Molecular Weight Analysis of Blue Shark (Prionace glauca) Collagen Hydrolysates by GPC-LS; Effect of High Molecular Weight Hydrolysates on Fibroblast Cultures: mRNA Collagen Type I Expression and Synthesis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Collagen Extraction
2.1.1. SDS-PAGE
2.2. Effect of Hydrolysis Conditions on BSCH Mw
2.2.1. GPC-LS Analysis
2.2.2. HPLC-SEC Analysis
2.3. Amino Acid Characterization of BSCH-C and CHC
2.4. Effect of HMW-BSCH and CHC on Cell Viability
2.5. Effect of High Mw BSCH and CHC on mRNA Collagen Synthesis of Fibroblast Culture
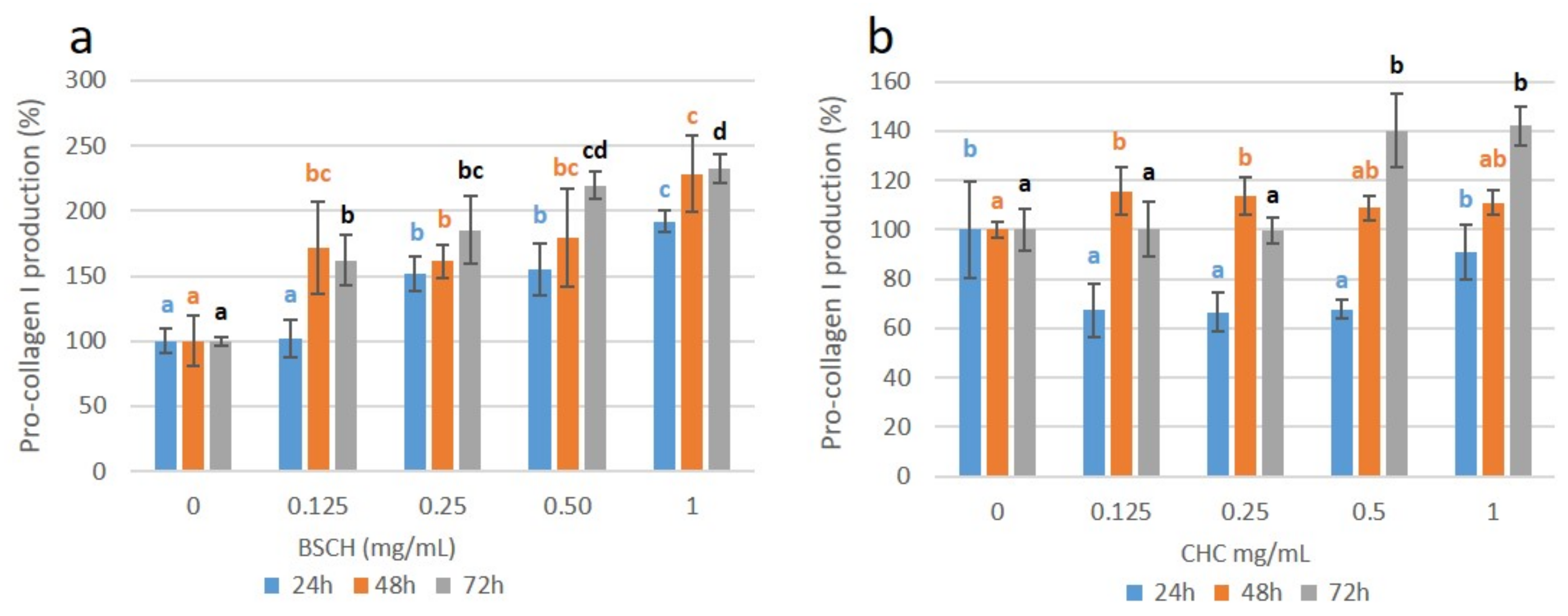
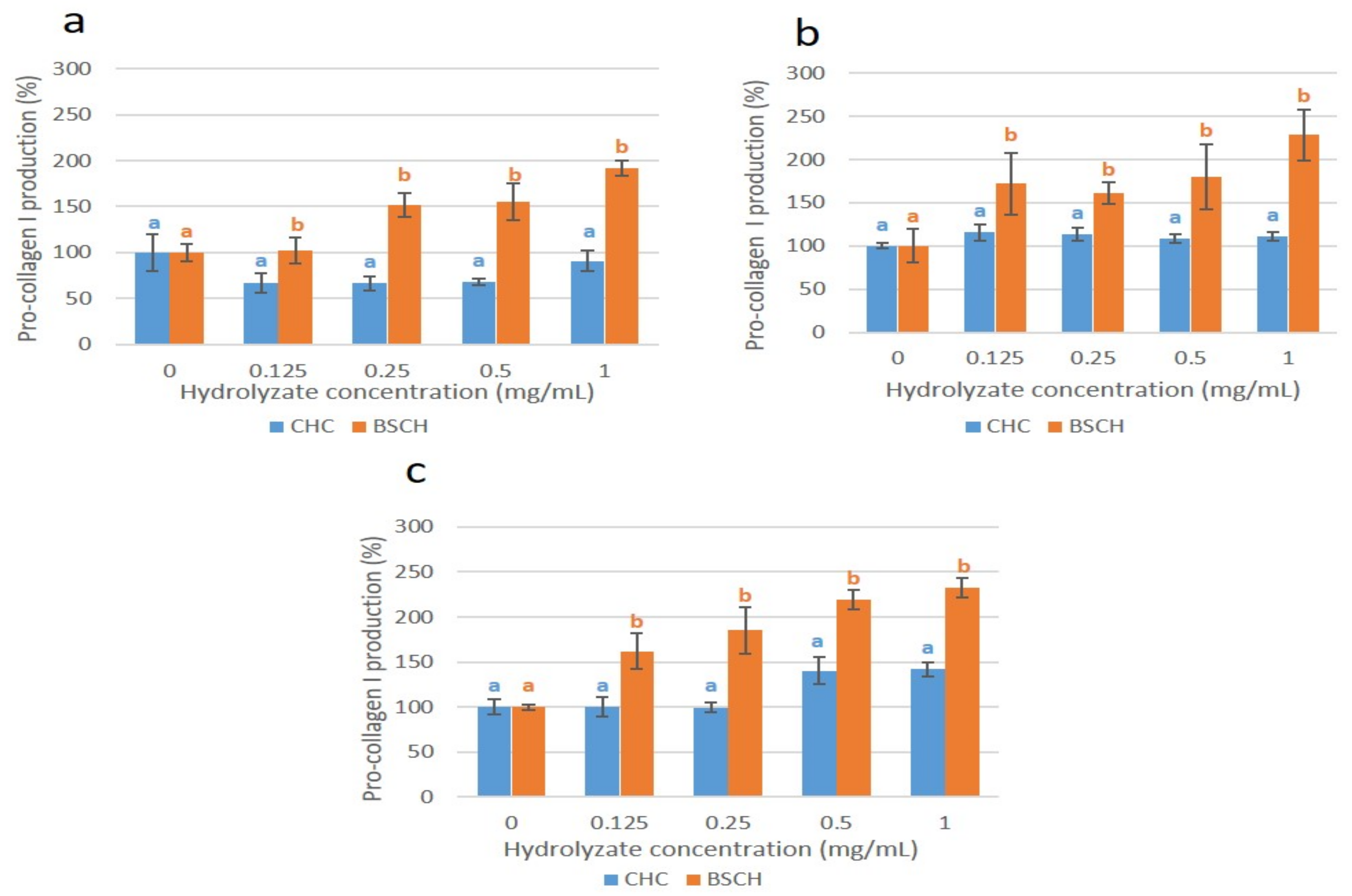
2.6. Effect of HMW-BSCH and CHC on Pro-Collagen I Synthesis (ELISA) of Fibroblast Culture
3. Materials and Methods
3.1. Biological Samples and Compositional Analysis
3.2. Extraction of Acid Soluble Collagen
3.2.1. Proximate Composition
3.2.2. SDS-PAGE
3.3. Enzymatic Hydrolysis of Acid Soluble Collagen
3.4. Characterization of Hydrolysates
3.4.1. Size-Exclusion Chromatography
3.4.2. GPC of Blue Shark Collagen Hydrolysates
3.4.3. Statistical Analysis
3.5. Amino Acid Characterization of BSCH and CHC
3.6. Cell Culture and Treatment with Blue Shark Collagen Hydrolysate
3.7. Cell Viability Assay
3.8. RNA Isolation and Quantitative Real-Time Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
