Benefits of Usage of Immobilized Silver Nanoparticles as Pseudomonas aeruginosa Antibiofilm Factors
Abstract
:1. Introduction
2. Results
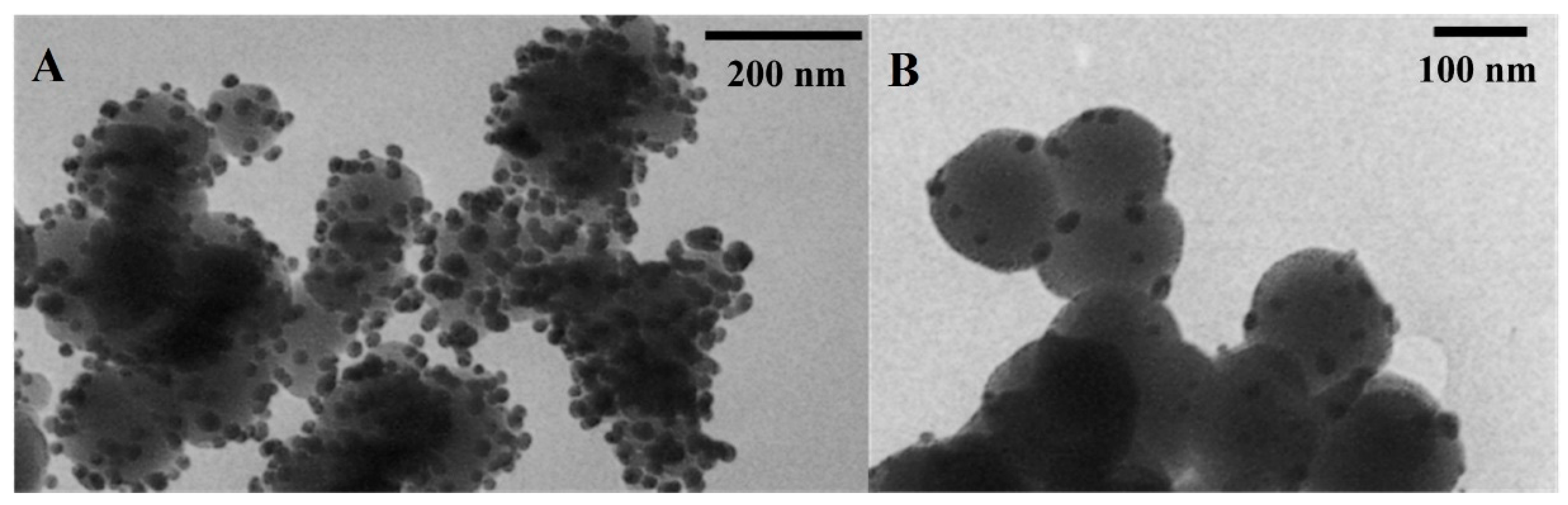
2.1. Silver Nanoparticles Immobilized on SiO2 and TiO2
2.2. Antibiotic Susceptibility
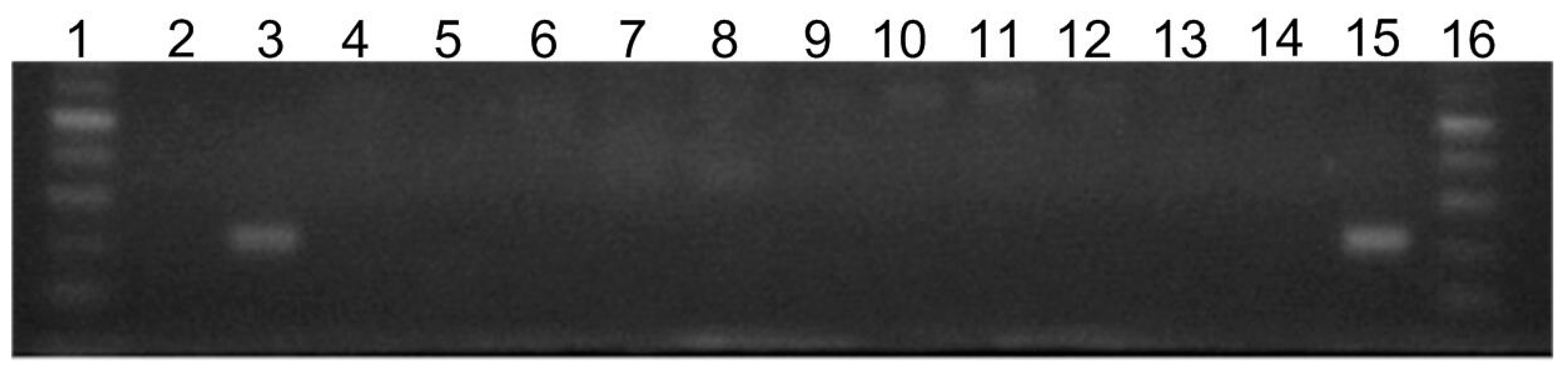
2.3. Prevalence of silE Gene in Tested Bacterial Strains
2.4. Activity of SiO2/Ag0, TiO2/Ag0, SiO2, and TiO2 against Planktonic Forms (Minimal Inhibitory Concentration (MIC) Determination)
2.5. Activity of SiO2/Ag0 and TiO2/Ag0 against Biofilm Forms (Minimal Biofilm Inhibitory Concentration (MBIC) Determination)
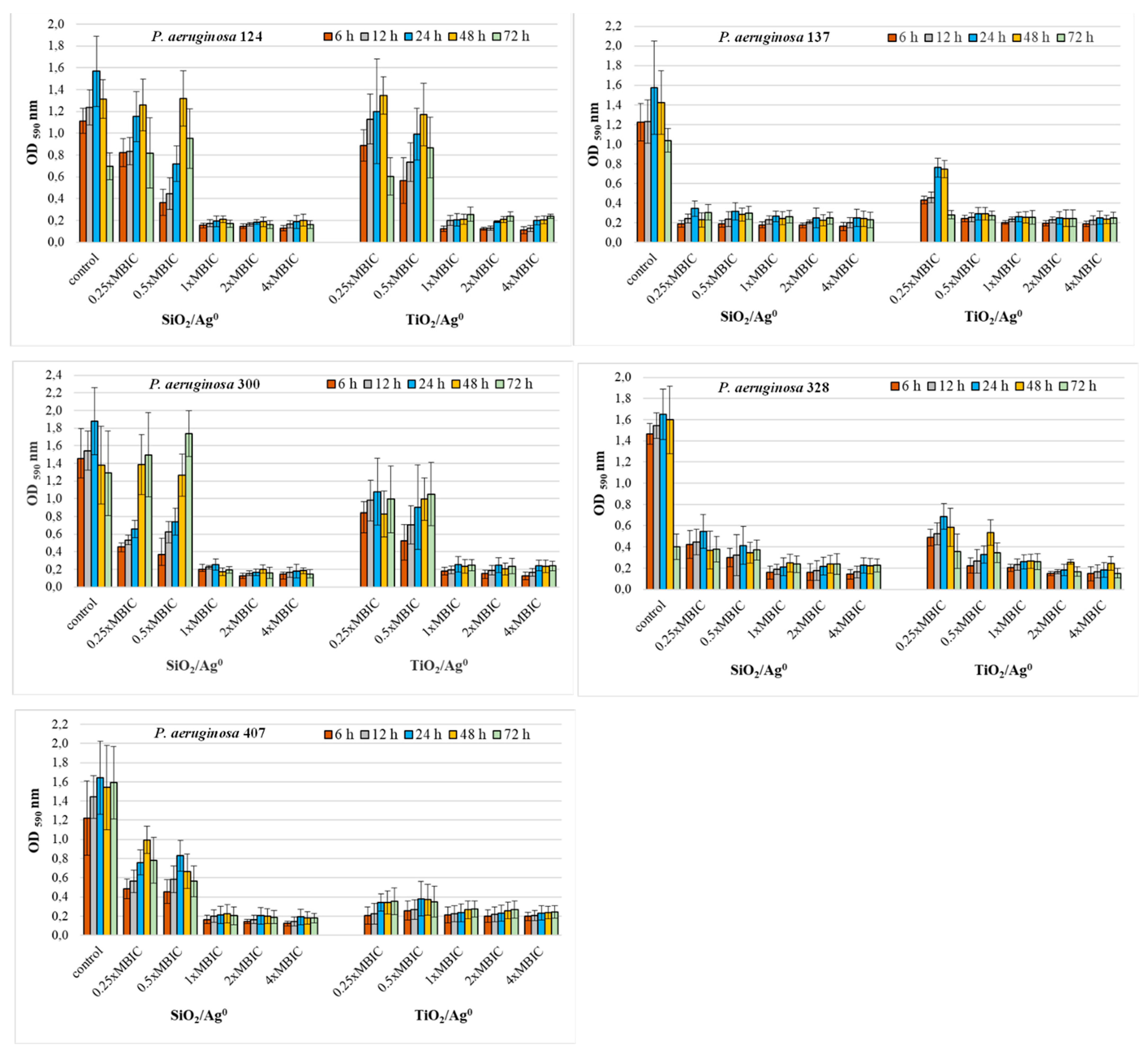
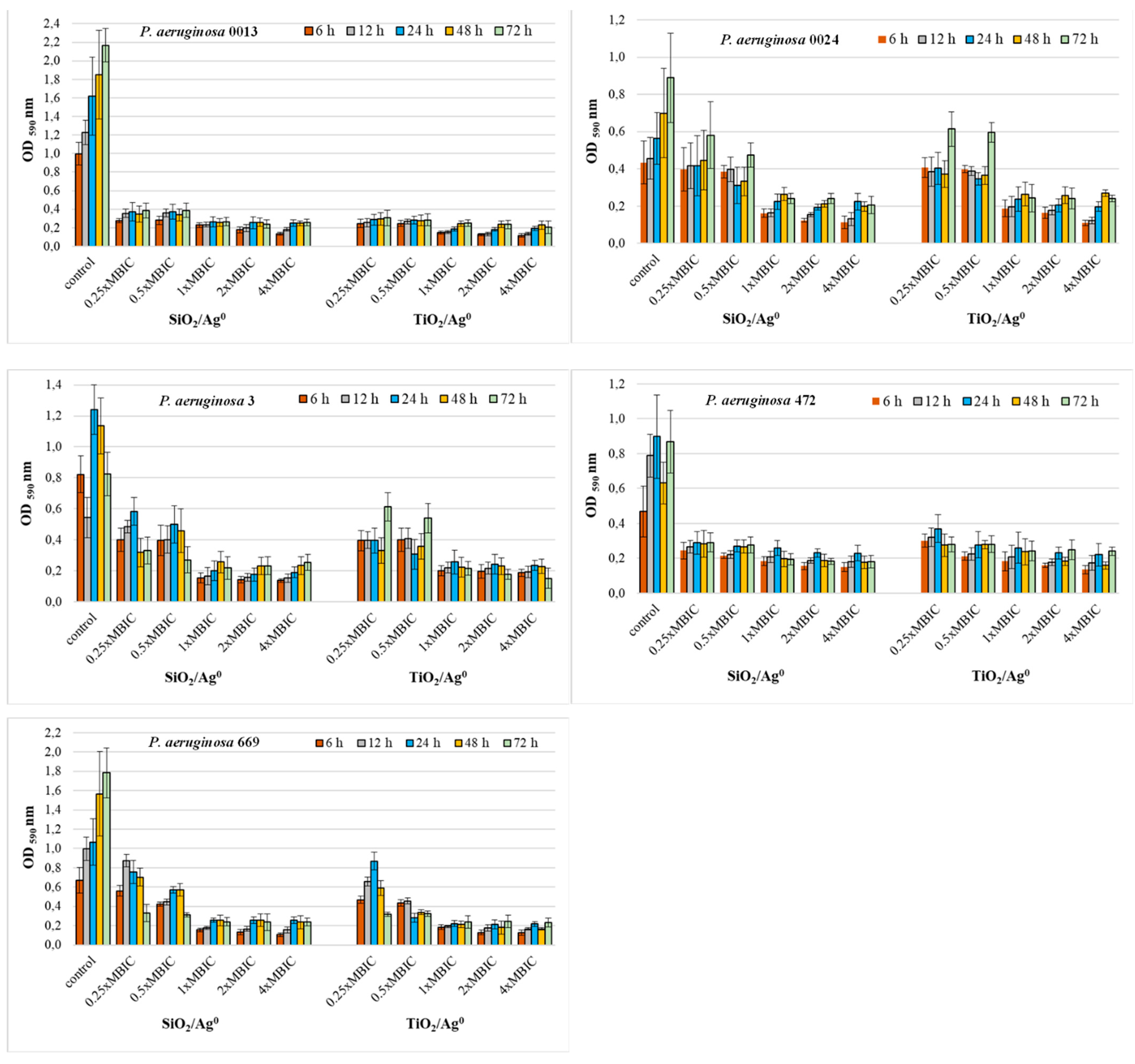
2.6. Antibiofilm Activity of SiO2/Ag0 and TiO2/Ag0
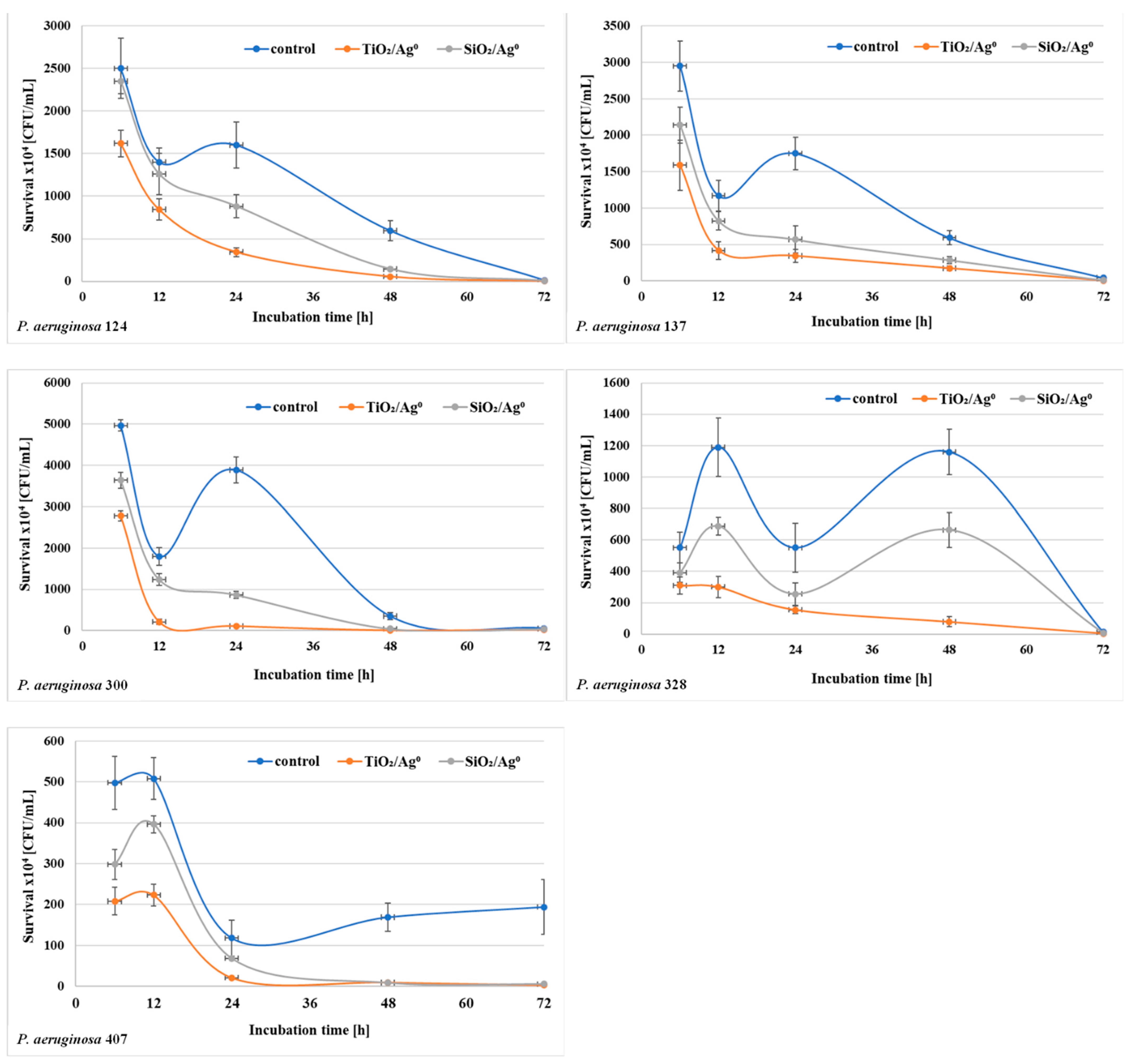
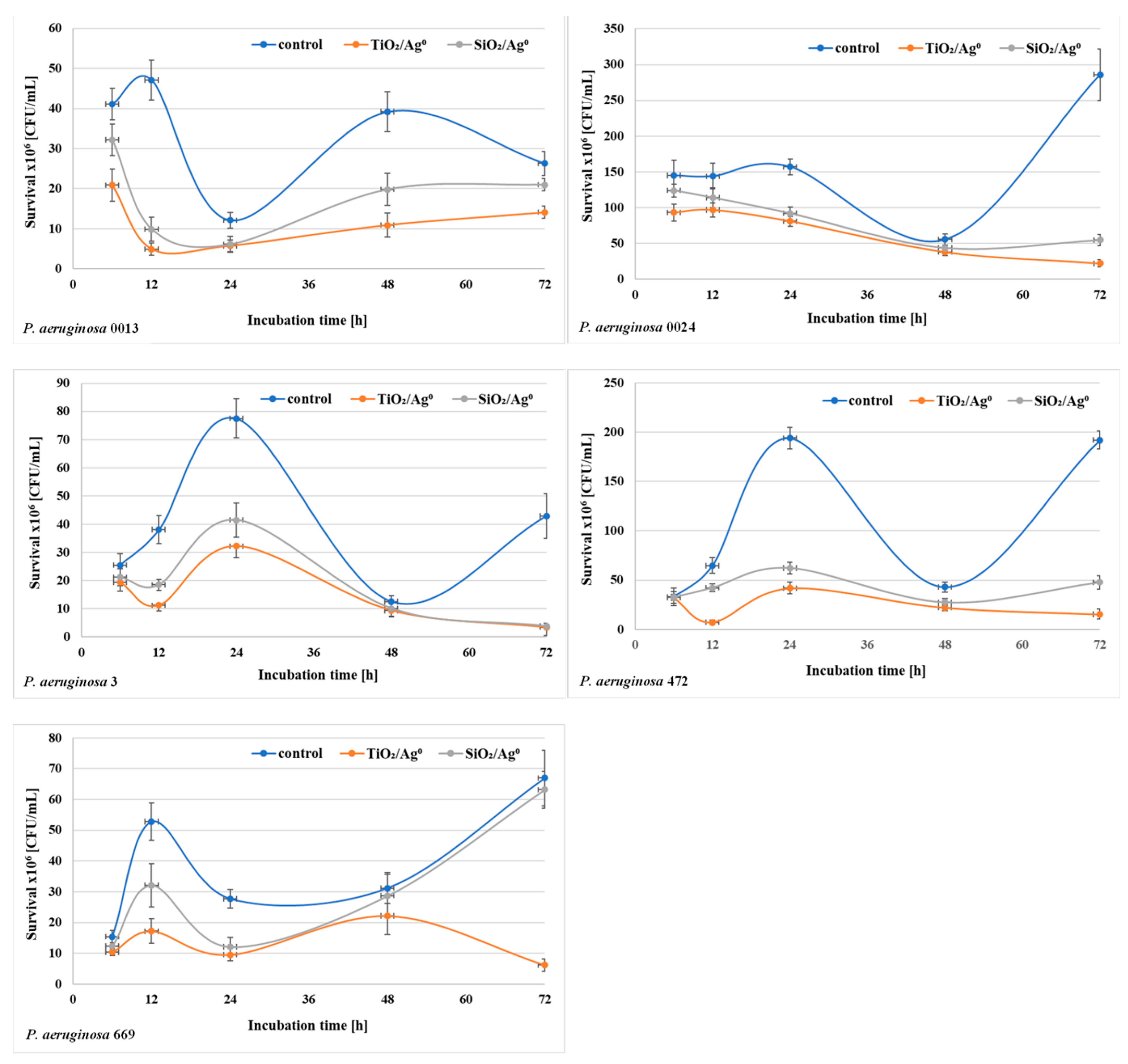
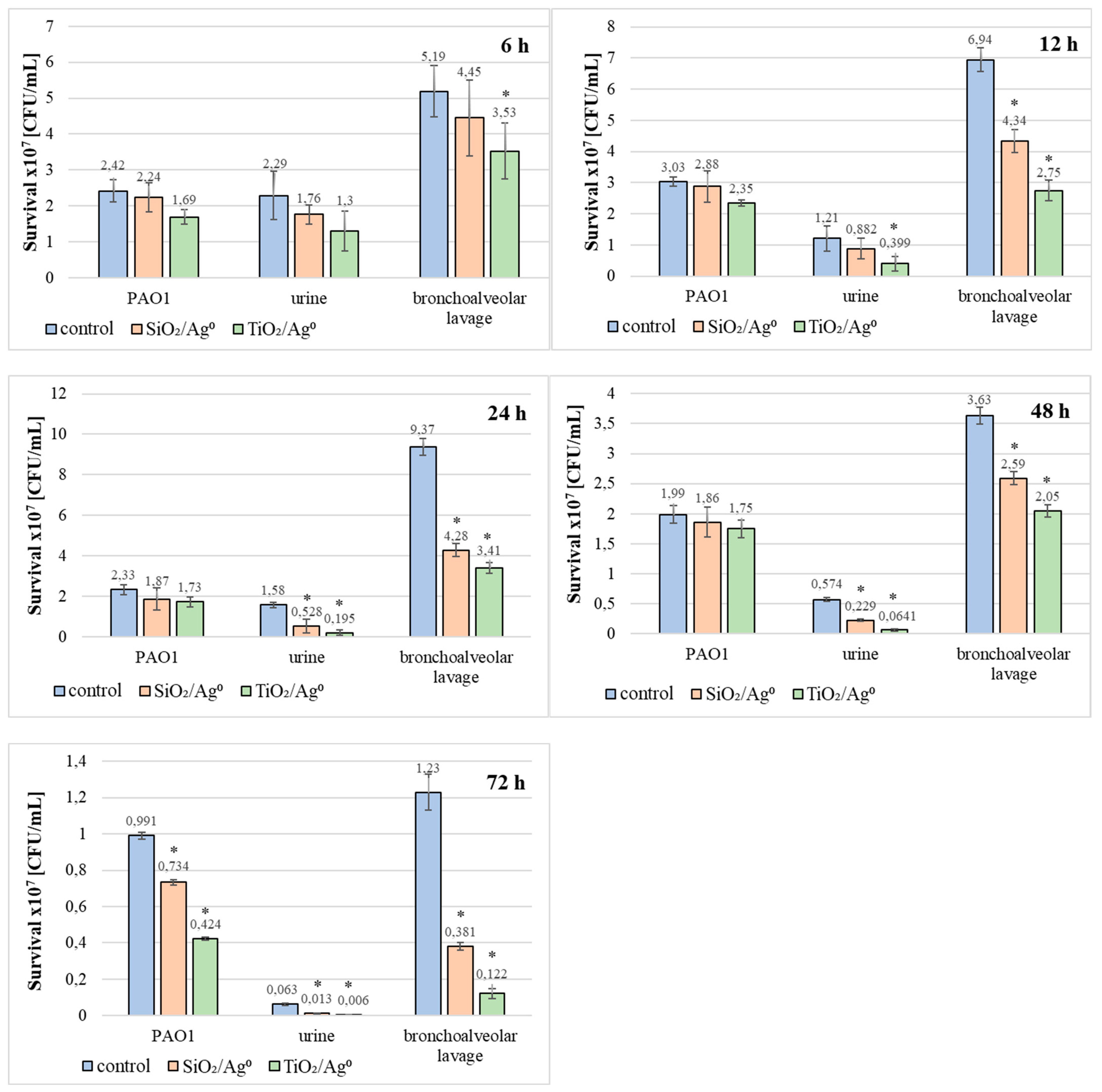
2.7. The Viability of P. aeruginosa Clinical Strains in the Biofilm in the Presence of SiO2/Ag0 and TiO2/Ag0
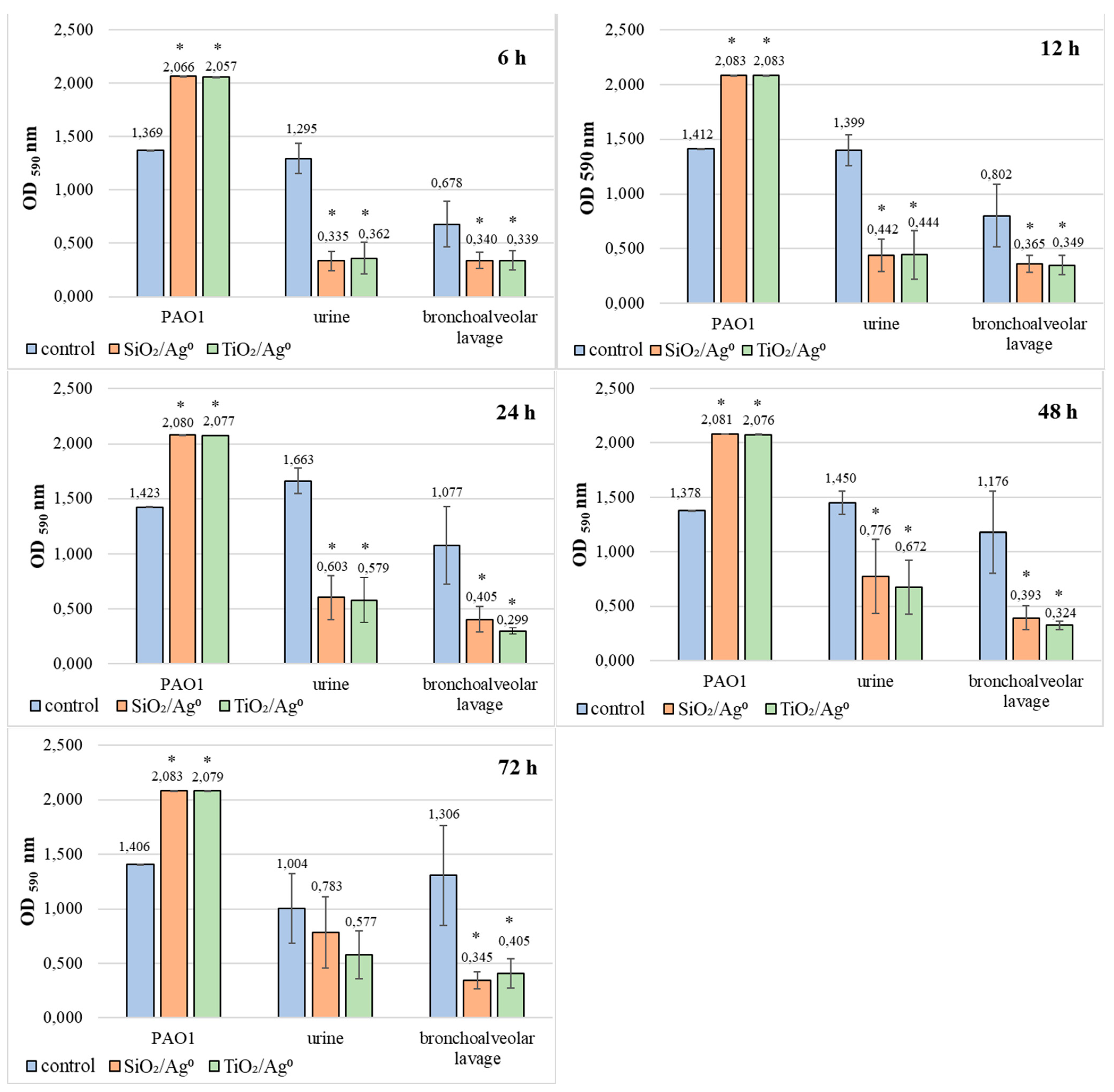
2.8. Comparison of SiO2/Ag0 and TiO2/Ag0 Antibiofilm Activity against the Reference PAO1 Strain and Clinical Strains of P. aeruginosa
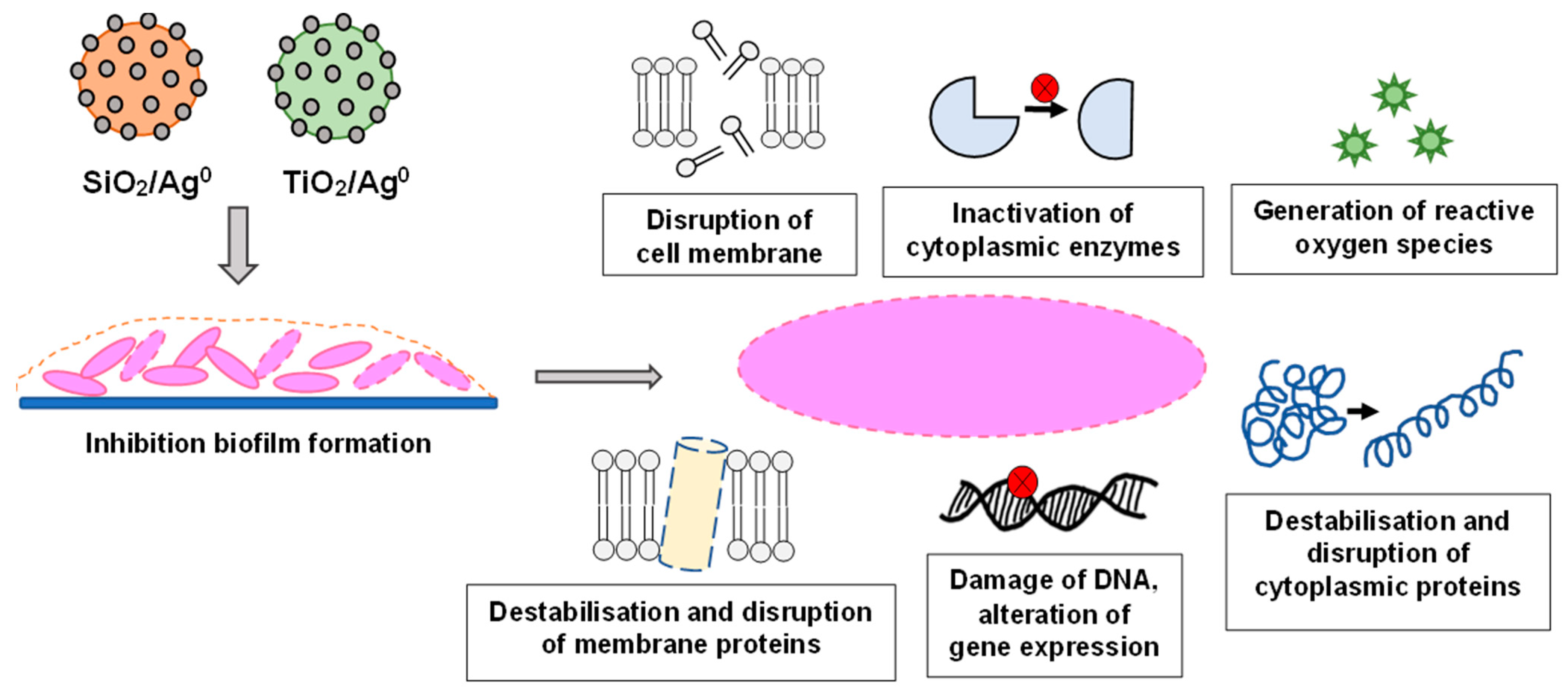
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains
4.2. Silver Nanoforms (SiO2/Ag0, TiO2/Ag0)
4.3. Antibiotic Susceptibility
4.4. Prevalence of Silver Resistance in Tested Bacteria Strains
4.5. Preparation of Bacterial Suspensions
4.6. Determination of the Minimal Inhibitory Concentration (MIC)
4.7. Determination of the Minimal Biofilm Inhibitory Concentration (MBIC)
4.8. Effects of SiO2/Ag0 and TiO2/Ag0 on Biofilm Formation
4.9. Effects of SiO2/Ag0 and TiO2/Ag0 on the Count of Live Bacteria in Biofilm
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Michiels, J.E.; Van den Bergh, B.; Verstraeten, N.; Fauvart, M.; Michiels, J. In vitro emergence of high persistence upon periodic aminoglycoside challenge in the ESKAPE pathogens. Antimicrob. Agents Chemother. 2016, 60, 4630–4637. [Google Scholar] [CrossRef] [Green Version]
- Rice, L.B. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: No ESKAPE. J. Infect. Dis. 2008, 19, 1079–1081. [Google Scholar] [CrossRef] [PubMed]
- Rice, L.B. Progress and challenges in implementing the research on ESKAPE pathogens. Infect. Control Hosp. Epidemiol. 2010, 31, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Streeter, K.; Katoul, M. Pseudomonas aeruginosa: A review of their pathogenesis and prevalence in clinical settings and the environment. Infect. Epidemiol. Med. 2016, 2, 25–32. [Google Scholar] [CrossRef]
