Inherent P2X7 Receptors Regulate Macrophage Functions during Inflammatory Diseases
Abstract
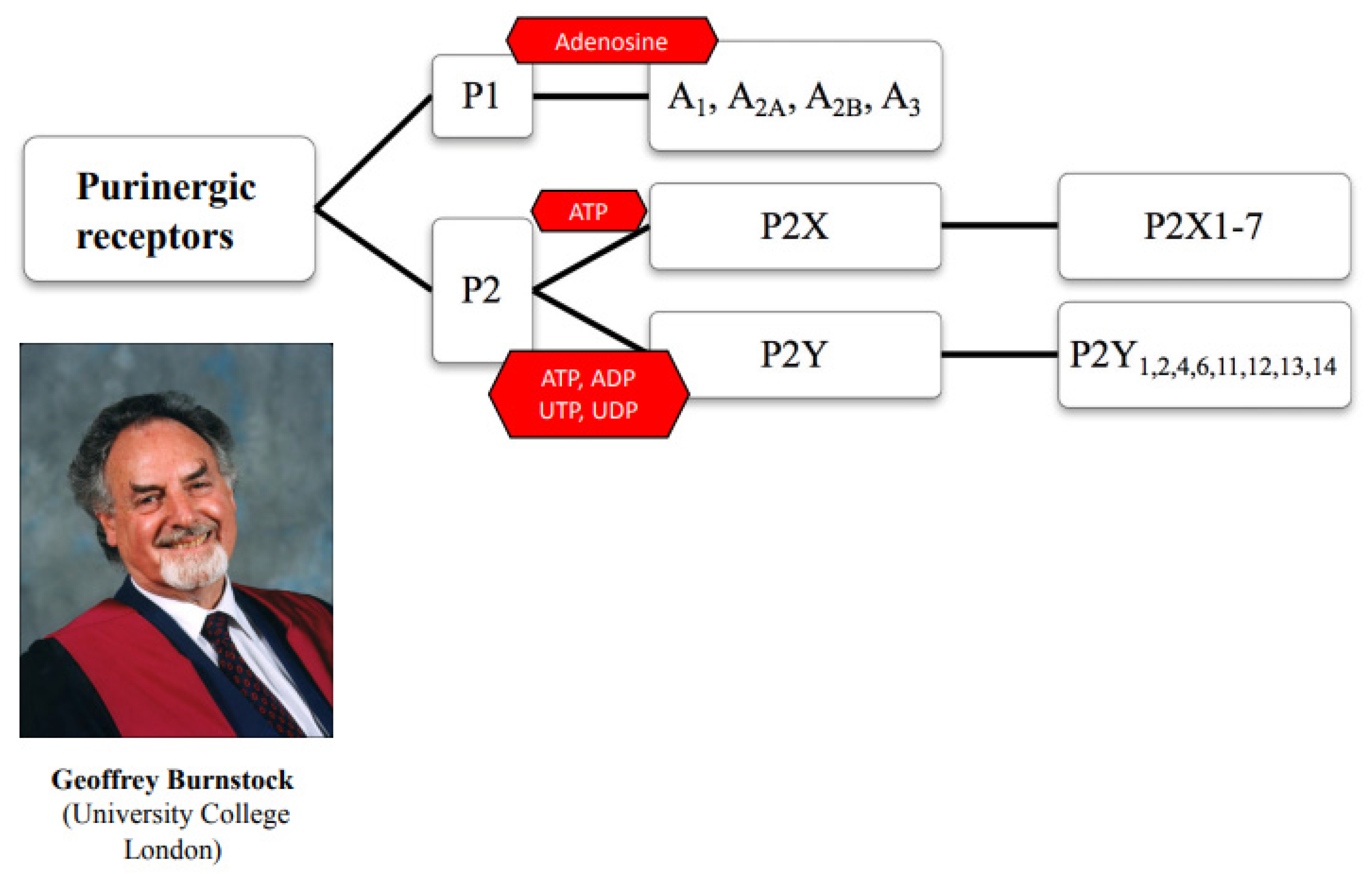
:1. Introduction
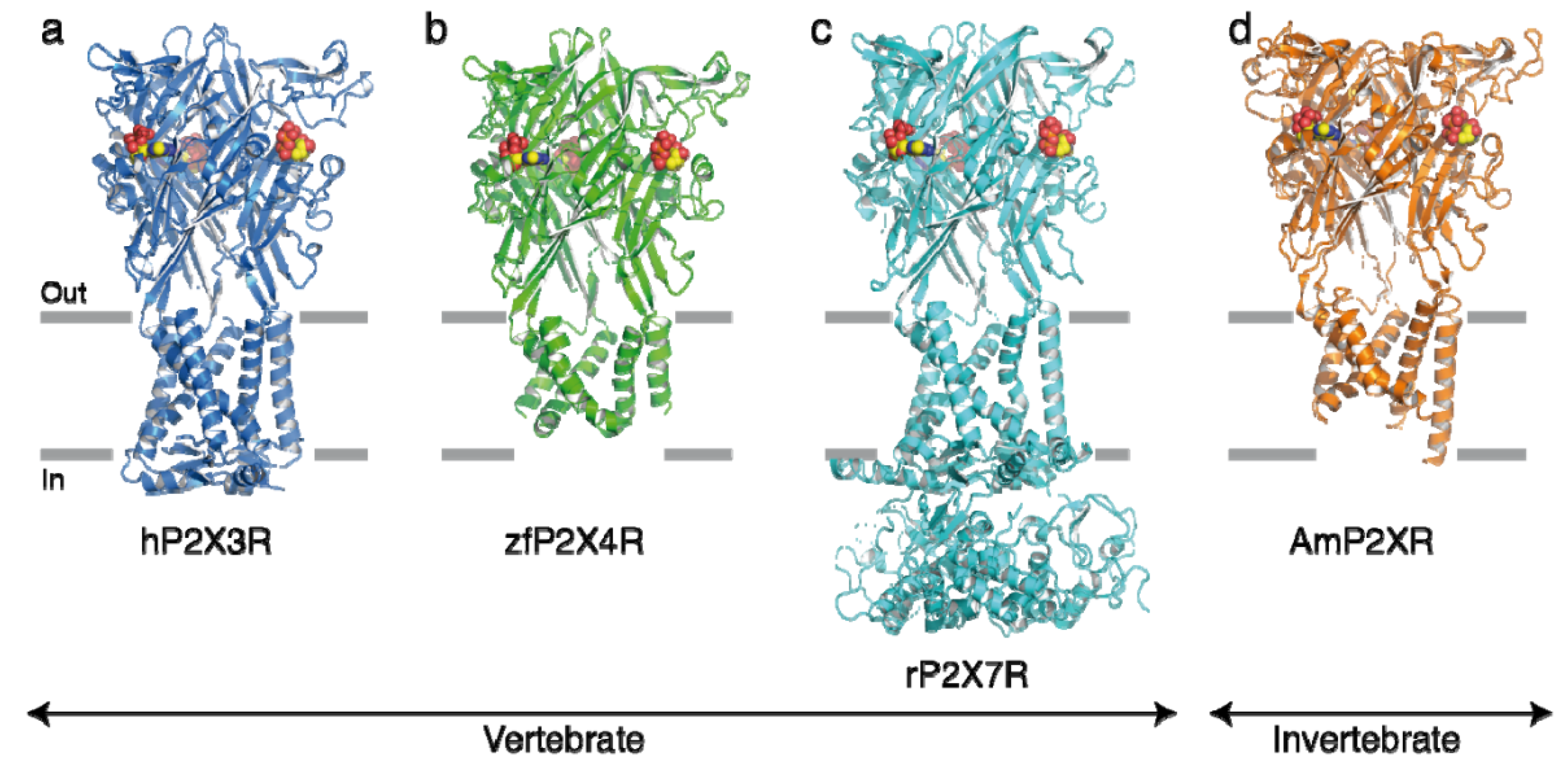
2. The P2X7R at Macrophages
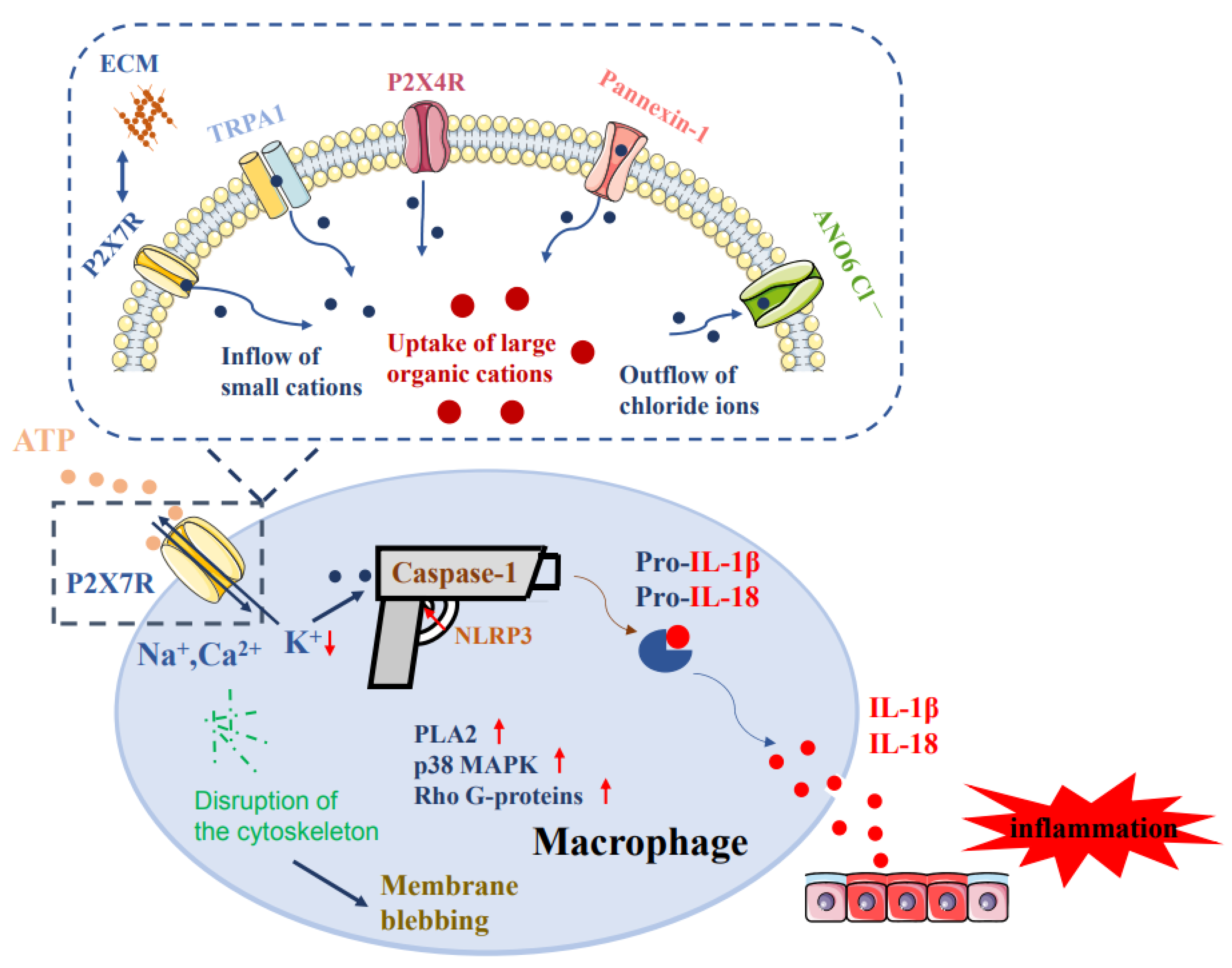
3. The Regulation of Pore Formation on Macrophages via P2X7Rs
4. The Role of P2X7Rs at Macrophages in Inflammatory Diseases
5. Peripheral Inflammatory Diseases
6. Neuroinflammation
7. P2X7R Splice Variants and Polymorphisms
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burnstock, G. Purinergic nerves. Pharmacol. Rev. 1972, 24, 509–581. [Google Scholar]
- Huang, Z.; Xie, N.; Illes, P.; Di Virgilio, F.; Ulrich, H.; Semyanov, A.; Verkhratsky, A.; Sperlagh, B.; Yu, S.-G.; Huang, C.; et al. From purines to purinergic signalling: Molecular functions and human diseases. Signal Transduct. Target. Ther. 2021, 6, 162. [Google Scholar] [CrossRef] [PubMed]
- Abbracchio, M.P.; Burnstock, G. Purinoceptors: Are there families of P2X and P2Y purinoceptors? Pharmacol. Ther. 1994, 64, 445–475. [Google Scholar] [CrossRef]
- Burnstock, G. Purinergic Signaling in the Cardiovascular System. Circ. Res. 2017, 120, 207–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarvis, M.F.; Khakh, B.S. ATP-gated P2X cation-channels. Neuropharmacology 2009, 56, 208–215. [Google Scholar] [CrossRef]
- Abbracchio, M.P.; Burnstock, G.; Boeynaems, J.M.; Barnard, E.A.; Boyer, J.L.; Kennedy, C.; Knight, G.E.; Fumagalli, M.; Gachet, C.; Jacobson, K.A.; et al. International Union of Pharmacology, L.V.III: Update on the P2Y G protein-coupled nucleotide receptors: From molecular mechanisms and pathophysiology to therapy. Pharmacol. Rev. 2006, 58, 281–341. [Google Scholar] [CrossRef]
- Jacobson, K.A.; Delicado, E.G.; Gachet, C.; Kennedy, C.; von Kügelgen, I.; Li, B.; Miras-Portugal, M.T.; Novak, I.; Schöneberg, T.; Perez-Sen, R.; et al. Update of P2Y receptor pharmacology: IUPHAR Review 27. Br. J. Pharmacol. 2020, 177, 2413–2433. [Google Scholar] [CrossRef]
- Fredholm, B.B.; IJzerman, A.P.; Jacobson, K.A.; Linden, J.; Müller, C.E. International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors—An update. Pharmacol. Rev. 2011, 63, 1–34. [Google Scholar] [CrossRef]
- Zimmermann, H.; Zebisch, M.; Sträter, N. Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal. 2012, 8, 437–502. [Google Scholar] [CrossRef] [Green Version]
- Yegutkin, G.G. Enzymes involved in metabolism of extracellular nucleotides and nucleosides: Functional implications and measurement of activities. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 473–497. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, H.; Braun, N. Ecto-nucleotidases--molecular structures, catalytic properties, and functional roles in the nervous system. Prog. Brain Res. 1999, 120, 371–385. [Google Scholar] [PubMed]
- Burnstock, G.; Boeynaems, J.M. Purinergic signalling and immune cells. Purinergic Signal. 2014, 10, 529–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Surprenant, A.; Rassendren, F.; Kawashima, E.; North, R.A.; Buell, G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7). Science 1996, 272, 735–738. [Google Scholar] [CrossRef]
- North, R.A. Molecular physiology of P2X receptors. Physiol. Rev. 2002, 82, 1013–1067. [Google Scholar] [CrossRef]
- Di Virgilio, F.; Dal Ben, D.; Sarti, A.C.; Giuliani, A.L.; Falzoni, S. The P2X7 Receptor in Infection and Inflammation. Immunity 2017, 47, 15–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Virgilio, F.; Schmalzing, G.; Markwardt, F. The Elusive P2X7 Macropore. Trends Cell Biol. 2018, 28, 392–404. [Google Scholar] [CrossRef]
- Illes, P.; Rubini, P.; Ulrich, H.; Zhao, Y.; Tang, Y. Regulation of Microglial Functions by Purinergic Mechanisms in the Healthy and Diseased CNS. Cells 2020, 9, 1108. [Google Scholar] [CrossRef]
- Miller, C.M.; Boulter, N.R.; Fuller, S.J.; Zakrzewski, A.M.; Lees, M.P.; Saunders, B.M.; Wiley, J.S.; Smith, N.C. The role of the P2X7 receptor in infectious diseases. PLoS Pathog. 2011, 7, e1002212. [Google Scholar] [CrossRef] [Green Version]
- Lavin, Y.; Merad, M. Macrophages: Gatekeepers of tissue integrity. Cancer Immunol. Res. 2013, 1, 201–209. [Google Scholar] [CrossRef] [Green Version]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- Jakubzick, C.V.; Randolph, G.J.; Henson, P.M. Monocyte differentiation and antigen-presenting functions. Nat. Rev. Immunol. 2017, 17, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Chawla, A.; Pollard, J.W. Macrophage biology in development, homeostasis and disease. Nature 2013, 496, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Ginhoux, F.; Jung, S. Monocytes and macrophages: Developmental pathways and tissue homeostasis. Nat. Rev. Immunol. 2014, 14, 392–404. [Google Scholar] [CrossRef]
