The Insulin-like Growth Factor Signalling Pathway in Cardiac Development and Regeneration
Abstract
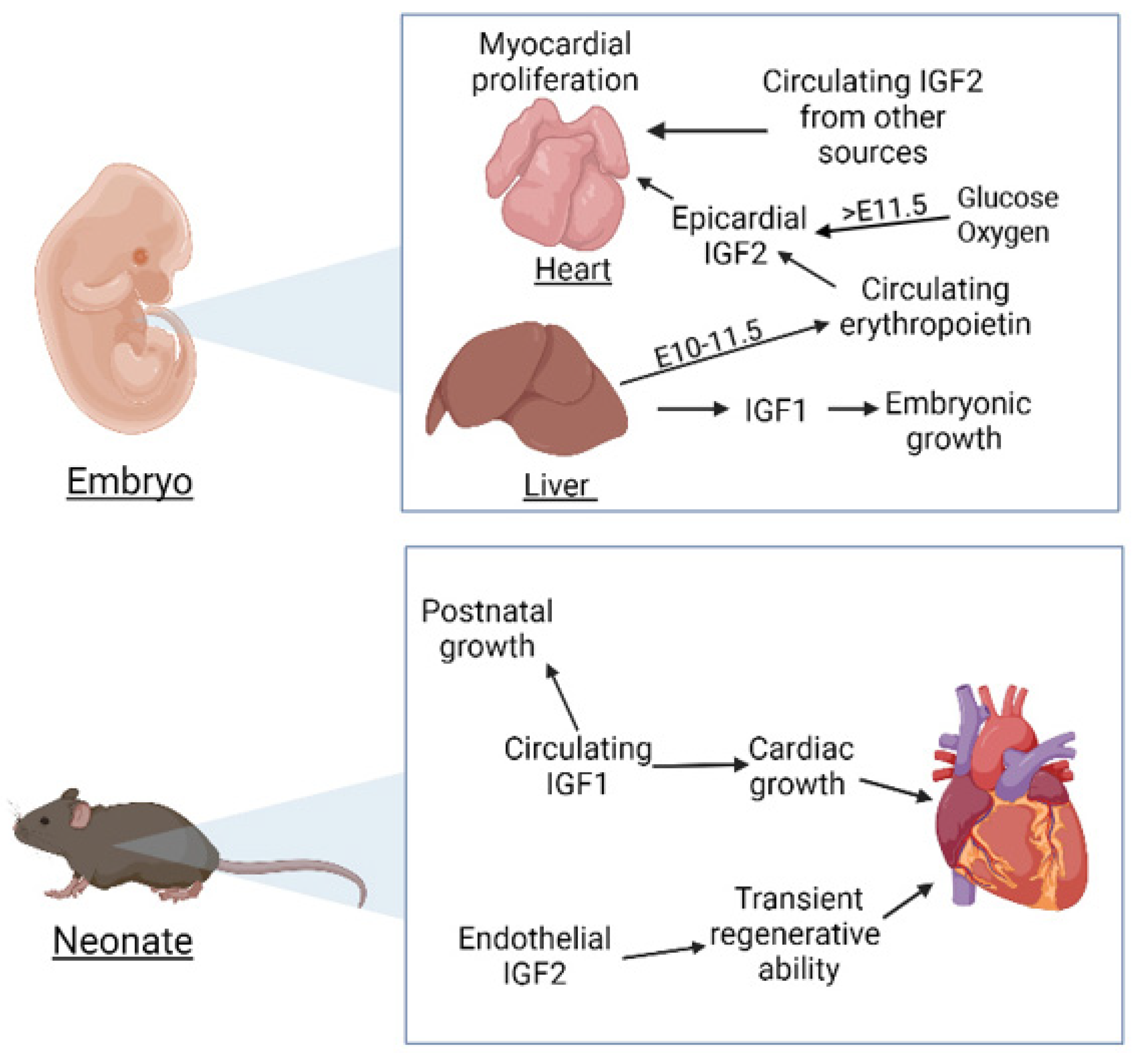
:1. Introduction
2. Insulin-like Growth Factors in Cardiac Development
3. Insulin and IGF Receptors in Cardiac Development
4. MicroRNA Regulation of the IGF Signalling Pathway
5. The IGF-Binding Proteins Family
6. The Insulin Receptor Substrate Family
7. Alternative IGF1R Ligands
8. IGF Signalling in Differentiation of Cardiomyocytes from Stem Cells
9. IGF Signalling in Cardiac Regeneration
10. Conclusions and Future Directions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CIRKO | Cardiomyocyte-restricted deletion of insulin receptors |
| CPC | Cardiac progenitor cell |
| DIPK2A | Divergent protein kinase domain 2A |
| DUSP5 | Dual Specificity Phosphatase 5 |
| E | Embryonic day |
| EPO | Erythropoietin |
| ERK | extracellular signal-regulated kinase 1 |
| ESC | Embryonic stem cells |
| FAK | Focal adhesion kinase |
| GSK3 | glycogen synthase kinase 3 |
| HASF | Hypoxia and Akt-induced stem cell factor |
| hESC | Human embryonic stem cells |
| IGF | Insulin-like growth factors |
| Igf | Insulin-like growth factors gene |
| IGFBP | Insulin-like growth factors binding proteins |
| IGFR | Insulin-like growth factor receptor |
| INSR | Insulin receptor |
| Insr | Insulin receptor gene |
| IRS | Insulin receptor substrate |
| M6P | Mannose-6-phosphate |
| MAPK | Mitogen activated protein kinases |
| MI2RKO | Double knockout of both receptors in cardiomyocytes |
| miR | MicroRNAs |
| miRNA | MicroRNAs |
| mRNA | Messenger RNA |
| PI3K | phosphatidylinositol 3-kinase |
| P | Postnatal day |
| RA | Retinoic acid |
| Raldh2 | Retinaldehyde dehydrogenase 2 gene |
| T3 | Thyroid hormone |
| YAP | Yesassociated protein |
References
- Tyser, R.C.; Miranda, A.M.; Chen, C.M.; Davidson, S.M.; Srinivas, S.; Riley, P.R. Calcium handling precedes cardiac differentiation to initiate the first heartbeat. Elife 2016, 5, e17113. [Google Scholar] [CrossRef]
- Oparil, S.; Bishop, S.P.; Clubb, F.J., Jr. Myocardial cell hypertrophy or hyperplasia. Hypertension 1984, 6 Pt 2, III38–III43. [Google Scholar] [CrossRef]
- Smith-Vikos, T.; Slack, F.J. MicroRNAs and their roles in aging. J. Cell Sci. 2012, 125 Pt 1, 7–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, H.J.; Suh, Y. Regulation of IGF -1 signaling by microRNAs. Front. Genet. 2015, 5, 472. [Google Scholar] [CrossRef] [Green Version]
- LeRoith, D.; Roberts, C.T., Jr. The insulin-like growth factor system and cancer. Cancer Lett. 2003, 195, 127–137. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.P.; Baker, J.; Perkins, A.S.; Robertson, E.J.; Efstratiadis, A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell 1993, 75, 59–72. [Google Scholar] [CrossRef]
- Powell-Braxton, L.; Hollingshead, P.; Warburton, C.; Dowd, M.; Pitts-Meek, S.; Dalton, D.; Gillett, N.; Stewart, T.A. IGF-I is required for normal embryonic growth in mice. Genes Dev. 1993, 7, 2609–2617. [Google Scholar] [CrossRef] [PubMed]
