First Report of Crown Gall of Kiwifruit (Actinidia deliciosa) Caused by Agrobacterium fabacearum in China and the Establishment of Loop-Mediated Isothermal Amplification Technique
Abstract
:1. Introduction
2. Results
2.1. Disease Occurrence
2.2. Pathogenicity Verification
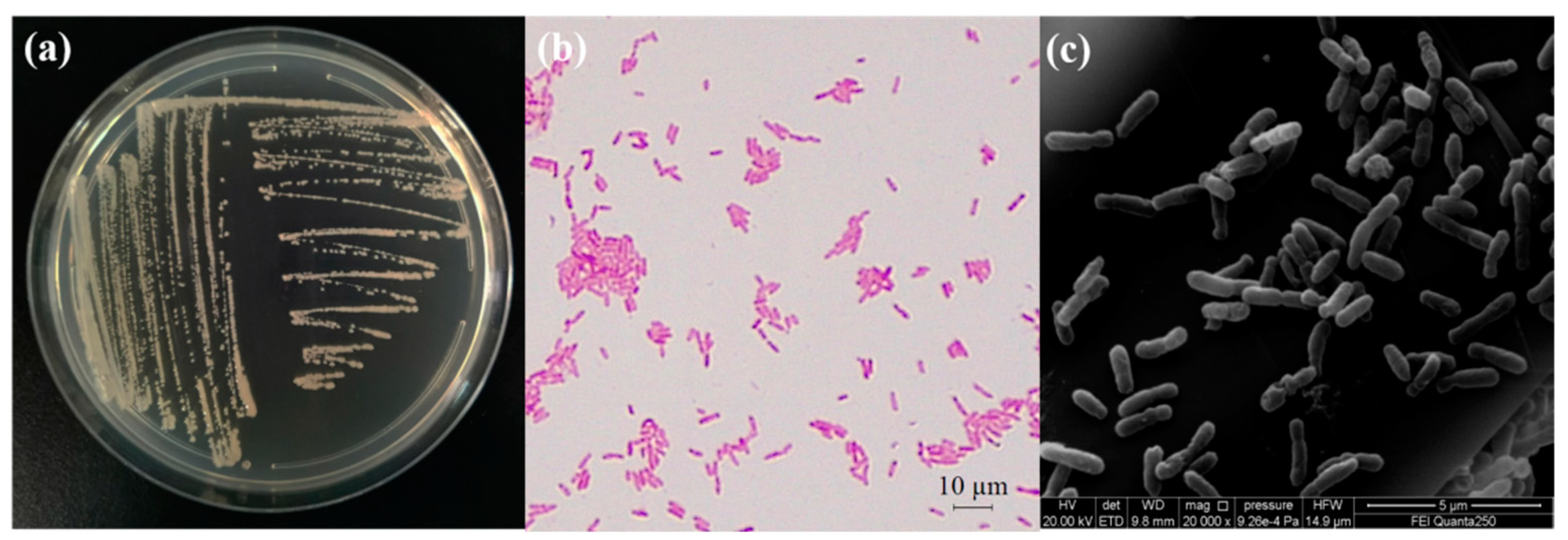
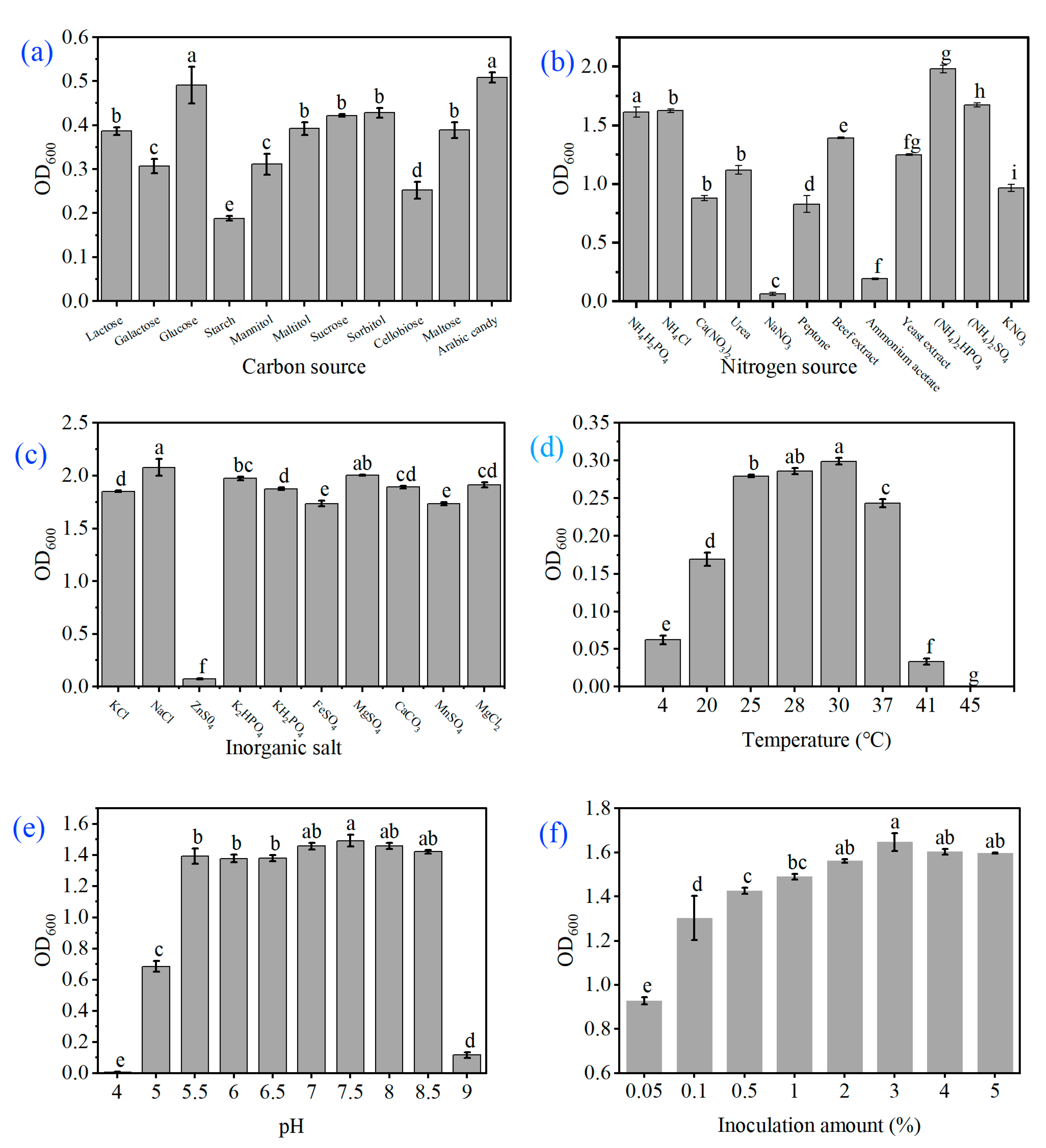
2.3. Study on Morphological and Biological Characteristics
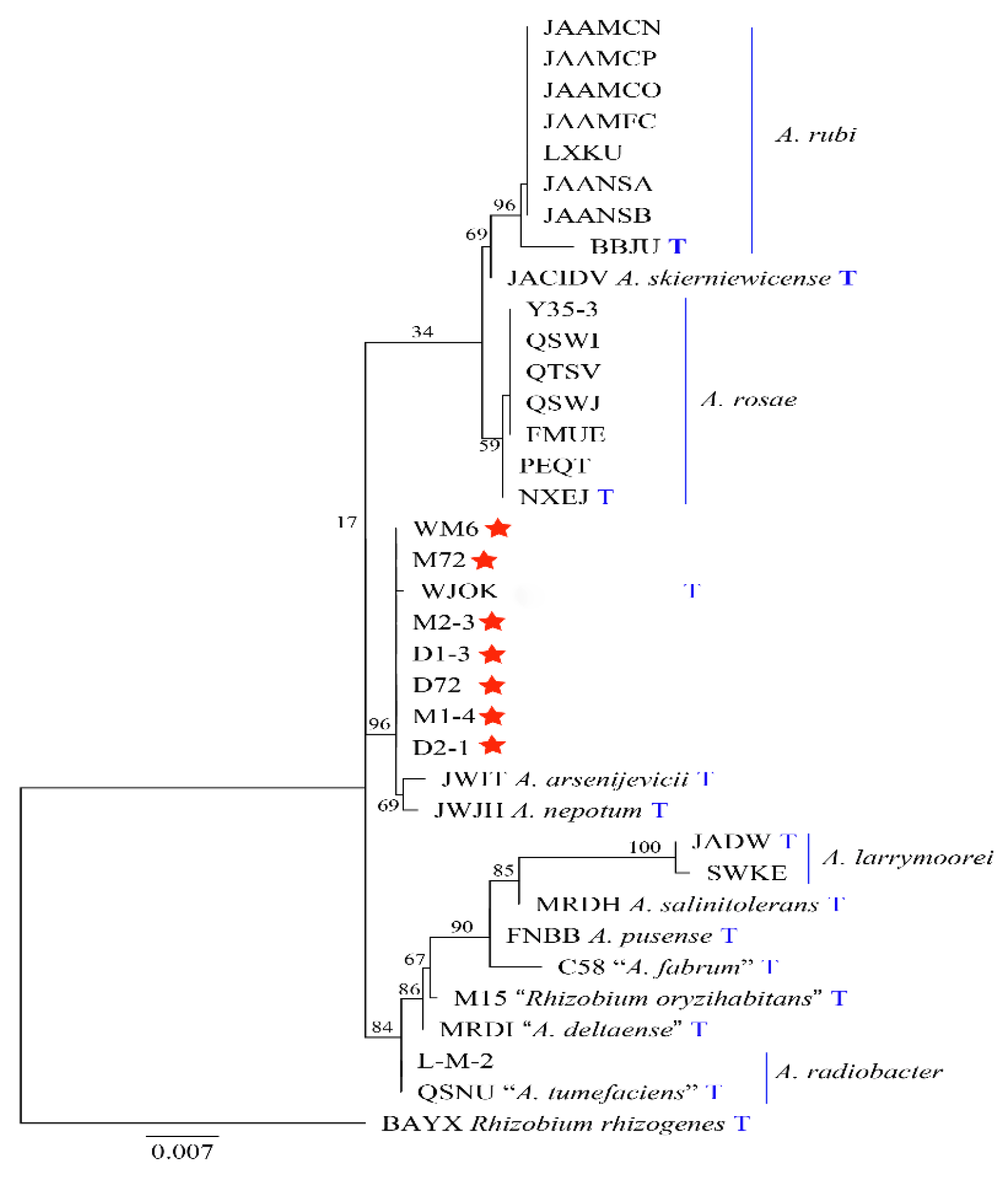
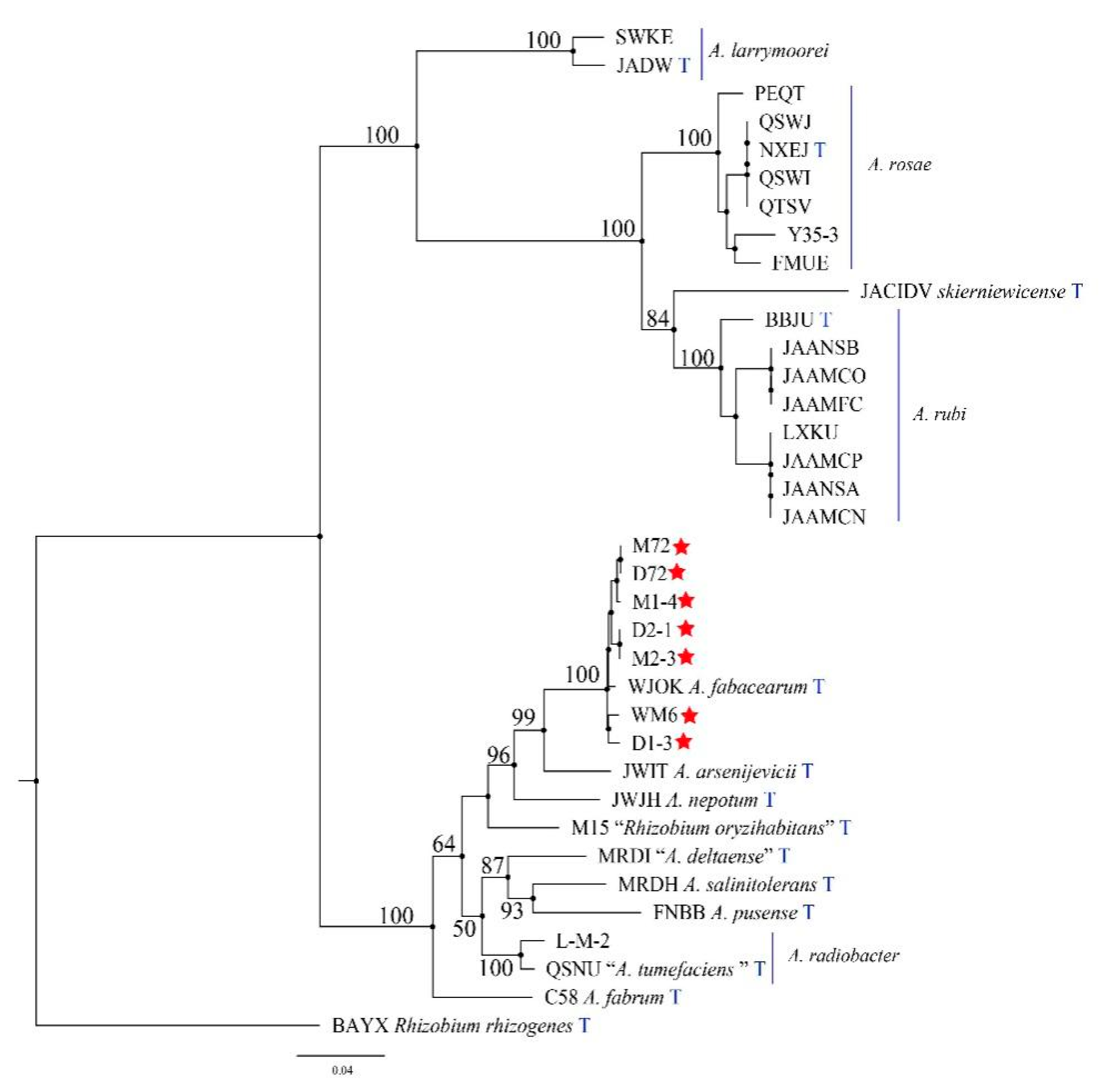
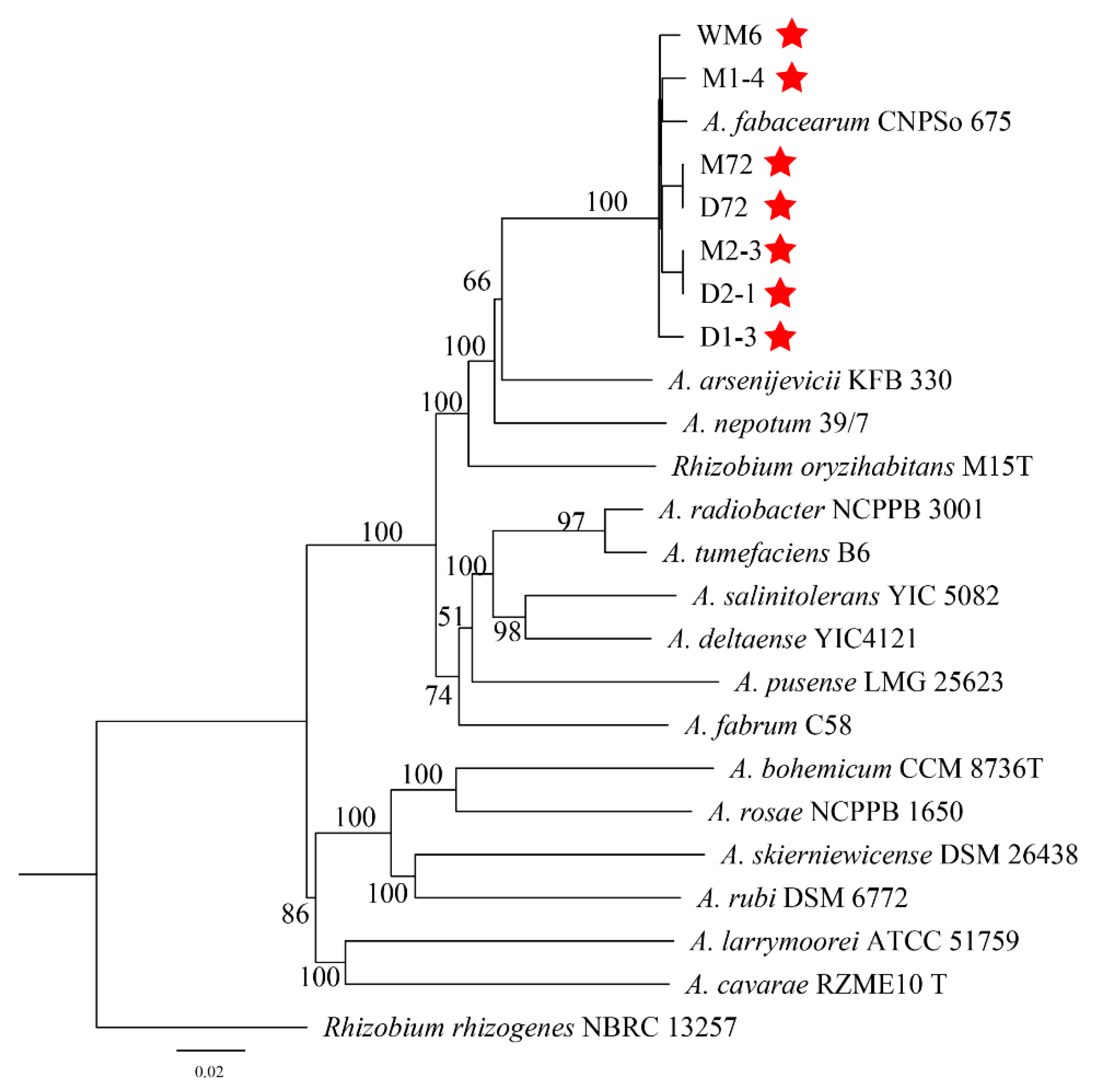
2.4. Molecular Biology Identification and Phylogeny
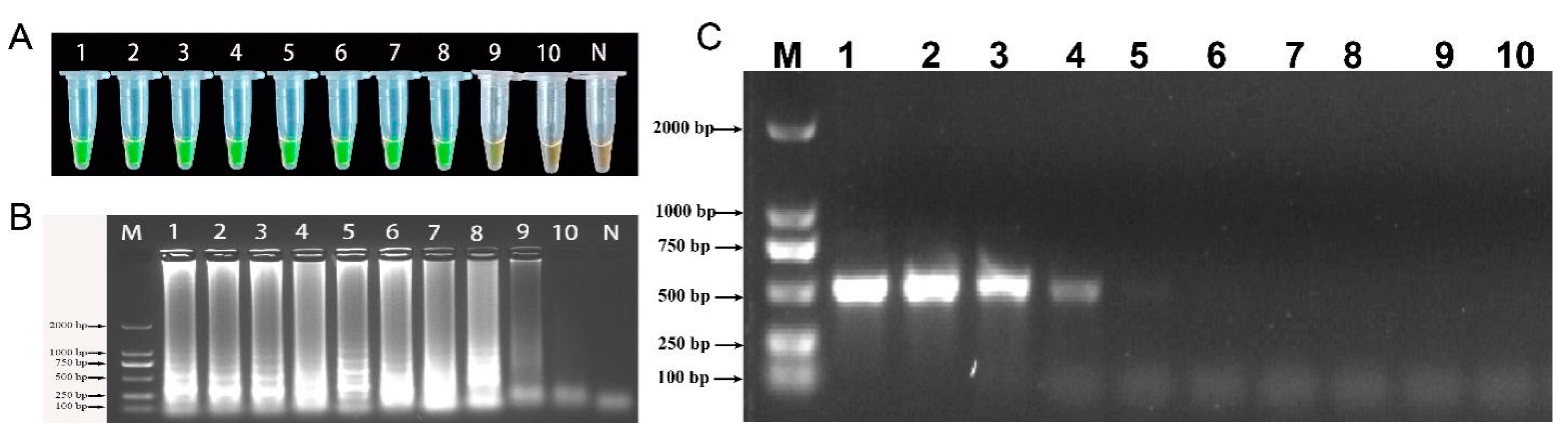
2.5. LAMP-Specific Detection
2.6. Sensitivity of LAMP and Conventional PCR Detection
2.7. Field Sample Testing
3. Discussion
4. Materials and Methods
4.1. Pathogenic Strain Isolated and Preservation
4.2. Pathogenicity Verification
4.3. The Study of Morphological and Biological Characteristics
4.4. Molecular Biology Identification and Phylogeny
4.5. Strain Source and Genomic DNA Extraction
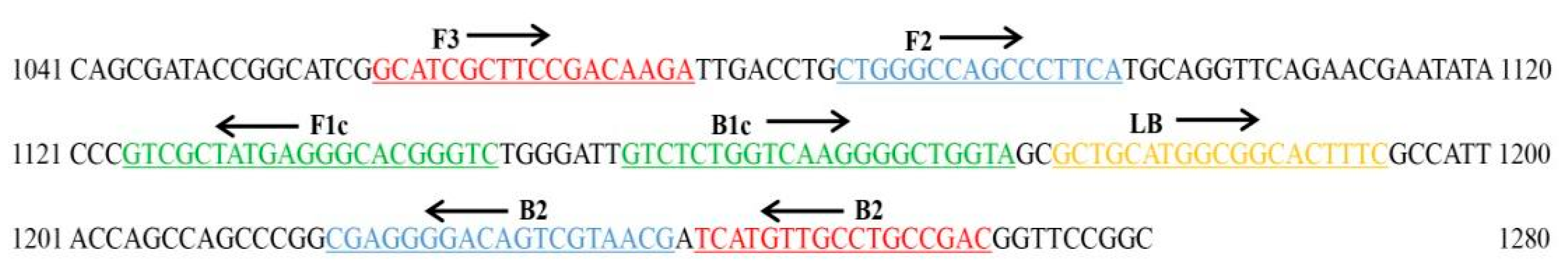
4.6. LAMP Primer Design
4.7. Reaction System Establishment
4.8. Specificity of the LAMP
4.9. Detection of A. fabacearum by LAMP and Conventional PCR
4.10. Test Field Samples
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sivakumaran, S.; Huffman, L.; Sivakumaran, S.; Drummond, L. The nutritional composition of Zespri® SunGold Kiwifruit and Zespri® Sweet Green Kiwifruit. Food Chem. 2018, 238, 195–202. [Google Scholar] [CrossRef]
- Ma, T.; Sun, X.; Zhao, J.; You, Y.; Lei, Y.; Gao, G.; Zhan, J. Nutrient compositions and antioxidant capacity of kiwifruit (Actinidia) and their relationship with flesh color and commercial value. Food Chem. 2017, 218, 294–304. [Google Scholar] [CrossRef]
- Pan, L.; Zhao, X.; Chen, M.; Fu, Y.; Xiang, M.; Chen, J. Effect of exogenous methyl jasmonate treatment on disease resistance of postharvest kiwifruit. Food Chem. 2020, 305, 125483. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.-R.; Vu, N.; Park, J.; Hwang, I.; Jeong, H.-J.; Cho, Y.-S.; Oh, C.-S. Phage PPPL-1, A New Biological Agent to Control Bacterial Canker Caused by Pseudomonas syringae pv. actinidiae in Kiwifruit. Antibiotics 2021, 10, 554. [Google Scholar] [CrossRef]
- Peşman, E.; Bak, F.E.; Konanç, M.U. The chemical characteristics and anatomy of Caucasian fir (Abies nordmanniana subsp. nordmanniana) crown gall tumors infected by Agrobacterium tumefaciens. Wood Sci. Technol. 2021, 55, 1025–1039. [Google Scholar] [CrossRef]