- Layhadi, J.A.; Fountain, S.J. P2X4 Receptor-Dependent Ca2+ Influx in Model Human Monocytes and Macrophages. Int. J. Mol. Sci. 2017, 18, 2261. [Google Scholar] [CrossRef] [Green Version]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Amici, S.A.; Dong, J.; Guerau-de-Arellano, M. Molecular Mechanisms Modulating the Phenotype of Macrophages and Microglia. Front. Immunol. 2017, 8, 1520. [Google Scholar] [CrossRef] [Green Version]
- Kigerl, K.A.; Gensel, J.C.; Ankeny, D.P.; Alexander, J.K.; Donnelly, D.J.; Popovich, P.G. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J. Neurosci. 2009, 29, 13435–13444. [Google Scholar] [CrossRef] [Green Version]
- Klaver, D.; Thurnher, M. Control of Macrophage Inflammation by P2Y Purinergic Receptors. Cells 2021, 10, 1098. [Google Scholar] [CrossRef] [PubMed]
- Duluc, D.; Delneste, Y.; Tan, F.; Moles, M.P.; Grimaud, L.; Lenoir, J.; Preisser, L.; Anegon, I.; Catala, L.; Ifrah, N.; et al. Tumor-associated leukemia inhibitory factor and IL-6 skew monocyte differentiation into tumor-associated macrophage-like cells. Blood 2007, 110, 4319–4330. [Google Scholar] [CrossRef]
- Martinez, F.O.; Sica, A.; Mantovani, A.; Locati, M. Macrophage activation and polarization. Front. Biosci. 2008, 13, 453–461. [Google Scholar] [CrossRef] [Green Version]
- Illes, P.; Müller, C.E.; Jacobson, K.A.; Grutter, T.; Nicke, A.; Fountain, S.J.; Kennedy, C.; Schmalzing, G.; Jarvis, M.F.; Stojilkovic, S.S.; et al. Update of P2X receptor properties and their pharmacology: IUPHAR Review 30. Br. J. Pharmacol. 2021, 178, 489–514. [Google Scholar] [CrossRef] [PubMed]
- Buell, G.; Chessell, I.P.; Michel, A.D.; Collo, G.; Salazzo, M.; Herren, S.; Gretener, D.; Grahames, C.; Kaur, R.; Kosco-Vilbois, M.H.; et al. Blockade of human P2X7 receptor function with a monoclonal antibody. Blood 1998, 92, 3521–3528. [Google Scholar] [CrossRef]
- Burnstock, G.; Knight, G.E. The potential of P2X7 receptors as a therapeutic target, including inflammation and tumour progression. Purinergic Signal. 2018, 14, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Mansoor, S.E.; Lü, W.; Oosterheert, W.; Shekhar, M.; Tajkhorshid, E.; Gouaux, E. X-ray structures define human P2X3 receptor gating cycle and antagonist action. Nature 2016, 538, 66–71. [Google Scholar] [CrossRef] [Green Version]
- Hattori, M.; Gouaux, E. Molecular mechanism of ATP binding and ion channel activation in P2X receptors. Nature 2012, 485, 207–212. [Google Scholar] [CrossRef] [Green Version]
- McCarthy, A.E.; Yoshioka, C.; Mansoor, S.E. Full-Length P2X7 Structures Reveal How Palmitoylation Prevents Channel Desensitization. Cell 2019, 179, 659–670. [Google Scholar] [CrossRef]
- Kasuya, G.; Fujiwara, Y.; Takemoto, M.; Dohmae, N.; Nakada-Nakura, Y.; Ishitani, R.; Hattori, M.; Nureki, O. Structural Insights into Divalent Cation Modulations of ATP-Gated P2X Receptor Channels. Cell Rep. 2016, 14, 932–944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gudipaty, L.; Humphreys, B.D.; Buell, G.; Dubyak, G.R. Regulation of P2X(7) nucleotide receptor function in human monocytes by extracellular ions and receptor density. Am. J. Physiol. Cell Physiol. 2001, 280, C943–C953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falzoni, S.; Munerati, M.; Ferrari, D.; Spisani, S.; Moretti, S.; Di Virgilio, F. The purinergic P2Z receptor of human macrophage cells. Characterization and possible physiological role. J. Clin. Investig. 1995, 95, 1207–1216. [Google Scholar] [CrossRef] [Green Version]
- Gu, B.J.; Zhang, W.Y.; Bendall, L.J.; Chessell, I.P.; Buell, G.N.; Wiley, J.S. Expression of P2X7 purinoceptors on human lymphocytes and monocytes: Evidence for nonfunctional P2X7 receptors. Am. J. Physiol. Cell Physiol. 2000, 279, C1189–C1197. [Google Scholar] [CrossRef] [PubMed]
- Monif, M.; Burnstock, G.; Williams, D.A. Microglia: Proliferation and activation driven by the P2X7 receptor. Int. J. Biochem. Cell Biol. 2010, 42, 1753–1756. [Google Scholar] [CrossRef]
- Qin, J.; Zhang, X.; Tan, B.; Zhang, S.; Yin, C.; Xue, Q.; Zhang, Z.; Ren, H.; Chen, J.; Liu, M.; et al. Blocking P2X7-Mediated Macrophage Polarization Overcomes Treatment Resistance in Lung Cancer. Cancer Immunol. Res. 2020, 8, 1426–1439. [Google Scholar] [CrossRef] [PubMed]
- Raneia E Silva, P.A.; de Lima, D.S.; Mesquita Luiz, J.P.; Camara, N.O.S.; Alves-Filho, J.C.F.; Pontillo, A.; Bortoluci, K.R.; Faquim-Mauro, E.L. Inflammatory effect of Bothropstoxin-I from Bothrops jararacussu venom mediated by NLRP3 inflammasome involves ATP and P2X7 receptor. Clin. Sci. 2021, 135, 687–701. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Fei, M.; Zhang, G.; Liang, W.C.; Lin, W.; Wu, Y.; Piskol, R.; Ridgway, J.; McNamara, E.; Huang, H.; et al. Blockade of the Phagocytic Receptor MerTK on Tumor-Associated Macrophages Enhances P2X7R-Dependent, STING Activation by Tumor-Derived cGAMP. Immunity 2020, 52, 357–373. [Google Scholar] [CrossRef] [PubMed]