3.9. Human Pro-Collagen I Quantification from Fibroblast Cell Culture Supernatants by Sandwich ELISAs
3.10. Cell Viability, RNA Expression, and Pro-Collagen I Biosynthesis Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sotelo, C.G.; Blanco, M.; Ariza, P.; Vázquez, J.A.; Pérez-Martín, R.I. Sustainable Sources from Aquatic Organisms for Cosmeceuticals Ingredients. Cosmetics 2021, 8, 48. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Blanco, M.; Massa, A.E.; Amado, I.T.; Pérez-Martín, R.I. Production of Fish Protein Hydrolysates from Scyliorhinus canicula Discards with Antihypertensive and Antioxidant Activities by Enzymatic Hydrolysis and Mathematical Optimization Using Response Surface Methodology. Mar. Drugs 2017, 15, 306. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, A.; Blanco, M.; Correa, B.; Pérez-Martín, R.I.; Sotelo, C. Effect of Fish Collagen Hydrolysates on Type I Collagen mRNA Levels of Human Dermal Fibroblast Culture. Mar. Drugs 2018, 16, 144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cleaver, G. Precise Determination of Protein Molecular Weight Using the Agilent 1260 Infinity Multi-Detector GPC/SEC System; Agilent Technologies, Inc.: Santa Clara, CA, USA, 2015. [Google Scholar]