- McMullen, J.R.; Shioi, T.; Huang, W.Y.; Zhang, L.; Tarnavski, O.; Bisping, E.; Schinke, M.; Kong, S.; Sherwood, M.C.; Brown, J.; et al. The insulin-like growth factor 1 receptor induces physiological heart growth via the phosphoinositide 3-kinase(p110alpha) pathway. J. Biol. Chem. 2004, 279, 4782–4793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Troncoso, R.; Ibarra, C.; Vicencio, J.M.; Jaimovich, E.; Lavandero, S. New insights into IGF-1 signaling in the heart. Trends Endocrinol. Metab. 2014, 25, 128–372. [Google Scholar] [CrossRef]
- Lee, W.S.; Kim, J. Insulin-like growth factor-1 signaling in cardiac aging. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864 Pt B, 1931–1938. [Google Scholar] [CrossRef]
- Gong, Y.; Yang, J.; Liu, Q.; Cai, J.; Zheng, Y.; Zhang, Y.; Yu, D.; Liu, H.; Zhang, Z. IGF1 Knockdown Hinders Myocardial Development through Energy Metabolism Dysfunction Caused by ROS-Dependent FOXO Activation in the Chicken Heart. Oxid. Med. Cell. Longev. 2019, 2019, 7838754. [Google Scholar] [CrossRef]
- Tsai, T.C.; Shih, C.C.; Chien, H.P.; Yang, A.H.; Lu, J.K.; Lu, J.H. Anti-apoptotic effects of IGF-I on mortality and dysmorphogenesis in tbx5-deficient zebrafish embryos. BMC Dev. Biol. 2018, 18, 5. [Google Scholar] [CrossRef] [Green Version]
- Chao, W.; D’Amore, P.A. IGF2: Epigenetic regulation and role in development and disease. Cytokine Growth Factor Rev. 2008, 19, 111–120. [Google Scholar] [CrossRef] [Green Version]
- Baral, K.; Rotwein, P. The insulin-like growth factor 2 gene in mammals: Organizational complexity within a conserved locus. PLoS ONE 2019, 14, e0219155. [Google Scholar] [CrossRef]
- DeChiara, T.M.; Robertson, E.J.; Efstratiadis, A. Parental imprinting of the mouse insulin-like growth factor II gene. Cell 1991, 64, 849–859. [Google Scholar] [CrossRef]
- Liu, J.L.; LeRoith, D. Insulin-like growth factor I is essential for postnatal growth in response to growth hormone. Endocrinology 1999, 140, 5178–5184. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, J.; Powell-Braxton, L.; Bondy, C. Effects of Igf1 gene deletion on postnatal growth patterns. Endocrinology 1999, 140, 3391–3394. [Google Scholar] [CrossRef]
- Engelmann, G.L.; Boehm, K.D.; Haskell, J.F.; Khairallah, P.A.; Ilan, J. Insulin-like growth factors and neonatal cardiomyocyte development: Ventricular gene expression and membrane receptor variations in normotensive and hypertensive rats. Mol. Cell. Endocrinol. 1989, 63, 1–14. [Google Scholar] [CrossRef]
- Li, P.; Cavallero, S.; Gu, Y.; Chen, T.H.; Hughes, J.; Hassan, A.B.; Brüning, J.C.; Pashmforoush, M.; Sucov, H.M. IGF signaling directs ventricular cardiomyocyte proliferation during embryonic heart development. Development 2011, 138, 1795–1805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meganathan, K.; Sotiriadou, I.; Natarajan, K.; Hescheler, J.; Sachinidis, A. Signaling molecules, transcription growth factors and other regulators revealed from in-vivo and in-vitro models for the regulation of cardiac development. Int. J. Cardiol. 2015, 183, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yan, H.; Dawes, N.J.; Mottino, G.A.; Frank, J.S.; Zhu, H. Insulin-like growth factor II induces DNA synthesis in fetal ventricular myocytes in vitro. Circ. Res. 1996, 79, 716–726. [Google Scholar] [CrossRef]