- Gallenga, C.E.; Lonardi, M.; Pacetti, S.; Violanti, S.S.; Tassinari, P.; Di Virgilio, F.; Tognon, M.; Perri, P. Molecular Mechanisms Related to Oxidative Stress in Retinitis Pigmentosa. Antioxidants 2021, 10, 848. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.L.; Lin, Y.; Liu, W.; Zhu, X.Z.; Liu, D.; Tong, M.L.; Liu, L.L.; Lin, L.R. The P2X7 receptor mediates NLRP3-dependent IL-1beta secretion and promotes phagocytosis in the macrophage response to Treponema pallidum. Int. Immunopharmacol. 2020, 82, 106344. [Google Scholar] [CrossRef]
- Janks, L.; Sprague, R.S.; Egan, T.M. ATP-Gated P2X7 Receptors Require Chloride Channels To Promote Inflammation in Human Macrophages. J. Immunol. 2019, 202, 883–898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hempel, C.; Nörenberg, W.; Sobottka, H.; Urban, N.; Nicke, A.; Fischer, W.; Schaefer, M. The phenothiazine-class antipsychotic drugs prochlorperazine and trifluoperazine are potent allosteric modulators of the human P2X7 receptor. Neuropharmacology 2013, 75, 365–379. [Google Scholar] [CrossRef]
- Nurkhametova, D.; Siniavin, A.; Streltsova, M.; Kudryavtsev, D.; Kudryavtsev, I.; Giniatullina, R.; Tsetlin, V.; Malm, T.; Giniatullin, R. Does Cholinergic Stimulation Affect the P2X7 Receptor-Mediated Dye Uptake in Mast Cells and Macrophages? Front. Cell. Neurosci. 2020, 14, 548376. [Google Scholar] [CrossRef] [PubMed]
- Schachter, J.; Motta, A.P.; de Souza, Z.A.; da Silva-Souza, H.A.; Guimaraes, M.Z.; Persechini, P.M. ATP-induced P2X7-associated uptake of large molecules involves distinct mechanisms for cations and anions in macrophages. J. Cell Sci. 2008, 121 Pt 19, 3261–3270. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Shi, S.; Su, Y.; Tong, J.S.; Li, L. P2X7R promotes angiogenesis and tumour-associated macrophage recruitment by regulating the, N.F.-κB signalling pathway in colorectal cancer cells. J. Cell. Mol. Med. 2020, 24, 10830–10841. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, M.; Cui, B.W.; Jin, C.H.; Wu, Y.L.; Shang, Y.; Yang, H.X.; Wu, M.; Liu, J.; Qiao, C.Y.; et al. P2X7 receptor-targeted regulation by tetrahydroxystilbene glucoside in alcoholic hepatosteatosis: A new strategy towards macrophage-hepatocyte crosstalk. Br. J. Pharmacol. 2020, 177, 2793–2811. [Google Scholar] [CrossRef]
- Dong, X.; Zheng, Z.; Lin, P.; Fu, X.; Li, F.; Jiang, J.; Jiang, J.; Zhu, P. ACPAs promote IL-1-beta production in rheumatoid arthritis by activating the NLRP3 inflammasome. Cell. Mol. Immunol. 2020, 17, 261–271. [Google Scholar] [CrossRef]
- Ousingsawat, J.; Wanitchakool, P.; Kmit, A.; Romao, A.M.; Jantarajit, W.; Schreiber, R.; Kunzelmann, K. Anoctamin 6 mediates effects essential for innate immunity downstream of P2X7 receptors in macrophages. Nat. Commun. 2015, 6, 6245. [Google Scholar] [CrossRef]
- Moreira-Souza, A.C.A.; Almeida-da-Silva, C.L.C.; Rangel, T.P.; Rocha, G.D.C.; Bellio, M.; Zamboni, D.S.; Vommaro, R.C.; Coutinho-Silva, R. The P2X7 Receptor Mediates Toxoplasma gondii Control in Macrophages through Canonical NLRP3 Inflammasome Activation and Reactive Oxygen Species Production. Front. Immunol. 2017, 8, 1257. [Google Scholar] [CrossRef] [Green Version]
- Marques-da-Silva, C.; Chaves, M.M.; Castro, N.G.; Coutinho-Silva, R.; Guimaraes, M.Z. Colchicine inhibits cationic dye uptake induced by ATP in P2X2 and P2X7 receptor-expressing cells: Implications for its therapeutic action. Br. J. Pharmacol. 2011, 163, 912–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janks, L.; Sharma, C.V.R.; Egan, T.M. A central role for P2X7 receptors in human microglia. J. Neuroinflammation 2018, 15, 325. [Google Scholar] [CrossRef]
- Yaron, J.R.; Gangaraju, S.; Rao, M.Y.; Kong, X.; Zhang, L.; Su, F.; Tian, Y.; Glenn, H.L.; Meldrum, D.R. K+ regulates Ca2+ to drive inflammasome signaling: Dynamic visualization of ion flux in live cells. Cell Death Dis. 2015, 6, e1954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelegrin, P.; Surprenant, A. Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J. 2006, 25, 5071–5082. [Google Scholar] [CrossRef] [Green Version]
- Pfeiffer, Z.A.; Aga, M.; Prabhu, U.; Watters, J.J.; Hall, D.J.; Bertics, P.J. The nucleotide receptor P2X7 mediates actin reorganization and membrane blebbing in, R.A.W 264.7 macrophages via p38 MAP kinase and Rho. J. Leukoc. Biol. 2004, 75, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, T.; Ishii, K.; Fukutomi, H.; Naguro, I.; Matsuzawa, A.; Takeda, K.; Ichijo, H. Requirement of reactive oxygen species-dependent activation of ASK1-p38 MAPK pathway for extracellular ATP-induced apoptosis in macrophage. J. Biol. Chem. 2008, 283, 7657–7665. [Google Scholar] [CrossRef] [Green Version]
- Le, S.H.; Raymond, M.N. P2X7 receptor-mediated phosphatidic acid production delays ATP-induced pore opening and cytolysis of, R.A.W 264.7 macrophages. Cell. Signal. 2007, 19, 1909–1918. [Google Scholar]
- Thomas, L.M.; Salter, R.D. Activation of macrophages by P2X7-induced microvesicles from myeloid cells is mediated by phospholipids and is partially dependent on, T.L.R4. J. Immunol. 2010, 185, 3740–3749. [Google Scholar] [CrossRef] [Green Version]