- Liu, T.; Ren, X.; Cao, G.; Zhou, X.; Jin, L. Transcriptome Analysis on the Mechanism of Ethylicin Inhibiting Pseudomonas syringae pv. actinidiae on Kiwifruit. Microorganisms 2021, 9, 724. [Google Scholar] [CrossRef]
- Moriya, S.; Iwanami, H.; Takahashi, S.; Kotoda, N.; Suzaki, K.; Yamamoto, T.; Abe, K. Genetic mapping of the crown gall resistance gene of the wild apple Malus sieboldii. Tree Genet. Genomes 2009, 6, 195–203. [Google Scholar] [CrossRef]
- Utkhede, R.S.; Smith, E.M. Evaluation of Biological and Chemical Treatments for Control of Crown Gall on Young Apple Trees in the Kootenay Valley of British Columbia. J. Phytopathol. 1993, 137, 265–271. [Google Scholar] [CrossRef]
- Opisa, O.M.; Achwanya, O.S.; Otaye, D.O.; Muthamia, J.M. The efficacy of sterilizing agents, copper oxychloride, vegetable oil and agrowipe (botanic neem extract) against crown gall disease of roses in Kericho, Kenya. Afr. J. Biol. Sci. 2020, 2, 115. [Google Scholar] [CrossRef]
- Kerr, A. Biological control of Crown Gall. Australas. Plant Pathol. 2016, 45, 15–18. [Google Scholar] [CrossRef]
- Tolba, I.; Soliman, M. Efficacy of native antagonistic bacterial isolates in biological control of crown gall disease in Egypt. Ann. Agric. Sci. 2013, 58, 43–49. [Google Scholar] [CrossRef] [Green Version]
- Kawaguchi, A.; Inoue, K.; Tanina, K.; Nita, M. Biological control for grapevine crown gall using nonpathogenic Rhizobium vitis strain ARK-1. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2017, 93, 547–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben Gharsa, H.; Bouri, M.; Hamdane, A.M.; Schuster, C.; Leclerque, A.; Rhouma, A. Bacillus velezensis strain MBY2, a potential agent for the management of crown gall disease. PLoS ONE 2021, 16, e0252823. [Google Scholar] [CrossRef] [PubMed]
- Asghari, S.; Harighi, B.; Ashengroph, M.; Clement, C.; Aziz, A.; Esmaeel, Q.; Barka, E.A. Induction of systemic resistance to Agrobacterium tumefaciens by endophytic bacteria in grapevine. Plant Pathol. 2020, 69, 827–837. [Google Scholar] [CrossRef]
- Ben Hsouna, A.; Touj, N.; Hammami, I.; Dridi, K.; Al-Ayed, A.S.; Hamdi, N. Chemical Composition and in vivo Efficacy of the Essential Oil of Mentha piperita L. in the Suppression of Crown Gall Disease on Tomato Plants. J. Oleo Sci. 2019, 68, 419–426. [Google Scholar] [CrossRef]