- Laustsen, P.G.; Russell, S.J.; Cui, L.; Entingh-Pearsall, A.; Holzenberger, M.; Liao, R.; Kahn, C.R. Essential role of insulin and insulin-like growth factor 1 receptor signaling in cardiac development and function. Mol. Cell. Biol. 2007, 27, 1649–1664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans-Anderson, H.J.; Alfieri, C.M.; Yutzey, K.E. Regulation of cardiomyocyte proliferation and myocardial growth during development by FOXO transcription factors. Circ. Res. 2008, 102, 686–694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colao, A.; Vitale, G.; Pivonello, R.; Ciccarelli, A.; Di Somma, C.; Lombardi, G. The heart: An end-organ of GH action. Eur. J. Endocrinol. 2004, 151 (Suppl. 1), S93–S101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subbarayan, S.K.; Fleseriu, M.; Gordon, M.B.; Brzana, J.A.; Kennedy, L.; Faiman, C.; Hatipoglu, B.A.; Prayson, R.A.; Delashaw, J.B.; Weil, R.J.; et al. Serum IGF-1 in the diagnosis of acromegaly and the profile of patients with elevated IGF-1 but normal glucose-suppressed growth hormone. Endocr. Pract. 2012, 18, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Brade, T.; Kumar, S.; Cunningham, T.J.; Chatzi, C.; Zhao, X.; Cavallero, S.; Li, P.; Sucov, H.M.; Ruiz-Lozano, P.; Duester, G. Retinoic acid stimulates myocardial expansion by induction of hepatic erythropoietin which activates epicardial Igf2. Development 2011, 138, 139–148. [Google Scholar] [CrossRef] [Green Version]
- Shen, H.; Cavallero, S.; Estrada, K.D.; Sandovici, I.; Kumar, S.R.; Makita, T.; Lien, C.L.; Constancia, M.; Sucov, H.M. Extracardiac control of embryonic cardiomyocyte proliferation and ventricular wall expansion. Cardiovasc. Res. 2015, 105, 271–278. [Google Scholar] [CrossRef] [Green Version]
- Barak, Y.; Hemberger, M.; Sucov, H.M. Phases and Mechanisms of Embryonic Cardiomyocyte Proliferation and Ventricular Wall Morphogenesis. Pediatr. Cardiol. 2019, 40, 1359–1366. [Google Scholar] [CrossRef]
- Federici, M.; Porzio, O.; Zucaro, L.; Fusco, A.; Borboni, P.; Lauro, D.; Sesti, G. Distribution of insulin/insulin-like growth factor-I hybrid receptors in human tissues. Mol. Cell. Endocrinol. 1997, 129, 121–126. [Google Scholar] [CrossRef]
- Federici, M.; Porzio, O.; Zucaro, L.; Giovannone, B.; Borboni, P.; Marini, M.A.; Lauro, D.; Sesti, G. Increased abundance of insulin/IGF-I hybrid receptors in adipose tissue from NIDDM patients. Mol. Cell. Endocrinol. 1997, 135, 41–47. [Google Scholar] [CrossRef]
- Werner, H.; Sarfstein, R.; Laron, Z. The role of nuclear insulin and igf1 receptors in metabolism and cancer. Biomolecules 2021, 11, 531. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Shen, H.; Gan, P.; Cavallero, S.; Kumar, S.R.; Lien, C.L.; Sucov, H.M. Differential roles of insulin like growth factor 1 receptor and insulin receptor during embryonic heart development. BMC Dev. Biol. 2019, 19, 5. [Google Scholar] [CrossRef] [Green Version]