- Matty, M.A.; Knudsen, D.R.; Walton, E.M.; Beerman, R.W.; Cronan, M.R.; Pyle, C.J.; Hernandez, R.E.; Tobin, D.M. Potentiation of P2RX7 as a host-directed strategy for control of mycobacterial infection. Elife 2019, 8, e39123. [Google Scholar] [CrossRef]
- Bomfim, C.C.B.; Amaral, E.P.; Cassado, A.D.A.; Salles, A.; do Nascimento, R.S.; Lasunskaia, E.; Hirata, M.H.; Álvarez, J.M.; D’Império-Lima, M.R. P2X7 Receptor in Bone Marrow-Derived Cells Aggravates Tuberculosis Caused by Hypervirulent Mycobacterium bovis. Front. Immunol. 2017, 8, 435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Illes, P.; Verkhratsky, A.; Tang, Y. Pathological ATPergic Signaling in Major Depression and Bipolar Disorder. Front. Mol. Neurosci. 2019, 12, 331. [Google Scholar] [CrossRef] [Green Version]
- Virginio, C.; MacKenzie, A.; Rassendren, F.A.; North, R.A.; Surprenant, A. Pore dilation of neuronal P2X receptor channels. Nat. Neurosci. 1999, 2, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Toombes, G.E.; Silberberg, S.D.; Swartz, K.J. Physical basis of apparent pore dilation of ATP-activated P2X receptor channels. Nat. Neurosci. 2015, 18, 1577–1583. [Google Scholar] [CrossRef] [PubMed]
- Pippel, A.; Stolz, M.; Woltersdorf, R.; Kless, A.; Schmalzing, G.; Markwardt, F. Localization of the gate and selectivity filter of the full-length P2X7 receptor. Proc. Natl. Acad. Sci. USA 2017, 114, E2156–E2165. [Google Scholar] [CrossRef] [Green Version]
- Markwardt, F. Human P2X7 receptors-Properties of single ATP-gated ion channels. Biochem. Pharmacol. 2021, 187, 114307. [Google Scholar] [CrossRef]
- Kopp, R.; Krautloher, A.; Ramirez-Fernandez, A.; Nicke, A. P2X7 Interactions and Signaling-Making Head or Tail of It. Front. Mol. Neurosci. 2019, 12, 183. [Google Scholar] [CrossRef]
- Smart, M.L.; Gu, B.; Panchal, R.G.; Wiley, J.; Cromer, B.; Williams, D.A.; Petrou, S. P2X7 receptor cell surface expression and cytolytic pore formation are regulated by a distal C-terminal region. J. Biol. Chem. 2003, 278, 8853–8860. [Google Scholar] [CrossRef] [Green Version]
- Panchin, Y.; Kelmanson, I.; Matz, M.; Lukyanov, K.; Usman, N.; Lukyanov, S. A ubiquitous family of putative gap junction molecules. Curr. Biol. 2000, 10, R473–R474. [Google Scholar] [CrossRef] [Green Version]
- Pelegrin, P.; Surprenant, A. The P2X7 receptor-pannexin connection to dye uptake and IL-1beta release. Purinergic Signal. 2009, 5, 129–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alberto, A.V.; Faria, R.X.; Couto, C.G.; Ferreira, L.G.; Souza, C.A.; Teixeira, P.C.; Fróes, M.M.; Alves, L.A. Is pannexin the pore associated with the P2X7 receptor? Naunyn Schmiedebergs Arch. Pharmacol. 2013, 386, 775–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, B.C.; Kim, S.H.; Lim, J.; Kim, Y.S. Activation of pannexin-1 mediates triglyceride-induced macrophage cell death. BMB Rep. 2020, 53, 588–593. [Google Scholar] [CrossRef]
- Garre, J.M.; Yang, G.; Bukauskas, F.F.; Bennett, M.V. FGF-1 Triggers Pannexin-1 Hemichannel Opening in Spinal Astrocytes of Rodents and Promotes Inflammatory Responses in Acute Spinal Cord Slices. J. Neurosci. 2016, 36, 4785–4801. [Google Scholar] [CrossRef] [PubMed]
- Perez-Flores, G.; Lévesque, S.A.; Pacheco, J.; Vaca, L.; Lacroix, S.; Pérez-Cornejo, P.; Arreola, J. The P2X7/P2X4 interaction shapes the purinergic response in murine macrophages. Biochem. Biophys. Res. Commun. 2015, 467, 484–490. [Google Scholar] [CrossRef]
- Karasawa, A.; Michalski, K.; Mikhelzon, P.; Kawate, T. The P2X7 receptor forms a dye-permeable pore independent of its intracellular domain but dependent on membrane lipid composition. Elife 2017, 6, e31186. [Google Scholar] [CrossRef]
- Dunning, K.; Martz, A.; Peralta, F.A.; Cevoli, F.; Boue-Grabot, E.; Compan, V.; Gautherat, F.; Wolf, P.; Chataigneau, T.; Grutter, T. P2X7 Receptors and, T.M.EM16 Channels Are Functionally Coupled with Implications for Macropore Formation and Current Facilitation. Int. J. Mol. Sci. 2021, 22, 6542. [Google Scholar] [CrossRef]
- Harkat, M.; Peverini, L.; Cerdan, A.H.; Dunning, K.; Beudez, J.; Martz, A.; Calimet, N.; Specht, A.; Cecchini, M.; Chataigneau, T.; et al. On the permeation of large organic cations through the pore of, ATP-gated P2X receptors. Proc. Natl. Acad. Sci. USA 2017, 114, E3786–E3795. [Google Scholar] [CrossRef] [Green Version]
- Babiychuk, E.B.; Monastyrskaya, K.; Potez, S.; Draeger, A. Blebbing confers resistance against cell lysis. Cell Death Differ. 2011, 18, 80–89. [Google Scholar] [CrossRef] [Green Version]
- Taylor, S.R.; Turner, C.M.; Elliott, J.I.; McDaid, J.; Hewitt, R.; Smith, J.; Pickering, M.C.; Whitehouse, D.L.; Cook, H.T.; Burnstock, G.; et al. P2X7 deficiency attenuates renal injury in experimental glomerulonephritis. J. Am. Soc. Nephrol. 2009, 20, 1275–1281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, L.H.; Rassendren, F.; MacKenzie, A.; Zhang, Y.H.; Surprenant, A.; North, R.A. N-methyl-D-glucamine and propidium dyes utilize different permeation pathways at rat P2X7 receptors. Am. J. Physiol. Cell Physiol. 2005, 289, C1295–C1302. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Wan, B.; Yang, Y.; Ren, X.; Guo, L.H.; Zhang, H. Crucial Role of P2X7 Receptor in Regulating Exocytosis of Single-Walled Carbon Nanotubes in Macrophages. Small 2016, 12, 5998–6011. [Google Scholar] [CrossRef] [PubMed]
