The Effects of Separate and Combined Treatment of Male Rats with Type 2 Diabetes with Metformin and Orthosteric and Allosteric Agonists of Luteinizing Hormone Receptor on Steroidogenesis and Spermatogenesis
Abstract
:1. Introduction
2. Results
2.1. Characterization of T2DM Model in Male Rats, and the Effect of Treatment with Metformin and LH/hCG-R-Agonists
2.2. Effects of Metformin and LH/hCG-R-Agonists and Their Combination on the Blood Testosterone Levels in Control and Diabetic Rats
2.3. Effects of Metformin and LH/hCG-R-Agonists and Their Combination on the Content of Testosterone, Estradiol and Their Precursors in the Testes of Control and Diabetic Rats
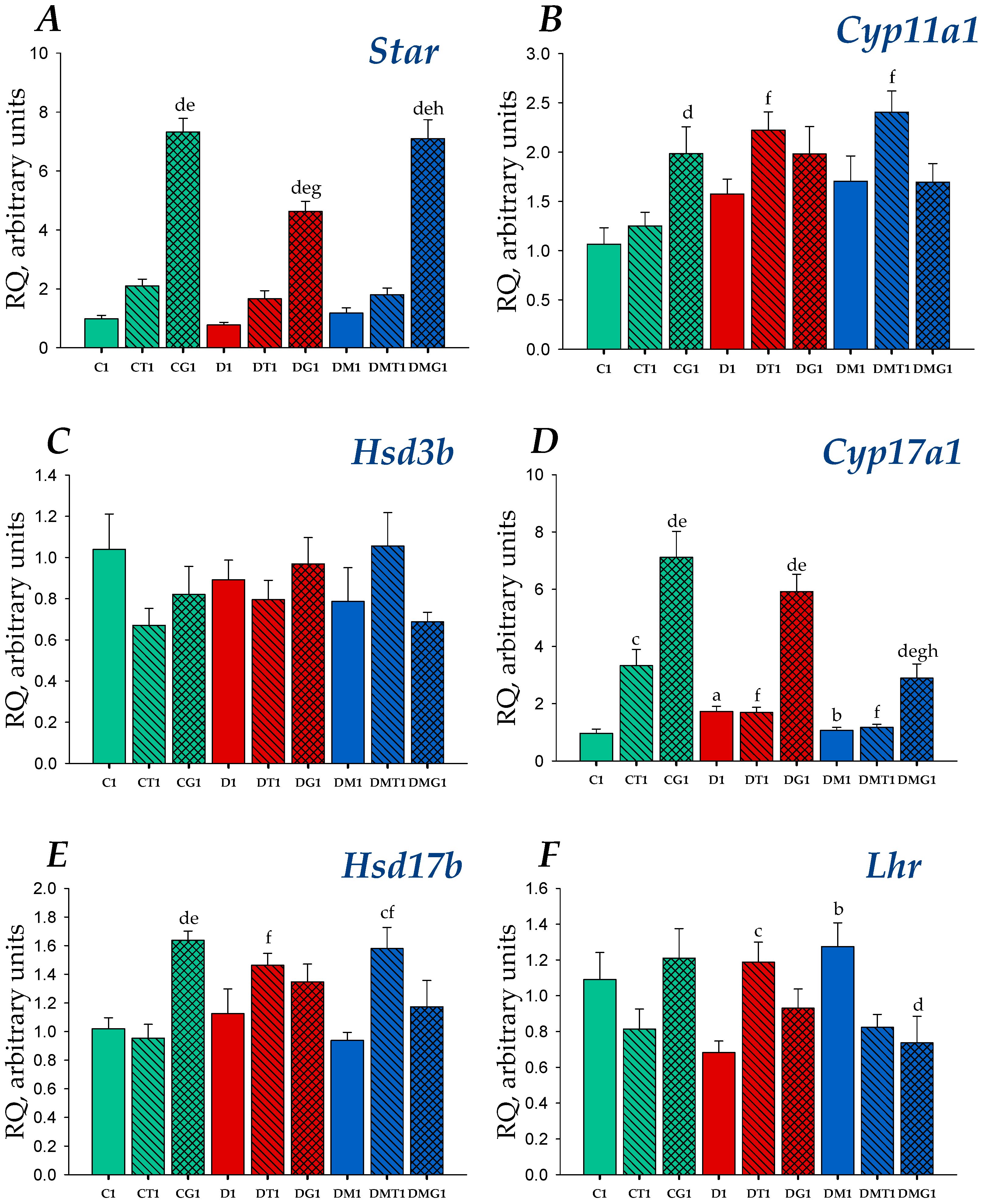
2.4. Effects of Metformin, LH/hCG-R-Agonists and Their Combination on Gene Expression in the Testes of Control and Diabetic Rats
2.5. The Effects of Metformin, LH/hCG-R-Agonists and Their Combination on the Sperm Parameters in Control and Diabetic Rats
2.6. The Effects of Metformin, LH/hCG-R-Agonists and Their Combination on Morphology of the Seminiferous Tubules in Control and Diabetic Rats
2.7. Immunohistochemical Analysis of LH/hCG-R Distribution in the Seminiferous Tubules of Control and Diabetic Rats, and the Effect of Five-Week Metformin Treatment, Five-Day Treatment with LH/hCG-R-Agonists and Their Combination
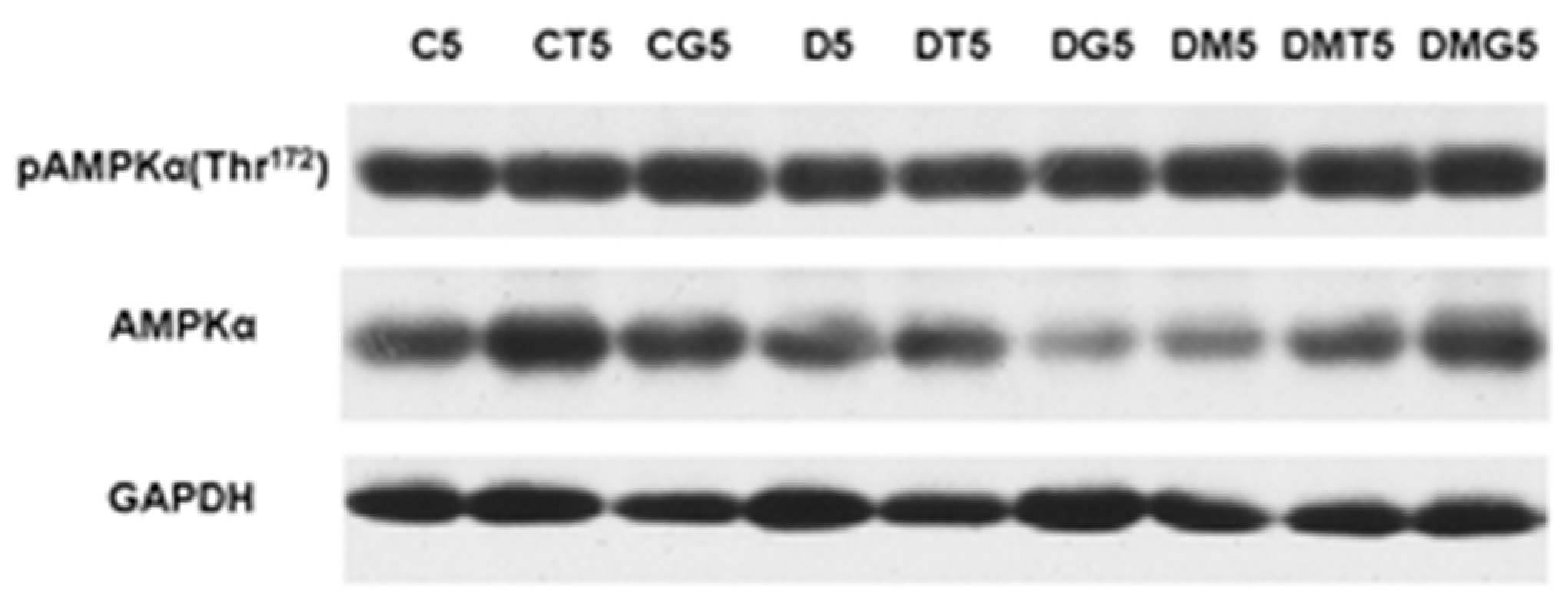
2.8. Western Blotting Analysis of α-AMPK and Its Active Thr172-Phosphorylated form in the Testes, and the Effect of Metformin and LH/hCG-R-Agonists Treatment
3. Discussion
4. Materials and Methods
4.1. The Drugs and Biochemical Reagents
4.2. The Experimental Animals
4.3. The Induction of Type 2 Diabetes in Male Wistar Rats
4.4. The Treatment of Diabetic Rats
4.5. Preparation of Tissue and Blood Samples for Analysis
4.6. The Determination of Blood Levels of Glucose, HbA1c, Insulin, Leptin, Total Cholesterol and Triglycerides
4.7. Glucose Tolerance Test
4.8. The Determination of Serum Testosterone Levels and Intratesticular Content of Testosterone, Estradiol and Their Precursors
4.9. The RNA Extraction and qRT-PCR Analysis of Testicular Genes
4.10. The Analysis of Epididymal Spermatozoa
4.11. Preparation of the Testis Sections for Histochemical and Immunohistochemical Analysis
4.12. Immunohistochemical Analysis of Intratesticular LH/hCG Receptor
4.13. Histochemical Analysis of the Seminiferous Tubules
4.14. The Estimation of AMP-Activated Protein Kinase with Western Blotting Analysis
4.15. Preparation of the Rat Blood and Tissues Samples and Determination of TP3 Content in Them Using Reverse-Phase HPLC
4.16. Statistical Analysis of the Experimental Data
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CYP11A1 (gene Cyp11a1) | cytochrome P450scc |
| CYP17A1 (gene Cyp17a1) | cytochrome P450 17A1/steroid 17α-monooxygenase |
| CYP19A1 (gene Cyp19a1) | cytochrome P450A1 (Aromatase) |
| hCG | human chorionic gonadotropin |
| HFD | high-fat diet |
| 3β-HSD (gene Hsd3b) | 3β-hydroxysteroid dehydrogenase |
| 17β-HSD (gene Hsd17b) | 17β-hydroxysteroid dehydrogenase |
| LH | luteinizing hormone |
| LH/hCG-R | luteinizing hormone/human chorionic gonadotropin receptor |
| MF | Metformin |
| StAR | steroidogenic acute regulatory protein |
| STZ | Streptozotocin |
| T2DM | type 2 diabetes mellitus |
| TP3 | 5-amino-N-tert-butyl-2-(methylsulfanyl)-4-(3-(nicotinamido)phenyl)thieno[2,3-d] pyrimidine-6-carboxamide |
References
- Sanchez-Rangel, E.; Inzucchi, S.E. Metformin: Clinical use in type 2 diabetes. Diabetologia 2017, 60, 1586–1593. [Google Scholar] [CrossRef]
- Madsen, K.S.; Chi, Y.; Metzendorf, M.I.; Richter, B.; Hemmingsen, B. Metformin for prevention or delay of type 2 diabetes mellitus and its associated complications in persons at increased risk for the development of type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2019, 12, CD008558. [Google Scholar] [CrossRef]
- Gnesin, F.; Thuesen, A.C.B.; Kähler, L.K.A.; Madsbad, S.; Hemmingsen, B. Metformin monotherapy for adults with type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2020, 6, CD012906. [Google Scholar] [CrossRef] [Green Version]
- Aras, M.; Tchang, B.G.; Pape, J. Obesity and Diabetes. Nurs. Clin. N. Am. 2021, 56, 527–541. [Google Scholar] [CrossRef] [PubMed]
- An, H.; He, L. Current understanding of metformin effect on the control of hyperglycemia in diabetes. J. Endocrinol. 2016, 228, 97–106. [Google Scholar] [CrossRef] [Green Version]
- Faure, M.; Bertoldo, M.J.; Khoueiry, R.; Bongrani, A.; Brion, F.; Giulivi, C.; Dupont, J.; Froment, P. Metformin in Reproductive Biology. Front. Endocrinol. 2018, 9, 675. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.Y.; Chang, T.C.; Lin, S.H.; Wu, S.T.; Cha, T.L.; Tsao, C.W. Metformin Ameliorates Testicular Function and Spermatogenesis in Male Mice with High-Fat and High-Cholesterol Diet-Induced Obesity. Nutrients 2020, 12, 1932. [Google Scholar] [CrossRef]
- Shpakov, A.O. Improvement effect of metformin on female and male reproduction in endocrine pathologies and its mechanisms. Pharmaceuticals 2021, 14, 42. [Google Scholar] [CrossRef]
- Tseng, C.H. The effect of metformin on male reproductive function and prostate: An updated review. World J. Mens Health 2021, 39, e15. [Google Scholar] [CrossRef]
- Lv, W.S.; Wen, J.P.; Li, L.; Sun, R.X.; Wang, J.; Xian, Y.X.; Cao, C.X.; Wang, Y.L.; Gao, Y.Y. The effect of metformin on food intake and its potential role in hypothalamic regulation in obese diabetic rats. Brain Res. 2012, 1444, 11–19. [Google Scholar] [CrossRef]
- Derkach, K.; Zakharova, I.; Zorina, I.; Bakhtyukov, A.; Romanova, I.; Bayunova, L.; Shpakov, A. The evidence of metabolic-improving effect of metformin in Ay/a mice with genetically-induced melanocortin obesity and the contribution of hypothalamic mechanisms to this effect. PLoS ONE 2019, 14, e0213779. [Google Scholar] [CrossRef]
