Colonic Mucosal Immune Activation in Mice with Ovalbumin-Induced Allergic Airway Disease: Association between Allergic Airway Disease and Irritable Bowel Syndrome
Abstract
:1. Introduction
2. Results
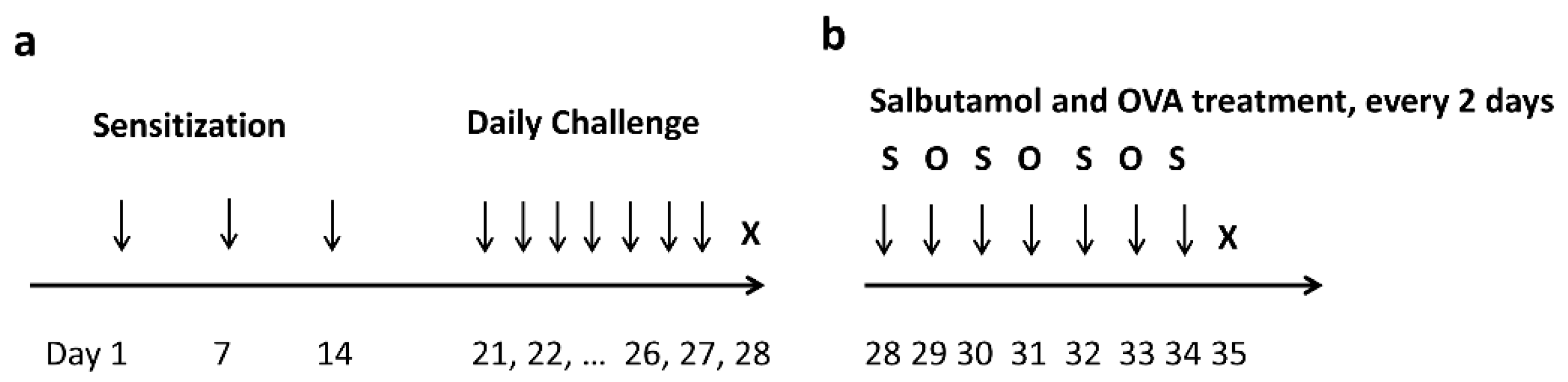
2.1. Allergic Airway Mouse Model Establishment
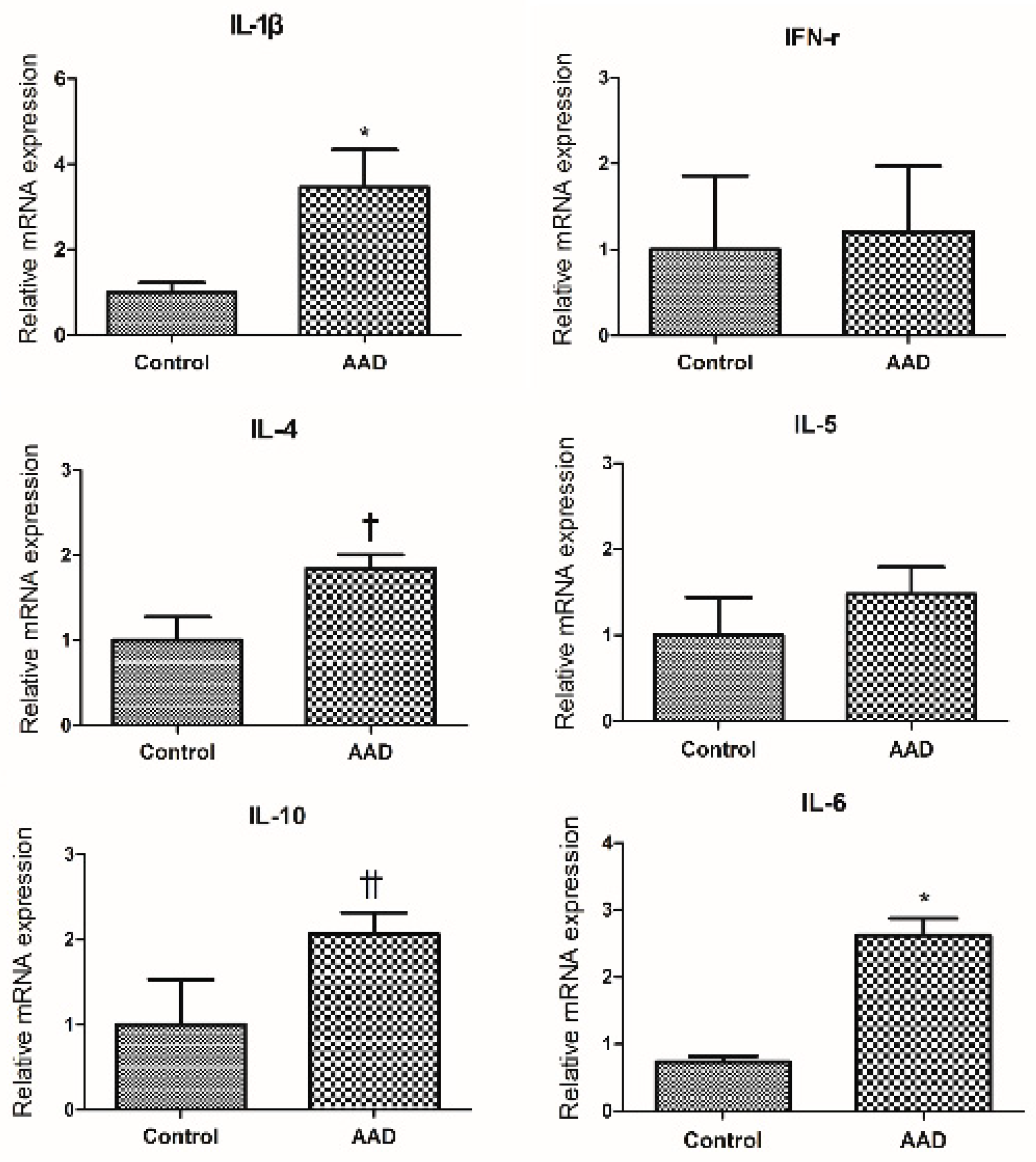
2.2. Evaluation of Cytokine Expression in Colonic Mucosa of AAD Mouse Using Quantitative RT-PCR Method (qRT-PCR)
2.3. Macroscopic Scoring of Colon Changes
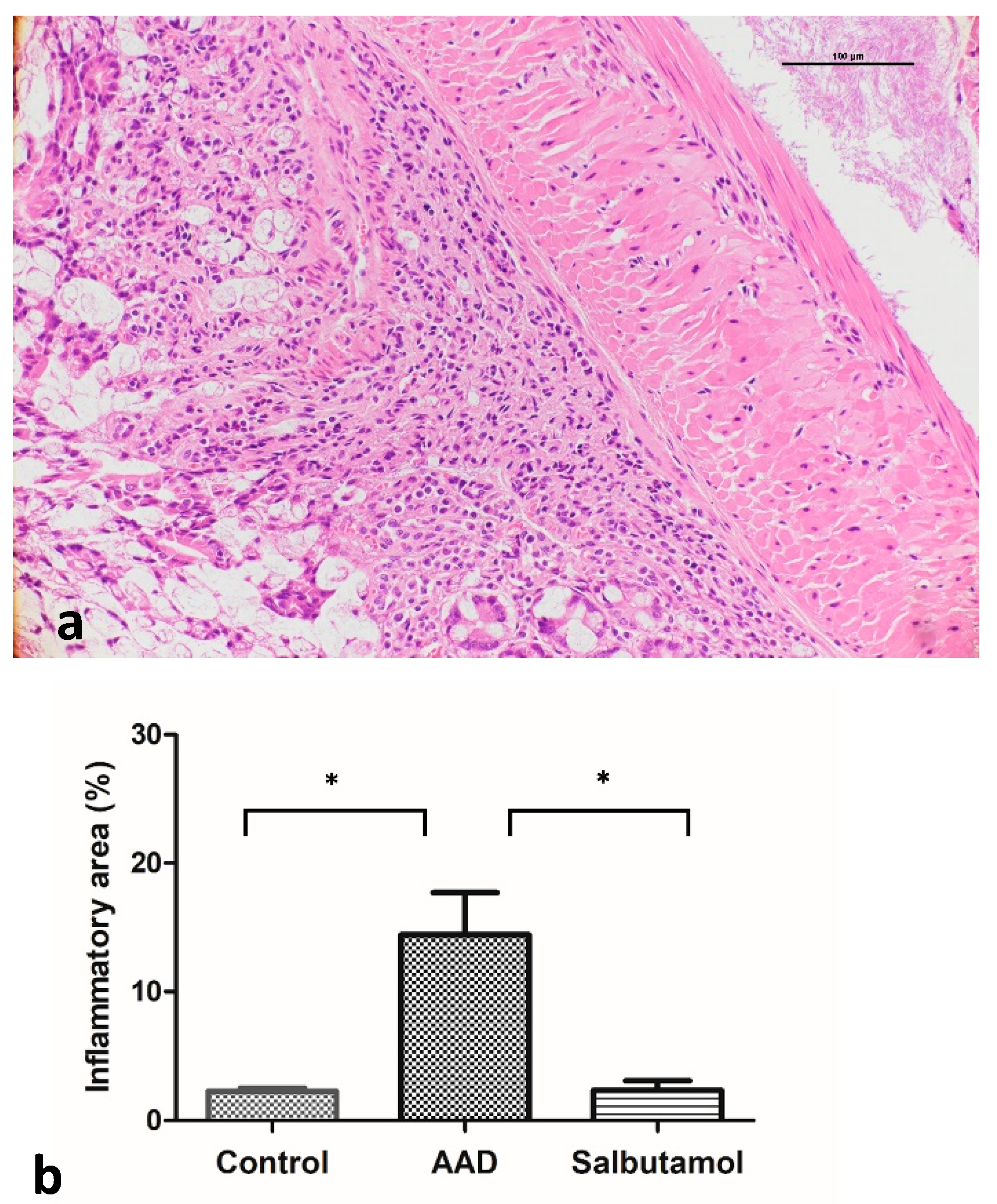
2.4. Histopathological Analysis of the Colonic Mucosa
2.5. Evaluation of Intestinal Cytokine Expression Using Enzyme-Linked Immunosorbent Assay (ELISA)
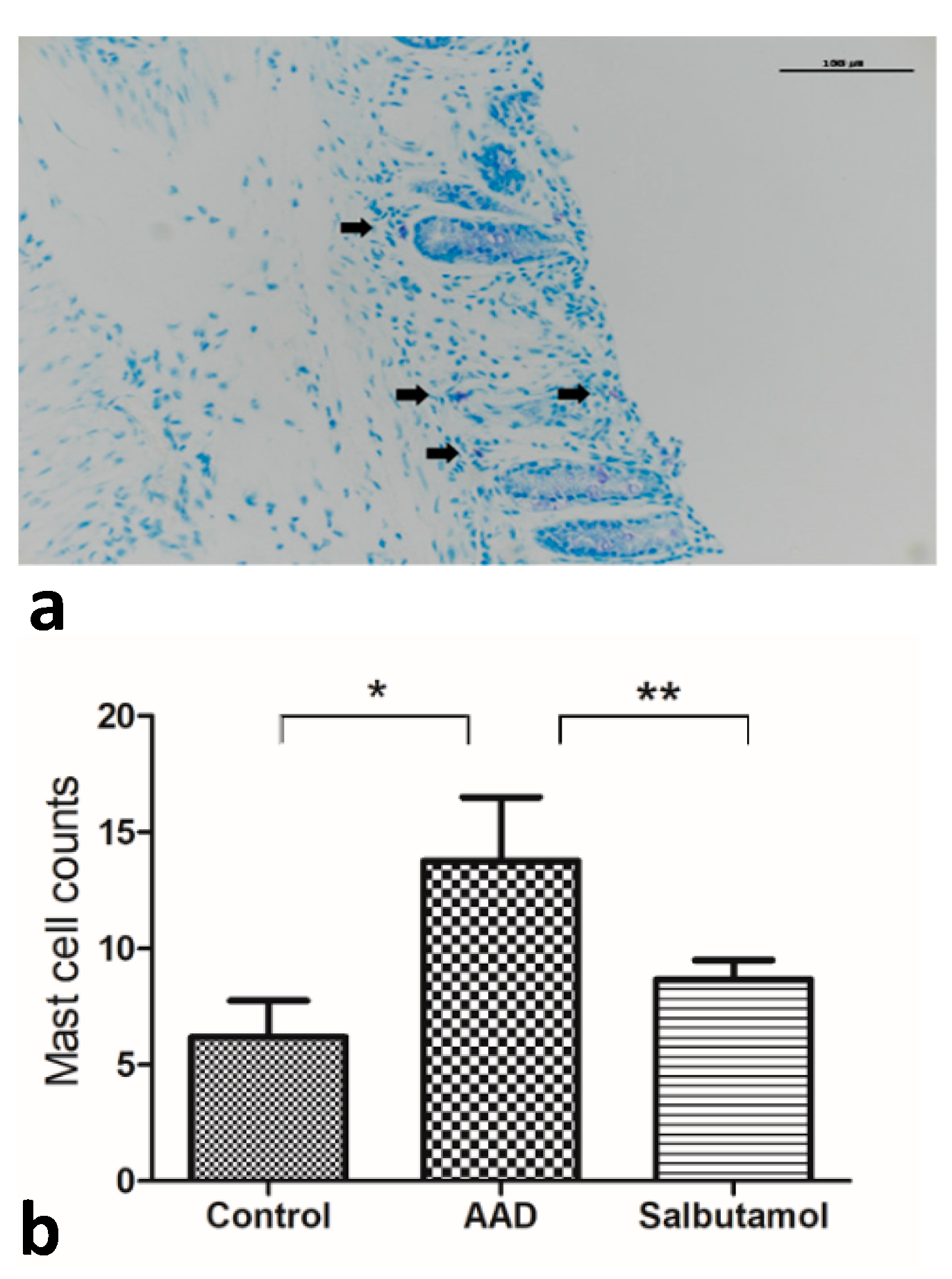
2.6. Mucosal MC Counts
2.7. Tryptase Western Blotting
3. Discussion
4. Materials and Methods
4.1. AAD Mouse Model
4.2. Macroscopic Evaluation of Inflammation
4.3. qRT-PCR for Inflammatory Cytokines in the Intestinal Mucosa
4.4. Salbutamol Treatment after OVA Challenge
4.5. Histological Evaluation of Inflammation
4.6. ELISA for Inflammatory Cytokines in the Intestinal Mucosa
4.7. Evaluation of MC Count
4.8. Western Blot Analysis of Tryptase
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Holtmann, G.J.; Ford, A.C.; Talley, N.J. Pathophysiology of irritable bowel syndrome. Lancet Gastroenterol. Hepatol. 2016, 1, 133–146. [Google Scholar] [CrossRef]
- Canavan, C.; West, J.; Card, T. The epidemiology of irritable bowel syndrome. Clin. Epidemiol. 2014, 6, 71–80. [Google Scholar] [PubMed] [Green Version]
- Vivinus-Nebot, M.; Dainese, R.; Anty, R.; Saint-Paul, M.C.; Nano, J.L.; Gonthier, N.; Marjoux, S.; Frin-Mathy, G.; Bernard, G.; Hebuterne, X.; et al. Combination of allergic factors can worsen diarrheic irritable bowel syndrome: Role of barrier defects and mast cells. Am. J. Gastroenterol. 