- Rigato, C.; Swinnen, N.; Buckinx, R.; Couillin, I.; Mangin, J.M.; Rigo, J.M.; Legendre, P.; Le Corronc, H. Microglia proliferation is controlled by P2X7 receptors in a Pannexin-1-independent manner during early embryonic spinal cord invasion. J. Neurosci. 2012, 32, 11559–11573. [Google Scholar] [CrossRef] [Green Version]
- Monif, M.; Reid, C.A.; Powell, K.L.; Drummond, K.J.; O’Brien, T.J.; Williams, D.A. Interleukin-1beta has trophic effects in microglia and its release is mediated by P2X7R pore. J. Neuroinflammation 2016, 13, 173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gheorghe, R.O.; Deftu, A.; Filippi, A.; Grosu, A.; Bica-Popi, M.; Chiritoiu, M.; Chiritoiu, G.; Munteanu, C.; Silvestro, L.; Ristoiu, V. Silencing the Cytoskeleton Protein Iba1 (Ionized Calcium Binding Adapter Protein 1) Interferes with, B.V.2 Microglia Functioning. Cell. Mol. Neurobiol. 2020, 40, 1011–1027. [Google Scholar] [CrossRef] [PubMed]
- Savio, L.E.B.; de Andrade, M.P.; da Silva, C.G.; Coutinho-Silva, R. The P2X7 Receptor in Inflammatory Diseases: Angel or Demon? Front. Pharmacol. 2018, 9, 52. [Google Scholar] [CrossRef] [Green Version]
- Shao, B.Z.; Xu, Z.Q.; Han, B.Z.; Su, D.F.; Liu, C. NLRP3 inflammasome and its inhibitors: A review. Front Pharmacol. 2015, 6, 262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, C.N.J.; Gorecki, D.C. P2RX7 Purinoceptor as a Therapeutic Target-The Second Coming? Front. Chem. 2018, 6, 248. [Google Scholar] [CrossRef] [Green Version]
- Perregaux, D.G.; Gabel, C.A. Post-translational processing of murine IL-1: Evidence that ATP-induced release of IL-1 alpha and IL-1 beta occurs via a similar mechanism. J. Immunol. 1998, 160, 2469–2477. [Google Scholar] [PubMed]
- Abderrazak, A.; Syrovets, T.; Couchie, D.; El, H.K.; Friguet, B.; Simmet, T.; Rouis, M. NLRP3 inflammasome: From a danger signal sensor to a regulatory node of oxidative stress and inflammatory diseases. Redox Biol. 2015, 4, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.H.; Walsh, M.C.; Yu, J.; Shen, H.; Wherry, E.J.; Choi, Y. Mice Lacking the Purinergic Receptor P2X5 Exhibit Defective Inflammasome Activation and Early Susceptibility to Listeria monocytogenes. J. Immunol. 2020, 205, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Li, L.H.; Chen, T.L.; Chiu, H.W.; Hsu, C.H.; Wang, C.C.; Tai, T.T.; Ju, T.C.; Chen, F.H.; Chernikov, O.V.; Tsai, W.C.; et al. Critical Role for the NLRP3 Inflammasome in Mediating IL-1beta Production in Shigella sonnei-Infected Macrophages. Front. Immunol. 2020, 11, 1115. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Planillo, R.; Kuffa, P.; Martinez-Colon, G.; Smith, B.L.; Rajendiran, T.M.; Nunez, G. K+ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity 2013, 38, 1142–1153. [Google Scholar] [CrossRef] [Green Version]
- Brough, D.; Le Feuvre, R.A.; Wheeler, R.D.; Solovyova, N.; Hilfiker, S.; Rothwell, N.J.; Verkhratsky, A. Ca2+ stores and Ca2+ entry differentially contribute to the release of IL-1 beta and IL-1 alpha from murine macrophages. J. Immunol. 2003, 170, 3029–3036. [Google Scholar] [CrossRef] [Green Version]
- Kahlenberg, J.M.; Dubyak, G.R. Mechanisms of caspase-1 activation by P2X7 receptor-mediated K+ release. Am. J. Physiol. Cell Physiol. 2004, 286, C1100–C1108. [Google Scholar] [CrossRef] [Green Version]
- Hanley, P.J.; Kronlage, M.; Kirschning, C.; Del, R.A.; Di Virgilio, F.; Leipziger, J.; Chessell, I.P.; Sargin, S.; Filippov, M.A.; Lindemann, O.; et al. Transient P2X7 receptor activation triggers macrophage death independent of Toll-like receptors 2 and 4, caspase-1, and pannexin-1 proteins. J. Biol. Chem. 2012, 287, 10650–10663. [Google Scholar] [CrossRef] [Green Version]
- Le Feuvre, R.A.; Brough, D.; Iwakura, Y.; Takeda, K.; Rothwell, N.J. Priming of macrophages with lipopolysaccharide potentiates P2X7-mediated cell death via a caspase-1-dependent mechanism, independently of cytokine production. J. Biol. Chem. 2002, 277, 3210–3218. [Google Scholar] [CrossRef] [Green Version]
- Csoka, B.; Németh, Z.H.; Törö, G.; Idzko, M.; Zech, A.; Koscsó, B.; Spolarics, Z.; Antonioli, L.; Cseri, K.; Erdélyi, K.; et al. Extracellular ATP protects against sepsis through macrophage P2X7 purinergic receptors by enhancing intracellular bacterial killing. FASEB J. 2015, 29, 3626–3637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Hu, D.; Feng, Y.; Wu, C.; Song, Y.; Liu, W.; Li, A.; Wang, Y.; Chen, K.; Tian, M.; et al. Paxillin mediates ATP-induced activation of P2X7 receptor and NLRP3 inflammasome. BMC Biol. 2020, 18, 182. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Han, X.; He, L.; Tang, F.; Huang, R.; Lin, Z.; Li, S.; Deng, S.; Xu, J.; Huang, H.; et al. Transient receptor potential ankyrin 1 contributes to the ATP-elicited oxidative stress and inflammation in THP-1-derived macrophage. Mol. Cell. Biochem. 2020, 473, 179–192. [Google Scholar] [CrossRef]