- Fakhkharia, P.; Tajeddinb, E.; Azimiradb, M.; Salmanzadeh-Ahrabi, S.; Abdi-Alia, A.; Nikmaneshd, B.; Eshratie, B.; Gouya, M.M.; Owliaf, P.; Zalig, M.R.; et al. Involvement of Pseudomonas aeruginosa in the occurrence of community and hospital acquired diarrhea, and its virulence diversity among the stool and the environmental samples. Int. J. Environ. Health Res. 2020, 19, 1–11. [Google Scholar] [CrossRef]
- Alhazmi, A. Pseudomonas aeruginosa—Pathogenesis and pathogenic mechanisms. Int. J. Biol. 2015, 7, 44–67. [Google Scholar] [CrossRef]
- Huang, Z.; Jiang, Y.; Liang, J. Pathogenesis could be one of the anti-cheating mechanisms for Pseudomonas aeruginosa society. Med. Hypotheses 2011, 76, 166–168. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Misba, L.; Khan, A.U. Antibiotics versus biofilm: An emerging battleground in microbial communities. Antimicrob. Resist. Infect. Control 2019, 8, 76. [Google Scholar] [CrossRef]
- Mikhailova, E.O. Silver nanoparticles: Mechanism of action and probable bio-application. J. Funct. Biomater. 2020, 11, 84. [Google Scholar] [CrossRef]
- O’Loughlin, C.T.; Miller, L.C.; Siryaporn, A.; Martin, K.; Semmelhack, F.; Bassler, B.L. A quorum-sensing inhibitor blocks Pseudomonas aeruginosa virulence and biofilm formation. Proc. Natl. Acad. Sci. USA 2013, 110, 17981–17986. [Google Scholar] [CrossRef] [Green Version]
- Ross, A.S.; Baliga, C.; Verma, P.; Duchin, J.; Gluck, M.A. Quarantine process for the resolution of uodenoscope-associated transmission of multidrug-resistant Escherichia coli. Gastrointest. Endosc. 2015, 82, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Thuptimdang, P.; Limpiyakorn, T.; McEvoy, J.; Prüß, B.M.; Khan, E. Effect of silver nanoparticles on Pseudomonas putida biofilms at different stages of maturity. J. Hazard. Mater. 2001, 290, 127–133. [Google Scholar] [CrossRef]
- Arancibia, F.; Bajer, T.T.; Ewig, S.; Mensa, J.; Gonzalez, J.; Niederman, M.S.; Torres, A. Community-acquired pneumonia due to gram-negative bacteria and Pseudomonas aeruginosa: Incidence, Risk and Prognosis. Arch. Intern. Med. 2002, 162, 1849–1858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalishwaralal, K.; BarathManiKanth, S.; Pandian, S.R.; Deepak, V.; Gurunathan, S. Silver nanoparticles impede the biofilm formation by Pseudomonas aeruginosa and Staphylococcus epidermidis. Colloids Surf. B Biointerfaces 2010, 79, 340–344. [Google Scholar] [CrossRef]