- Asghari, S.; Harighi, B.; Mozafari, A.A.; Esmaeel, Q.; Barka, E.A. Screening of endophytic bacteria isolated from domesticated and wild growing grapevines as potential biological control agents against crown gall disease. BioControl 2019, 64, 723–735. [Google Scholar] [CrossRef]
- Escobar, M.A.; Civerolo, E.L.; Summerfelt, K.R.; Dandekar, A.M. RNAi-mediated oncogene silencing confers resistance to crown gall tumorigenesis. Proc. Natl. Acad. Sci. USA 2001, 98, 13437–13442. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Humann, J.L.; Pitrak, J.S.; Cuperus, J.; Parks, T.D.; Whistler, C.A.; Mok, M.C.; Ream, L.W. Translation Start Sequences Affect the Efficiency of Silencing of Agrobacterium tumefaciens T-DNA Oncogenes. Plant Physiol. 2003, 133, 966–977. [Google Scholar] [CrossRef] [Green Version]
- Krastanova, S.V.; Balaji, V.; Holden, M.R.; Sekiya, M.; Xue, B.; Momol, E.A.; Burr, T.J. Resistance to crown gall disease in transgenic grapevine rootstocks containing truncated virE2 of Agrobacterium. Transgenic Res. 2010, 19, 949–958. [Google Scholar] [CrossRef]
- Moriya, S.; Iwanami, H.; Haji, T.; Okada, K.; Shimizu, T.; Suzaki, K.; Kitamoto, N.; Katayose, Y.; Wu, J.; Yamamoto, T.; et al. QTL analysis of crown gall disease resistance in apple: First plant R gene candidates effective against Rhizobium rhizogenes (Ti). Tree Genet. Genomes 2021, 17, 1–15. [Google Scholar] [CrossRef]
- Kahla, Y.; Zouari-Bouassida, K.; Rezgui, F.; Trigui, M.; Tounsi, S. Efficacy of Eucalyptus cinereaas a Source of Bioactive Compounds for Curative Biocontrol of Crown Gall Caused by Agrobacterium tumefaciens Strain B6. BioMed Res. Int. 2017, 2017, 9308063. [Google Scholar] [CrossRef] [PubMed]
- Delamuta, J.R.M.; Scherer, A.J.; Ribeiro, R.A.; Hungria, M. Genetic diversity of Agrobacterium species isolated from nodules of common bean and soybean in Brazil, Mexico, Ecuador and Mozambique, and description of the new species Agrobacterium fabacearum sp. nov. Int. J. Syst. Evol. Microbiol. 2020, 70, 4233–4244. [Google Scholar] [CrossRef] [PubMed]
- Mafakheri, H.; Taghavi, S.M.; Puławska, J.; de Lajudie, P.; Lassalle, F.; Osdaghi, E. Two Novel Genomospecies in the Agrobacterium tumefaciens Species Complex Associated with Rose Crown Gall. Phytopathology 2019, 109, 1859–1868. [Google Scholar] [CrossRef] [PubMed]
