Fructose-Rich Diet Is a Risk Factor for Metabolic Syndrome, Proximal Tubule Injury and Urolithiasis in Rats
Abstract
:1. Introduction
2. Results
2.1. Assessment of Nutritional and Metabolic Status
2.2. Assessment of Urine
2.3. Analysis of Urinary Electrolytes Excretion as a Percentage of Dietary Intake
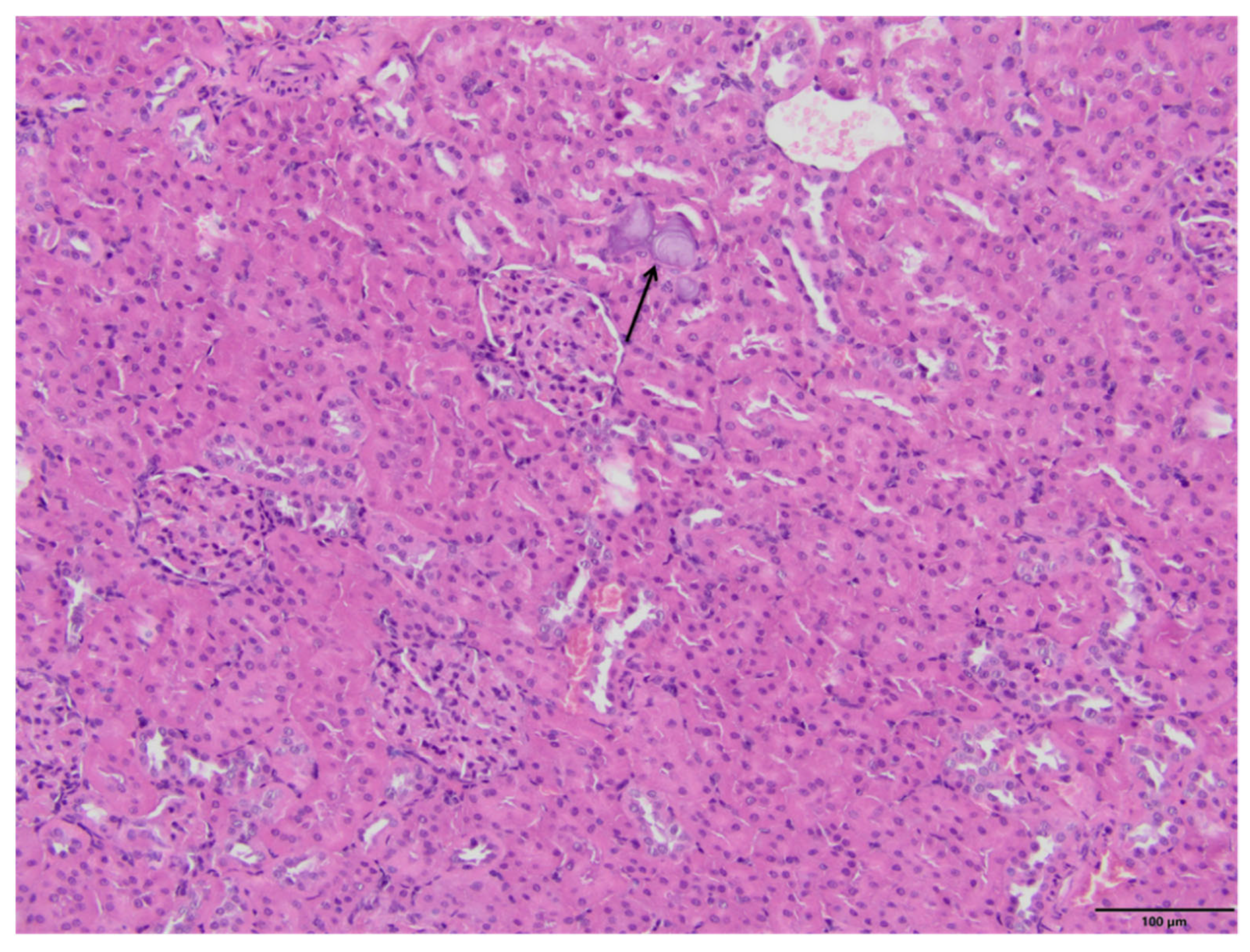
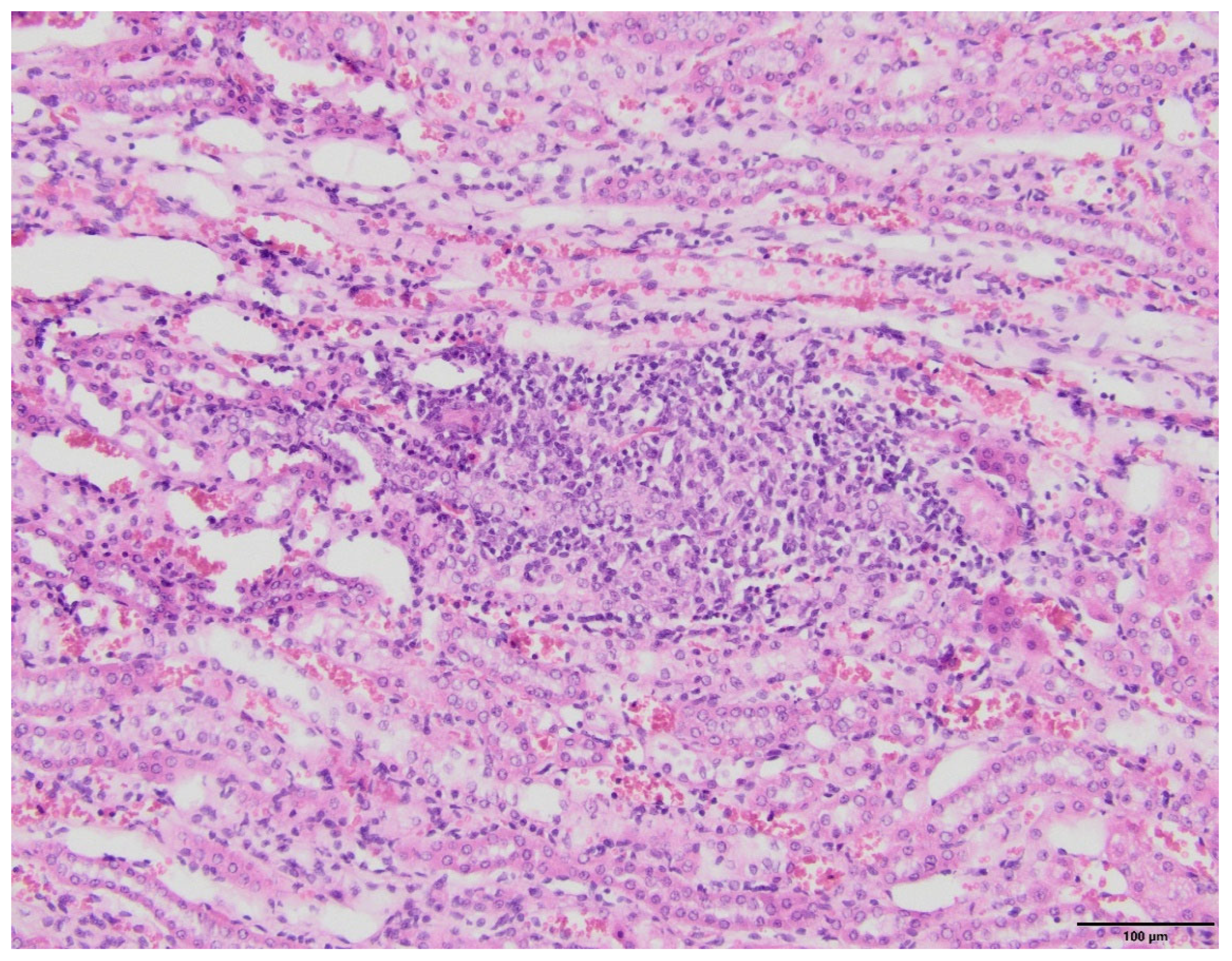
2.4. Kidney Histopathology
3. Discussion
3.1. Fructose Induced Metabolic Syndrome and Inflammation
3.2. Fructose Effects on Macro-Mineral Homeostasis
4. Materials and Methods
4.1. Animal Model
4.2. Laboratory Tests
FEx(x) = (U(x) × SCr/UCr × S(x)) × 100
4.3. Histology
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gross, L.S.; Li, L.; Ford, E.S.; Liu, S. Increased Consumption of Refined Carbohydrates and the Epidemic of Type 2 Diabetes in the United States: An Ecologic Assessment. Am. J. Clin. Nutr. 2004, 79, 774–779. [Google Scholar] [CrossRef]
- Johnson, R.J.; Sanchez-Lozada, L.G.; Nakagawa, T. The Effect of Fructose on Renal Biology and Disease. J. Am. Soc. Nephrol. JASN 2010, 21, 2036–2039. [Google Scholar] [CrossRef]
- Azaïs-Braesco, V.; Sluik, D.; Maillot, M.; Kok, F.; Moreno, L.A. A Review of Total & Added Sugar Intakes and Dietary Sources in Europe. Nutr. J. 2017, 16, 6. [Google Scholar] [CrossRef] [Green Version]
- Brymora, A.; Flisiński, M.; Johnson, R.J.; Goszka, G.; Stefańska, A.; Manitius, J. Low-Fructose Diet Lowers Blood Pressure and Inflammation in Patients with Chronic Kidney Disease. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc.—Eur. Ren. Assoc. 2012, 27, 608–612. [Google Scholar] [CrossRef]
- Van den Berghe, G. Fructose: Metabolism and Short-Term Effects on Carbohydrate and Purine Metabolic Pathways. Prog. Biochem. Pharmacol. 1986, 21, 1–32. [Google Scholar]
- Mäenpää, P.H.; Raivio, K.O.; Kekomäki, M.P. Liver Adenine Nucleotides: Fructose-Induced Depletion and Its Effect on Protein Synthesis. Science 1968, 161, 1253–1254. [Google Scholar] [CrossRef]
- Kretowicz, M.; Johnson, R.J.; Ishimoto, T.; Nakagawa, T.; Manitius, J. The Impact of Fructose on Renal Function and Blood Pressure. Int. J. Nephrol. 2011, 2011, 315879. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, T.; Tuttle, K.R.; Short, R.A.; Johnson, R.J. Hypothesis: Fructose-Induced Hyperuricemia as a Causal Mechanism for the Epidemic of the Metabolic Syndrome. Nat. Clin. Pract. Nephrol. 2005, 1, 80–86. [Google Scholar] [CrossRef]