- Brüning, J.C.; Michael, M.D.; Winnay, J.N.; Hayashi, T.; Hörsch, D.; Accili, D.; Goodyear, L.J.; Kahn, C.R. A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol. Cell. 1998, 2, 559–569. [Google Scholar] [CrossRef]
- Belke, D.D.; Betuing, S.; Tuttle, M.J.; Graveleau, C.; Young, M.E.; Pham, M.; Zhang, D.; Cooksey, R.C.; McClain, D.A.; Litwin, S.E.; et al. Insulin signaling coordinately regulates cardiac size, metabolism, and contractile protein isoform expression. J. Clin. Investig. 2002, 109, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Xin, M.; Kim, Y.; Sutherland, L.B.; Qi, X.; McAnally, J.; Schwartz, R.J.; Richardson, J.A.; Bassel-Duby, R.; Olson, E.N. Regulation of insulin-like growth factor signaling by Yap governs cardiomyocyte proliferation and embryonic heart size. Sci. Signal. 2011, 4, ra70. [Google Scholar] [CrossRef] [Green Version]
- Von Gise, A.; Lin, Z.; Schlegelmilch, K.; Honor, L.B.; Pan, G.M.; Buck, J.N.; Ma, Q.; Ishiwata, T.; Zhou, B.; Camargo, F.D.; et al. YAP1, the nuclear target of Hippo signaling, stimulates heart growth through cardiomyocyte proliferation but not hypertrophy. Proc. Natl. Acad. Sci. USA 2012, 109, 2394–2399. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.C.; Zhang, L.; McMillen, I.C.; Botting, K.J.; Duffield, J.A.; Zhang, S.; Suter, C.M.; Brooks, D.A.; Morrison, J.L. Fetal growth restriction and the programming of heart growth and cardiac insulin-like growth factor 2 expression in the lamb. J. Physiol. 2011, 589 Pt 19, 4709–4722. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, T.; Eggenschwiler, J.; Fisher, P.; D’Ercole, A.J.; Davenport, M.L.; Efstratiadis, A. Mouse mutants lacking the type 2 IGF receptor (IGF2R) are rescued from perinatal lethality in Igf2 and Igf1r null backgrounds. Dev. Biol. 1996, 177, 517–535. [Google Scholar] [CrossRef] [Green Version]
- Hawkes, C.; Jhamandas, J.H.; Harris, K.H.; Fu, W.; MacDonald, R.G.; Kar, S. Single transmembrane domain insulin-like growth factor-II/mannose-6-phosphate receptor regulates central cholinergic function by activating a G-protein-sensitive, protein kinase C-dependent pathway. J. Neurosci. 2006, 26, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Qin, Q.; Wu, L.; Jing, X.; Deng, S.; She, Q. Insulin-like growth factor 1 receptor signaling regulates embryonic epicardial cell proliferation through focal adhesion kinase pathway. Acta Biochim. Biophys. Sin. 2018, 50, 976–983. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Ransom, J.F.; Li, A.; Vedantham, V.; von Drehle, M.; Muth, A.N.; Tsuchihashi, T.; McManus, M.T.; Schwartz, R.J.; Srivastava, D. Dysregulation of cardiogenesis, cardiac conduction, and cell cycle in mice lacking miRNA-1-2. Cell 2007, 129, 303–317. [Google Scholar] [CrossRef] [Green Version]