2012, 107, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Cremon, C.; Gargano, L.; Morselli-Labate, A.M.; Santini, D.; Cogliandro, R.F.; De Giorgio, R.; Stanghellini, V.; Corinaldesi, R.; Barbara, G. Mucosal immune activation in irritable bowel syndrome: Gender-dependence and association with digestive symptoms. Am. J. Gastroenterol. 2009, 104, 392–400. [Google Scholar] [CrossRef]
- Loo, E.X.L.; Wang, Y.; Siah, K.T.H. Association between Irritable Bowel Syndrome and Allergic Diseases: To Make a Case for Aeroallergen. Int. Arch. Allergy Immunol. 2020, 181, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Powell, N.; Huntley, B.; Beech, T.; Knight, W.; Knight, H.; Corrigan, C.J. Increased prevalence of gastrointestinal symptoms in patients with allergic disease. Postgrad. Med. J. 2007, 83, 182–186. [Google Scholar] [CrossRef] [Green Version]
- Tobin, M.C.; Moparty, B.; Farhadi, A.; DeMeo, M.T.; Bansal, P.J.; Keshavarzian, A. Atopic irritable bowel syndrome: A novel subgroup of irritable bowel syndrome with allergic manifestations. Ann. Allergy Asthma Immunol. 2008, 100, 49–53. [Google Scholar] [CrossRef]
- Russo, C.; Zeng, D.; Prosperini, G.; Spicuzza, L.; Guarino, F.; Polosa, R. Effect of salbutamol on nasal symptoms and mast cell degranulation induced by adenosine 5’ monophosphate nasal challenge. Clin. Exp. Allergy 2005, 35, 1192–1196. [Google Scholar] [CrossRef] [PubMed]
- Kimball, E.S.; Palmer, J.M.; D’Andrea, M.R.; Hornby, P.J.; Wade, P.R. Acute colitis induction by oil of mustard results in later development of an IBS-like accelerated upper GI transit in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 288, G1266–G1273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guilarte, M.; Santos, J.; de Torres, I.; Alonso, C.; Vicario, M.; Ramos, L.; Martinez, C.; Casellas, F.; Saperas, E.; Malagelada, J.R. Diarrhoea-predominant IBS patients show mast cell activation and hyperplasia in the jejunum. Gut 2007, 56, 203–209. [Google Scholar] [CrossRef] [Green Version]