- Shams, M.; Vial, L.; Chapulliot, D.; Nesme, X.; Lavire, C. Rapid and accurate species and genomic species identification and exhaustive population diversity assessment of Agrobacterium spp. using recA-based PCR. Syst. Appl. Microbiol. 2013, 36, 351–358. [Google Scholar] [CrossRef]
- Kuzmanović, N.; Pulawska, J.; Prokić, A.; Ivanović, M.; Zlatković, N.; Jones, J.B.; Obradovic, A. Agrobacterium arsenijevicii sp. nov., isolated from crown gall tumors on raspberry and cherry plum. Syst. Appl. Microbiol. 2015, 38, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Miles, T.D.; Martin, F.N.; Coffey, M.D. Development of Rapid Isothermal Amplification Assays for Detection of Phytophthora spp. in Plant Tissue. Phytopathology 2015, 105, 265–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Wang, C.; Wang, L.; Zhang, X.; Yan, J.; Wang, J.; Wang, M. Development of loop-mediated isothermal amplification (LAMP) assay for rapid detection of Fusarium proliferatum causing ear and kernel rot on maize. Crop. Prot. 2020, 132, 105142. [Google Scholar] [CrossRef]
- Chavhan, R.; Mondal, K.K.; Karuppayil, S.; Chakrabarty, P. Evolution of biotypes within race 18 population of Xanthomonas citri subsp. malvacearum and their predominance in Indian cotton belts. Physiol. Mol. Plant Pathol. 2021, 116, 101721. [Google Scholar] [CrossRef]
- Kong, L.; Wang, H.-B.; Wang, S.-S.; Xu, P.-P.; Zhang, R.-F.; Dong, S.; Zheng, X.-B. Rapid detection of potato late blight using a loop-mediated isothermal amplification assay. J. Integr. Agric. 2020, 19, 1274–1282. [Google Scholar] [CrossRef]
- Zhang, X.; Harrington, T.C.; Batzer, J.C.; Kubota, R.; Peres, N.A.; Gleason, M.L. Detection of Colletotrichum acutatum Sensu Lato on Strawberry by Loop-Mediated Isothermal Amplification. Plant Dis. 2016, 100, 1804–1812. [Google Scholar] [CrossRef] [Green Version]
- Notomi, T.; Okayama, H.; Masubuchai, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, E63. [Google Scholar] [CrossRef] [Green Version]
- Niessen, L.; Vogel, R.F. Detection of Fusarium graminearum DNA using a loop-mediated isothermal amplification (LAMP) assay. Int. J. Food Microbiol. 2010, 140, 183–191. [Google Scholar] [CrossRef]
- Yang, X.; Gu, C.-Y.; Abid, M.; Al-Attala, M.N.; Qin, G.-H.; Xu, Y.-L.; Phyo, S.S.M.; Zhang, A.-F.; Zang, H.-Y.; Chen, Y. Development of loop-mediated isothermal amplification assay for rapid diagnosis of pomegranate twig blight and crown rot disease caused by Coniella granati. Crop. Prot. 2020, 135, 105190. [Google Scholar] [CrossRef]