- Elia, L.; Contu, R.; Quintavalle, M.; Varrone, F.; Chimenti, C.; Russo, M.A.; Cimino, V.; De Marinis, L.; Frustaci, A.; Catalucci, D.; et al. Reciprocal regulation of microRNA-1 and insulin-like growth factor-1 signal transduction cascade in cardiac and skeletal muscle in physiological and pathological conditions. Circulation 2009, 120, 2377–2385. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, I.; Patel, A.; Sundaresan, N.R.; Gupta, M.P.; Solaro, R.J.; Nagalingam, R.S.; Gupta, M. A novel cardiomyocyte-enriched microRNA, miR-378, targets insulin-like growth factor 1 receptor: Implications in postnatal cardiac remodeling and cell survival. J. Biol. Chem. 2012, 287, 12913–12926. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Zhou, H.; Tang, Q. miR-133: A Suppressor of Cardiac Remodeling? Front. Pharmacol. 2018, 9, 903. [Google Scholar] [CrossRef]
- Huang, M.B.; Xu, H.; Xie, S.J.; Zhou, H.; Qu, L.H. Insulin-like growth factor-1 receptor is regulated by microRNA-133 during skeletal myogenesis. PLoS ONE 2011, 6, e29173. [Google Scholar] [CrossRef] [Green Version]
- Hua, Y.; Zhang, Y.; Ren, J. IGF-1 deficiency resists cardiac hypertrophy and myocardial contractile dysfunction: Role of microRNA-1 and microRNA-133a. J. Cell. Mol. Med. 2012, 16, 83–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, E.K.; Gorospe, M. Minireview: Posttranscriptional regulation of the insulin and insulin-like growth factor systems. Endocrinology 2010, 151, 1403–1408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, L.; Xiong, X.; Liu, Y.; Wang, J. miRNA-1: Functional roles and dysregulation in heart disease. Mol. Biosyst. 2014, 10, 2775–2782. [Google Scholar] [CrossRef] [PubMed]
- Bach, L.A. IGF-binding proteins. J. Mol. Endocrinol. 2018, 61, T11–T28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slater, T.; Haywood, N.J.; Matthews, C.; Cheema, H.; Wheatcroft, S.B. Insulin-like growth factor binding proteins and angiogenesis: From cancer to cardiovascular disease. Cytokine Growth Factor Rev. 2019, 46, 28–35. [Google Scholar] [CrossRef] [Green Version]
- Clemmons, D.R. Insulin-like growth factor binding proteins and their role in controlling IGF actions. Cytokine Growth Factor Rev. 1997, 8, 45–626. [Google Scholar] [CrossRef] [Green Version]
- Kamei, H.; Duan, C. Alteration of organ size and allometric scaling by organ-specific targeting of IGF signaling. Gen. Comp. Endocrinol. 2021, 314, 113922. [Google Scholar] [CrossRef]
- Rhee, S.; Paik, D.T.; Yang, J.Y.; Nagelberg, D.; Williams, I.; Tian, L.; Roth, R.; Chandy, M.; Ban, J.; Belbachir, N.; et al. Endocardial/endothelial angiocrines regulate cardiomyocyte development and maturation and induce features of ventricular non-compaction. Eur. Heart J. 2021, 42, 4264–4276. [Google Scholar] [CrossRef]
- Hoffmann, S.; Schmitteckert, S.; Raedecke, K.; Rheinert, D.; Diebold, S.; Roeth, R.; Weiss, B.; Granzow, M.; Niesler, B.; Griesbeck, A.; et al. Network-driven discovery yields new insight into Shox2-dependent cardiac rhythm control. Biochim. Biophys. Acta Gene Regul. Mech. 2021, 1864, 194702. [Google Scholar] [CrossRef]