- Piche, T.; Saint-Paul, M.C.; Dainese, R.; Marine-Barjoan, E.; Iannelli, A.; Montoya, M.L.; Peyron, J.F.; Czerucka, D.; Cherikh, F.; Filippi, J.; et al. Mast cells and cellularity of the colonic mucosa correlated with fatigue and depression in irritable bowel syndrome. Gut 2008, 57, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Buhner, S.; Li, Q.; Vignali, S.; Barbara, G.; De Giorgio, R.; Stanghellini, V.; Cremon, C.; Zeller, F.; Langer, R.; Daniel, H.; et al. Activation of human enteric neurons by supernatants of colonic biopsy specimens from patients with irritable bowel syndrome. Gastroenterology 2009, 137, 1425–1434. [Google Scholar] [CrossRef] [PubMed]
- Barbara, G.; Stanghellini, V.; De Giorgio, R.; Cremon, C.; Cottrell, G.S.; Santini, D.; Pasquinelli, G.; Morselli-Labate, A.M.; Grady, E.F.; Bunnett, N.W.; et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology 2004, 126, 693–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, M.P.; Walker, M.M.; Ford, A.C.; Talley, N.J. The overlap of atopy and functional gastrointestinal disorders among 23,471 patients in primary care. Aliment. Pharmacol. Ther. 2014, 40, 382–391. [Google Scholar] [CrossRef]
- Tan, T.K.; Chen, A.C.; Lin, C.L.; Shen, T.C.; Li, T.C.; Wei, C.C. Preschoolers With Allergic Diseases Have an Increased Risk of Irritable Bowel Syndrome When Reaching School Age. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 26–30. [Google Scholar] [CrossRef]