- Richardson, M.C.; Ingamells, S.; Simonis, C.D.; Cameron, I.T.; Sreekumar, R.; Vijendren, A.; Sellahewa, L.; Coakley, S.; Byrne, C.D. Stimulation of lactate production in human granulosa cells by metformin and potential involvement of adenosine 5’ monophosphate-activated protein kinase. J. Clin. Endocrinol. Metab. 2009, 94, 670–677. [Google Scholar] [CrossRef]
- Alves, M.G.; Martins, A.D.; Vaz, C.V.; Correia, S.; Moreira, P.I.; Oliveira, P.F.; Socorro, S. Metformin and male reproduction: Effects on Sertoli cell metabolism. Br. J. Pharmacol. 2014, 171, 1033–1042. [Google Scholar] [CrossRef] [Green Version]
- Foretz, M.; Guigas, B.; Bertrand, L.; Pollak, M.; Viollet, B. Metformin: From mechanisms of action to therapies. Cell Metab. 2014, 20, 953–966. [Google Scholar] [CrossRef] [Green Version]
- Foretz, M.; Guigas, B.; Viollet, B. Understanding the glucoregulatory mechanisms of metformin in type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2019, 15, 569–589. [Google Scholar] [CrossRef] [Green Version]
- Rena, G.; Hardie, D.G.; Pearson, E.R. The mechanisms of action of metformin. Diabetologia 2017, 60, 1577–1585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drzewoski, J.; Hanefeld, M. The current and potential therapeutic use of metformin-the good old drug. Pharmaceuticals 2021, 14, 122. [Google Scholar] [CrossRef] [PubMed]
- Bosman, E.; Esterhuizen, A.D.; Rodrigues, F.A.; Becker, P.J.; Hoffmann, W.A. Effect of metformin therapy and dietary supplements on semen parameters in hyperinsulinaemic males. Andrologia 2015, 47, 974–979. [Google Scholar] [CrossRef] [Green Version]
- Yan, W.J.; Mu, Y.; Yu, N.; Yi, T.L.; Zhang, Y.; Pang, X.L.; Cheng, D.; Yang, J. Protective effects of metformin on reproductive function in obese male rats induced by high-fat diet. J. Assist. Reprod. Genet. 2015, 32, 1097–1104. [Google Scholar] [CrossRef] [Green Version]
- Casarini, L.; Lispi, M.; Longobardi, S.; Milosa, F.; La Marca, A.; Tagliasacchi, D.; Pignatti, E.; Simoni, M. LH and hCG action on the same receptor results in quantitatively and qualitatively different intracellular signaling. PLoS ONE 2012, 7, e46682. [Google Scholar] [CrossRef] [Green Version]
- Riccetti, L.; de Pascali, F.; Gilioli, L.; Potì, F.; Giva, L.B.; Marino, M.; Tagliavini, S.; Trenti, T.; Fanelli, F.; Mezzullo, M.; et al. Human LH and hCG stimulate differently the early signalling pathways but result in equal testosterone synthesis in mouse Leydig cells in vitro. Reprod. Biol. Endocrinol. 2017, 15, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riccetti, L.; Yvinec, R.; Klett, D.; Gallay, N.; Combarnous, Y.; Reiter, E.; Simoni, M.; Casarini, L.; Ayoub, M.A. Human luteinizing hormone and chorionic gonadotropin display biased agonism at the LH and LH/CG receptors. Sci. Rep. 2017, 7, 940. [Google Scholar] [CrossRef]
- De Pascali, F.; Reiter, E. β-arrestins and biased signaling in gonadotropin receptors. Minerva Ginecol. 2018, 70, 525–538. [Google Scholar] [CrossRef]
- Walker, W.H. Non-classical actions of testosterone and spermatogenesis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 1557–1569. [Google Scholar] [CrossRef] [Green Version]
- Smith, L.B.; Walker, W.H. The regulation of spermatogenesis by androgens. Semin. Cell Dev. Biol. 2014, 30, 2–13. [Google Scholar] [CrossRef] [Green Version]
- Handelsman, D.J. Testosterone, Spermatogenesis, and Unravelling the Mysteries of Puberty. Endocrinology 2020, 161, bqaa120. [Google Scholar] [CrossRef]
- Ezcurra, D.; Humaidan, P. A review of luteinising hormone and human chorionic gonadotropin when used in assisted reproductive technology. Reprod. Biol. Endocrinol. 2014, 12, 95. [Google Scholar] [CrossRef] [Green Version]
- Casarini, L.; Santi, D.; Brigante, G.; Simoni, M. Two Hormones for One Receptor: Evolution, Biochemistry, Actions, and Pathophysiology of LH and hCG. Endocr. Rev. 2018, 39, 549–592. [Google Scholar] [CrossRef]
- Carson, S.A.; Kallen, A.N. Diagnosis and Management of Infertility: A Review. JAMA 2021, 326, 65–76. [Google Scholar] [CrossRef]
- Cailleux-Bounacer, A.; Reznik, Y.; Cauliez, B.; Menard, J.F.; Duparc, C.; Kuhn, J.M. Evaluation of endocrine testing of Leydig cell function using extractive and recombinant human chorionic gonadotropin and different doses of recombinant human LH in normal men. Eur. J. Endocrinol. 2008, 159, 171–178. [Google Scholar] [CrossRef] [Green Version]
- Veldhuis, J.D.; Liu, P.Y.; Takahashi, P.Y.; Keenan, D.M. Dynamic testosterone responses to near-physiological LH pulses are determined by the time pattern of prior intravenous LH infusion. Am. J. Physiol. Endocrinol. Metab. 2012, 303, 720–728. [Google Scholar] [CrossRef]
- Latronico, A.C.; Arnhold, I.J.P. Gonadotropin resistance. Endocr. Dev. 2013, 24, 25–32. [Google Scholar] [CrossRef]
- Camperi, J.; Combes, A.; Guibourdenche, J.; Guillarme, D.; Pichon, V.; Fournier, T.; Delaunay, N. An attempt to characterize the human Chorionic Gonadotropin protein by reversed phase liquid chromatography coupled with high-resolution mass spectrometry at the intact level. J. Pharm. Biomed. Anal. 2018, 161, 35–44. [Google Scholar] [CrossRef] [Green Version]
- van Straten, N.C.; Timmers, C.M. Non-Peptide ligands for the gonadotropin receptors. Annu. Rep. Med. Chem. 2009, 44, 171–188. [Google Scholar] [CrossRef]
- Van Straten, N.C.; Schoonus-Gerritsma, G.G.; van Someren, R.G.; Draaijer, J.; Adang, A.E.; Timmers, C.M.; Hanssen, R.G.; van Boeckel, C.A. The first orally active low molecular weight agonists for the LH receptor: Thienopyr(im)idines with therapeutic potential for ovulation induction. ChemBioChem 2002, 3, 1023–1026. [Google Scholar] [CrossRef]