- Sullivan, M.; Folytn-Arfa Kia, A.; Long, M.; Walsh, M.; Kavanagh, K.; McClean, S.; Creaven, B.S. Isolation and characterization of silver(I) complexes of substituted coumarin-4-carboxylates which are effective against Pseudomonas aeruginosa biofilms. Polyhedron 2014, 67, 549–559. [Google Scholar] [CrossRef] [Green Version]
- Parasuraman, P.; Devadatha, B.; Sarma, V.V.; Ranganathan, S.; Ampasala, D.R.; Reddy, D.; Kumavath, R.; Kim, I.W.; Patel, S.K.S.; Kalia, V.C.; et al. Inhibition of microbial quorum sensing mediated virulence factors by Pestalotiopsis sydowiana. J. Microbiol. Biotechnol. 2020, 30, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Dhanalekshmi, K.I.; Meena, K.S. Comparison of antibacterial activities of Ag@TiO2 and Ag@SiO2 core—Shell nanoparticles. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 128, 887–890. [Google Scholar] [CrossRef]
- Joshi, A.S.; Singh, P.; Mijakovic, I. Interactions of gold and silver nanoparticles with bacterial biofilms: Molecular interactions behind inhibition and resistance. Int. J. Mol. Sci. 2020, 21, 7658. [Google Scholar] [CrossRef]
- Otari, S.V.; Patel, S.K.S.; Kalia, V.C.; Kim, I.W.; Lee, J.K. Antimicrobial activity of biosynthesized silver nanoparticles decorated silica nanoparticles. Indian J. Microbiol. 2019, 59, 379–382. [Google Scholar] [CrossRef]
- Fletcher, P.D.; Holt, B.L. Controlled silanization of silica nanoparticles to stabilize foams, climbing films, and liquid marbles. Langmuir 2011, 27, 12869–12876. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liu, W.; Xue, C.; Zhou, S.; Lan, F.; Bi, L.; Xu, H.; Yang, X.; Zeng, F.D. Toxicity and penetration of TiO2 nanoparticles in hairless mice and porcine skin after subchronic dermal exposure. Toxicol. Lett. 2009, 191, 1–8. [Google Scholar] [CrossRef]
- Athinarayanan, J.; Periasamy, V.S.; Alsaif, M.A.; Al-Warthan, A.A.; Alshatwi, A.A. Presence of nanosilica (E551) in commercial food products: TNF-mediated oxidative stress and altered cell cycle progression in human lung fibroblast cells. Cell Biol. Toxicol. 2014, 30, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Percival, S.L.; Woods, E.; Nutekpor, M.; Bowler, P.; Radford, A.; Cochrane, C. Prevalence of silver resistance in bacteria isolated from diabetic foot ulcers and efficacy of silver-containing wound dressings. Ostomy Wound Manag. 2008, 54, 30–40. [Google Scholar]