- Pokrywczynska, M.; Flisinski, M.; Jundzill, A.; Krzyzanowska, S.; Brymora, A.; Deptula, A.; Bodnar, M.; Kloskowski, T.; Stefanska, A.; Marszalek, A.; et al. Impact of Fructose Diet and Renal Failure on the Function of Pancreatic Islets. Pancreas 2014, 43, 801–808. [Google Scholar] [CrossRef]
- Johnson, R.J.; Perez-Pozo, S.E.; Sautin, Y.Y.; Manitius, J.; Sanchez-Lozada, L.G.; Feig, D.I.; Shafiu, M.; Segal, M.; Glassock, R.J.; Shimada, M.; et al. Hypothesis: Could Excessive Fructose Intake and Uric Acid Cause Type 2 Diabetes? Endocr. Rev. 2009, 30, 96–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, R.J.; Segal, M.S.; Sautin, Y.; Nakagawa, T.; Feig, D.I.; Kang, D.-H.; Gersch, M.S.; Benner, S.; Sánchez-Lozada, L.G. Potential Role of Sugar (Fructose) in the Epidemic of Hypertension, Obesity and the Metabolic Syndrome, Diabetes, Kidney Disease, and Cardiovascular Disease. Am. J. Clin. Nutr. 2007, 86, 899–906. [Google Scholar] [CrossRef]
- Sánchez-Lozada, L.G.; Tapia, E.; Jiménez, A.; Bautista, P.; Cristóbal, M.; Nepomuceno, T.; Soto, V.; Avila-Casado, C.; Nakagawa, T.; Johnson, R.J.; et al. Fructose-Induced Metabolic Syndrome Is Associated with Glomerular Hypertension and Renal Microvascular Damage in Rats. Am. J. Physiol. Renal Physiol. 2007, 292, F423–F429. [Google Scholar] [CrossRef] [Green Version]
- Scales, C.D.; Smith, A.C.; Hanley, J.M.; Saigal, C.S. Urologic Diseases in America Project Prevalence of Kidney Stones in the United States. Eur. Urol. 2012, 62, 160–165. [Google Scholar] [CrossRef] [Green Version]
- Sas, D.J. An Update on the Changing Epidemiology and Metabolic Risk Factors in Pediatric Kidney Stone Disease. Clin. J. Am. Soc. Nephrol. CJASN 2011, 6, 2062–2068. [Google Scholar] [CrossRef] [Green Version]
- Taylor, E.N.; Curhan, G.C. Fructose Consumption and the Risk of Kidney Stones. Kidney Int. 2008, 73, 207–212. [Google Scholar] [CrossRef] [Green Version]
- Koh, E.T.; Reiser, S.; Fields, M. Dietary Fructose as Compared to Glucose and Starch Increases the Calcium Content of Kidney of Magnesium-Deficient Rats. J. Nutr. 1989, 119, 1173–1178. [Google Scholar] [CrossRef]