- Van de Lagemaat, R.; Timmers, C.M.; Kelder, J.; van Koppen, C.; Mosselman, S.; Hanssen, R.G. Induction of ovulation by a potent, orally active, low molecular weight agonist (Org 43553) of the luteinizing hormone receptor. Hum. Reprod. 2009, 24, 640–648. [Google Scholar] [CrossRef] [Green Version]
- Van de Lagemaat, R.; Raafs, B.C.; van Koppen, C.; Timmers, C.M.; Mulders, S.M.; Hanssen, R.G. Prevention of the onset of ovarian hyperstimulation syndrome (OHSS) in the rat after ovulation induction with a low molecular weight agonist of the LH receptor compared with hCG and rec-LH. Endocrinology 2011, 152, 4350–4357. [Google Scholar] [CrossRef] [PubMed]
- Bakhtyukov, A.A.; Derkach, K.V.; Gureev, M.A.; Dar’in, D.V.; Sorokoumov, V.N.; Romanova, I.V.; Morina, I.Y.; Stepochkina, A.M.; Shpakov, A.O. Comparative Study of the Steroidogenic Effects of Human Chorionic Gonadotropin and Thieno[2,3-D]pyrimidine-Based Allosteric Agonist of Luteinizing Hormone Receptor in Young Adult, Aging and Diabetic Male Rats. Int. J. Mol. Sci. 2020, 21, 7493. [Google Scholar] [CrossRef]
- Shpakov, A.O.; Dar’in, D.V.; Derkach, K.V.; Lobanov, P.S. The stimulating influence of thienopyrimidine compounds on the adenylyl cyclase systems in the rat testes. Dokl. Biochem. Biophys. 2014, 456, 104–107. [Google Scholar] [CrossRef]
- Derkach, K.V.; Dar’in, D.V.; Bakhtyukov, A.A.; Lobanov, P.S.; Shpakov, A.O. In vitro and in vivo studies of functional activity of new low molecular weight agonists of the luteinizing hormone receptor. Biochem. Mosc. Suppl. Ser. B 2016, 10, 294–300. [Google Scholar] [CrossRef]
- Bakhtyukov, A.A.; Derkach, K.V.; Dar’in, D.V.; Sharova, T.S.; Shpakov, A.O. Decrease in the basal and luteinizing hormone receptor agonist-stimulated testosterone production in aging male rats. Adv. Gerontol. 2019, 9, 179–185. [Google Scholar] [CrossRef]
- Bakhtyukov, A.A.; Derkach, K.V.; Dar’in, D.V.; Stepochkina, A.M.; Shpakov, A.O. A low molecular weight agonist of the luteinizing hormone receptor stimulates adenylyl cyclase in the testicular membranes and steroidogenesis in the testes of rats with type 1 diabetes. Biochem. Mosc. Suppl. Ser. A 2019, 13, 301–309. [Google Scholar] [CrossRef]
- Derkach, K.V.; Bakhtyukov, A.A.; Bayunova, L.V.; Zorina, I.I.; Shpakov, A.O. Normalization of testicular steroidogenesis and spermatogenesis in male rats with type 2 diabetes mellitus under the conditions of metformin therapy. Dokl. Biol. Sci. 2020, 493, 110–113. [Google Scholar] [CrossRef]
- Van Koppen, C.J.; Zaman, G.J.; Timmers, C.M.; Kelder, J.; Mosselman, S.; van de Lagemaat, R.; Smit, M.J.; Hanssen, R.G. A signaling-selective, nanomolar potent allosteric low molecular weight agonist for the human luteinizing hormone receptor. Naunyn Schmiedebergs Arch. Pharmacol. 2008, 378, 503–514. [Google Scholar] [CrossRef]
- Galano, M.; Li, Y.; Li, L.; Sottas, C.; Papadopoulos, V. Role of Constitutive STAR in Leydig Cells. Int. J. Mol. Sci. 2021, 22, 2021. [Google Scholar] [CrossRef] [PubMed]
- Eacker, S.M.; Agrawal, N.; Qian, K.; Dichek, H.L.; Gong, E.Y.; Lee, K.; Braun, R.E. Hormonal regulation of testicular steroid and cholesterol homeostasis. Mol. Endocrinol. 2008, 22, 623–635. [Google Scholar] [CrossRef] [Green Version]
- Dyson, M.T.; Kowalewski, M.P.; Manna, P.R.; Stocco, D.M. The differential regulation of steroidogenic acute regulatory protein-mediated steroidogenesis by type I and type II PKA in MA-10 cells. Mol. Cell. Endocrinol. 2009, 300, 94–103. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Chen, F.; Ye, L.; Zirkin, B.; Chen, H. Steroidogenesis in Leydig cells: Effects of aging and environmental factors. Reproduction 2017, 154, 111–122. [Google Scholar] [CrossRef] [Green Version]
- Zirkin, B.R.; Papadopoulos, V. Leydig cells: Formation, function, and regulation. Biol. Reprod. 2018, 99, 101–111. [Google Scholar] [CrossRef]
- Derkach, K.V.; Bakhtyukov, A.A.; Romanova, I.V.; Zorina, I.I.; Bayunova, L.V.; Bondareva, V.M.; Morina, I.Y.; Kumar Roy, V.; Shpakov, A.O. The effect of metformin treatment on the basal and gonadotropin-stimulated steroidogenesis in male rats with type 2 diabetes mellitus. Andrologia 2020, 52, e13816. [Google Scholar] [CrossRef]
- Kapoor, D.; Aldred, H.; Clark, S.; Channer, K.S.; Jones, T.H. Clinical and biochemical assessment of hypogonadism in men with type 2 diabetes: Correlations with bioavailable testosterone and visceral adiposity. Diabetes Care 2007, 30, 911–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandel, A.; Dhindsa, S.; Topiwala, S.; Chaudhuri, A.; Dandona, P. Testosterone concentration in young patients with diabetes. Diabetes Care 2008, 31, 2013–2017. [Google Scholar] [CrossRef] [Green Version]
- Le, M.T.; Nguyen, H.; Dang, H.; Nguyen, T.; Van Nguyen, T.; Nguyen, Q. Impact of metabolic syndrome on the viability of human spermatozoa: A cross-sectional descriptive study in men from infertile couples. Basic Clin. Androl. 2021, 31, 22. [Google Scholar] [CrossRef]
- Grossmann, M.; Thomas, M.C.; Panagiotopoulos, S.; Sharpe, K.; MacIsaac, R.J.; Clarke, S.; Zajac, J.D.; Jerums, G. Low testosterone levels are common and associated with insulin resistance in men with diabetes. J. Clin. Endocrinol. Metab. 2008, 93, 1834–1840. [Google Scholar] [CrossRef] [Green Version]
- Grossmann, M. Low testosterone in men with type 2 diabetes: Significance and treatment. J. Clin. Endocrinol. Metab. 2011, 96, 2341–2353. [Google Scholar] [CrossRef]
- Rezvani, M.R.; Saadatjou, S.A.; Sorouri, S.; Hassanpour Fard, M. Comparison of serum free testosterone, luteinizing hormone and follicle stimulating hormone levels in diabetics and non-diabetics men-a case-control study. J. Res. Health Sci. 2012, 12, 98–100. [Google Scholar]
- Al Hayek, A.A.; Khader, Y.S.; Jafal, S.; Khawaja, N.; Robert, A.A.; Ajlouni, K. Prevalence of low testosterone levels in men with type 2 diabetes mellitus: A cross-sectional study. J. Fam. Commun. Med. 2013, 20, 179–186. [Google Scholar] [CrossRef] [Green Version]