- Finley, P.J.; Norton, R.; Austin, C.; Mitchell, A.; Zank, S.; Durham, P. Unprecedented silver resistance in clinically isolated Enterobacteriaceae: Major implications for burn and wound management. Antimicrob. Agents Chemother. 2015, 59, 4734–4741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosny, A.E.M.; Rasmy, S.A.; Aboul-Magd, D.S.; Kashef, M.T.; El-Bazza, Z.E. The increasing threat of silver-resistance in clinical isolates from wounds and burns. Infect. Drug Resist. 2019, 12, 1985–2001. [Google Scholar] [CrossRef] [Green Version]
- Kędziora, A.; Gorzelańczyk, K.; Bugla-Płoskońska, G. Positive and negative aspects of silver nanoparticles usage. Biol. Int. 2013, 53, 67–76. [Google Scholar]
- Kędziora, A.; Stręk, W.; Kepiński, L.; Bugla-Płoskońska, G.; Doroszkiewicz, W. Synthesis and antibacterial activity of novel titanium dioxide doped with silver. J. Solgel. Sci. Technol. 2012, 62, 79–86. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Zhao, C.; Zhang, Y.; Yao, J.; Yang, W.; Hu, Q.; Wang, C.; Cao, C. Effect of stable antimicrobial nano-silver packaging on inhibiting mildew and in storage of rice. Food Chem. 2017, 215, 477–482. [Google Scholar] [CrossRef]
- Mohandas, A.; Krishnan, A.G.; Biswas, R.; Menon, D.; Nair, M.B. Antibacterial and cytocompatible nanotextured surface incorporating silver via single step hydrothermal processing. Mat. Sci. Eng. C Mater. Biol. Appl. 2017, 75, 115–124. [Google Scholar] [CrossRef]
- Wiglusz, R.J.; Kędziora, A.; Łukowiak, A.; Doroszkiewicz, W.; Stręk, W. Hydroxyapatites and europium (III) doped hydroxyapatites as a carrier of silver nanoparticles and their antimicrobial activity. J. Biomed. Nanotechnol. 2012, 8, 605–612. [Google Scholar] [CrossRef]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver nanoparticles as potential antibacterial agents. Molecules 2015, 18, 8856–8874. [Google Scholar] [CrossRef] [Green Version]
- Yin, I.X.; Zhao, I.S.; Mei, M.L.; Li, Q.; Yu, O.Y.; Chu, C.H. Use of silver nanomaterials for caries prevention: A concise review. Int. J. Nanomedicine. 2020, 6, 3181–3191. [Google Scholar] [CrossRef]
- Arokiyaraj, S.; Vincent, S.; Saravanan, M.; Lee, Y.; Oh, Y.K.; Kim, K.H. Green synthesis of silver nanoparticles using Rheum palmatum root extract and their antibacterial activity against Staphylococcus aureus and Pseudomonas aeruginosa. Artif. Cell Nanomed. 2017, 45, 372–379. [Google Scholar] [CrossRef] [Green Version]
