Optimal Efficacy and Safety of Humanized Anti-Scg3 Antibody to Alleviate Oxygen-Induced Retinopathy
Abstract
:1. Introduction
2. Results
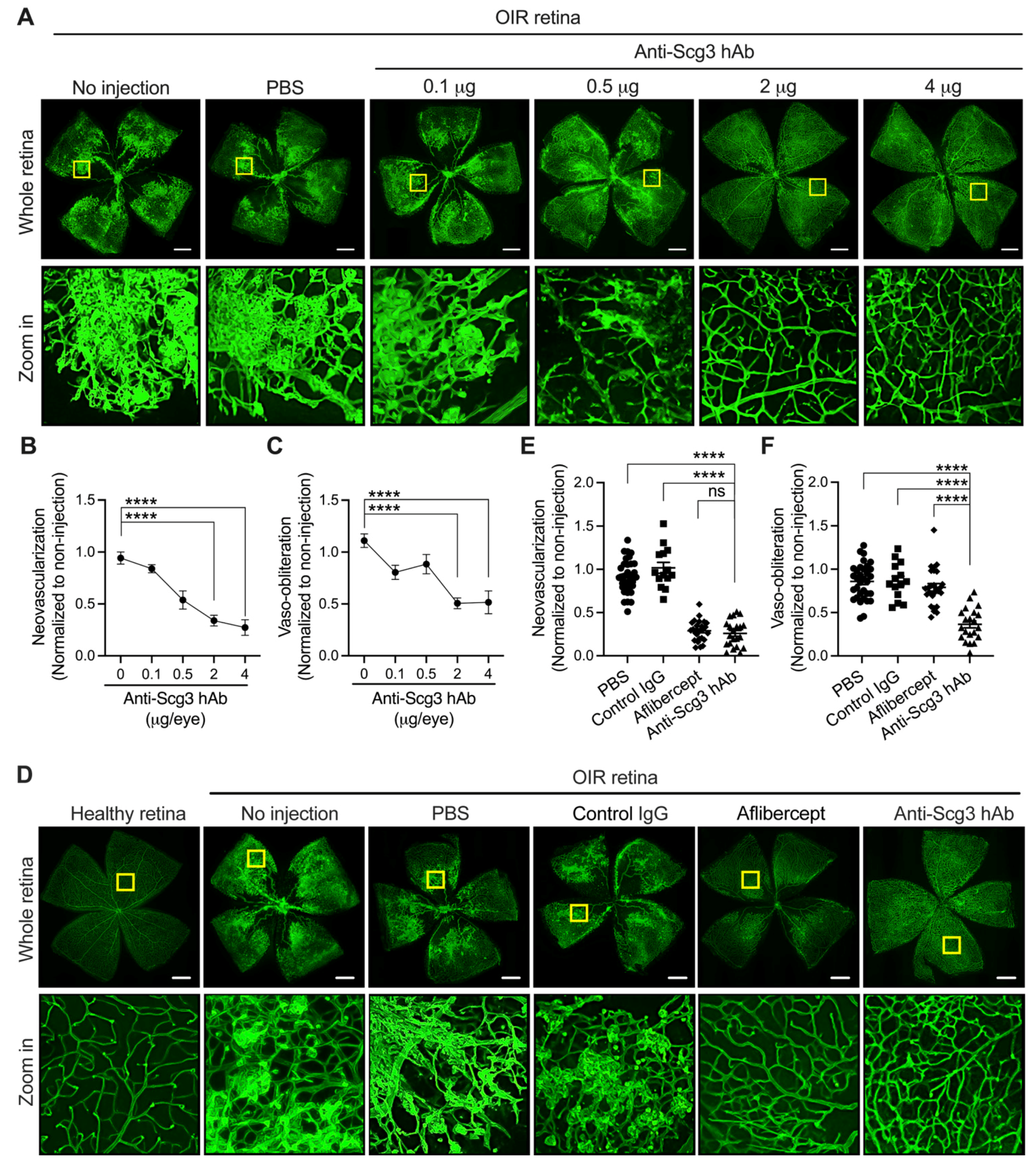
2.1. Alleviation of Pathological RNV in OIR Mice by Anti-Scg3 hAb
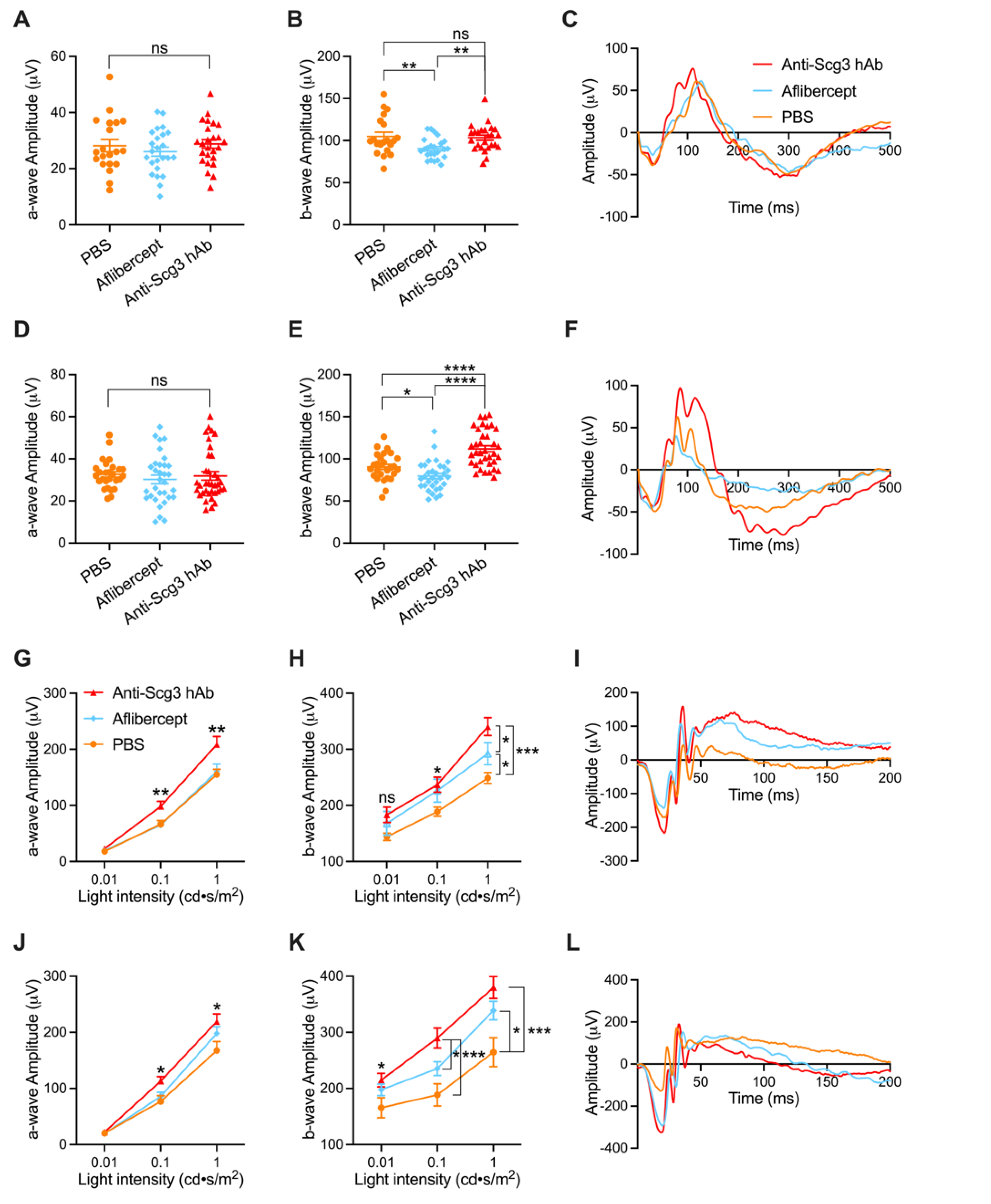
2.2. Improved Visual Function of OIR Mice by Anti-Scg3 hAb Therapy
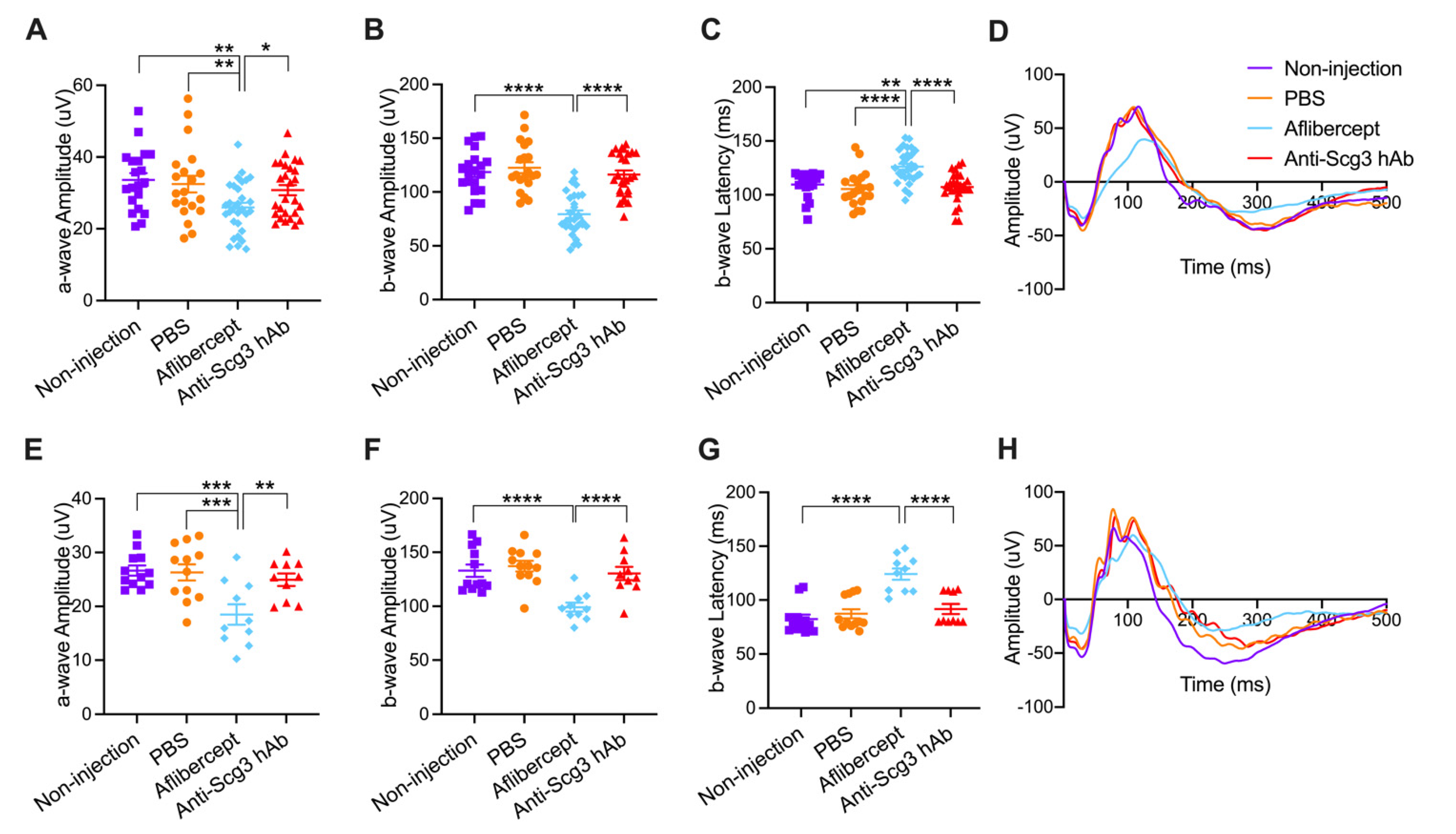
2.3. Adverse Effects on Visual Function of Healthy Neonatal Mice
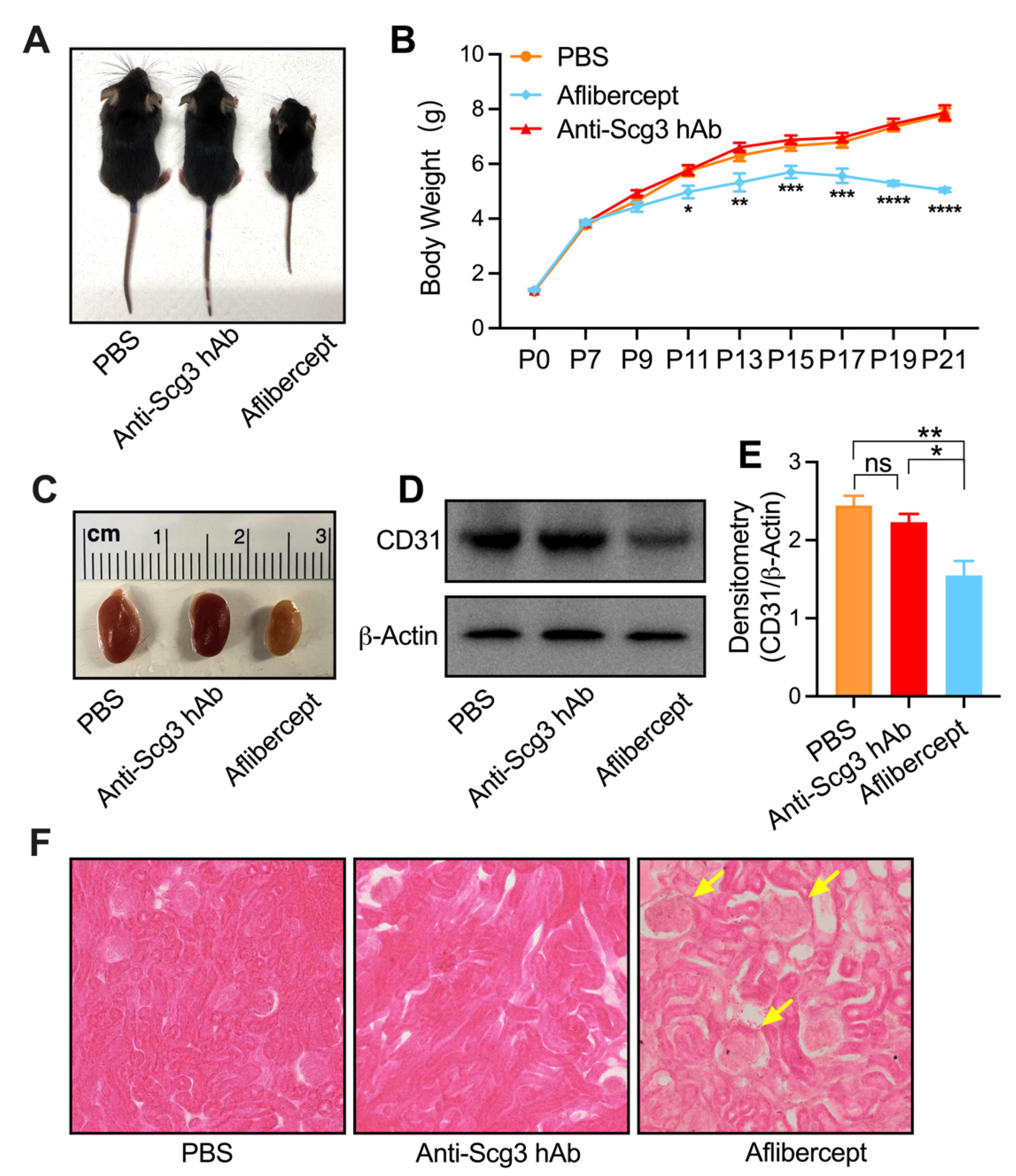
2.4. Adverse Systemic Effects in Neonatal Mice
3. Discussion
4. Materials and Methods
4.1. OIR Mouse Model
4.2. Anti-Angiogenic Therapy
4.3. Histopathology and Immunohistochemistry
4.4. FA
4.5. ERG
4.6. IOP Measurement
4.7. OCT
4.8. Systemic Toxicity
4.9. Western Blot
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hellström, A.; Smith, L.E.H.; Dammann, O. Retinopathy of Prematurity. Lancet 2013, 382, 1445–1457. [Google Scholar] [CrossRef] [Green Version]
- Lepore, D.; Quinn, G.E.; Molle, F.; Baldascino, A.; Orazi, L.; Sammartino, M.; Purcaro, V.; Giannantonio, C.; Papacci, P.; Romagnoli, C. Intravitreal Bevacizumab versus Laser Treatment in Type 1 Retinopathy of Prematurity: Report on Fluorescein Angiographic Findings. Ophthalmology 2014, 121, 2212–2219. [Google Scholar] [CrossRef] [PubMed]
- Lepore, D.; Quinn, G.E.; Molle, F.; Orazi, L.; Baldascino, A.; Ji, M.H.; Sammartino, M.; Sbaraglia, F.; Ricci, D.; Mercuri, E. Follow-up to Age 4 Years of Treatment of Type 1 Retinopathy of Prematurity Intravitreal Bevacizumab Injection versus Laser: Fluorescein Angiographic Findings. Ophthalmology 2018, 125, 218–226. [Google Scholar] [CrossRef]