- Jangir, R.N.; Jain, G.C. Diabetes mellitus induced impairment of male reproductive functions: A review. Curr. Diabetes Rev. 2014, 10, 147–157. [Google Scholar] [CrossRef]
- Fink, J.; Matsumoto, M.; Tamura, Y. Potential application of testosterone replacement therapy as treatment for obesity and type 2 diabetes in men. Steroids 2018, 138, 161–166. [Google Scholar] [CrossRef]
- Zhou, L.; Han, L.; Liu, M.; Lu, J.; Pan, S. Impact of metabolic syndrome on sex hormones and reproductive function: A meta-analysis of 2923 cases and 14062 controls. Aging 2020, 13, 1962–1971. [Google Scholar] [CrossRef]
- Mansour, M.; Coleman, E.; Dennis, J.; Akingbemi, B.; Schwartz, D.; Braden, T.; Judd, R.; Plaisance, E.; Stewart, L.K.; Morrison, E. Activation of PPARγ by Rosiglitazone does not negatively impact male sex steroid hormones in diabetic rats. PPAR Res. 2009, 2009, 101857. [Google Scholar] [CrossRef] [Green Version]
- Rato, L.; Alves, M.G.; Duarte, A.I.; Santos, M.S.; Moreira, P.I.; Cavaco, J.E.; Oliveira, P.F. Testosterone deficiency induced by progressive stages of diabetes mellitus impairs glucose metabolism and favors glycogenesis in mature rat Sertoli cells. Int. J. Biochem. Cell Biol. 2015, 66, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zha, W.; Bai, Y.; Xu, L.; Liu, Y.; Yang, Z.; Gao, H.; Li, J. Curcumin attenuates testicular injury in rats with streptozotocin-induced diabetes. BioMed Res. Int. 2018, 2018, 7468019. [Google Scholar] [CrossRef]
- Allam, M.A.; Khowailed, A.A.; Elattar, S.; Mahmoud, A.M. Umbelliferone ameliorates oxidative stress and testicular injury, improves steroidogenesis and upregulates peroxisome proliferator-activated receptor gamma in type 2 diabetic rats. J. Pharm. Pharmacol. 2021, rgab083. [Google Scholar] [CrossRef]
- Samir, S.M.; Elalfy, M.; Nashar, E.M.E.; Alghamdi, M.A.; Hamza, E.; Serria, M.S.; Elhadidy, M.G. Cardamonin exerts a protective effect against autophagy and apoptosis in the testicles of diabetic male rats through the expression of Nrf2 via p62-mediated Keap-1 degradation. Korean J. Physiol. Pharmacol. 2021, 25, 341–354. [Google Scholar] [CrossRef]
- Ding, G.L.; Liu, Y.; Liu, M.E.; Pan, J.X.; Guo, M.X.; Sheng, J.Z.; Huang, H.F. The effects of diabetes on male fertility and epigenetic regulation during spermatogenesis. Asian J. Androl. 2015, 17, 948–953. [Google Scholar] [CrossRef]
- Maresch, C.C.; Stute, D.C.; Alves, M.G.; Oliveira, P.F.; de Kretser, D.M.; Linn, T. Diabetes-induced hyperglycemia impairs male reproductive function: A systematic review. Hum. Reprod. Update 2018, 24, 86–105. [Google Scholar] [CrossRef] [Green Version]
- Shpakov, A.O.; Ryzhov, J.R.; Bakhtyukov, A.A.; Derkach, K.V. The regulation of the male hypothalamic-pituitary-gonadal axis and testosterone production by adipokines. In Advances in Testosterone Action; Estrada, M., Ed.; Intech Open Access Publisher: Rijeka, Croatia, 2018; pp. 25–57. ISBN 978-953-51-6241-4. [Google Scholar] [CrossRef] [Green Version]
- Derkach, K.V.; Bondareva, V.M.; Chistyakova, O.V.; Berstein, L.M.; Shpakov, A.O. The effect of long-term intranasal serotonin treatment on metabolic parameters and hormonal signaling in rats with high-fat diet/low-dose streptozotocin-induced type 2 diabetes. Int. J. Endocrinol. 2015, 2015, 245459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Annie, L.; Jeremy, M.; Gurusubramanian, G.; Derkach, K.V.; Shpakov, A.O.; Roy, V.K. Effect of metformin on testicular expression and localization of leptin receptor and levels of leptin in the diabetic mice. Mol. Reprod. Dev. 2020, 87, 620–629. [Google Scholar] [CrossRef] [PubMed]
- Jakubík, J.; Randáková, A.; El-Fakahany, E.E.; Doležal, V. Analysis of equilibrium binding of an orthosteric tracer and two allosteric modulators. PLoS ONE 2019, 14, e0214255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, K.; Lazari, M.F.; Li, S.; Korgaonkar, C.; Ascoli, M. Role of the rate of internalization of the agonist-receptor complex on the agonist-induced down-regulation of the lutropin/choriogonadotropin receptor. Mol. Endocrinol. 1999, 13, 1295–1304. [Google Scholar] [CrossRef] [PubMed]
- Amsterdam, A.; Hanoch, T.; Dantes, A.; Tajima, K.; Strauss, J.F.; Seger, R. Mechanisms of gonadotropin desensitization. Mol. Cell. Endocrinol. 2002, 187, 69–74. [Google Scholar] [CrossRef]
- Gudermann, T.; Birnbaumer, M.; Birnbaumer, L. Homologous desensitization of the murine luteinizing hormone receptor expressed in L cells. Mol. Cell. Endocrinol. 1995, 110, 125–135. [Google Scholar] [CrossRef]
- Wang, L.; Menon, K.M. Regulation of luteinizing hormone/chorionic gonadotropin receptor messenger ribonucleic acid expression in the rat ovary: Relationship to cholesterol metabolism. Endocrinology 2005, 146, 423–431. [Google Scholar] [CrossRef] [Green Version]
- Byambaragchaa, M.; Seong, H.K.; Choi, S.H.; Kim, D.J.; Kang, M.H.; Min, K.S. Constitutively Activating Mutants of Equine LH/CGR Constitutively Induce Signal Transduction and Inactivating Mutations Impair Biological Activity and Cell-Surface Receptor Loss In Vitro. Int. J. Mol. Sci. 2021, 22, 10723. [Google Scholar] [CrossRef]
- Gholizadeh, F.; Dastghaib, S.; Koohpeyma, F.; Bayat, E.; Mokarram, P. The protective effect of Stevia rebaudiana Bertoni on serum hormone levels, key steroidogenesis enzymes, and testicular damage in testes of diabetic rats. Acta Histochem. 2019, 121, 833–840. [Google Scholar] [CrossRef]
- Nna, V.U.; Bakar, A.B.A.; Ahmad, A.; Eleazu, C.O.; Mohamed, M. Oxidative Stress, NF-κB-Mediated Inflammation and Apoptosis in the Testes of Streptozotocin-Induced Diabetic Rats: Combined Protective Effects of Malaysian Propolis and Metformin. Antioxidants 2019, 8, 465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasiri, K.; Akbari, A.; Nimrouzi, M.; Ruyvaran, M.; Mohamadian, A. Safflower seed oil improves steroidogenesis and spermatogenesis in rats with type II diabetes mellitus by modulating the genes expression involved in steroidogenesis, inflammation and oxidative stress. J. Ethnopharmacol. 2021, 275, 114139. [Google Scholar] [CrossRef]