- Shah, S.; Gaikwad, S.; Nagar, S.; Kulshrestha, S.; Vaidya, V.; Nawani, N.; Pawar, S. Biofilm inhibition and anti-quorum sensing activity of phytosynthesized silver nanoparticles against the nosocomial pathogen Pseudomonas aeruginosa. Biofouling 2019, 35, 34–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, P.; Pandit, S.; Garnæs, J.; Tunjic, S.; Mokkapati, V.R.S.S.; Sultan, A.; Thygesen, A.; Mackevica, A.; Mateiu, R.V.; Daugaard, A.E.; et al. Green synthesis of gold and silver nanoparticles from Cannabis sativa (industrial hemp) and their capacity for biofilm inhibition. Int. J. Nanomed. 2018, 13, 3571–3591. [Google Scholar] [CrossRef]
- Mann, R.; Holmes, A.; McNeilly, O.; Cavaliere, R.; Sotiriou, G.A.; Rice, S.A.; Gunawan, C. Evolution of biofilm-forming pathogenic bacteria in the presence of nanoparticles and antibiotic: Adaptation phenomena and cross-resistance. J. Nanobiotechnol. 2021, 19, 291. [Google Scholar] [CrossRef] [PubMed]
- de Lacerda Coriolano, D.; de Souza, J.B.; Bueno, E.V.; Medeiros, S.M.F.R.D.S.; Cavalcanti, I.D.L.; Cavalcanti, I.M.F. Antibacterial and antibiofilm potential of silver nanoparticles against antibiotic-sensitive and multidrug-resistant Pseudomonas aeruginosa strains. Braz. J. Microbiol. 2021, 52, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Silver, S. Bacterial silver resistance: Molecular biology and uses and misuses of silver compounds. FEMS Microbiol. Rev. 2003, 27, 341–353. [Google Scholar] [CrossRef] [Green Version]
- Silver, S.; Gupta, A.; Matsui, K.; Lo, J.F. Resistance to Ag(I) cations in bacteria: Environments, genes and proteins. Met. Based Drugs 1999, 6, 315–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kedziora, A.; Wernecki, M.; Korzekwa, K.; Speruda, M.; Gerasymchuk, Y.; Lukowiak, A.; Bugla-Płoskońska, G. Consequences of long-term bacteria’s exposure to silver nanoformulations with different physico-chemical properties. Int. J. Nanomed. 2020, 15, 199–213. [Google Scholar] [CrossRef] [Green Version]
- Kiedrowski, M.R.; Horswill, A.R. New approaches for treating staphylococcal biofilm infections. Ann. N. Y. Acad. Sci. 2011, 1241, 104–121. [Google Scholar] [CrossRef] [PubMed]
- Alemayehu, D.; Casey, P.G.; McAuliffe, O.; Guinane, C.M.; Martin, J.G.; Shanahan, F.; Coffey, A.; Ross, R.P.; Hill, C. Bacteriophages ϕMR299-2 and ϕNH-4 can eliminate Pseudomonas aeruginosa in the murine lung and on cystic fibrosis lung airway cells. mBio 2012, 3, e00029-12. [Google Scholar] [CrossRef] [Green Version]
- Bugla-Płoskońska, G.; Leszkiewicz, A.; Borak, B.H.; Jasiorski, M.; Drulis-Kawa, Z.; Baszczuk, A.; Maruszewski, K.; Doroszkiewicz, W. Bactericidal properties of silica particles with silver islands located the surface. Int. J. Antimicrob. Agents 2007, 29, 746–748. [Google Scholar] [CrossRef] [PubMed]