- Quoc, N.; Xuan, N.; Phuong, N.; Trang, H.; Chau, N.; Duong, C.; Dickinson, M. Development of loop mediated isothermal amplification assays for the detection of sugarcane white leaf disease. Physiol. Mol. Plant Pathol. 2021, 113, 101595. [Google Scholar] [CrossRef]
- Ghosh, R.; Tarafdar, A.; Sharma, M. Rapid and sensitive diagnoses of dry root rot pathogen of chickpea (Rhizoctonia bataticola (Taub.) Butler) using loop-mediated isothermal amplification assay. Sci. Rep. 2017, 7, srep42737. [Google Scholar] [CrossRef] [PubMed]
- Thiessen, L.D.; Keune, J.A.; Neill, T.M.; Turechek, W.W.; Grove, G.G.; Mahaffee, W.F. Development of a grower-conducted inoculum detection assay for management of grape powdery mildew. Plant Pathol. 2016, 65, 238–249. [Google Scholar] [CrossRef]
- Khan, M.; Wang, R.; Li, B.; Liu, P.; Weng, Q.; Chen, Q. Comparative Evaluation of the LAMP Assay and PCR-Based Assays for the Rapid Detection of Alternaria solani. Front. Microbiol. 2018, 9, 2089. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Otsubo, K.; Hieno, A.; Suga, H.; Kageyama, K. A simple loop-mediated isothermal amplification assay to detect Phytophthoracolocasiae in infected taro plants. J. Gen. Plant Pathol. 2019, 85, 337–346. [Google Scholar] [CrossRef]
- Xiao, C.; Li, R. Detection and Control of Fusarium oxysporum from Soft Rot in Dendrobium officinale by Loop-Mediated Isothermal Amplification Assays. Biology 2021, 10, 1136. [Google Scholar] [CrossRef]
- Suzaki, K.; Sawada, H.; Kisaki, G. Loop-mediated isothermal amplification of bacterial effector genes to detect Pseudomonas syringae pv. actinidiae biovars 1 and 3. J. Gen. Plant Pathol. 2021, 1–8. [Google Scholar] [CrossRef]
- Pirc, M.; Alič, Š.; Dreo, T. Rapid Loop-Mediated Isothermal Amplification for Detection of the Ralstonia solanacearum Species Complex Bacteria in Symptomatic Potato Tubers and Plants. Methods Mol. Biol. 2021, 2354, 401–413. [Google Scholar] [CrossRef]
- Nair, S.; Manimekalai, R.; Raj, P.G.; Hegde, V. Loop mediated isothermal amplification (LAMP) assay for detection of coconut root wilt disease and arecanut yellow leaf disease phytoplasma. World J. Microbiol. Biotechnol. 2016, 32, 108. [Google Scholar] [CrossRef]