- Tokunaga, C.C.; Mitton, K.P.; Dailey, W.; Massoll, C.; Roumayah, K.; Guzman, E.; Tarabishy, N.; Cheng, M.; Drenser, K.A. Effects of Anti-VEGF Treatment on the Recovery of the Developing Retina Following Oxygen-Induced Retinopathy. Investig. Ophthalmol. Vis. Sci. 2014, 55, 1884–1892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lutty, G.A.; McLeod, D.S.; Bhutto, I.; Wiegand, S.J. Effect of VEGF Trap on Normal Retinal Vascular Development and Oxygen-Induced Retinopathy in the Dog. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4039–4047. [Google Scholar] [CrossRef] [Green Version]
- Tang, F.; LeBlanc, M.E.; Wang, W.; Liang, D.; Chen, P.; Chou, T.-H.; Tian, H.; Li, W. Anti-Secretogranin III Therapy of Oxygen-Induced Retinopathy with Optimal Safety. Angiogenesis 2019, 22, 369–382. [Google Scholar] [CrossRef]
- Sato, T.; Wada, K.; Arahori, H.; Kuno, N.; Imoto, K.; Iwahashi-Shima, C.; Kusaka, S. Serum Concentrations of Bevacizumab (Avastin) and Vascular Endothelial Growth Factor in Infants with Retinopathy of Prematurity. Am. J. Ophthalmol. 2012, 153, 327–333.e1. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.-C.; Lien, R.; Liao, P.-J.; Wang, N.-K.; Chen, Y.-P.; Chao, A.-N.; Chen, K.-J.; Chen, T.-L.; Hwang, Y.-S.; Lai, C.-C. Serum Levels of Vascular Endothelial Growth Factor and Related Factors after Intravitreous Bevacizumab Injection for Retinopathy of Prematurity. JAMA Ophthalmol. 2015, 133, 391–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.-Y.; Lien, R.; Wang, N.-K.; Chao, A.