- Jasiorski, M.; Leszkiewicz, A.; Brzeziński, S.; Bugla-Płoskońska, G.; Malinowska, G.; Borak, B.; Karbownik, I.; Baszczuk, A.; Stręk, W.; Doroszkiewicz, W. Textile with silver silica spheres: Its antimicrobial activity against Escherichia coli and Staphylococcus aureus. J. Solgel. Sci. Technol. 2009, 51, 330–334. [Google Scholar] [CrossRef]
- Kędziora, A.; Korzekwa, K.; Strek, W.; Pawlak, A.; Doroszkiewicz, W.; Bugla-PLoSkonska, G. Silver nanoforms as a therapeutic agent for killing Escherichia coli and certain ESKAPE pathogens. Curr. Microbiol. 2016, 73, 139–147. [Google Scholar] [CrossRef] [Green Version]
- Gerasymchuk, Y.; Lukowiak, A.; Wedzynska, A.; Kedziora, A.; Bugla-PLoSkonska, G.; Piatek, D.; Bachanek, T.; Chernii, V.; Tomachynski, L.; Strek, W. New photosensitive nanometric graphite oxide composites as antimicrobial material with prolonged action. J. Inorg. Biochem. 2016, 159, 142–148. [Google Scholar] [CrossRef]
- Kulshrestha, S.; Qayyum, S.; Khan, A.U. Antibiofilm efficacy of green synthesized graphene oxide-silver nanocomposite using Lagerstroemia speciosa floral extract: A comparative study on inhibition of gram-positive and gram-negative biofilms. Microb. Pathog. 2017, 103, 167–177. [Google Scholar] [CrossRef]
- Ravishankar, R.V.; Jamuna, B.A. Nanoparticles and their potential application as antimicrobials. In Science against Microbial Pathogens: Communicating Current Research and Technological Advances; Mendez-Vilas, A., Ed.; Formatex: Badajoz, Spain, 2011; pp. 197–209. [Google Scholar]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramirez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef] [Green Version]
- Fatima, S.; Ali, K.; Ahmed, B.; Al Kheraif, A.A.; Syed, A.; Elgorban, A.M.; Musarrat, J.; Lee, J. Titanium dioxide nanoparticles Induce inhibitory effects against planktonic cells and biofilms of human oral cavity isolates of Rothia mucilaginosa, Georgenia sp. and Staphylococcus saprophyticus. Pharmaceutics 2021, 13, 1564. [Google Scholar] [CrossRef]
- Altaf, M.; Zeyad, M.T.; Hashmi, M.A.; Manoharadas, S.; Hussain, S.A.; Abuhasile, M.S.A.; Almuzainid, M.A.M. Effective inhibition and eradication of pathogenic biofilms by titanium dioxide nanoparticles synthesized using Carum copticum extract. RSC Adv. 2021, 11, 19248. [Google Scholar] [CrossRef]