- Suleiman, J.B.; Abu Bakar, A.B.; Noor, M.M.; Nna, V.U.; Othman, Z.A.; Zakaria, Z.; Eleazu, C.O.; Mohamed, M. Bee bread mitigates downregulation of steroidogenic genes, decreased spermatogenesis, and epididymal oxidative stress in male rats fed with high-fat diet. Am. J. Physiol. Endocrinol. Metab. 2021, 321, e351–e366. [Google Scholar] [CrossRef] [PubMed]
- Wagner, I.V.; Klöting, N.; Savchuk, I.; Eifler, L.; Kulle, A.; Kralisch-Jäcklein, S.; Dötsch, J.; Hiort, O.; Svechnikov, K.; Söder, O. Diabetes Type 1 Negatively Influences Leydig Cell Function in Rats, which is Partially Reversible by Insulin Treatment. Endocrinology 2021, 162, bqab017. [Google Scholar] [CrossRef]
- Papadopoulos, V.; Aghazadeh, Y.; Fan, J.; Campioli, E.; Zirkin, B.; Midzak, A. Translocator protein-mediated pharmacology of cholesterol transport and steroidogenesis. Mol. Cell. Endocrinol. 2015, 408, 90–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manzella, D.; Grella, R.; Esposito, K.; Giugliano, D.; Barbagallo, M.; Paolisso, G. Blood pressure and cardiac autonomic nervous system in obese type 2 diabetic patients: Effect of metformin administration. Am. J. Hypertens. 2004, 17, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.C.; Wahlqvist, M.L.; Lee, M.S.; Tsai, H.N. Incidence of dementia is increased in type 2 diabetes and reduced by the use of sulfonylureas and metformin. J. Alzheimers Dis. 2011, 24, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.P.; Feng, L.; Yap, K.B.; Lee, T.S.; Tan, C.H.; Winblad, B. Long-term metformin usage and cognitive function among older adults with diabetes. J. Alzheimers Dis. 2014, 41, 61–68. [Google Scholar] [CrossRef]
- De Broe, M.E.; Kajbaf, F.; Lalau, J.D. Renoprotective Effects of Metformin. Nephron 2018, 138, 261–274. [Google Scholar] [CrossRef] [Green Version]
- Zilov, A.V.; Abdelaziz, S.I.; AlShammary, A.; Al Zahrani, A.; Amir, A.; Assaad Khalil, S.H.; Brand, K.; Elkafrawy, N.; Hassoun, A.A.K.; Jahed, A.; et al. Mechanisms of action of metformin with special reference to cardiovascular protection. Diabetes Metab. Res. Rev. 2019, 35, e3173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, Y.; Zhou, Y.; Ling, P.; Feng, X.; Luo, S.; Zheng, X.; Little, P.J.; Xu, S.; Weng, J. Metformin in cardiovascular diabetology: A focused review of its impact on endothelial function. Theranostics 2021, 11, 9376–9396. [Google Scholar] [CrossRef]
- Morin-Papunen, L.; Rantala, A.S.; Unkila-Kallio, L.; Tiitinen, A.; Hippeläinen, M.; Perheentupa, A.; Tinkanen, H.; Bloigu, R.; Puukka, K.; Ruokonen, A.; et al. Metformin improves pregnancy and live-birth rates in women with polycystic ovary syndrome (PCOS): A multicenter, double-blind, placebo-controlled randomized trial. J. Clin. Endocrinol. Metab. 2012, 97, 1492–1500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sam, S.; Ehrmann, D.A. Metformin therapy for the reproductive and metabolic consequences of polycystic ovary syndrome. Diabetologia 2017, 60, 1656–1661. [Google Scholar] [CrossRef]
- Hyer, S.; Balani, J.; Shehata, H. Metformin in pregnancy: Mechanisms and clinical applications. Int. J. Mol. Sci. 2018, 19, 1954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costello, M.; Garad, R.; Hart, R.; Homer, H.; Johnson, L.; Jordan, C.; Mocanu, E.; Qiao, J.; Rombauts, L.; Teede, H.J.; et al. A Review of first line infertility treatments and supporting evidence in women with polycystic ovary syndrome. Med. Sci. 2019, 7, 95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benham, J.L.; Donovan, L.E.; Yamamoto, J.M. Metformin in pregnancy for women with type 2 diabetes: A review. Curr. Diabetes Rep. 2021, 21, 36. [Google Scholar] [CrossRef]
- Casulari, L.A.; Caldas, A.D.; Domingues Casulari Motta, L.; Lofrano-Porto, A. Effects of metformin and short-term lifestyle modification on the improvement of male hypogonadism associated with metabolic syndrome. Minerva Endocrinol. 2010, 35, 145–151. [Google Scholar] [PubMed]
- Morgante, G.; Tosti, C.; Orvieto, R.; Musacchio, M.C.; Piomboni, P.; De Leo, V. Metformin improves semen characteristics of oligo-terato-asthenozoospermic men with metabolic syndrome. Fertil. Steril. 2011, 95, 2150–2152. [Google Scholar] [CrossRef] [PubMed]
- Stokes, V.J.; Anderson, R.A.; George, J.T. How does obesity affect fertility in men–and what are the treatment options? Clin. Endocrinol. 2015, 82, 633–638. [Google Scholar] [CrossRef]
- Ozata, M.; Oktenli, C.; Bingol, N.; Ozdemir, I.C. The effects of metformin and diet on plasma testosterone and leptin levels in obese men. Obes. Res. 2001, 9, 662–667. [Google Scholar] [CrossRef]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I. Erectile dysfunction and low sex drive in men with type 2 DM: The potential role of diabetic pharmacotherapy. J. Clin. Diagn. Res. 2016, 10, FC21–FC26. [Google Scholar] [CrossRef]
- Pelusi, C.; Giagulli, V.A.; Baccini, M.; Fanelli, F.; Mezzullo, M.; Fazzini, A.; Bianchi, N.; Carbone, M.D.; De Pergola, G.; Mastroroberto, M.; et al. Clomiphene citrate effect in obese men with low serum testosterone treated with metformin due to dysmetabolic disorders: A randomized, double-blind, placebo-controlled study. PLoS ONE 2017, 12, e0183369. [Google Scholar] [CrossRef] [Green Version]
- Attia, G.R.; Rainey, W.E.; Carr, B.R. Metformin directly inhibits androgen production in human thecal cells. Fertil. Steril. 2001, 76, 517–524. [Google Scholar] [CrossRef]