- Folta-Stogniew, E.; Williams, K.R. Determination of molecular masses of proteins in solution: Implementation of an HPLC Size Exclusion Chromatography and laser light scattering service in a core laboratory. J. Biomol. Tech. 1999, 10, 51–63. [Google Scholar] [PubMed]
- Blanco, M.; Sanz, N.; Valcarcel, J.; Pérez-Martín, R.I.; Sotelo, C.G. Does Subunit Composition Influence the Intermolecular Crosslinking of Fish Collagen? A Study with Hake and Blue Shark Skin Collagens. Polymers 2020, 12, 1734. [Google Scholar] [CrossRef] [PubMed]
- Blanco, M.; Vázquez, J.A.; Pérez-Martín, R.I.; Sotelo, C. Hydrolysates of Fish Skin Collagen: An Opportunity for Valorizing Fish Industry Byproducts. Mar. Drugs 2017, 15, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, C.N.; Yates, J. The International Organization for Standardization (ISO): Global Governance through Voluntary Consensus; Routledge: London, UK; New York, NY, USA, 2009. [Google Scholar]
- Chotphruethipong, L.; Aluko, R.E.; Benjakul, S. Hydrolyzed collagen from porcine lipase-defatted seabass skin: Antioxidant, fibroblast cell proliferation, and collagen production activities. J. Food Biochem. 2019, 43, e12825. [Google Scholar] [CrossRef] [PubMed]