- Maurer-Jones, M.A.; Gunsolus, I.L.; Meyer, B.M.; Christenson, C.J.; Haynes, C.L. Impact of TiO2 nanoparticles on growth, biofilm formation, and flavin secretion in Shewanella oneidensis. Anal. Chem. 2013, 85, 5810–5818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, J.; Qin, S.; Wei, Y.; Liu, S.; Peng, H.; Li, Q.; Luo, L.; Lv, M. Silver nanoparticles exert concentration-dependent influences on biofilm development and architecture. Cell Prolif. 2019, 52, e12616. [Google Scholar] [CrossRef]
- Palanisamy, N.K.; Ferina, N.; Amirulhusni, A.N.; Mohd-Zain, Z.; Hussaini, J.; Ping, L.J.; Durairaj, R. Antibiofilm properties of chemically synthesized silver nanoparticles found against Pseudomonas aeruginosa. J. Nanobiotechnol. 2014, 12, 2. [Google Scholar] [CrossRef] [Green Version]
- Poulin, M.B.; Kuperman, L.L. Regulation of Biofilm Exopolysaccharide Production by Cyclic Di-Guanosine Monophosphate. Front. Microbiol. 2021, 12, 730980. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, C.; Hou, J.; Wang, P.; You, G.; Miao, L. Effects of cerium oxide nanoparticles on bacterial growth and behaviors:induction of biofilm formation and stress respons. Environ. Sci. Pollut. Res. 2019, 26, 9293–9304. [Google Scholar] [CrossRef] [PubMed]
- Kamaraj, K.; George, R.P.; Anandkumar, B.; Parvathavarthini, N.; Kamachi Mudali, U. A Silver nanoparticle loaded TiO2 nanoporous layer for visible light induced antimicrobial applications. Bioelectrochemistry 2015, 106, 290–297. [Google Scholar] [CrossRef]
- Yao, Y.; Ohko, Y.; Sekiguchi, Y.; Fujishima, A.; Kubota, Y. Self-sterilization using silicone catheters coated with Ag and TiO2 nanocomposite thin film. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 2, 453–460. [Google Scholar] [CrossRef]
- Naik, K.; Kowshik, M. Anti-Biofilm efficacy of low temperature processed AgCl–TiO2 nanocomposite coating. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 34, 62–68. [Google Scholar] [CrossRef]
- Flores, C.Y.; Diaz, C.; Rubert, A.; Benítez, G.A.; Moreno, M.S.; Fernández Lorenzo de Mele, M.A.; Salvarezza, R.C.; Schilardi, P.L.; Vericat, C. Spontaneous adsorption of silver nanoparticles on Ti/TiO2 surfaces. Antibacterial effect on Pseudomonas aeruginosa. J. Colloid Interface Sci. 2010, 350, 402–408. [Google Scholar] [CrossRef]
- The European Committee on Antimicrobial Susceptibility Testing. Routine and extended internal quality control for MIC determination and disk diffusion as recommended by EUCAST. Ver-sion 11.0. 2021. Available online: http://www.eucast.org (accessed on 9 December 2021).