- Minato, A.; Ise, H.; Goto, M.; Akaike, T. Cardiac differentiation of embryonic stem cells by substrate immobilization of insulin-like growth factor binding protein 4 with elastin-like polypeptides. Biomaterials 2012, 33, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Yan, Y.; Gong, H.; Fang, B.; Zhou, Y.; Ding, Z.; Yin, P.; Zhang, G.; Ye, Y.; Yang, C.; et al. Insulin-like growth factor binding protein 4 enhances cardiomyocytes induction in murine-induced pluripotent stem cells. J. Cell Biochem. 2014, 115, 1495–1504. [Google Scholar] [CrossRef]
- Zhu, W.; Fan, Y.; Frenzel, T.; Gasmi, M.; Bartus, R.T.; Young, W.L.; Yang, G.Y.; Chen, Y. Insulin growth factor-1 gene transfer enhances neurovascular remodeling and improves long-term stroke outcome in mice. Stroke 2008, 39, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Shaw, L.M. The insulin receptor substrate (IRS) proteins: At the intersection of metabolism and cancer. Cell Cycle 2011, 10, 1750–1756. [Google Scholar] [CrossRef] [Green Version]
- White, M.F. IRS proteins and the common path to diabetes. Am. J. Physiol. Endocrinol. Metab. 2002, 283, E413–E422. [Google Scholar] [CrossRef] [Green Version]
- Qi, Y.; Xu, Z.; Zhu, Q.; Thomas, C.; Kumar, R.; Feng, H.; Dostal, D.E.; White, M.F.; Baker, K.M.; Guo, S. Myocardial loss of IRS1 and IRS2 causes heart failure and is controlled by p38α MAPK during insulin resistance. Diabetes 2013, 62, 3887–3900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beigi, F.; Schmeckpeper, J.; Pow-Anpongkul, P.; Payne, J.A.; Zhang, L.; Zhang, Z.; Huang, J.; Mirotsou, M.; Dzau, V.J. C3orf58, a novel paracrine protein, stimulates cardiomyocyte cell-cycle progression through the PI3K-AKT-CDK7 pathway. Circ. Res. 2013, 113, 372–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.; Guo, J.; Beigi, F.; Hodgkinson, C.P.; Facundo, H.T.; Zhang, Z.; Espinoza-Derout, J.; Zhou, X.; Pratt, R.E.; Mirotsou, M.; et al. HASF is a stem cell paracrine factor that activates PKC epsilon mediated cytoprotection. J. Mol. Cell. Cardiol. 2014, 66, 157–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bareja, A.; Hodgkinson, C.P.; Payne, A.J.; Pratt, R.E.; Dzau, V.J. HASF (C3orf58) is a novel ligand of the insulin-like growth factor 1 receptor. Biochem. J. 2017, 474, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Engels, M.C.; Rajarajan, K.; Feistritzer, R.; Sharma, A.; Nielsen, U.B.; Schalij, M.J.; de Vries, A.A.; Pijnappels, D.A.; Wu, S.M. Insulin-like growth factor promotes cardiac lineage induction in vitro by selective expansion of early mesoderm. Stem Cells 2014, 32, 1493–1502. [Google Scholar] [CrossRef] [Green Version]
- McDevitt, T.C.; Laflamme, M.A.; Murry, C.E. Proliferation of cardiomyocytes derived from human embryonic stem cells is mediated via the IGF/PI 3-kinase/Akt signaling pathway. J. Mol. Cell. Cardiol. 2005, 39, 865–873. [Google Scholar] [CrossRef] [Green Version]
- Didié, M.; Christalla, P.; Rubart, M.; Muppala, V.; Döker, S.; Unsöld, B.; El-Armouche, A.; Rau, T.; Eschenhagen, T.; Schwoerer, A.P.; et al. Parthenogenetic stem cells for tissue-engineered heart repair. J. Clin. Investig. 2013, 123, 1285–1298. [Google Scholar] [CrossRef] [Green Version]
