pregnancy | The qPCR diagnostic approach had a sensitivity and specificity of respectively 93% and 93% for Nugent-defined BV |
| Franasiaket al. [25] | 2016 | United States | To characterize the microbiome at the time of embryo transfer | 33 infertile women 18 ongoing pregnancies 15 not | Distal part of transfer catheter used for embryo transfer | 16S ribosomal subunit hypervariable region analysis with next-generation sequencing | There were a total of 278 different genus calls present across patient samples; Flavobacterium and Lactobacillus represent the majority of the bacterium seen in both groups | Lactobacillus was the most often found species for both pregnant and not; there were no differences in microbiomes between ongoing and non-ongoing pregnancy | |
| Mishra et al. [26] | 2017 | India | To know the prevalence of microorganisms in the infertile couples of a tertiary caring teaching hospital | 288 infertile couples 68% primary 32% secondary Male factor—27%, Female factor—50% Both—5% Unexplained—18% | The endocervical swabs or high vagina swabs | Bacteria and yeast culturing | 65% of women from infertile with cultures growth Escherichia coli—27% Staphylococcus aureus—23% Pseudomonas sp. 21% Coagulase (−) Staphylococci—17%, Candida albicans—9% Klebsiella sp.—3% | - | - |
| Graspeuntner et al. [27] | 2017 | Germany | To characterize the microbial pattern in females diagnosed with ININF in comparison to females with nININF, FSW and healthy controls | 47 infertile women 26 females women with nININF 21 women with ININF | Three swabs from the cervix and serum samples | 16S amplicon sequencing and anti-chlamydial antibodies immunobloting | Females with ININF had higher frequency of previous Chlamydia trachomatis infections in comparison to fertile and nININF; while on average 78.34% of all sequence reads in fertile females belong to Lactobacillus, the percentage is reduced to 69% in nININF, 58% in ININF; the relative read count of the genus Gardnerella increased from 5% in fertile females, to 6% in nININF and up to 10% in ININF and the same trend is observed for the genera Prevotella and Sneathia | Authors established a model to predict the underlying cause of infertility by using the following parameters: (1) detection of pathogens by PCR and cultivation, (2) serological status of Chlamydia trachomatis IgG/IgA and (3) the first ten taxa found in microbiota sequencing; it correctly predicted 17 of 18 ININF—Area under curve: 0.978 | - |
| Babu et al. [28] | 2017 | India | To compare the vaginal flora and analyse the incidence of asymptomatic vaginosis among healthy women and in women with infertility problems | 116 infertile women | Vaginal swabs | Bacteria culturing | The most dominant flora was Candida spp. (27%), Enterococcus (23%) followed by Gram negative bacilli such as Escherichia coli (14%). The percentage of Lactobacillus was relatively low (4%). Asymptomatic vaginosis was present in 28% | ||
| Tao et al. [29] | 2017 | United States | To establish the validation of taxonomic identification and characterize endometrium microbiome by analyzing embryo transfer catheter tips | 70 infertile women | Distal part of transfer catheter used for embryo transfer | PCR analysis RNA sequencing | Lactobacillus spp. were detected in all of the samples; Among 70 samples, 33 samples contained over 90% of Lactobacillus abundance and 50 samples contained over 70% of Lactobacillus abundance, which was consistent with the dominance of Lactobacillus in the lower reproductive tracts; Corynebacterium spp. were detected in 40 out of 70 patients. Bifidobacterium spp. were detected in 15 out of 70 patients and Staphylococcus spp. were detected in 38 out of 70 patients. Other lactic acid-producing bacteria from the genera Streptococcus were present in 38 out of 70 patients | - | The metagenomics workflow provides a rapid and sensitive method to identify bacteria in clinical embryo transfer specimens |