-N.; Chen, K.-J.; Chen, T.-L.; Hwang, Y.-S.; Lai, C.-C.; Wu, W.-C. Changes in Systemic Vascular Endothelial Growth Factor Levels after Intravitreal Injection of Aflibercept in Infants with Retinopathy of Prematurity. Graefes. Arch. Clin. Exp. Ophthalmol. 2018, 256, 479–487. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, L.; Zhang, Q.; Xu, Y.; Zhao, P.; Xia, H. Serum Vascular Endothelial Growth Factor Levels before and after Intravitreous Ranibizumab Injection for Retinopathy of Prematurity. J. Ophthalmol. 2019, 2019, 2985161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morin, J.; Luu, T.M.; Superstein, R.; Ospina, L.H.; Lefebvre, F.; Simard, M.-N.; Shah, V.; Shah, P.S.; Kelly, E.N.; Canadian Neonatal Network and the Canadian Neonatal Follow-Up Network Investigators. Neurodevelopmental Outcomes Following Bevacizumab Injections for Retinopathy of Prematurity. Pediatrics 2016, 137, e20153218. [Google Scholar] [CrossRef] [Green Version]
- Stahl, A.; Lepore, D.; Fielder, A.; Fleck, B.; Reynolds, J.D.; Chiang, M.F.; Li, J.; Liew, M.; Maier, R.; Zhu, Q.; et al. Ranibizumab versus Laser Therapy for the Treatment of Very Low Birthweight Infants with Retinopathy of Prematurity (RAINBOW): An Open-Label Randomised Controlled Trial. Lancet 2019, 394, 1551–1559. [Google Scholar] [CrossRef]
- LeBlanc, M.E.; Wang, W.; Chen, X.; Caberoy, N.B.; Guo, F.; Shen, C.; Ji, Y.; Tian, H.; Wang, H.; Chen, R.; et al. Secretogranin III as a Disease-Associated Ligand for Antiangiogenic Therapy of Diabetic Retinopathy. J. Exp. Med. 2017, 214, 1029–1047. [Google Scholar] [CrossRef]