- Chotphruethipong, L.; Binlateh, T.; Hutamekalin, P.; Sukketsiri, W.; Aluko, R.E.; Benjakul, S. In vitro antioxidant and wound-healing activities of hydrolyzed collagen from defatted asian sea bass skin as influenced by different enzyme types and hydrolysis processes. RSC Adv. 2021, 11, 18144–18151. [Google Scholar] [CrossRef]
- Benjakul, S.; Karnjanapratum, S.; Visessanguan, W. Hydrolysed collagen from lates calcarifer skin: Its acute toxicity and impact on cell proliferation and collagen production of fibroblasts. Int. J. Food Sci. 2018, 53, 1871–1879. [Google Scholar] [CrossRef]
- Je, H.J.; Han, Y.K.; Lee, H.G.; Bae, I.Y. Anti-aging potential of fish collagen hydrolysates subjected to simulated gastrointestinal digestion and Caco-2 cell permeation. J. Appl. Biol. Chem. 2019, 62, 101–107. [Google Scholar] [CrossRef] [Green Version]
- Ding, D.; Yu, T.; Du, B.; Huang, Y. Collagen hydrolysate from Thunnus orientalis bone induces osteoblast proliferation and differentiation. Chem. Eng. Sci. 2019, 205, 143–150. [Google Scholar] [CrossRef]
- Tokudome, Y.; Nakamura, K.; Kage, M.; Todo, H.; Sugibayashi, K.; Hashimoto, F. Effects of soybean peptide and collagen peptide on collagen synthesis in normal human dermal fibroblasts. Int. J. Food Sci. Nutr. 2012, 63, 689–695. [Google Scholar] [CrossRef] [Green Version]