- Ng, H.-Y.; Lee, Y.-T.; Kuo, W.-H.; Huang, P.-C.; Lee, W.-C.; Lee, C.-T. Alterations of Renal Epithelial Glucose and Uric Acid Transporters in Fructose Induced Metabolic Syndrome. Kidney Blood Press. Res. 2018, 43, 1822–1831. [Google Scholar] [CrossRef]
- Nguyen, N.U.; Dumoulin, G.; Henriet, M.T.; Regnard, J. Increase in Urinary Calcium and Oxalate after Fructose Infusion. Horm. Metab. Res. Horm. Stoffwechselforsch. Horm. Metab. 1995, 27, 155–158. [Google Scholar] [CrossRef]
- Abate, N.; Chandalia, M.; Cabo-Chan, A.V.; Moe, O.W.; Sakhaee, K. The Metabolic Syndrome and Uric Acid Nephrolithiasis: Novel Features of Renal Manifestation of Insulin Resistance. Kidney Int. 2004, 65, 386–392. [Google Scholar] [CrossRef] [Green Version]
- Asselman, M.; Verkoelen, C.F. Fructose Intake as a Risk Factor for Kidney Stone Disease. Kidney Int. 2008, 73, 139–140. [Google Scholar] [CrossRef] [Green Version]
- Sakhaee, K.; Capolongo, G.; Maalouf, N.M.; Pasch, A.; Moe, O.W.; Poindexter, J.; Adams-Huet, B. Metabolic Syndrome and the Risk of Calcium Stones. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc.—Eur. Ren. Assoc. 2012, 27, 3201–3209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siener, R. Nutrition and Kidney Stone Disease. Nutrients 2021, 13, 1917. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.R.; Glenton, P.A. Calcium Oxalate Crystal Deposition in Kidneys of Hypercalciuric Mice with Disrupted Type IIa Sodium-Phosphate Cotransporter. Am. J. Physiol. Renal Physiol. 2008, 294, F1109–F1115. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.R. Nephrocalcinosis in Animal Models with and without Stones. Urol. Res. 2010, 38, 429–438. [Google Scholar] [CrossRef] [Green Version]
- Oron-Herman, M.; Rosenthal, T.; Sela, B.-A. Hyperhomocysteinemia as a Component of Syndrome X. Metabolism 2003, 52, 1491–1495. [Google Scholar] [CrossRef]
- Hwang, S.-Y.; Woo, C.W.H.; Au-Yeung, K.K.W.; Siow, Y.L.; Zhu, T.Y.; Karmin, O. Homocysteine Stimulates Monocyte Chemoattractant Protein-1 Expression in the Kidney via Nuclear Factor-KappaB Activation. Am. J. Physiol. Renal Physiol. 2008, 294, F236–F244. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, P.; Gersch, M.S.; Mu, W.; Scherer, P.M.; Kim, K.M.; Gesualdo, L.; Henderson, G.N.; Johnson, R.J.; Sautin, Y.Y. Ketohexokinase-Dependent Metabolism of Fructose Induces Proinflammatory Mediators in Proximal Tubular Cells. J. Am. Soc. Nephrol. JASN 2009, 20, 545–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kizhner, T.; Werman, M.J. Long-Term Fructose Intake: Biochemical Consequences and Altered Renal Histology in the Male Rat. Metabolism 2002, 51, 1538–1547. [Google Scholar] [CrossRef]
- Gersch, M.S.; Mu, W.; Cirillo, P.; Reungjui, S.; Zhang, L.; Roncal, C.; Sautin, Y.Y.; Johnson, R.J.; Nakagawa, T. Fructose, but Not Dextrose, Accelerates the Progression of Chronic Kidney Disease. Am. J. Physiol. Renal Physiol. 2007, 293, F1256–F1261. [Google Scholar] [CrossRef] [Green Version]
- Nakayama, T.; Kosugi, T.; Gersch, M.; Connor, T.; Sanchez-Lozada, L.G.; Lanaspa, M.A.; Roncal, C.; Perez-Pozo, S.E.; Johnson, R.J.; Nakagawa, T. Dietary Fructose Causes Tubulointerstitial Injury in the Normal Rat Kidney. Am. J. Physiol. Renal Physiol. 2010, 298, F712–F720. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, T.; Hu, H.; Zharikov, S.; Tuttle, K.R.; Short, R.A.; Glushakova, O.; Ouyang, X.; Feig, D.I.; Block, E.R.; Herrera-Acosta, J.; et al. A Causal Role for Uric Acid in Fructose-Induced Metabolic Syndrome. Am. J. Physiol. Renal Physiol. 2006, 290, F625–F631. [Google Scholar] [CrossRef] [Green Version]
- Spatola, L.; Ferraro, P.M.; Gambaro, G.; Badalamenti, S.; Dauriz, M. Metabolic Syndrome and Uric Acid Nephrolithiasis: Insulin Resistance in Focus. Metabolism 2018, 83, 225–233. [Google Scholar] [CrossRef]
- Hu, Q.-H.; Wang, C.; Li, J.-M.; Zhang, D.-M.; Kong, L.-D. Allopurinol, Rutin, and Quercetin Attenuate Hyperuricemia and Renal Dysfunction in Rats Induced by Fructose Intake: Renal Organic Ion Transporter Involvement. Am. J. Physiol. Renal Physiol. 2009, 297, F1080–F1091. [Google Scholar] [CrossRef] [Green Version]
- Milne, D.B.; Nielsen, F.H. The Interaction between Dietary Fructose and Magnesium Adversely Affects Macromineral Homeostasis in Men. J. Am. Coll. Nutr. 2000, 19, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Ng, R.C.; Rouse, D.; Suki, W.N. Calcium Transport in the Rabbit Superficial Proximal Convoluted Tubule. J. Clin. Investig. 1984, 74, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Girardi, A.C.; Titan, S.M.; Malnic, G.; Rebouças, N.A. Chronic Effect of Parathyroid Hormone on NHE3 Expression in Rat Renal Proximal Tubules. Kidney Int. 2000, 58, 1623–1631. [Google Scholar] [CrossRef] [Green Version]