- Vogel, R.N.; Strampe, M.; Fagbemi, O.E.; Visotcky, A.; Tarima, S.; Carroll, J.; Costakos, D.M. Foveal Development in Infants Treated with Bevacizumab or Laser Photocoagulation for Retinopathy of Prematurity. Ophthalmology 2018, 125, 444–452. [Google Scholar] [CrossRef]
- Clark, A.; Wright, T.; Isaac, M.; Westall, C.; Mireskandari, K.; Tehrani, N.N. Macular Morphology Following Unilateral Bevacizumab Injection for Retinopathy of Prematurity: An OCT Study. J. AAPOS 2017, 21, 499–501.e1. [Google Scholar] [CrossRef]
- Hoerster, R.; Muether, P.; Dahlke, C.; Mehler, K.; Oberthür, A.; Kirchhof, B.; Fauser, S. Serum Concentrations of Vascular Endothelial Growth Factor in an Infant Treated with Ranibizumab for Retinopathy of Prematurity. Acta Ophthalmol. 2013, 91, e74–e75. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, M.; Razak, A.; Patel, W.; Pullattayil, A.K.; Kaushal, A. Neurodevelopmental Outcomes Following Bevacizumab Treatment for Retinopathy of Prematurity: A Systematic Review and Meta-Analysis. J. Perinatol. 2020, 41, 1225–1235. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, G.; Shankaran, S.; Nolen, T.L.; Sridhar, A.; Kennedy, K.A.; Hintz, S.R.; Phelps, D.L.; DeMauro, S.B.; Carlo, W.A.; Gantz, M.G.; et al. Neurodevelopmental Outcomes of Preterm Infants With Retinopathy of Prematurity by Treatment. Pediatrics 2019, 144, e20183537. [Google Scholar] [CrossRef]
- Geisen, P.; Peterson, L.J.; Martiniuk, D.; Uppal, A.; Saito, Y.; Hartnett, M.E. Neutralizing Antibody to VEGF Reduces Intravitreous Neovascularization and May Not Interfere with Ongoing Intraretinal Vascularization in a Rat Model of Retinopathy of Prematurity. Mol. Vis. 2008, 14, 345–357. [Google Scholar]