- Lee, K.; Lim, Y.S.; Yong, D.; Yum, J.H.; Chong, Y. Evaluation of the Hodge test and the imipenem-EDTA double-disk synergy test for differentiating metallo-beta-lactamase producing isolates of Pseudomonas spp. and Acinetobacter spp. J. Clin. Microbiol. 2003, 41, 4623–4629. [Google Scholar] [CrossRef] [Green Version]
- National Committee for Clinical Laboratory Standards. Performance Standards for Antimicrobial Disc Susceptibility Tests, 6th ed.; National Committee for Clinical Laboratory Standards: Malvern, PA, USA, 1997. [Google Scholar]
- O’Toole, G.A.; Kolter, R. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signaling pathways: A genetic analysis. Mol. Microbiol. 1998, 28, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Stepanovic, S.; Vulcovic, D.; Hola, V.; Bonaventura, G.D.; Djukić, S.; Cirković, I.; Ruzicka, F. Quantification of biofilm in microtiter plates: Overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS 2007, 13, 891–899. [Google Scholar] [CrossRef] [PubMed]
| Sample | Average Size of SiO2, TiO2 (nm) | Average Size of Ag0 (nm) | Surface Area (m2/g) | Pore Size (nm) | Content of Silver (% w/w) |
|---|---|---|---|---|---|
| SiO2/Ag0 | 123.73 (±25.86) | 17.91 (±3.37) | 332.0 ± 14.0 | 8.0 ± 2.0 | 10.0 |
| TiO2/Ag0 | 154.33 (±15.98) | 16.57 (±5.01) | 424.0 ± 8.0 | 4.0 ± 2.0 | 10.0 |
| Origin | Strain Number * | AN | CAZ | CIP | CL | IPM | MEM | TZP | MBL |
|---|---|---|---|---|---|---|---|---|---|
| broncho-alveolar lavage fluid | 0013 | S | R | R | S | R | R | R | - |
| 0024 | R | R | R | I | R | R | R | - | |
| 3 | R | R | R | S | R | R | R | + | |
| 472 | R | R | S | S | R | I | R | - | |
| 669 | R | S | R | S | R | R | R | - | |
| urine | 124 | S | R | I | S | I | S | I | - |
| 137 | S | R | I | S | I | S | I | - | |
| 300 | R | I | R | S | R | I | R | - | |
| 328 | R | I | R | S | R | R | I | - | |
| 407 | S | I | I | S | I | I | I | - | |
| ATCC collection | PAO1 (15692) | S | R | R | S | R | S | R | - |
| Origin | Strain Number | MIC (µg/mL) | |||
|---|---|---|---|---|---|
| SiO2/Ag0 | TiO2/Ag0 | SiO2 | TiO2 | ||
| PAO1 (ATCC 15692) | 256 | 256 | >8192 | >8192 | |
| bronchoalveolar lavage fluid | 0013 | 32 | 32 | >8192 | >8192 |
| 0024 | 32 | 16 | |||
| 3 | 32 | 16 | |||
| 472 | 32 | 16 | |||
| 669 | 16 | 16 | |||
| urine | 124 | 128 | 64 | >8192 | >8192 |
| 137 | 32 | 32 | |||
| 300 | 64 | 64 | |||
| 328 | 16 | 16 | |||
| 407 | 32 | 32 | |||
| Origin | Strain Number | MBIC (µg/mL) | |
|---|---|---|---|
| SiO2/Ag0 | TiO2/Ag0 | ||
| PAO1 (ATCC 15692) | 512 | 512 | |
| bronchoalveolar lavage fluid | 0013 | 64 | 128 |
| 0024 | 128 | 512 | |
| 3 | 128 | 512 | |
| 472 | 64 | 128 | |
| 669 | 32 | 64 | |
| urine | 124 | 256 | 128 |
| 137 | 64 | 128 | |
| 300 | 128 | 128 | |
| 328 | 64 | 64 | |
| 407 | 64 | 256 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korzekwa, K.; Kędziora, A.; Stańczykiewicz, B.; Bugla-Płoskońska, G.; Wojnicz, D. Benefits of Usage of Immobilized Silver Nanoparticles as Pseudomonas aeruginosa Antibiofilm Factors. Int. J. Mol. Sci. 2022, 23, 284. https://doi.org/10.3390/ijms23010284
Korzekwa K, Kędziora A, Stańczykiewicz B, Bugla-Płoskońska G, Wojnicz D. Benefits of Usage of Immobilized Silver Nanoparticles as Pseudomonas aeruginosa Antibiofilm Factors. International Journal of Molecular Sciences. 2022; 23(1):284. https://doi.org/10.3390/ijms23010284
Chicago/Turabian StyleKorzekwa, Kamila, Anna Kędziora, Bartłomiej Stańczykiewicz, Gabriela Bugla-Płoskońska, and Dorota Wojnicz. 2022. "Benefits of Usage of Immobilized Silver Nanoparticles as Pseudomonas aeruginosa Antibiofilm Factors" International Journal of Molecular Sciences 23, no. 1: 284. https://doi.org/10.3390/ijms23010284