- Uke, A.; Khin, S.; Kobayashi, K.; Satou, T.; Kim, O.-K.; Hoat, T.X.; Natsuaki, K.T.; Ugaki, M. Detection of Sri Lankan cassava mosaic virus by loop-mediated isothermal amplification using dried reagents. J. Virol. Methods 2022, 299, 114336. [Google Scholar] [CrossRef] [PubMed]
- Panno, S.; Matić, S.; Tiberini, A.; Caruso, A.G.; Bella, P.; Torta, L.; Stassi, R.; Davino, A.S. Loop Mediated Isothermal Amplification: Principles and Applications in Plant Virology. Plants 2020, 9, 461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, C.; Wan, T.; Wu, R.; Zhao, M.; Zhao, Y.; Cai, Y. Resistance analysis of cherry rootstock ‘CDR-1’ (Prunus mahaleb) to crown gall disease. BMC Plant Biol. 2020, 20, 516. [Google Scholar] [CrossRef] [PubMed]
- Padilla, R.; Gaillard, V.; Le, T.N.; Bellvert, F.; Chapulliot, D.; Nesme, X.; Dessaux, Y.; Vial, L.; Lavire, C.; Kerzaon, I. Development and validation of a UHPLC-ESI-QTOF mass spectrometry method to analyze opines, plant biomarkers of crown gall or hairy root diseases. J. Chromatogr. B 2021, 1162, 122458. [Google Scholar] [CrossRef]
- Kerr, A.; Bullard, G. Biocontrol of Crown Gall by Rhizobium rhizogenes: Challenges in Biopesticide Commercialisation. Agronomy 2020, 10, 1126. [Google Scholar] [CrossRef]
- Li, H.; Zhang, H.; Liu, Z.; Lin, Z.; Qiu, Y.; Tang, H.; Qiu, S. Rapid diagnosis of Ralstonia solanacearum infection sweet potato in China by loop-mediated isothermal amplification. Arch. Microbiol. 2021, 203, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Gomes, Y.; Caterino-De-Araujo, A.; Campos, K.; Gonçalves, M.G.; Leite, A.C.; Lima, M.A.; Araújo, A.; Silva, M.T.; Espíndola, O. Loop-Mediated Isothermal Amplification (LAMP) Assay for Rapid and Accurate Confirmatory Diagnosis of HTLV-1/2 Infection. Viruses 2020, 12, 981. [Google Scholar] [CrossRef]
- Frisch, L.M.; Mann, M.A.; Marek, D.N.; Niessen, L. Development and optimization of a loop-mediated isothermal amplification (LAMP) assay for the species-specific detection of Penicillium expansum. Food Microbiol. 2021, 95, 103681. [Google Scholar] [CrossRef]