- Wang, H.; Liu, J.S.; Peng, S.H.; Deng, X.Y.; Zhu, D.M.; Javidiparsijani, S.; Wang, G.R.; Li, D.Q.; Li, L.X.; Wang, Y.C.; et al. Gut-lung crosstalk in pulmonary involvement with inflammatory bowel diseases. World J. Gastroenterol. 2013, 19, 6794–6804. [Google Scholar] [CrossRef]
- Sampson, H.A. Update on food allergy. J. Allergy Clin. Immunol. 2004, 113, 805–819. [Google Scholar] [CrossRef]
- Powell, N.; Walker, M.M.; Talley, N.J. Gastrointestinal eosinophils in health, disease and functional disorders. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 146–156. [Google Scholar] [CrossRef]
- Brandt, E.B.; Scribner, T.A.; Akei, H.S.; Rothenberg, M.E. Experimental gastrointestinal allergy enhances pulmonary responses to specific and unrelated allergens. J. Allergy Clin. Immunol. 2006, 118, 420–427. [Google Scholar] [CrossRef]
- Ruane, D.; Brane, L.; Reis, B.S.; Cheong, C.; Poles, J.; Do, Y.; Zhu, H.; Velinzon, K.; Choi, J.H.; Studt, N.; et al. Lung dendritic cells induce migration of protective T cells to the gastrointestinal tract. J. Exp. Med. 2013, 210, 1871–1888. [Google Scholar] [CrossRef] [Green Version]
- Tham, E.H.; Lee, A.J.; Bever, H.V. Aeroallergen sensitization and allergic disease phenotypes in Asia. Asian. Pac. J. Allergy Immunol. 2016, 34, 181–189. [Google Scholar]
- Rayapudi, M.; Mavi, P.; Zhu, X.; Pandey, A.K.; Abonia, J.P.; Rothenberg, M.E.; Mishra, A. Indoor insect allergens are potent inducers of experimental eosinophilic esophagitis in mice. J. Leukoc. Biol. 2010, 88, 337–346. [Google Scholar] [CrossRef] [Green Version]
- Magnusson, J.; Lin, X.P.; Dahlman-Hoglund, A.; Hanson, L.L.; Telemo, E.; Magnusson, O.; Bengtsson, U.; Ahlstedt, S. Seasonal intestinal inflammation in patients with birch pollen allergy. J. Allergy Clin. Immunol. 2003, 112, 45–50. [Google Scholar] [CrossRef]