| Kyono et al. [30] | 2018 | Japan | To analyze the endometrial and vaginal microbiome among a Japanese infertile population and its impact on implantation | 102 infertile patients 79 IVF 23 non-IVF | Endometrial fluid and vaginal discharge samples collected using an intrauterine insemination catheter | PCR and pyrosequencing of V4 of the bacterial 16S rRNA genes | The Lactobacillus-dominated microbiota (>90% Lactobacillus spp.) in the endometrium vs vagina was 38% vs. 44% in the IVF patients, 74% vs. 74% in the non-IVF patients, and 86% vs. 86% in the healthy volunteers. The major taxonomies were Gardnerella, Streptococcus, Atopobium, Bifidobacterium, Sneathia, Prevotella, and Staphylococcus; the median percentage of the endometrial Lactobacilli in the IVF patients was lower than that of the non-IVF patients and healthy volunteers (64% vs. 96% vs. 99.5%); the percentage of LD endometrial status was also the lowest in the IVF group (38% vs 74% vs 86%) | A considerable percentage of non-Lactobacillus- dominated (NLD) microbiota was found in the endometrium of Japanese infertile women. During the study, 18 patients achieved pregnancy: 3 natural conceptions and 15 by single vitrified-warmed blastocyst transfer. The median percentages of the endometrial and vaginal Lactobacilli in those pregnant cases were 96% and 98%. There were seven NLD cases (six IVF and one non-IVF) who achieved pregnancy—5 ongoing, 1 early miscarriage and 1 lost to follow-up | |
| Wee et al. [31] | 2018 | Australia | To examine the vaginal, cervical, and endometrial microbiota for women with a history of infertility compared to women with a history of fertility | 15 infertile patients | Vaginal, cervical, and endometrial samples | PCR analysis RNA sequencing | The genus Lactobacillus was most frequently observed. The most abundant taxonomic units were the same in both cervical and vaginal specimens for all 24 of the 33 women where both were sequenced | Infertile women more often had Ureaplasma in the vagina and Gardnerella in the cervix | Tenascin-C expression correlated with a history of miscarriage |
| Koedooder et al. [32] | 2019 | Netherlands | To determine if the presence or absence of certain vaginal bacteria associated with failure or success to become pregnant after an in IVF or IVF-ICSI) treatments. | 192 women undergoing IVF or IVF-ICSI treatment | Self-collected vaginal swab | Microbiome profiling with the use of interspace profiling (IS-pro) | A relatively low load of Lactobacillus, a high load of Proteobacteria or Lactobacillus jensenii was correlated with failure to become pregnant. We found that Gardnerella vaginalis strains were characterized by two distinct ISprofiles. Only one of these IS-types was correlated with low pregnancy rate, namely IS-pro type 1 (IST1). Combined observations resulted in a predictive algorithm for failure to become pregnant with the following parameters: relative Lactobacillus load <20%, relative load of Lactobacillus jensenii >35%, presence of Gardnerella vaginalis IST1 or Proteobacteria >28% of total bacterial load and such situation was called unfavourable microbiome profile—6% of these women became pregnant after a fresh ET In the group with Lactobacillus crispatus abundance of ≥60%, 24% of women became pregnant. In the group with Lactobacillus crispatus abundance <60%, 53% (50 of 95) of women became pregnant. All women who did have a favourable profile (158/192 women, 82%) could be stratified into groups with a high and an average chance of pregnancy based on relative abundance of Lactobacillus crispatus alone. The prediction model identified a subgroup of women (18%) who had a low chance to become pregnant following fresh ET. This failure was correctly predicted in 32 out of 34 women based on the vaginal microbiota composition, resulting in a accuracy of 94% (sensitivity, 26%; specificity, 97%). Additionally, the degree of dominance of Lactobacillus crispatus was an important factor in predicting pregnancy. Women who had a favourable profile as well as <60% Lactobacillus crispatus had a high chance of pregnancy: more than half became pregnant. | ||
| Bernabeu et al. [33] | 2019 | Spain | To investigate if the vaginal microbiome influences the IVF outcome | 31 patients undergoing ART | Vaginal samples | V3 V4 region of 16S rRNA analysis | Lactobacillus spp. standing out as the most prevalent genus. It was majorly represented by Lactobacillus crispatus (47%), Lactobacillus helveticus (23%), Lactobacillus iners (22%), and Lactobacillus jenseii (4%); The cluster analysis identified two main clusters: the first encompassed the genera Lactobacillus, Gardnerella, Clostridium, Staphylococcus, and Dialister, and the second included all other genera. | Samples from women who achieved pregnancy showed a greater presence of Lactobacillus spp.; In women who achieved pregnancy, the microorganisms mainly from the first cluster were detected. | |