- Shan, L.; Haseeb, H.A.; Zhang, J.; Zhang, D.; Jeffers, D.P.; Dai, X.; Guo, W. A loop-mediated isothermal amplification (LAMP) assay for the rapid detection of toxigenic Fusarium temperatum in maize stalks and kernels. Int. J. Food Microbiol. 2019, 291, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Lu, C.; Wang, S.; Xiong, Q.; Zhang, H.; Wang, Y.; Zheng, X. Rapid diagnosis of soybean anthracnose caused by Colletotrichum truncatum using a loop-mediated isothermal amplification (LAMP) assay. Eur. J. Plant Pathol. 2017, 148, 785–793. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, Z.; Zheng, X.; Wang, Y. Detection of Phytophthora melonisin samples of soil, water, and plant tissue with polymerase chain reaction. Can. J. Plant Pathol. 2007, 29, 172–181. [Google Scholar] [CrossRef]
- Rouhrazi, K.; Rahimian, H. Biochemical and genetic characterisation of Agrobacterium tumefaciens the causal agent of walnut crown gall disease in Iran. Arch. Phytopathol. Plant Prot. 2014, 47, 2493–2500. [Google Scholar] [CrossRef]
- Genov, N.; Llop, P.; López, M.M.; Bobev, S.G.; Álvarez, B. Molecular and phenotypic characterization of Agrobacterium species from vineyards allows identification of typical Agrobacterium vitis and atypical biovar 1 strains. J. Appl. Microbiol. 2015, 118, 1465–1477. [Google Scholar] [CrossRef]
- Zhao, T.; Qiao, K.; Wang, L.; Zhang, W.; Meng, W.; Liu, F.; Gao, X.; Zhu, J. Isolation and characterization of a strain with high microbial attachment in aerobic granular sludge. J. Environ. Sci. 2021, 106, 194–203. [Google Scholar] [CrossRef]




| Species | Strain Genome Numbers |
|---|---|
| ‘A. fabrum’ | C58 |
| arsenijevicii | JWIT |
| ‘A. deltaense’ | MRDI |
| A. fabacearum | WJOK |
| A. nepotum | JWJH |
| A. larrymoorei | JADW |
| A. larrymoorei | SWKE |
| A. radiobacter | QSNU |
| A. radiobacter | L-M-2 |
| A. pusense | FNBB |
| A. salinitolerans | MRDH |
| A. rosae | NXEJ |
| A. rosae | PEQT |
| A. rosae | QSWJ |
| A. rosae | QTSV |
| A. rosae | FMUE |
| A. rosae | QSWI |
| A. rosae | Y35-3 |
| A. rubi | JAAMCO |
| A. rubi | JAAMFC |
| A. rubi | JAAMCP |
| A. rubi | JAAMCN |
| A. rubi | JAANSA |
| A. rubi | JAANSB |
| A. rubi | BBJU |
| A. rubi | LXKU |
| A. skierniewicense | JACIDV |
| ‘Rhizobium oryzihabitans’ | M15 |
| Rhizobium rhizogenes | BAYX |
| Serial Number | Species | Sources |
|---|---|---|
| 1–7 | A. fabacearum | Roots of kiwifruit plant |
| 8 | A. radiobacter | Amygdalus persica L. |
| 9 | Rhizobium rhizogenes | Amygdalus persica L. |
| 10 | A. rubi | Amygdalus persica L. |
| 11–12 | A. radiobacter | Roots of Prunus salicina Lindl. |
| 13 | A. rosae | Roots of Cerasus spp. |
| 14–15 | A. radiobacter | Kiwifruit root system soil |
| 16–20 | Arthrobacter sp. | Kiwifruit root system soil |