- Rabbani, S.I.; Devi, K.; Khanam, S. Role of pioglitazone with metformin or glimepiride on oxidative stress-induced nuclear damage and reproductive toxicity in diabetic rats. Malays. J. Med. Sci. 2010, 17, 3–11. [Google Scholar]
- Ghasemnejad-Berenji, M.; Ghazi-Khansari, M.; Yazdani, I.; Nobakht, M.; Abdollahi, A.; Ghasemnejad-Berenji, H.; Mohajer Ansari, J.; Pashapour, S.; Dehpour, A.R. Effect of metformin on germ cell-specific apoptosis, oxidative stress and epididymal sperm quality after testicular torsion/detorsion in rats. Andrologia 2018, 50, e12846. [Google Scholar] [CrossRef] [PubMed]
- Nna, V.U.; Bakar, A.B.A.; Ahmad, A.; Mohamed, M. Diabetes-induced testicular oxidative stress, inflammation, and caspase-dependent apoptosis: The protective role of metformin. Arch. Physiol. Biochem. 2018, 126, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Nna, V.U.; Bakar, A.B.A.; Ahmad, A.; Mohamed, M. Down-regulation of steroidogenesis-related genes and its accompanying fertility decline in streptozotocin-induced diabetic male rats: Ameliorative effect of metformin. Andrology 2019, 7, 110–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasrolahi, O.; Khaneshi, F.; Rahmani, F.; Razi, M. Honey and metformin ameliorated diabetes-induced damages in testes of rat; correlation with hormonal changes. Iran. J. Reprod. Med. 2013, 11, 1013–1020. [Google Scholar] [PubMed]
- Nna, V.U.; Bakar, A.B.A.; Ahmad, A.; Umar, U.Z.; Suleiman, J.B.; Zakaria, Z.; Othman, Z.A.; Mohamed, M. Malaysian propolis and metformin mitigate subfertility in streptozotocin-induced diabetic male rats by targeting steroidogenesis, testicular lactate transport, spermatogenesis and mating behaviour. Andrology 2020, 8, 731–746. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.L.; Strauss, J.F., III. Molecular pathology and mechanism of action of the steroidogenic acute regulatory protein, StAR. J. Steroid Biochem. Mol. Biol. 1999, 69, 131–141. [Google Scholar] [CrossRef]
- Shoorei, H.; Khaki, A.; Shokoohi, M.; Khaki, A.A.; Alihemmati, A.; Moghimian, M.; Abtahi-Eivary, S.H. Evaluation of carvacrol on pituitary and sexual hormones and their receptors in the testicle of male diabetic rats. Hum. Exp. Toxicol. 2020, 39, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- Shetty, G.; Wilson, G.; Huhtaniemi, I.; Boettger-Tong, H.; Meistrich, M.L. Testosterone inhibits spermatogonial differentiation in juvenile spermatogonial depletion mice. Endocrinology 2001, 142, 2789–2795. [Google Scholar] [CrossRef] [PubMed]
- Huhtaniemi, I. Mechanisms in Endocrinology: Hormonal regulation of spermatogenesis: Mutant mice challenging old paradigms. Eur. J. Endocrinol. 2018, 179, 143–150. [Google Scholar] [CrossRef]
- Amory, J.K.; Coviello, A.D.; Page, S.T.; Anawalt, B.D.; Matsumoto, A.M.; Bremner, W.J. Serum 17-hydroxyprogesterone strongly correlates with intratesticular testosterone in gonadotropin-suppressed normal men receiving various dosages of human chorionic gonadotropin. Fertil. Steril. 2008, 89, 380–386. [Google Scholar] [CrossRef] [Green Version]
- Crosnoe, L.E.; Grober, E.; Ohl, D.; Kim, E.D. Exogenous testosterone: A preventable cause of male infertility. Transl. Androl. Urol. 2013, 2, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Beattie, M.C.; Adekola, L.; Papadopoulos, V.; Chen, H.; Zirkin, B.R. Leydig cell aging and hypogonadism. Exp. Gerontol. 2015, 68, 87–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Meliegy, A.; Motawi, A.; Abd El Salam, M.A. Systematic review of hormone replacement therapy in the infertile man. Arab. J. Urol. 2018, 16, 140–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keeney, D.S.; Sprando, R.L.; Robaire, B.; Zirkin, B.R.; Ewing, L.L. Reversal of long-term LH deprivation on testosterone secretion and Leydig cell volume, number and proliferation in adult rats. J. Endocrinol. 1990, 127, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zirkin, B.R. Long-term suppression of Leydig cell steroidogenesis prevents Leydig cell aging. Proc. Natl. Acad. Sci. USA 1999, 96, 14877–14881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffman, Y.M.; Peegel, H.; Sprock, M.J.; Zhang, Q.Y.; Menon, K.M. Evidence that human chorionic gonadotropin/luteinizing hormone receptor down-regulation involves decreased levels of receptor messenger ribonucleic acid. Endocrinology 1991, 128, 388–393. [Google Scholar] [CrossRef]
- Wang, H.; Segaloff, D.L.; Ascoli, M. Lutropin/choriogonadotropin down-regulates its receptor by both receptor-mediated endocytosis and a cAMP-dependent reduction in receptor mRNA. J. Biol. Chem. 1991, 266, 780–785. [Google Scholar] [CrossRef]
- El-Hefnawy, T.; Huhtaniemi, I. Progesterone can participate in down-regulation of the luteinizing hormone receptor gene expression and function in cultured murine Leydig cells. Mol. Cell. Endocrinol. 1998, 137, 127–138. [Google Scholar] [CrossRef]
- Fejes, I.; Koloszár, S.; Závaczki, Z.; Daru, J.; Szöllösi, J.; Pál, A. Effect of body weight on testosterone/estradiol ratio in oligozoospermic patients. Arch. Androl. 2006, 52, 97–102. [Google Scholar] [CrossRef] [Green Version]
- Leisegang, K.; Sengupta, P.; Agarwal, A.; Henkel, R. Obesity and male infertility: Mechanisms and management. Andrologia 2021, 53, e13617. [Google Scholar] [CrossRef]
- Rice, S.; Pellatt, L.; Ramanathan, K.; Whitehead, S.A.; Mason, H.D. Metformin inhibits aromatase via an extracellular signal-regulated kinase-mediated pathway. Endocrinology 2009, 150, 4794–4801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuhrmeister, I.P.; Branchini, G.; Pimentel, A.M.; Ferreira, G.D.; Capp, E.; Brum, I.S.; von Eye Corleta, H. Human granulosa cells: Insulin and insulin-like growth factor-1 receptors and aromatase expression modulation by metformin. Gynecol. Obstet. Investig. 2014, 77, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Tosca, L.; Chabrolle, C.; Uzbekova, S.; Dupont, J. Effects of metformin on bovine granulosa cells steroidogenesis: Possible involvement of adenosine 5′ monophosphate-activated protein kinase (AMPK). Biol. Reprod. 2007, 76, 368–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertoldo, M.J.; Faure, M.; Dupont, J.; Froment, P. AMPK: A master energy regulator for gonadal function. Front. Neurosci. 2015, 9, 235. [Google Scholar] [CrossRef] [Green Version]