- McCloskey, M.; Wang, H.; Jiang, Y.; Smith, G.W.; Strange, J.; Hartnett, M.E. Anti-VEGF Antibody Leads to Later Atypical Intravitreous Neovascularization and Activation of Angiogenic Pathways in a Rat Model of Retinopathy of Prematurity. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2020–2026. [Google Scholar] [CrossRef] [Green Version]
- Mezu-Ndubuisi, O.J.; Wang, Y.; Schoephoerster, J.; Falero-Perez, J.; Zaitoun, I.S.; Sheibani, N.; Gong, S. Intravitreal Delivery of VEGF-A165-Loaded PLGA Microparticles Reduces Retinal Vaso-Obliteration in an In Vivo Mouse Model of Retinopathy of Prematurity. Curr. Eye Res. 2019, 44, 275–286. [Google Scholar] [CrossRef] [Green Version]
- Davitt, B.V.; Wallace, D.K. Plus Disease. Surv. Ophthalmol. 2009, 54, 663–670. [Google Scholar] [CrossRef]
- Hartnett, M.E.; Martiniuk, D.; Byfield, G.; Geisen, P.; Zeng, G.; Bautch, V.L. Neutralizing VEGF Decreases Tortuosity and Alters Endothelial Cell Division Orientation in Arterioles and Veins in a Rat Model of ROP: Relevance to plus Disease. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3107–3114. [Google Scholar] [CrossRef]
- Solarte, C.E.; Awad, A.H.; Wilson, C.M.; Ells, A. Plus Disease: Why Is It Important in Retinopathy of Prematurity? Middle East Afr. J. Ophthalmol. 2010, 17, 148–155. [Google Scholar] [CrossRef] [Green Version]