- Binswanger, U.; Helmle-Kolb, C.; Forgo, J.; Mrkic, B.; Murer, H. Rapid Stimulation of Na+/H+ Exchange by 1,25-Dihydroxyvitamin D3; Interaction with Parathyroid-Hormone-Dependent Inhibition. Pflugers Arch. 1993, 424, 391–397. [Google Scholar] [CrossRef]
- Lemann, J.; Piering, W.F.; Lennon, E.J. Possible Role of Carbohydrate-Induced Calciuria in Calcium Oxalate Kidney-Stone Formation. N. Engl. J. Med. 1969, 280, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Kohri, K.; Garside, J.; Blacklock, N.J. The Role of Magnesium in Calcium Oxalate Urolithiasis. Br. J. Urol. 1988, 61, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Li, M.K.; Blacklock, N.J.; Garside, J. Effects of Magnesium on Calcium Oxalate Crystallization. J. Urol. 1985, 133, 123–125. [Google Scholar] [CrossRef]
- Fetner, C.D.; Barilla, D.E.; Townsend, J.; Pak, C.Y. Effects of Magnesium Oxide on the Crystallization of Calcium Salts in Urine in Patients with Recurrent Nephrolithiasis. J. Urol. 1978, 120, 399–401. [Google Scholar] [CrossRef]
- Bergstra, A.E.; Lemmens, A.G.; Beynen, A.C. Dietary Fructose vs. Glucose Stimulates Nephrocalcinogenesis in Female Rats. J. Nutr. 1993, 123, 1320–1327. [Google Scholar] [CrossRef] [PubMed]
- Manterys, A.; Filipiak-Florkiewicz, A.; Florkiewicz, A.; Franczyk-Zarow, M.; Kus, E.; Sady, M.; Kostogrys, R. Short-Term Feeding with High Fructose Diet Impairs Bone Mineralization in Growing Rats. Prog. Nutr. 2018, 20, 629–634. [Google Scholar] [CrossRef]
- Knox, F.G.; Osswald, H.; Marchand, G.R.; Spielman, W.S.; Haas, J.A.; Berndt, T.; Youngberg, S.P. Phosphate Transport along the Nephron. Am. J. Physiol.-Ren. Physiol. 1977, 233, F261–F268. [Google Scholar] [CrossRef]
- Douard, V.; Sabbagh, Y.; Lee, J.; Patel, C.; Kemp, F.W.; Bogden, J.D.; Lin, S.; Ferraris, R.P. Excessive Fructose Intake Causes 1,25-(OH)(2)D(3)-Dependent Inhibition of Intestinal and Renal Calcium Transport in Growing Rats. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E1303–E1313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, R.J.; Perez-Pozo, S.E.; Lillo, J.L.; Grases, F.; Schold, J.D.; Kuwabara, M.; Sato, Y.; Hernando, A.A.; Garcia, G.; Jensen, T.; et al. Fructose Increases Risk for Kidney Stones: Potential Role in Metabolic Syndrome and Heat Stress. BMC Nephrol. 2018, 19, 315. [Google Scholar] [CrossRef]