- Weaver, E.A.; Ramachandran, R. Metformin attenuates steroidogenesis in ovarian follicles of the broiler breeder hen. Reproduction 2020, 160, 659–672. [Google Scholar] [CrossRef]
- Estienne, A.; Bongrani, A.; Ramé, C.; Kurowska, P.; Błaszczyk, K.; Rak, A.; Ducluzeau, P.H.; Froment, P.; Dupont, J. Energy sensors and reproductive hypothalamo-pituitary ovarian axis (HPO) in female mammals: Role of mTOR (mammalian target of rapamycin), AMPK (AMP-activated protein kinase) and SIRT1 (Sirtuin 1). Mol. Cell. Endocrinol. 2021, 521, 111113. [Google Scholar] [CrossRef]
- Przygrodzka, E.; Hou, X.; Zhang, P.; Plewes, M.R.; Franco, R.; Davis, J.S. PKA and AMPK signaling pathways differentially regulate luteal steroidogenesis. Endocrinology 2021, 162, bqab015. [Google Scholar] [CrossRef]
- Newton, C.L.; Anderson, R.C. Pharmacoperones for misfolded gonadotropin receptors. Handb. Exp. Pharmacol. 2018, 245, 111–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanssen, R.G.J.M.; Timmers, C.M. Thieno[2,3-d]pyrimidines with Combined LH and FSH Agonistic Activity. Patent WO2003020726, 13 March 2003. [Google Scholar]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Narayana, K.; D’Souza, U.J.; Seetharama Rao, K.P. Ribavirin-induced sperm shape abnormalities in Wistar rat. Mutat. Res. 2002, 513, 193–196. [Google Scholar] [CrossRef]
- Silva, J.; Basso, J.; Sousa, J.; Fortuna, A.; Vitorino, C. Development and full validation of an HPLC methodology to quantify atorvastatin and curcumin after their intranasal co-delivery to mice. Biomed. Chromatogr. 2019, 33, e4621. [Google Scholar] [CrossRef] [PubMed]
- Gautam, D.K.; Misro, M.M.; Chaki, S.P.; Chandra, M.; Sehgal, N. hCG treatment raises H2O2 levels and induces germ cell apoptosis in rat testis. Apoptosis 2007, 12, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Kim, T.S.; Park, C.K.; Lee, S.H.; Kim, J.M.; Lee, K.S.; Lee, I.K.; Park, J.W.; Lawson, M.A.; Lee, D.S. hCG-induced endoplasmic reticulum stress triggers apoptosis and reduces steroidogenic enzyme expression through activating transcription factor 6 in Leydig cells of the testis. J. Mol. Endocrinol. 2013, 50, 151–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]










| Parameter | Control, n = 30 | Diabetes, n = 30 | Diabetes + MF, n = 30 |
|---|---|---|---|
| Body weight, g | 352.2 ± 3.9 | 402.2 ± 5.2 a | 361.3 ± 5.5 b |
| Fasting glucose, mM | 4.34 ± 0.08 | 6.57 ± 0.12 a | 5.64 ± 0.11 a,b |
| Glucose (120′, GTT), mM | 5.37 ± 0.09 | 9.77 ± 0.26 a | 6.48 ± 0.19 a,b |
| HbA1c, % | 4.52 ± 0.08 | 7.07 ± 0.14 a | 5.56 ± 0.14 a,b |
| Fasting insulin, ng/mL | 0.61 ± 0.04 | 0.96 ± 0.05 a | 0.66 ± 0.04 b |
| Insulin (120, GTT), ng/mL | 0.77 ± 0.07 | 1.30 ± 0.09 a | 0.86 ± 0.06 b |
| IR (fasting), rel.units | 2.68 ± 0.18 | 6.41 ± 0.42 a | 3.80 ± 0.28 a,b |
| IR (120, GTT), rel.units | 4.21 ± 0.40 | 13.19 ± 1.22 a | 5.69 ± 0.48 b |
| Fasting leptin, ng/mL | 1.81 ± 0.09 | 3.49 ± 0.11 a | 2.00 ± 0.07 b |
| Leptin (120, GTT), ng/mL | 2.51 ± 0.13 | 4.70 ± 0.18 a | 3.17 ± 0.15 a,b |
| Triglycerides, mM | 0.75 ± 0.01 | 1.18 ± 0.05 a | 0.84 ± 0.03 b |
| Total cholesterol, mM | 4.31 ± 0.06 | 6.38 ± 0.11 a | 4.91 ± 0.08 a,b |
| Testosterone, nM | 12.01 ± 0.70 | 7.02 ± 0.39 a | 8.82 ± 0.43 a,b |
| Group | Testosterone Concentration, nM | |||
|---|---|---|---|---|
| 0 (before) | 1 (11 a.m.) | 3 (1 p.m.) | 5 (3 p.m.) | |
| C1 | 13.53 ± 1.45 | 16.34 ± 2.52 | 17.66 ± 2.82 | 14.96 ± 1.60 |
| CT1 | 11.11 ± 1.42 | 23.52 ± 3.43 | 34.58 ± 3.82 c | 33.35 ± 3.89 c |
| CG1 | 13.14 ± 1.42 | 35.36 ± 3.17 d,e | 70.88 ± 4.77 d,e | 62.20 ± 1.71 d,e |
| D1 | 5.69 ± 0.80 a | 7.20 ± 0.68 | 6.72 ± 0.47 a | 7.08 ± 0.84 a |
| DT1 | 5.73 ± 0.64 f | 10.06 ± 0.73 f | 19.02 ± 1.98 c,f | 22.28 ± 1.75 c,f |
| DG1 | 6.98 ± 0.58 g | 19.59 ± 2.11 d,e,g | 39.01 ± 2.08 d,e,g | 32.70 ± 2.53 d,e,g |
| DM1 | 8.99 ± 0.89 a | 16.40 ± 3.64 | 11.85 ± 0.96 | 10.44 ± 1.39 |
| DMT1 | 9.61 ± 1.36 | 21.94 ± 2.21 h | 29.68 ± 2.29 c,h | 33.03 ± 1.40 c,h |
| DMG1 | 9.59 ± 0.76 | 32.70 ± 2.41 d,e,h | 60.74 ± 3.90 d,e,h | 58.13 ± 1.12 d,e,h |
| Group | Testosterone Concentration, nM | |||||
|---|---|---|---|---|---|---|
| 0 (before) | 1st Day | 2nd Day | 3rd Day | 4th Day | 5th Day | |
| C5 | 12.52 ± 1.55 | 12.45 ± 1.07 | 15.18 ± 1.07 | 13.40 ± 1.68 | 11.55 ± 1.35 | 12.64 ± 1.57 |
| CT5 | 12.30 ± 1.56 | 32.72 ± 4.26 c | 35.30 ± 5.61 c | 31.87 ± 4.19 c | 32.20 ± 2.95 c | 33.96 ± 3.94 c |
| CG5 | 13.03 ± 1.73 | 72.66 ± 5.89 d,e | 33.11 ± 2.32 d | 34.14 ± 3.72 d | 40.09 ± 1.09 d,e | 39.48 ± 2.95 d |
| D5 | 6.62 ± 0.79 a | 6.67 ± 1.34 a | 6.88 ± 0.89 a | 6.06 ± 0.99 a | 6.61 ± 0.43 a | 6.37 ± 0.94 a |
| DT5 | 6.89 ± 0.92 f | 19.32 ± 1.49 c,f | 27.40 ± 4.48 c | 26.32 ± 4.76 c | 27.41 ± 4.67 c | 29.93 ± 3.80 c,f |
| DG5 | 6.27 ± 1.03 g | 44.84 ± 4.67 d,e,g | 33.15 ± 5.89 d | 29.19 ± 3.47 d | 32.16 ± 6.18 d | 35.37 ± 5.86 d |
| DM5 | 9.91 ± 0.40 | 8.71 ± 0.83 a | 10.81 ± 0.92 a,b | 12.59 ± 1.72 b | 10.65 ± 0.81 b | 11.11 ± 1.23 |
| DMT5 | 10.38 ± 1.27 | 30.41 ± 2.52 c | 29.77 ± 2.18 c | 25.42 ± 2.80 c | 26.42 ± 2.38 c | 23.62 ± 3.15 c |
| DMG5 | 12.06 ± 2.13 | 65.37 ± 7.09 d,e | 38.06 ± 5.70 d | 28.77 ± 2.66 d | 28.55 ± 2.40 d | 27.92 ± 2.37 d |
| Group | Progestrerone, nmol/g | 17-OH-Progesterone, pmol/g | Androstenedione, ng/g | Testosterone, nmol/g | Estradiol, pmol/g |
|---|---|---|---|---|---|
| C1 | 0.314 ± 0.018 | 61.92 ± 10.00 | 59.07 ± 7.50 | 1.079 ± 0.045 | 49.39 ± 2.97 |