- Zague, V. Influência da Suplementação com Colágeno Hidrolisado no Metabolismo da Matriz Extracelular e Proliferação de Fibroblastos Dérmicos Humanos Derivados de áreas Fotoprotegida e Fotoexposta, Cultivados em Monocamada e Equivalente Dérmico. Ph.D. Thesis, Universidade de São Paulo, São Paulo, Brazil, 2015. [Google Scholar]
- Bodin, J.; Adrien, A.; Bodet, P.E.; Dufour, D.; Baudouin, S.; Maugard, T.; Bridiau, N. Ulva intestinalis protein extracts promote in vitro collagen and hyaluronic acid production by human dermal fibroblasts. Molecules 2020, 25, 2091. [Google Scholar] [CrossRef]
- Zague, V.; de Freitas, V.; da Costa Rosa, M.; de Castro, G.A.; Jaeger, R.G.; Machado-Santelli, G.M. Collagen hydrolysate intake increases skin collagen expression and suppresses matrix metalloproteinase 2 activity. J. Med. Food 2011, 14, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Nikoo, M.; Boran, G.; Zhou, P.; Regenstein, J.M. Collagen and gelatin. Annu. Rev. Food Sci. Technol. 2015, 6, 527–557. [Google Scholar] [CrossRef] [PubMed]
- Bissell, M.J.; Hall, H.G.; Parry, G. How does the extracellular matrix direct gene expression? J. Theor. Biol. 1982, 99, 31–68. [Google Scholar] [CrossRef]
- Petreaca, M.; Martins-Green, M. Handbook of Stem Cells, 2nd ed.; Academic Press: Cambridge, MA, USA, 2013; pp. 191–226. [Google Scholar]