- Herlitz, L.C.; D’Agati, V.D.; Markowitz, G.S. Crystalline Nephropathies. Arch. Pathol. Lab. Med. 2012, 136, 713–720. [Google Scholar] [CrossRef] [Green Version]





| Determined Parameters | RD (I) | F10 (II) | F60 (III) | ANOVA | P |
|---|---|---|---|---|---|
| Food consumption [g/day] | 37.9 ± 2.5 | 27.2 ± 4.5 | 28.6 ± 2.4 | <0.001 | I vs. II I vs. III |
| Water consumption [mL/day] | 56 ± 9 | 90 ± 24.5 | 50 ± 14 | <0.01 | I vs. II II vs. III |
| Weight gain [gram] | 275 ± 23 | 275 ± 30 | 219 ± 37 | <0.01 | I vs. III II vs. III |
| Total energy value [kcal per day] | 99.6 ± 6.6 | 96.6 ± 10.3 | 102.8 ± 8.9 | NS | |
| Energy from fructose [kcal/day] | 3 ± 0.2 | 27.2 ± 5.5 | 61.7 ± 5.3 | <0.001 | I vs. II I vs. III II vs. III |
| Albumin [g/dL] | 3.3 ± 0.1 | 3.2 ± 0.2 | 3.1 ± 0.2 | NS | - |
| Fructose [mg/dL] | 0.59 ± 0.05 | 1.26 ± 0.7 | 1.04 ± 0.5 | NS | - |
| BUN [mg/dL] | 20.8 ± 1.4 | 18.1 ± 5.2 | 18.7 ± 2.6 | NS | - |
| HCS [µmol/L] | 4.5 ± 0.2 | 5.8 ± 1.4 | 6.4 ± 1.5 | < 0.05 | I vs. III |
| Creatinine [mg/dL] | 0.54 ± 0.04 | 0.59 ± 0.09 | 0.47 ± 0.04 | < 0.05 | II vs. III |
| Creatinine clearance [mL/min/100 g] | 0.47 ± 0.08 | 0.40 ± 0.08 | 0.44 ± 0.09 | NS | - |
| Erythropoietin [mIU/mL] | 0.65 ± 0.56 | 1.72 ± 2.01 | 1.33 ± 0.84 | NS | - |
| Uric Acid [mg/dL] | 1.76 ± 0.4 | 1.45 ± 0.3 | 1.56 ± 0.1 | NS | - |
| Insulin [ng/mL] | 3.87 ± 2.0 | 5.54 ± 2.4 | 5.31 ± 1.2 | NS | - |
| HOMA [AU] | 1.94 ± 0.95 | 3.13 ± 1.036 | 2.36 ± 0.81 | NS | - |
| Triglicerides [mg/dL] | 158 ± 34 | 180 ± 27 | 221 ± 83 | NS | - |
| Cholesterol [mg/dL] | 74 ± 11 | 60 ± 9 | 66 ± 9 | NS | - |
| PTH [pg/mL] | 225 ± 112 | 283 ± 136 | 492 ± 340 | NS | - |
| Vitamin 25(OH)D3 [ng/mL] | 52 ± 11 | 19 ± 17 | 31 ±44 | NS | - |
| FGF-23 [pg/mL] | 212 ± 66 | 379 ±324 | 216 ± 51 | NS | - |
| Calcium [mmol/L] | 2.52 ± 0.05 | 2.53 ± 0.11 | 2.44 ± 0.09 | NS | - |
| Phosphate [mmol/L] | 2.32 ± 0.1 | 2.39 ± 0.2 | 2.60 ± 0.2 | NS | - |
| CaxPi [mmol2/L2] | 5.85 ± 0.36 | 6.04 ± 0.49 | 6.34 ± 0.59 | NS | - |
| Magnesium [mmol/L] | 2.26 ± 0.1 | 2.54 ± 0.4 | 2.23 ± 0.3 | NS | - |