| Liu et al. [34] | 2019 | China | To systematically compare the endometrial microbiota in infertile women with and without chronic endometritis, as diagnosed by a quantitative and reference range-based method | 130 infertile women | Endometrial biopsy and fluid | RNA sequencing | The median relative abundance of Lactobacillus was 2% and 81% in the chronic endometritis and non-chronic endometritis microbiotas, respectively. Lactobacillus crispatus was less abundant in the chronic endometritis microbiota. Eighteen non-Lactobacillus taxa including Dialister, Bifidobacterium, Prevotella, Gardnerella, and Anaerococcus were more abundant in the chronic endometritis microbiota and of these, Anaerococcus and Gardnerella were negatively correlated in relative abundance with Lactobacillus | Chronic endometritis was associated with a higher abundance of 18 bacterial taxa in the endometrial cavity | |
| Kitaya et al. [35] | 2019 | Japan | To characterize the microbiota in the endometrial fluid and vaginal secretions in women with RIF | 46 infertile patients 28 with with a history of RIF 18 infertile patients undergoing the first IVF attempt | Vaginal swab and endometrial fluid | PCR analysis | There were no significant differences in the detection rate of the specific bacterial species in the VS microbiota between the two groups. Lactobacillus dominated endometrial fluid microbiota, defined by >90%; Lactobacillus genus status, was observed at a higher rate in the RIF group (64%) than in the control group (39%) without statistical significance; In vagina 67.9% women in the RIF group and 44.4% in the control group represented Lactobacillus-dominated microbiota. The detection rate of Gardnerella in the EF microbiota was 39.3% in the RIF group and 27.7% in the control group. Burkholderia was not detected in any of the EF microbiota in the control group but was detectable in 25% of the RIF group. Burkholderia was not detectable in infertile women undergoing the first IVF-ET attempt but in a quarter of those with a history of RIF | ||
| Cheong et al. [36] | 2019 | Malaysia | To evaluate the alteration of endocervical microbiome in association with Chlamydia trachomatis infection among a cohort of women in Malaysia | 34 infertile women | Endocervical swabs | 16S rRNA metagenomic sequencing | A total of 40 out of 70 participants were infected with genital Chlamydia trachomatis based on the diagnostic test result; higher level of Delftia, Streptococcus, Pseudomonas, Cloacibacterium, Prevotella, Veillonella, Megasphaera, Ureaplasma, and Ralstonia were obvious among the subjects with Chlamydia trachomatis infection (+) compared to the group without chlamydial infection; elevated level of phyla Bacteroidetes was detected in the Chlamydia trachomatis-infected samples (+) in infertile group | 88% of the subjects from the infertile group was infected by Chlamydia trachomatis, as opposed to only 28% in the fertile group | No significant correlation between chlamydial infection was detected with demographics such as age, marital status, as well as ethnicity A lower prevalence of genus Megasphaera was detected among the subjects with Chlamyidia trachomatis infection (+) in comparison to the non-infected group |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitale, S.G.; Ferrari, F.; Ciebiera, M.; Zgliczyńska, M.; Rapisarda, A.M.C.; Vecchio, G.M.; Pino, A.; Angelico, G.; Knafel, A.; Riemma, G.; et al. The Role of Genital Tract Microbiome in Fertility: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 180. https://doi.org/10.3390/ijms23010180
Vitale SG, Ferrari F, Ciebiera M, Zgliczyńska M, Rapisarda AMC, Vecchio GM, Pino A, Angelico G, Knafel A, Riemma G, et al. The Role of Genital Tract Microbiome in Fertility: A Systematic Review. International Journal of Molecular Sciences. 2022; 23(1):180. https://doi.org/10.3390/ijms23010180
Chicago/Turabian StyleVitale, Salvatore Giovanni, Federico Ferrari, Michał Ciebiera, Magdalena Zgliczyńska, Agnese Maria Chiara Rapisarda, Giada Maria Vecchio, Alessandra Pino, Giuseppe Angelico, Anna Knafel, Gaetano Riemma, and et al. 2022. "The Role of Genital Tract Microbiome in Fertility: A Systematic Review" International Journal of Molecular Sciences 23, no. 1: 180. https://doi.org/10.3390/ijms23010180
APA StyleVitale, S. G., Ferrari, F., Ciebiera, M., Zgliczyńska, M., Rapisarda, A. M. C., Vecchio, G. M., Pino, A., Angelico, G., Knafel, A., Riemma, G., De Franciscis, P., & Cianci, S. (2022). The Role of Genital Tract Microbiome in Fertility: A Systematic Review. International Journal of Molecular Sciences, 23(1), 180. https://doi.org/10.3390/ijms23010180