- Porciatti, V.; Saleh, M.; Nagaraju, M. The Pattern Electroretinogram as a Tool to Monitor Progressive Retinal Ganglion Cell Dysfunction in the DBA/2J Mouse Model of Glaucoma. Investig. Ophthalmol. Vis. Sci. 2007, 48, 745–751. [Google Scholar] [CrossRef] [Green Version]
- Brandli, A.; Stone, J. Using the Electroretinogram to Assess Function in the Rodent Retina and the Protective Effects of Remote Limb Ischemic Preconditioning. J. Vis. Exp. 2015, e52658. [Google Scholar] [CrossRef] [Green Version]
- Benchorin, G.; Calton, M.A.; Beaulieu, M.O.; Vollrath, D. Assessment of Murine Retinal Function by Electroretinography. Bio. Protoc. 2017, 7, e2218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, C.B.; D’Amore, P.A.; Connor, K.M. Revisiting the Mouse Model of Oxygen-Induced Retinopathy. Eye Brain 2016, 8, 67–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Connor, K.M.; Krah, N.M.; Dennison, R.J.; Aderman, C.M.; Chen, J.; Guerin, K.I.; Sapieha, P.; Stahl, A.; Willett, K.L.; Smith, L.E.H. Quantification of Oxygen-Induced Retinopathy in the Mouse: A Model of Vessel Loss, Vessel Regrowth and Pathological Angiogenesis. Nat. Protoc. 2009, 4, 1565–1573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, S.; Imai, S.; Ogishima, H.; Tsuruma, K.; Shimazawa, M.; Hara, H. Morphological and Functional Changes in the Retina after Chronic Oxygen-Induced Retinopathy. PLoS ONE 2012, 7, e32167. [Google Scholar] [CrossRef] [Green Version]
- Jeon, C.J.; Strettoi, E.; Masland, R.H. The Major Cell Populations of the Mouse Retina. J. Neurosci. 1998, 18, 8936–8946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akkoyun, I.; Karabay, G.; Haberal, N.; Dagdeviren, A.; Yilmaz, G.; Oto, S.; Erkanli, L.; Akova, Y.A. Structural Consequences after Intravitreal Bevacizumab Injection without Increasing Apoptotic Cell Death in a Retinopathy of Prematurity Mouse Model. Acta Ophthalmol. 2012, 90, 564–570. [Google Scholar] [CrossRef]