- AOAC. Official Methods of Analysis of the AOAC; Association of Official Analytical Chemists Inc.: Arlington, VA, USA, 2000. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Phys. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.; Morgenstern, B. Characterization of gelatin and acid soluble collagen by size exclusion chromatography coupled with multi angle light scattering. Biomacromolecules 2003, 4, 1727–1732. [Google Scholar] [CrossRef] [PubMed]
- MyAssays Ltd. Four Parameter Logistic Curve. 25 October 2012. Available online: https://www.myassays.com/four-parameter-logistic-curve.assay (accessed on 1 July 2019).
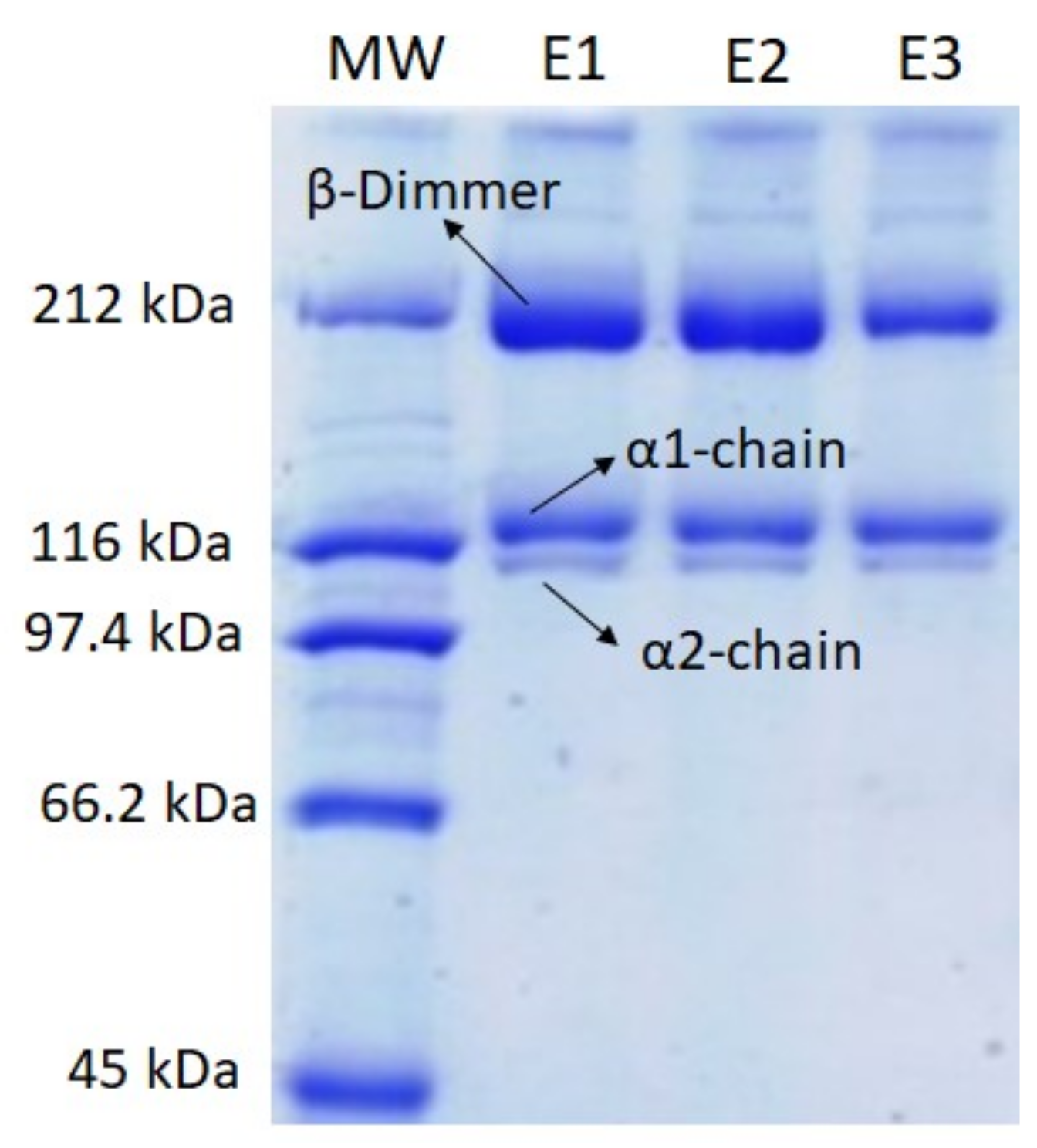

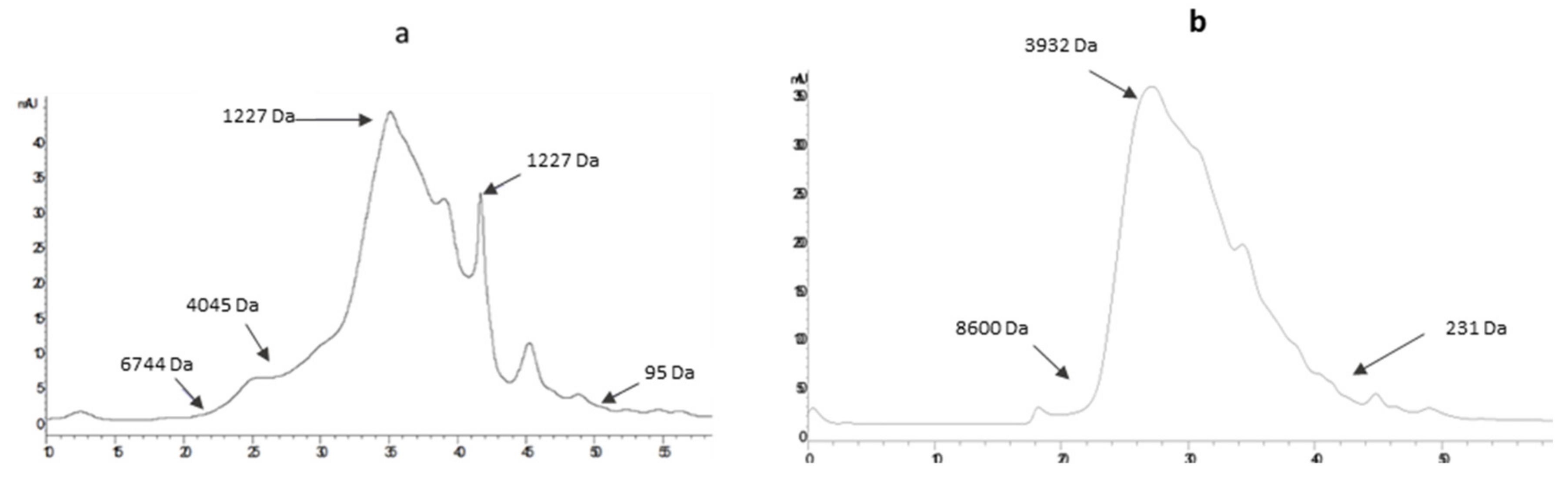
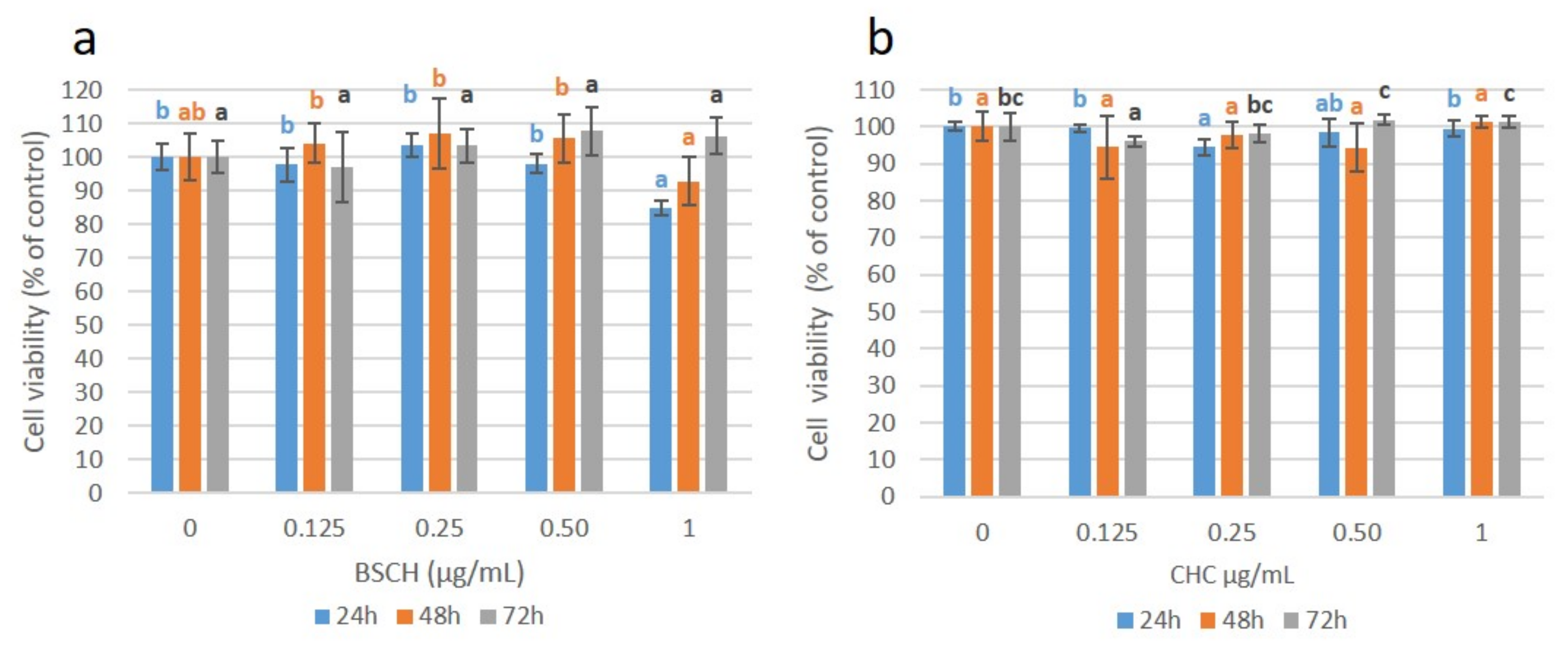
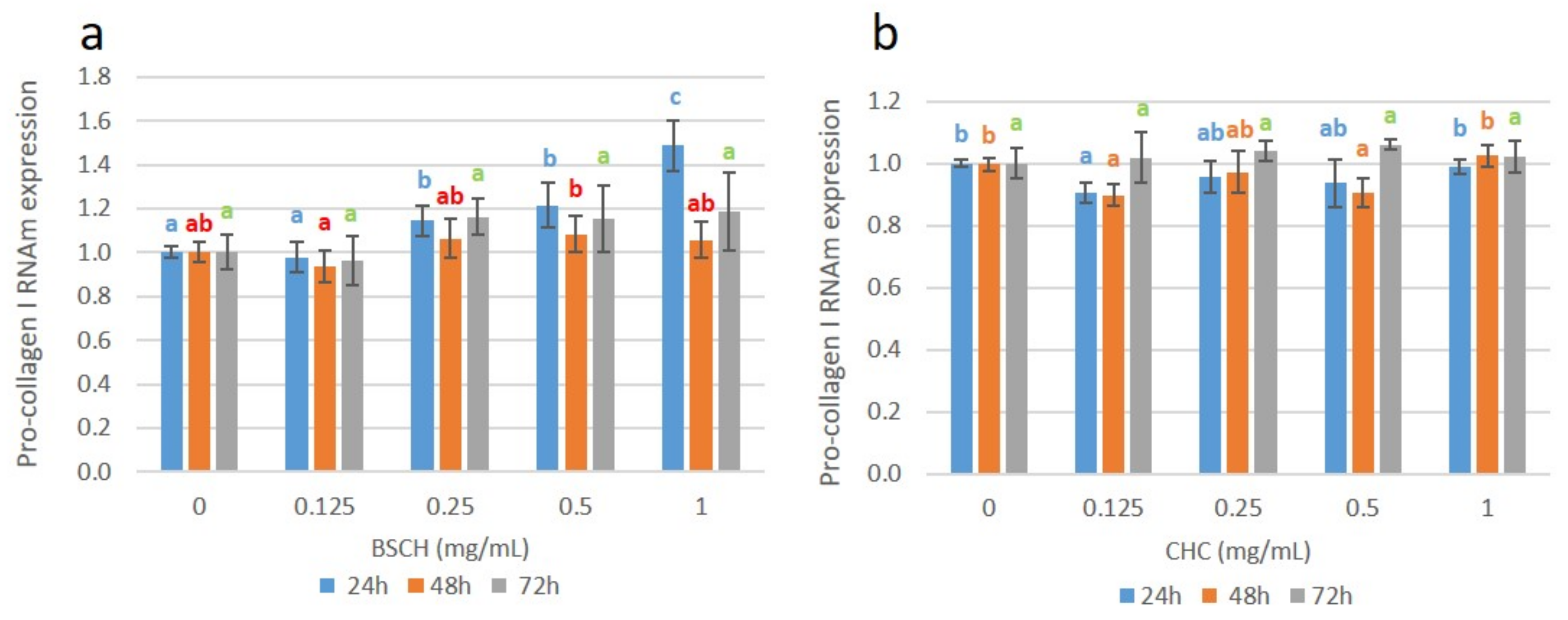

| Lyophilized BSASC | Proximate Composition (%) | ||
| Moisture | Protein | Ash | |
| 12.93 ± 0.70 | 84.46 ± 0.65 | 1.32 ± 0.54 | |
| Blue Shark Collagen Hydrolysis Conditions | Mw (kDa) | |||
|---|---|---|---|---|
| Experiments | Enzyme | E/S Rate | Time (h) | |
| A | Papain | 1/5 | 6 | 11.6 ± 5.4 |
| B | Alcalase | 1/20 | 6 | 3.5 ± 0.2 |
| C | Papain | 1/20 | 0.5 | 34.43 ± 10.2 |
| D | Alcalase | 1/20 | 0.5 | 3.2 ± 0.7 |
| E | Alcalase | 1/5 | 6 | 8 ± 5.0 |
| F | Papain | 1/5 | 0.5 | 20.72 ± 4.2 |
| G | Papain | 1/20 | 6 | 9.26 ± 5.3 |
| H | Alcalase | 1/5 | 0.5 | 4.3 ± 0.0 |