| Sodium [mmol/L] | 140.02 ± 1.68 | 139.34 ± 3.50 | 140.03 ± 2.54 | NS | - |
| Potassium [mmol/L] | 5.14 ± 0.95 | 5.42 ± 0.72 | 5.07 ± 0.48 | NS | - |
| Determined Parameters | RD (I) | F10 (II) | F60 (III) | ANOVA | P |
|---|---|---|---|---|---|
| Urine output [mL/day] | 26 ± 9.6 | 56 ± 27 | 25 ± 13 | < 0.001 | I vs. II II vs. III |
| Urine pH | 8.5 ± 0.57 | 8.2 ± 0.83 | 5.6 ± 0.51 | < 0.001 | I vs. III II vs. III |
| Urine specific gravity [g/L] | 1.0125 ± 0.003 | 1.014 ± 0.004 | 1.026 ± 0.004 | < 0.001 | I vs. III II vs. III |
| PCR [mg/mg Cr] | 1.0 ± 0.4 | 1.1 ± 0.5 | 0.8 ± 0.5 | NS | - |
| MCP-1/Cr [ng/mg Cr] | 3.5 ± 0.6 | 4.4 ± 4.0 | 11.2 ± 2.5 | < 0.01 | I vs. III II vs. III |
| NAG/Cr [U/g Cr] | 8.6 ± 5 | 15.1 ± 7.6 | 20.8 ± 5 | < 0.05 | I vs. III |
| uUAEx [mg/day] | 3.28 ± 0.51 | 2.58 ± 0.57 | 2.18 ± 0.66 | < 0.05 | I vs. III |
| UACl (mL/min) | 0.10 ± 0.04 | 0.13 ± 0.04 | 0.10 ± 0.03 | NS | - |
| UAFEx [%] | 4.4 ± 1.2 | 5.2 ± 1.6 | 4.1 ± 1.6 | NS | - |
| uNaEx [mg/day] | 33.3 ± 8.5 | 40.9 ± 10.8 | 166.8 ± 27.2 | < 0.001 | I vs. III II vs. III |
| NaCl [mL/min] | 0.007 ± 0.002 | 0.008 ± 0.002 | 0.031 ± 0.013 | < 0.001 | I vs. III II vs. III |
| NaFEx [%] | 0.24 ± 0.08 | 0.34 ± 0.06 | 1.16 ± 0.45 | < 0.001 | I vs. III II vs. III |
| uPiEx [mg/day] | 1.85 ± 1.61 | 4.42 ± 5.14 | 75.30 ± 21.95 | < 0.001 | I vs. III II vs. III |
| PiCl [mL/min] | 0.017 ± 0.015 | 0.044 ± 0.056 | 0.567 ± 0.284 | < 0.001 | I vs. III II vs. III |
| PiFEx [%] | 0.6 ± 0.6 | 1.7 ± 0.2 | 21 ± 5 | < 0.001 | I vs. III II vs. III |
| uCaEx [mg/day] | 2.02 ± 0.89 | 5.71 ± 3.05 | 3.08 ± 1.92 | < 0.05 | I vs. II |
| CaCl [mL/min] | 0.014 ± 0.006 | 0.038 ± 0.020 | 0.019 ± 0.014 | < 0.05 | I vs. II |
| CaFEx [%] | 0.45 ± 0.2 | 1.46 ± 0.7 | 0.75 ± 0.5 | < 0.05 | I vs. II |
| uMgEx [mg/day] | 5.2 ± 1.9 | 5.9 ± 2.1 | 3.9 ± 1.4 | NS | - |
| MgCl [mL/min] | 0.19 ± 0 | 0.17 ± 0.07 | 0.11 ± 0.3 | NS | - |
| MgFEx [%] | 5.4 ± 0 | 6.7 ± 2.5 | 4.4 ± 0.7 | NS | - |
| uKEx [mg/day] | 174.3 ± 19.9 | 152.1 ± 26.1 | 124.9 ± 45.3 | NS | - |
| KCl [mL/min] | 0.61 ± 0.1 | 0.51 ± 0.1 | 0.45 ± 0.17 | NS | - |
| KFEx [%] | 19.74 ± 2.15 | 19.92 ± 4.73 | 16.85 ± 5.46 | NS | - |
| Determined Parameters | RD (I) | F10 (II) | F60 (III) | ANOVA | P |
|---|---|---|---|---|---|