- Inan, U.U.; Avci, B.; Kusbeci, T.; Kaderli, B.; Avci, R.; Temel, S.G. Preclinical Safety Evaluation of Intravitreal Injection of Full-Length Humanized Vascular Endothelial Growth Factor Antibody in Rabbit Eyes. Investig. Ophthalmol. Vis. Sci. 2007, 48, 1773–1781. [Google Scholar] [CrossRef] [Green Version]
- Avci, B.; Avci, R.; Inan, U.U.; Kaderli, B. Comparative Evaluation of Apoptotic Activity in Photoreceptor Cells after Intravitreal Injection of Bevacizumab and Pegaptanib Sodium in Rabbits. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3438–3446. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Cao, Z.; Ji, H.; Yang, X.; Iwamoto, H.; Wahlberg, E.; Länne, T.; Sun, B.; Cao, Y. Anti-VEGF- and Anti-VEGF Receptor-Induced Vascular Alteration in Mouse Healthy Tissues. Proc. Natl. Acad. Sci. USA 2013, 110, 12018–12023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mintz-Hittner, H.A.; Kennedy, K.A.; Chuang, A.Z. BEAT-ROP Cooperative Group Efficacy of Intravitreal Bevacizumab for Stage 3+ Retinopathy of Prematurity. N. Engl. J. Med. 2011, 364, 603–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabbinavar, F.; Hurwitz, H.I.; Fehrenbacher, L.; Meropol, N.J.; Novotny, W.F.; Lieberman, G.; Griffing, S.; Bergsland, E. Phase II, Randomized Trial Comparing Bevacizumab plus Fluorouracil (FU)/Leucovorin (LV) with FU/LV Alone in Patients with Metastatic Colorectal Cancer. J. Clin. Oncol. 