| CT1 | 0.480 ± 0.036 | 70.81 ± 3.64 | 76.20 ± 13.27 | 1.522 ± 0.103 | 44.00 ± 2.26 |
| CG1 | 1.312 ± 0.085 d,e | 119.98 ± 13.37 d,e | 90.48 ± 7.35 | 1.830 ± 0.214 d | 35.82 ± 1.69 d |
| D1 | 0.283 ± 0.017 | 50.30 ± 5.33 | 59.32 ± 4.46 | 0.716 ± 0.061 a | 57.18 ± 2.53 |
| DT1 | 0.419 ± 0.049 | 77.70 ± 13.02 | 60.28 ± 9.49 | 1.297 ± 0.177 c | 36.56 ± 5.21 c |
| DG1 | 0.717 ± 0.070 d,e,g | 79.69 ± 8.10 g | 62.22 ± 6.11 | 1.456 ± 0.138 d | 35.93 ± 2.50 d |
| DM1 | 0.285 ± 0.019 | 54.23 ± 10.92 | 69.93 ± 10.25 | 0.999 ± 0.149 | 39.08 ± 2.48 a,b |
| DMT1 | 0.355 ± 0.032 | 72.03 ± 5.96 | 92.03 ± 8.74 | 1.681 ± 0.152 | 35.09 ± 3.71 |
| DMG1 | 0.808 ± 0.092 d,e,g | 123.05 ± 8.65 d,e,h | 114.27 ± 15.48 h | 2.373 ± 0.341 d | 29.24 ± 2.38 |
| Group | Progestrerone, nmol/g | 17-OH-Progesterone, pmol/g | Androstenedione, ng/g | Testosterone, nmol/g | Estradiol, pmol/g |
|---|---|---|---|---|---|
| C5 | 0.325 ± 0.023 | 69.0 ± 6.9 | 60.8 ± 5.5 | 1.150 ± 0.095 | 51.25 ± 3.50 |
| CT5 | 0.463 ± 0.090 | 111.5 ± 10.8 c | 120.1 ± 13.9 c | 1.597 ± 0.153 | 50.57 ± 1.32 |
| CG5 | 0.743 ± 0.064 d,e | 211.6 ± 13.0 d,e | 158.7 ± 16.8 d | 1.723 ± 0.103 d | 37.81 ± 6.25 |
| D5 | 0.262 ± 0.025 | 51.7 ± 4.9 | 61.3 ± 4.8 | 0.676 ± 0.080 a | 55.95 ± 1.51 |
| DT5 | 0.333 ± 0.036 | 78.1 ± 9.2 | 120.0 ± 15.2 c | 1.462 ± 0.077 c | 48.92 ± 4.17 |
| DG5 | 0.688 ± 0.125 d,e | 355.7 ± 38.2 d,e,g | 190.5 ± 9.6 d,e | 1.598 ± 0.111 d | 39.13 ± 3.57 d |
| DM5 | 0.287 ± 0.021 | 60.8 ± 4.7 | 63.5 ± 6.0 | 1.265 ± 0.155 b | 36.37 ± 5.28 a,b |
| DMT5 | 0.322 ± 0.032 | 77.1 ± 6.7 f | 122.4 ± 23.6 c | 1.170 ± 0.107 | 55.28 ± 3.72 c |
| DMG5 | 0.858 ± 0.128 d,e | 370.1 ± 31.2 d,e,g | 167.5 ± 6.0 d | 1.469 ± 0.151 | 45.98 ± 3.79 |
| Group | Thickness of the Seminiferous Epithelium, μm | Number of Spermatogonia, Units | Number of Pachytene Spermatocytes, Units |
|---|---|---|---|
| C5 | 66.20 ± 0.92 | 54.06 ± 1.19 | 49.74 ± 0.84 |
| CT5 | 68.35 ± 0.84 | 57.64 ± 0.92 c | 53.96 ± 0.75 c |
| CG5 | 67.65 ± 0.99 | 62.02 ± 0.89 d,e | 56.34 ± 0.74d |
| D5 | 58.38 ± 0.71 a | 47.04 ± 0.86 a | 37.28 ± 0.77 a |
| DT5 | 69.97 ± 1.04 c | 57.72 ± 1.05 c | 51.26 ± 0.89 cf |
| DG5 | 66.65 ± 1.10 d,e | 60.82 ± 0.89 d | 53.80 ± 1.24 d |
| DM5 | 62.68 ± 1.14 a,b | 59.08 ± 0.77 a,b | 49.54 ± 1.05 b |
| DMT5 | 66.44 ± 1.07 c,h | 59.36 ± 0.64 | 50.12 ± 0.74 f |
| DMG5 | 64.79 ± 0.70 | 58.76 ± 0.83 g | 51.28 ± 0.79 g |
| Group | pAMPKα(Thr172)/ tAMPKα | pAMPKα(Thr172)/ GAPDH | tAMPKα/GAPDH |
|---|---|---|---|
| C5 | 0.502 ± 0.045 | 0.884 ± 0.091 | 1.862 ± 0.324 |
| CT5 | 0.491 ± 0.071 | 0.781 ± 0.036 | 1.717 ± 0.233 |
| CG5 | 0.541 ± 0.113 | 0.825 ± 0.107 | 1.707 ± 0.292 |
| D5 | 0.687 ± 0.085 | 0.731 ± 0.065 | 1.093 ± 0.102 |
| DT5 | 0.773 ± 0.111 | 0.780 ± 0.067 | 1.076 ± 0.161 |
| DG5 | 0.864 ± 0.092 | 0.807 ± 0.100 | 1.000 ± 0.185 |
| DM5 | 1.081 ± 0.203 a | 0.868 ± 0.051 | 0.917 ± 0.180 a |
| DMT5 | 0.771 ± 0.113 | 0.909 ± 0.147 | 1.306 ± 0.320 |
| DMG5 | 0.502 ± 0.067 d,h | 0.680 ± 0.073 | 1.443 ± 0.189 |
| Genes | Forward/Reverse Sequence | PS | AT | Genbank |
|---|---|---|---|---|
| Lhr | (For) CTGCGCTGTCCTGGCC | 103 | 55 | NM_012978.1 |
| (Rev) CGACCTCATTAAGTCCCCTGAA | ||||
| Star | (For) AAGGCTGGAAGAAGGAAAGC | 66 | 55 | NM_031558.3 |
| (Rev) CACCTGGCACCACCTTACTT | ||||
| Cyp11a1 | (For) TATTCCGCTTTGCCTTTGAG | 74 | 55 | NM_017286.3 |
| (Rev) CACGATCTCCTCCAACATCC | ||||
| Hsd3b | (For) AGGCCTGTGTCCAAGCTAGTGT | 161 | 55 | XM_017591325.1 |
| (Rev) CTCGGCCATCTTTTTGCTGTAT | ||||
| Cyp17a1 | (For) CATCCCCCACAAGGCTAAC | 61 | 55 | XM_006231435.3 |
| (Rev) TGTGTCCTTGGGGACAGTAAA | ||||
| Hsd17b | (For) CCTTTGGCTTTGCCATGAGA | 68 | 55 | NM_024392.2 |
| (Rev) CAATCCATCCTGCTCCAACCT | ||||
| Cyp19a1 | (For) GGTATCAGCCTGTCGTGGAC | 118 | 56 | NM_017085.2 |
| (Rev) AGCCTGTGCATTCTTCCGAT | ||||
| Actb | (For) CTGGCACCACACCTTCTACA | 125 | 55 | NM_031144.3 |
| (Rev) AGGTCTCAAACATGATCTGGGT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakhtyukov, A.A.; Derkach, K.V.; Sorokoumov, V.N.; Stepochkina, A.M.; Romanova, I.V.; Morina, I.Y.; Zakharova, I.O.; Bayunova, L.V.; Shpakov, A.O. The Effects of Separate and Combined Treatment of Male Rats with Type 2 Diabetes with Metformin and Orthosteric and Allosteric Agonists of Luteinizing Hormone Receptor on Steroidogenesis and Spermatogenesis. Int. J. Mol. Sci. 2022, 23, 198. https://doi.org/10.3390/ijms23010198
Bakhtyukov AA, Derkach KV, Sorokoumov VN, Stepochkina AM, Romanova IV, Morina IY, Zakharova IO, Bayunova LV, Shpakov AO. The Effects of Separate and Combined Treatment of Male Rats with Type 2 Diabetes with Metformin and Orthosteric and Allosteric Agonists of Luteinizing Hormone Receptor on Steroidogenesis and Spermatogenesis. International Journal of Molecular Sciences. 2022; 23(1):198. https://doi.org/10.3390/ijms23010198
Chicago/Turabian StyleBakhtyukov, Andrey A., Kira V. Derkach, Viktor N. Sorokoumov, Anna M. Stepochkina, Irina V. Romanova, Irina Yu. Morina, Irina O. Zakharova, Liubov V. Bayunova, and Alexander O. Shpakov. 2022. "The Effects of Separate and Combined Treatment of Male Rats with Type 2 Diabetes with Metformin and Orthosteric and Allosteric Agonists of Luteinizing Hormone Receptor on Steroidogenesis and Spermatogenesis" International Journal of Molecular Sciences 23, no. 1: 198. https://doi.org/10.3390/ijms23010198
APA StyleBakhtyukov, A. A., Derkach, K. V., Sorokoumov, V. N., Stepochkina, A. M., Romanova, I. V., Morina, I. Y., Zakharova, I. O., Bayunova, L. V., & Shpakov, A. O. (2022). The Effects of Separate and Combined Treatment of Male Rats with Type 2 Diabetes with Metformin and Orthosteric and Allosteric Agonists of Luteinizing Hormone Receptor on Steroidogenesis and Spermatogenesis. International Journal of Molecular Sciences, 23(1), 198. https://doi.org/10.3390/ijms23010198