| Sodium intake from diet [mg/day] | 113.7 ± 7.6 | 81.6 ± 13.6 | 142.0 ± 12.4 | <0.001 | I vs. II I vs. III II vs. III |
| uNaEx/Na consumed in diet [%] | 29.2 ± 6.8 | 50.7 ± 12.7 | 117.0 ± 10.4 | <0.001 | I vs. II I vs. III II vs. III |
| Calcium intake from diet [mg/day] | 417 ± 28 | 299 ± 50 | 174 ± 15 | <0.001 | I vs. II I vs. III II vs. III |
| uCaEx/Ca consumed in diet [%] | 0.5 ± 0.2 | 1.9 ± 0.9 | 1.8 ± 1.1 | <0.05 | I vs. II I vs. III |
| Phosphate intake from diet [mg/day] | 227 ± 15 | 163 ± 27 | 156 ± 14 | <0.001 | I vs. II I vs. III |
| uPiEx/Pi consumed in diet [%] | 0.8 ± 0.7 | 3.0 ± 3.6 | 47.7 ± 11.1 | <0.001 | I vs. III II vs. III |
| Metabolic Characteristics | Rats without Kidney Deposits (N = 7) | Rats with Kidney Deposits (N = 4) | ANOVA |
|---|---|---|---|
| Insulin [ng/mL] | 4.60 ± 1.60 | 6.86 ± 0.83 | <0.05 |
| uCaEx [mg/day] | 2.02 ± 0.89 | 5.71 ± 3.05 | <0.01 |
| CaCl [mL/min] | 0.018 ± 0.011 | 0.047 ± 0.015 | <0.01 |
| CaFEx [%] | 0.73 ± 0.42 | 1.67 ± 0.58 | <0.05 |
| uMgEx [mg/day] | 3.93 ± 1.22 | 6.39 ± 2.16 | <0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flisiński, M.; Brymora, A.; Skoczylas-Makowska, N.; Stefańska, A.; Manitius, J. Fructose-Rich Diet Is a Risk Factor for Metabolic Syndrome, Proximal Tubule Injury and Urolithiasis in Rats. Int. J. Mol. Sci. 2022, 23, 203. https://doi.org/10.3390/ijms23010203
Flisiński M, Brymora A, Skoczylas-Makowska N, Stefańska A, Manitius J. Fructose-Rich Diet Is a Risk Factor for Metabolic Syndrome, Proximal Tubule Injury and Urolithiasis in Rats. International Journal of Molecular Sciences. 2022; 23(1):203. https://doi.org/10.3390/ijms23010203
Chicago/Turabian StyleFlisiński, Mariusz, Andrzej Brymora, Natalia Skoczylas-Makowska, Anna Stefańska, and Jacek Manitius. 2022. "Fructose-Rich Diet Is a Risk Factor for Metabolic Syndrome, Proximal Tubule Injury and Urolithiasis in Rats" International Journal of Molecular Sciences 23, no. 1: 203. https://doi.org/10.3390/ijms23010203
APA StyleFlisiński, M., Brymora, A., Skoczylas-Makowska, N., Stefańska, A., & Manitius, J. (2022). Fructose-Rich Diet Is a Risk Factor for Metabolic Syndrome, Proximal Tubule Injury and Urolithiasis in Rats. International Journal of Molecular Sciences, 23(1), 203. https://doi.org/10.3390/ijms23010203