- Sui, Y.; Zhang, W.; Tang, T.; Gao, L.; Cao, T.; Zhu, H.; You, Q.; Yu, B.; Yang, T. Insulin-like growth factor-II overexpression accelerates parthenogenetic stem cell differentiation into cardiomyocytes and improves cardiac function after acute myocardial infarction in mice. Stem Cell Res. Ther. 2020, 11, 86. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Harrison, M.R.; Osorio, A.; Kim, J.; Baugh, A.; Duan, C.; Sucov, H.M.; Lien, C.L. Igf Signaling is Required for Cardiomyocyte Proliferation during Zebrafish Heart Development and Regeneration. PLoS ONE 2013, 8, e67266. [Google Scholar] [CrossRef] [Green Version]
- Shen, H.; Gan, P.; Wang, K.; Darehzereshki, A.; Wang, K.; Kumar, S.R.; Lien, C.L.; Patterson, M.; Tao, G.; Sucov, H.M. Mononuclear diploid cardiomyocytes support neonatal mouse heart regeneration in response to paracrine IGF2 signaling. Elife 2020, 9, e53071. [Google Scholar] [CrossRef]
- Tan, L.; Bogush, N.; Naib, H.; Perry, J.; Calvert, J.W.; Martin, D.I.K.; Graham, R.M.; Naqvi, N.; Husain, A. Redox activation of JNK2α2 mediates thyroid hormone-stimulated proliferation of neonatal murine cardiomyocytes. Sci. Rep. 2019, 9, 17731. [Google Scholar] [CrossRef]
- Bogush, N.; Tan, L.; Naib, H.; Faizullabhoy, E.; Calvert, J.W.; Iismaa, S.E.; Gupta, A.; Ramchandran, R.; Martin, D.I.K.; Graham, R.M.; et al. DUSP5 expression in left ventricular cardiomyocytes of young hearts regulates thyroid hormone (T3)-induced proliferative ERK1/2 signaling. Sci. Rep. 2020, 10, 21918. [Google Scholar] [CrossRef] [PubMed]
- Andrade, D.; Oliveira, G.; Menezes, L.; Nascimento, A.L.; Carvalho, S.; Stumbo, A.C.; Thole, A.; Garcia-Souza, É.; Moura, A.; Carvalho, L.; et al. Insulin-like growth factor-1 short-period therapy improves cardiomyopathy stimulating cardiac progenitor cells survival in obese mice. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 151–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xin, M.; Kim, Y.; Sutherland, L.B.; Murakami, M.; Qi, X.; McAnally, J.; Porrello, E.R.; Mahmoud, A.I.; Tan, W.; Shelton, J.M.; et al. Hippo pathway effector Yap promotes cardiac regeneration. Proc. Natl. Acad. Sci. USA 2013, 110, 13839–13844. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz del Moral, S.; Benaouicha, M.; Muñoz-Chápuli, R.; Carmona, R. The Insulin-like Growth Factor Signalling Pathway in Cardiac Development and Regeneration. Int. J. Mol. Sci. 2022, 23, 234. https://doi.org/10.3390/ijms23010234
Díaz del Moral S, Benaouicha M, Muñoz-Chápuli R, Carmona R. The Insulin-like Growth Factor Signalling Pathway in Cardiac Development and Regeneration. International Journal of Molecular Sciences. 2022; 23(1):234. https://doi.org/10.3390/ijms23010234
Chicago/Turabian StyleDíaz del Moral, Sandra, Maha Benaouicha, Ramón Muñoz-Chápuli, and Rita Carmona. 2022. "The Insulin-like Growth Factor Signalling Pathway in Cardiac Development and Regeneration" International Journal of Molecular Sciences 23, no. 1: 234. https://doi.org/10.3390/ijms23010234