| Retention Time (min) | Mw (kDa) | % Area | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BSCH | CHC | |||||||||
| A | B | C | D | E | F | G | H | |||
| 20–30 | 17–3 | 37 ± 7 | 2 ± 0 | 9.33 ± 2 | 4 ± 1 | 3.5 ± 2 | 18.3 ± 7 | 30 ± 0 | 2.67 ± 2 | 50.6 ± 0.6 |
| 30–40 | 3–0.5 | 52 ± 9 | 84.5 ± 3 | 71.7 ± 3 | 88.6 ± 1 | 82 ± 2 | 69.3± 6 | 55 ± 13 | 89 ± 2 | 42.4 ± 0.2 |
| 40–50 | 0.5–0.09 | 11 ± 2 | 13 ± 4 | 19 ± 2 | 7.3 ± 2 | 14.5 ± 4 | 12.3 ± 4 | 15 ± 14 | 8.33 ± 2 | 6.9 ± 0.4 |
| Amino Acid | BSCH-C | CHC |
|---|---|---|
| Hydroxyproline | 70.249 ± 0.05 | 89.85 ± 0.33 |
| Aspartic acid | 44.761 ± 0.02 | 43.27 ± 0.07 |
| Threonine | 23.567 ± 0.02 | 15.91 ± 0.06 |
| Serine | 43.928 ± 0.07 | 35.31 ± 0.12 |
| Glutamic acid | 76.674 ± 0.08 | 70.18 ± 0.05 |
| Proline | 112.081 ± 0.08 | 115.41 ± 0.67 |
| Glycine | 306.533 ± 0.32 | 296.87 ± 1.48 |
| Alanine | 118.869 ± 0.04 | 106.385 ± 0.31 |
| Cysteine | 3.696 ± 0.004 | 3.383 ± 0.11 |
| Valine | 24.817 ± 0.030 | 20.650 ± 0.20 |
| Methionine | 14.3 ± 0.006 | 6.137 ± 0.00 |
| Isoleucine | 21.357 ± 0.04 | 11.158 ± 0.00 |
| Leucine | 24.856 ± 0.02 | 23.058 ± 0.03 |
| Tyrosine | 4.4 ± 0.01 | 2.086 ± 0.02 |
| Phenylalanine | 16.699 ± 0.06 | 12.299 ± 0.05 |
| Hydroxylysine | 4.518 ± 0.02 | 5.977 ± 0.04 |
| Histdine | 7.543 ± 0.002 | 5.543 ± 0.016 |