- Wanke, D.; Mauch-Mücke, K.; Holler, E.; Hehlgans, T. Human beta-defensin-2 and -3 enhance pro-inflammatory cytokine expression induced by, T.L.R ligands via ATP-release in a P2X7R dependent manner. Immunobiology 2016, 221, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- de Torre-Minguela, C.; Barbera-Cremades, M.; Gomez, A.I.; Martin-Sanchez, F.; Pelegrin, P. Macrophage activation and polarization modify P2X7 receptor secretome influencing the inflammatory process. Sci. Rep. 2016, 6, 22586. [Google Scholar] [CrossRef]
- Pelegrin, P.; Surprenant, A. Dynamics of macrophage polarization reveal new mechanism to inhibit IL-1beta release through pyrophosphates. EMBO J. 2009, 28, 2114–2127. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Castejon, G.; Baroja-Mazo, A.; Pelegrin, P. Novel macrophage polarization model: From gene expression to identification of new anti-inflammatory molecules. Cell. Mol. Life Sci. 2011, 68, 3095–3107. [Google Scholar] [CrossRef]
- Neves, A.R.; Castelo-Branco, M.T.; Figliuolo, V.R.; Bernardazzi, C.; Buongusto, F.; Yoshimoto, A.; Nanini, H.F.; Coutinho, C.M.; Carneiro, A.J.; Coutinho-Silva, R.; et al. Overexpression of ATP-activated P2X7 receptors in the intestinal mucosa is implicated in the pathogenesis of Crohn’s disease. Inflamm. Bowel Dis. 2014, 20, 444–457. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Zhang, Y.; Zheng, J.H.; Li, X.; Yao, Y.L.; Wu, Y.L.; Song, S.Z.; Sun, P.; Nan, J.X.; Lian, L.H. Potentiation of hepatic stellate cell activation by extracellular ATP is dependent on P2X7R-mediated NLRP3 inflammasome activation. Pharmacol. Res. 2017, 117, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Cui, B.W.; Wu, Y.L.; Zhang, Y.; Shang, Y.; Liu, J.; Yang, H.X.; Qiao, C.Y.; Zhan, Z.Y.; Ye, H.; et al. P2X7R orchestrates the progression of murine hepatic fibrosis by making a feedback loop from macrophage to hepatic stellate cells. Toxicol. Lett. 2020, 333, 22–32. [Google Scholar] [CrossRef]
- Wu, X.; Ren, J.; Chen, G.; Wu, L.; Song, X.; Li, G.; Deng, Y.; Wang, G.; Gu, G.; Li, J. Systemic blockade of P2X7 receptor protects against sepsis-induced intestinal barrier disruption. Sci. Rep. 2017, 7, 4364. [Google Scholar] [CrossRef] [Green Version]
- Pereira, J.M.S.; Barreira, A.L.; Gomes, C.R.; Ornellas, F.M.; Ornellas, D.S.; Miranda, L.C.; Cardoso, L.R.; Coutinho-Silva, R.; Schanaider, A.; Morales, M.M.; et al. Brilliant blue, G.; a P2X7 receptor antagonist, attenuates early phase of renal inflammation, interstitial fibrosis and is associated with renal cell proliferation in ureteral obstruction in rats. BMC Nephrol. 2020, 21, 206. [Google Scholar] [CrossRef]
- Koo, T.Y.; Lee, J.G.; Yan, J.J.; Jang, J.Y.; Ju, K.D.; Han, M.; Oh, K.H.; Ahn, C.; Yang, J. The P2X7 receptor antagonist, oxidized adenosine triphosphate, ameliorates renal ischemia-reperfusion injury by expansion of regulatory T cells. Kidney Int. 2017, 92, 415–431. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.J.; Jo, S.G.; Kim, D.J.; Park, J.H. NLRP3 inflammasome mediates interleukin-1beta production in immune cells in response to Acinetobacter baumannii and contributes to pulmonary inflammation in mice. Immunology 2017, 150, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Wang, B.; Mao, J. The pathogenesis and treatment of the ‘Cytokine Storm’ in, C.O.VID-19. J. Infect. 2020, 80, 607–613. [Google Scholar] [CrossRef]
- Wang, C.; Xie, J.; Zhao, L.; Fei, X.; Zhang, H.; Tan, Y.; Nie, X.; Zhou, L.; Liu, Z.; Ren, Y.; et al. Alveolar macrophage dysfunction and cytokine storm in the pathogenesis of two severe, C.O.VID-19 patients. EBioMedicine 2020, 57, 102833. [Google Scholar] [CrossRef]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Monteagudo, L.A.; Boothby, A.; Gertner, E. Continuous Intravenous Anakinra Infusion to Calm the Cytokine Storm in Macrophage Activation Syndrome. ACR Open Rheumatol. 2020, 2, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Di Virgilio, F.; Tang, Y.; Sarti, A.C.; Rossato, M. A rationale for targeting the P2X7 receptor in Coronavirus disease 19. Br. J. Pharmacol. 2020, 177, 4990–4994. [Google Scholar] [CrossRef] [PubMed]
- Illes, P. P2X7 Receptors Amplify CNS Damage in Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 5996. [Google Scholar] [CrossRef]
- Illes, P.; Rubini, P.; Huang, L.; Tang, Y. The P2X7 receptor: A new therapeutic target in Alzheimer’s disease. Expert Opin. Ther. Targets 2019, 23, 165–176. [Google Scholar] [CrossRef]
- Francistiova, L.; Bianchi, C.; Di Lauro, C.; Sebastian-Serrano, A.; de Diego-Garcia, L.; Kobolak, J.; Dinnyés, A.; Díaz-Hernández, M. The Role of P2X7 Receptor in Alzheimer’s Disease. Front. Mol. Neurosci. 2020, 13, 94. [Google Scholar] [CrossRef]