| 21–22 | Bacillus sp. | Kiwifruit root system soil |
| 23–27 | Streptomyces sp. | Kiwifruit root system soil |
| 28–31 | Pseudomonas sp. | Kiwifruit root system soil |
| 32 | Pestalotiopsis microspora | Kiwifruit root system soil |
| 33–34 | Phomopsis vaccinii | Kiwifruit root system soil |
| 35 | Trametes hirsuta | Kiwifruit root system soil |
| 36 | Rhodanobacter thiooxydans | Kiwifruit root system soil |
| 37 | Verticillium dahliae | potato root system soil |
| Primer Name | Sequence (5′~3′) |
|---|---|
| F3 | GCATCGCTTCCGACAAGA |
| B3 | GTCGGCAGGCAACATGA |
| FIP | GACCCGTGCCCTCATAGCGACCTGGGCCAGCCCTTCA |
| BIP | GTCTCTGGTCAAGGGGCTGGTACGTTACGACTGTCCCCTCG |
| LB | GCTGCATGGCGGCACTTTC |
| Component | Dosage | Final Concentration |
|---|---|---|
| 10 × Isothermal Amplification Buffer | 2.5 μL | 1 × (Contains 2 mM MgSO4) |
| MgSO4 | 1.5 μL | 6 mM (Total 8 mM) |
| dNTPs Mix | 3.5 μL | 1.4 mM each |
| FIP | 1 μL | 1.6 μM |
| BIP | 1 μL | 1.6 μM |
| F3 | 1 μL | 0.2 μM |
| B3 | 1 μL | 0.2 μM |
| LB | 1 μL | 0.4 μM |
| Bst 2.0 DNA Polymerase (8000 U/mL) | 1 μL | 320 U/mL |
| DNA template | 1 μL | |
| ddH2O | to 25 μL | |
| Total reaction volume | 25 μL | |
| Paraffin oil | 20 μL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, L.; Shi, J.; Zhao, Z.; Ran, F.; Mo, F.; Long, Y.; Yin, X.; Li, W.; Chen, T.; Chen, J. First Report of Crown Gall of Kiwifruit (Actinidia deliciosa) Caused by Agrobacterium fabacearum in China and the Establishment of Loop-Mediated Isothermal Amplification Technique. Int. J. Mol. Sci. 2022, 23, 207. https://doi.org/10.3390/ijms23010207
He L, Shi J, Zhao Z, Ran F, Mo F, Long Y, Yin X, Li W, Chen T, Chen J. First Report of Crown Gall of Kiwifruit (Actinidia deliciosa) Caused by Agrobacterium fabacearum in China and the Establishment of Loop-Mediated Isothermal Amplification Technique. International Journal of Molecular Sciences. 2022; 23(1):207. https://doi.org/10.3390/ijms23010207
Chicago/Turabian StyleHe, Linan, Jinqiao Shi, Zhibo Zhao, Fei Ran, Feixu Mo, Youhua Long, Xianhui Yin, Wenzhi Li, Tingting Chen, and Jia Chen. 2022. "First Report of Crown Gall of Kiwifruit (Actinidia deliciosa) Caused by Agrobacterium fabacearum in China and the Establishment of Loop-Mediated Isothermal Amplification Technique" International Journal of Molecular Sciences 23, no. 1: 207. https://doi.org/10.3390/ijms23010207