| Lysine | 28.206 ± 0.06 | 27.158 ± 0.08 |
| Arginine | 52.948 ± 0.02 | 46.090 ± 0.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blanco, M.; Sanz, N.; Sánzhez, A.C.; Correa, B.; Pérez-Martín, R.I.; Sotelo, C.G. Molecular Weight Analysis of Blue Shark (Prionace glauca) Collagen Hydrolysates by GPC-LS; Effect of High Molecular Weight Hydrolysates on Fibroblast Cultures: mRNA Collagen Type I Expression and Synthesis. Int. J. Mol. Sci. 2022, 23, 32. https://doi.org/10.3390/ijms23010032
Blanco M, Sanz N, Sánzhez AC, Correa B, Pérez-Martín RI, Sotelo CG. Molecular Weight Analysis of Blue Shark (Prionace glauca) Collagen Hydrolysates by GPC-LS; Effect of High Molecular Weight Hydrolysates on Fibroblast Cultures: mRNA Collagen Type I Expression and Synthesis. International Journal of Molecular Sciences. 2022; 23(1):32. https://doi.org/10.3390/ijms23010032
Chicago/Turabian StyleBlanco, María, Noelia Sanz, Ana C. Sánzhez, Begoña Correa, Ricardo I. Pérez-Martín, and Carmen G. Sotelo. 2022. "Molecular Weight Analysis of Blue Shark (Prionace glauca) Collagen Hydrolysates by GPC-LS; Effect of High Molecular Weight Hydrolysates on Fibroblast Cultures: mRNA Collagen Type I Expression and Synthesis" International Journal of Molecular Sciences 23, no. 1: 32. https://doi.org/10.3390/ijms23010032