- Wang, X.H.; Xie, X.; Luo, X.G.; Shang, H.; He, Z.Y. Inhibiting purinergic P2X7 receptors with the antagonist brilliant blue G is neuroprotective in an intranigral lipopolysaccharide animal model of Parkinson’s disease. Mol. Med. Rep. 2017, 15, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Giacomelli, A.; Albino, M.; de Souza, H.D.N.; Correa-Velloso, J.; de Jesus Santos, A.P.; Baranova, J.; Ulrich, H. P2Y6 and P2X7 Receptor Antagonism Exerts Neuroprotective/ Neuroregenerative Effects in an Animal Model of Parkinson’s Disease. Front. Cell. Neurosci. 2019, 13, 476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apolloni, S.; Amadio, S.; Parisi, C.; Matteucci, A.; Potenza, R.L.; Armida, M.; Popoli, P.; D’Ambrosi, N.; Volonté, C. Spinal cord pathology is ameliorated by P2X7 antagonism in a, S.O.D1-mutant mouse model of amyotrophic lateral sclerosis. Dis. Models Mech. 2014, 7, 1101–1109. [Google Scholar]
- Fabbrizio, P.; Amadio, S.; Apolloni, S.; Volonte, C. P2X7 Receptor Activation Modulates Autophagy in, S.O.D1-G93A Mouse Microglia. Front. Cell. Neurosci. 2017, 11, 249. [Google Scholar] [CrossRef] [Green Version]
- Beaino, W.; Janssen, B.; Kooij, G.; van der Pol, S.M.A.; van Het, H.B.; van Horssen, J.; Windhorst, A.D.; de Vries, H.E. Purinergic receptors P2Y12R and P2X7R: Potential targets for, P.E.T imaging of microglia phenotypes in multiple sclerosis. J. Neuroinflammation 2017, 14, 259. [Google Scholar] [CrossRef]
- Grygorowicz, T.; Struzynska, L. Early P2X7R-dependent activation of microglia during the asymptomatic phase of autoimmune encephalomyelitis. Inflammopharmacology 2019, 27, 129–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jimenez-Pacheco, A.; Diaz-Hernandez, M.; Arribas-Blazquez, M.; Sanz-Rodriguez, A.; Olivos-Ore, L.A.; Artalejo, A.R.; Alves, M.; Letavic, M.; Miras-Portugal, M.T.; Conroy, R.M.; et al. Transient P2X7 Receptor Antagonism Produces Lasting Reductions in Spontaneous Seizures and Gliosis in Experimental Temporal Lobe Epilepsy. J. Neurosci. 2016, 36, 5920–5932. [Google Scholar] [CrossRef]
- Beamer, E.; Fischer, W.; Engel, T. The ATP-Gated P2X7 Receptor As a Target for the Treatment of Drug-Resistant Epilepsy. Front. Neurosci. 2017, 11, 21. [Google Scholar] [CrossRef] [Green Version]
- Melani, A.; Amadio, S.; Gianfriddo, M.; Vannucchi, M.G.; Volonte, C.; Bernardi, G.; Pedata, F.; Sancesario, G. P2X7 receptor modulation on microglial cells and reduction of brain infarct caused by middle cerebral artery occlusion in rat. J. Cereb. Blood Flow Metab. 2006, 26, 974–982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartlett, R.; Stokes, L.; Sluyter, R. The P2X7 receptor channel: Recent developments and the use of P2X7 antagonists in models of disease. Pharmacol. Rev. 2014, 66, 638–675. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.; Chu, J.; Singh, S.; Salter, R.D. Identification and characterization of a novel variant of the human P2X7 receptor resulting in gain of function. Purinergic Signal. 2010, 6, 31–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sluyter, R.; Shemon, A.N.; Wiley, J.S. Glu496 to Ala polymorphism in the P2X7 receptor impairs, ATP-induced IL-1 beta release from human monocytes. J. Immunol. 2004, 172, 3399–3405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, B.J.; Zhang, W.; Worthington, R.A.; Sluyter, R.; Dao-Ung, P.; Petrou, S.; Barden, J.A.; Wiley, J.S. A Glu-496 to Ala polymorphism leads to loss of function of the human P2X7 receptor. J. Biol. Chem. 2001, 276, 11135–11142. [Google Scholar] [CrossRef] [Green Version]
- Stokes, L.; Fuller, S.J.; Sluyter, R.; Skarratt, K.K.; Gu, B.J.; Wiley, J.S. Two haplotypes of the P2X7 receptor containing the Ala-348 to Thr polymorphism exhibit a gain-of-function effect and enhanced interleukin-1beta secretion. FASEB J. 2010, 24, 2916–2927. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.J.; Sluyter, R.; Skarratt, K.K.; Shemon, A.N.; Dao-Ung, L.P.; Fuller, S.J.; Barden, J.A.; Clarke, A.L.; Petrou, S.; Wiley, J.S. An Arg307 to Gln polymorphism within the ATP-binding site causes loss of function of the human P2X7 receptor. J. Biol. Chem. 2004, 279, 31287–31295. [Google Scholar] [CrossRef] [Green Version]
- Xiao, J.; Sun, L.; Yan, H.; Jiao, W.; Miao, Q.; Feng, W.; Wu, X.; Gu, Y.; Jiao, A.; Guo, Y.; et al. Metaanalysis of P2X7 gene polymorphisms and tuberculosis susceptibility. FEMS Immunol. Med. Microbiol. 2010, 60, 165–170. [Google Scholar] [CrossRef] [Green Version]
- Britton, W.J.; Fernando, S.L.; Saunders, B.M.; Sluyter, R.; Wiley, J.S. The genetic control of susceptibility to Mycobacterium tuberculosis. Novartis Found. Symp. 2007, 281, 79–89. [Google Scholar]
- Lees, M.P.; Fuller, S.J.; McLeod, R.; Boulter, N.R.; Miller, C.M.; Zakrzewski, A.M.; Mui, E.J.; Witola, W.H.; Coyne, J.J.; Hargrave, A.C.; et al. P2X7 receptor-mediated killing of an intracellular parasite, Toxoplasma gondii, by human and murine macrophages. J. Immunol. 2010, 184, 7040–7046. [Google Scholar] [CrossRef] [Green Version]