- Nybacka, S.; Ohman, L.; Storsrud, S.; Mybeck, M.; Bohn, L.; Wilpart, K.; Winkvist, A.; Bengtsson, U.; Tornblom, H.; Simren, M. Neither self-reported atopy nor IgE-mediated allergy are linked to gastrointestinal symptoms in patients with irritable bowel syndrome. Neurogastroenterol. Motil. 2018, 30, e13379. [Google Scholar] [CrossRef]
- Carroll, S.Y.; O’Mahony, S.M.; Grenham, S.; Cryan, J.F.; Hyland, N.P. Disodium cromoglycate reverses colonic visceral hypersensitivity and influences colonic ion transport in a stress-sensitive rat strain. PLoS ONE 2013, 8, e84718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serna, H.; Porras, M.; Vergara, P. Mast cell stabilizer ketotifen [4-(1-methyl-4-piperidylidene)-4h-benzo[4,5]cyclohepta[1,2-b]thiophen-10(9H)-one fumarate] prevents mucosal mast cell hyperplasia and intestinal dysmotility in experimental Trichinella spiralis inflammation in the rat. J. Pharmacol. Exp. Ther. 2006, 319, 1104–1111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camilleri, M. Current and future pharmacological treatments for diarrhea-predominant irritable bowel syndrome. Expert Opin. Pharmacother. 2013, 14, 1151–1160. [Google Scholar] [CrossRef] [PubMed]
- Klooker, T.K.; Braak, B.; Koopman, K.E.; Welting, O.; Wouters, M.M.; van der Heide, S.; Schemann, M.; Bischoff, S.C.; van den Wijngaard, R.M.; Boeckxstaens, G.E. The mast cell stabiliser ketotifen decreases visceral hypersensitivity and improves intestinal symptoms in patients with irritable bowel syndrome. Gut 2010, 59, 1213–1221. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Oh, E.Y.; Park, Y.H.; Yang, M.; Park, K.H.; Park, J.W.; Lee, J.H. Potential of serum soluble CD93 as a biomarker for asthma in an ovalbumin-induced asthma murine model. Biomarkers 2018, 23, 446–452. [Google Scholar] [CrossRef]