2003, 21, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Mezu-Ndubuisi, O.J. In Vivo Angiography Quantifies Oxygen-Induced Retinopathy Vascular Recovery. Optom. Vis. Sci. 2016, 93, 1268–1279. [Google Scholar] [CrossRef] [Green Version]
- Patel, A.K.; Akinsoji, E.; Hackam, A.S. Defining the Relationships Among Retinal Function, Layer Thickness and Visual Behavior During Oxidative Stress-Induced Retinal Degeneration. Curr. Eye. Res. 2016, 41, 977–986. [Google Scholar] [CrossRef]
- Kokona, D.; Jovanovic, J.; Ebneter, A.; Zinkernagel, M.S. In Vivo Imaging of Cx3cr1gfp/Gfp Reporter Mice with Spectral-Domain Optical Coherence Tomography and Scanning Laser Ophthalmoscopy. J. Vis. Exp. 2017. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Tian, H.; Dai, C.; Wen, R.; Li, X.; Webster, K.A.; Li, W. Optimal Efficacy and Safety of Humanized Anti-Scg3 Antibody to Alleviate Oxygen-Induced Retinopathy. Int. J. Mol. Sci. 2022, 23, 350. https://doi.org/10.3390/ijms23010350
He Y, Tian H, Dai C, Wen R, Li X, Webster KA, Li W. Optimal Efficacy and Safety of Humanized Anti-Scg3 Antibody to Alleviate Oxygen-Induced Retinopathy. International Journal of Molecular Sciences. 2022; 23(1):350. https://doi.org/10.3390/ijms23010350
Chicago/Turabian StyleHe, Ye, Hong Tian, Chang Dai, Rong Wen, Xiaorong Li, Keith A. Webster, and Wei Li. 2022. "Optimal Efficacy and Safety of Humanized Anti-Scg3 Antibody to Alleviate Oxygen-Induced Retinopathy" International Journal of Molecular Sciences 23, no. 1: 350. https://doi.org/10.3390/ijms23010350