- Wiley, J.S.; Sluyter, R.; Gu, B.J.; Stokes, L.; Fuller, S.J. The human P2X7 receptor and its role in innate immunity. Tissue Antigens 2011, 78, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.J.; Baird, P.N.; Vessey, K.A.; Skarratt, K.K.; Fletcher, E.L.; Fuller, S.J.; Richardson, A.J.; Guymer, R.H.; Wiley, J.S. A rare functional haplotype of the P2RX4 and P2RX7 genes leads to loss of innate phagocytosis and confers increased risk of age-related macular degeneration. FASEB J. 2013, 27, 1479–1487. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, N.R. Role of the purinergic P2X receptors in osteoclast pathophysiology. Curr. Opin. Pharmacol. 2019, 47, 97–101. [Google Scholar] [CrossRef]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef]
- Jorgensen, N.R.; Syberg, S.; Ellegaard, M. The role of P2X receptors in bone biology. Curr. Med. Chem. 2015, 22, 902–914. [Google Scholar] [CrossRef] [PubMed]
- Varley, I.; Hughes, D.C.; Greeves, J.P.; Fraser, W.D.; Sale, C. SNPs in the vicinity of P2X7R, RANK/RANKL/OPG and Wnt signalling pathways and their association with bone phenotypes in academy footballers. Bone 2018, 108, 179–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McQuillin, A.; Bass, N.J.; Choudhury, K.; Puri, V.; Kosmin, M.; Lawrence, J.; Curtis, D.; Gurling, H.M. Case-control studies show that a non-conservative amino-acid change from a glutamine to arginine in the P2RX7 purinergic receptor protein is associated with both bipolar- and unipolar-affective disorders. Mol. Psychiatry 2009, 14, 614–620. [Google Scholar] [CrossRef]
- Czamara, D.; Müller-Myhsok, B.; Lucae, S. The P2RX7 polymorphism rs2230912 is associated with depression: A meta-analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 82, 272–277. [Google Scholar] [CrossRef]
- Green, E.K.; Grozeva, D.; Raybould, R.; Elvidge, G.; Macgregor, S.; Craig, I.; Farmer, A.; McGuffin, P.; Forty, L.; Jones, L.; et al. P2RX7: A bipolar and unipolar disorder candidate susceptibility gene? Am. J. Med. Genet. B Neuropsychiatr. Genet. 2009, 150B, 1063–1069. [Google Scholar] [CrossRef]
- Feng, W.P.; Zhang, B.; Li, W.; Liu, J. Lack of association of P2RX7 gene rs2230912 polymorphism with mood disorders: A meta-analysis. PLoS ONE 2014, 9, e88575. [Google Scholar] [CrossRef] [Green Version]
- Roger, S.; Mei, Z.Z.; Baldwin, J.M.; Dong, L.; Bradley, H.; Baldwin, S.A.; Surprenant, A.; Jiang, L.H. Single nucleotide polymorphisms that were identified in affective mood disorders affect ATP-activated P2X7 receptor functions. J. Psychiatr. Res. 2010, 44, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Aprile-Garcia, F.; Metzger, M.W.; Paez-Pereda, M.; Stadler, H.; Acuna, M.; Liberman, A.C.; Senin, S.A.; Gerez, J.; Hoijman, E.; Refojo, D.; et al. Co-Expression of Wild-Type P2X7R with Gln460Arg Variant Alters Receptor Function. PLoS ONE 2016, 11, e0151862. [Google Scholar] [CrossRef] [PubMed]
- Metzger, M.W.; Walser, S.M.; Dedic, N.; Aprile-Garcia, F.; Jakubcakova, V.; Adamczyk, M.; Webb, K.J.; Uhr, M.; Refojo, D.; Schmidt, M.V.; et al. Heterozygosity for the Mood Disorder-Associated Variant Gln460Arg Alters P2X7 Receptor Function and Sleep Quality. J. Neurosci. 2017, 37, 11688–11700. [Google Scholar] [CrossRef] [PubMed]
- Sluyter, R.; Stokes, L.; Fuller, S.J.; Skarratt, K.K.; Gu, B.J.; Wiley, J.S. Functional significance of P2RX7 polymorphisms associated with affective mood disorders. J. Psychiatr. Res. 2010, 44, 1116–1117. [Google Scholar] [CrossRef]
- Backlund, L.; Nikamo, P.; Hukic, D.S.; Ek, I.R.; Träskman-Bendz, L.; Landén, M.; Edman, G.; Schalling, M.; Frisén, L.; Osby, U. Cognitive manic symptoms associated with the P2RX7 gene in bipolar disorder. Bipolar Disord. 2011, 13, 500–508. [Google Scholar] [CrossRef]
- Gu, B.J.; Field, J.; Dutertre, S.; Ou, A.; Kilpatrick, T.J.; Lechner-Scott, J.; Scott, R.; Lea, R.; Taylor, B.V.; Stankovich, J.; et al. A rare P2X7 variant Arg307Gln with absent pore formation function protects against neuroinflammation in multiple sclerosis. Hum. Mol. Genet. 2015, 24, 5644–5654. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, W.; Rubini, P.; Tang, Y.; Engel, T.; Illes, P. Inherent P2X7 Receptors Regulate Macrophage Functions during Inflammatory Diseases. Int. J. Mol. Sci. 2022, 23, 232. https://doi.org/10.3390/ijms23010232
Ren W, Rubini P, Tang Y, Engel T, Illes P. Inherent P2X7 Receptors Regulate Macrophage Functions during Inflammatory Diseases. International Journal of Molecular Sciences. 2022; 23(1):232. https://doi.org/10.3390/ijms23010232
Chicago/Turabian StyleRen, Wenjing, Patrizia Rubini, Yong Tang, Tobias Engel, and Peter Illes. 2022. "Inherent P2X7 Receptors Regulate Macrophage Functions during Inflammatory Diseases" International Journal of Molecular Sciences 23, no. 1: 232. https://doi.org/10.3390/ijms23010232
APA StyleRen, W., Rubini, P., Tang, Y., Engel, T., & Illes, P. (2022). Inherent P2X7 Receptors Regulate Macrophage Functions during Inflammatory Diseases. International Journal of Molecular Sciences, 23(1), 232. https://doi.org/10.3390/ijms23010232