- Erben, U.; Loddenkemper, C.; Doerfel, K.; Spieckermann, S.; Haller, D.; Heimesaat, M.M.; Zeitz, M.; Siegmund, B.; Kuhl, A.A. A guide to histomorphological evaluation of intestinal inflammation in mouse models. Int. J. Clin. Exp. Pathol. 2014, 7, 4557–4576. [Google Scholar]
- Fu, S.; Ni, S.; Wang, D.; Hong, T. Coptisine Suppresses Mast Cell Degranulation and Ovalbumin-Induced Allergic Rhinitis. Molecules 2018, 23, 3039. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Parameter | Control | AAD Group | Salbutamol Group |
|---|---|---|---|
| Colon length, cm | 11.50 ± 0.27 | 9.171 ± 0.22 * | 11.40 ± 0.29 |
| Colon weight, g | 0.491 ± 0.014 | 0.498 ± 0.015 | 0.479 ± 0.018 |
| Stool score | 0.2 ± 0.133 | 1.3 ± 0.26 * | 0.5 ± 0.167 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Keum, B.; Byun, J.; Kim, B.; Lee, K.; Yeon, J.; Lee, J.; Choi, H.; Kim, E.; Jeen, Y.; et al. Colonic Mucosal Immune Activation in Mice with Ovalbumin-Induced Allergic Airway Disease: Association between Allergic Airway Disease and Irritable Bowel Syndrome. Int. J. Mol. Sci. 2022, 23, 181. https://doi.org/10.3390/ijms23010181
Kim S, Keum B, Byun J, Kim B, Lee K, Yeon J, Lee J, Choi H, Kim E, Jeen Y, et al. Colonic Mucosal Immune Activation in Mice with Ovalbumin-Induced Allergic Airway Disease: Association between Allergic Airway Disease and Irritable Bowel Syndrome. International Journal of Molecular Sciences. 2022; 23(1):181. https://doi.org/10.3390/ijms23010181
Chicago/Turabian StyleKim, Sanghyun, Bora Keum, Junhyoung Byun, Byoungjae Kim, Kijeong Lee, Jiwoo Yeon, Jaemin Lee, Hyuksoon Choi, Eunsun Kim, Yoontae Jeen, and et al. 2022. "Colonic Mucosal Immune Activation in Mice with Ovalbumin-Induced Allergic Airway Disease: Association between Allergic Airway Disease and Irritable Bowel Syndrome" International Journal of Molecular Sciences 23, no. 1: 181. https://doi.org/10.3390/ijms23010181






