A Molecular Analysis of Cytokine Content across Extracellular Vesicles, Secretions, and Intracellular Space from Different Site-Specific Adipose-Derived Stem Cells
Abstract
:1. Introduction
2. Results
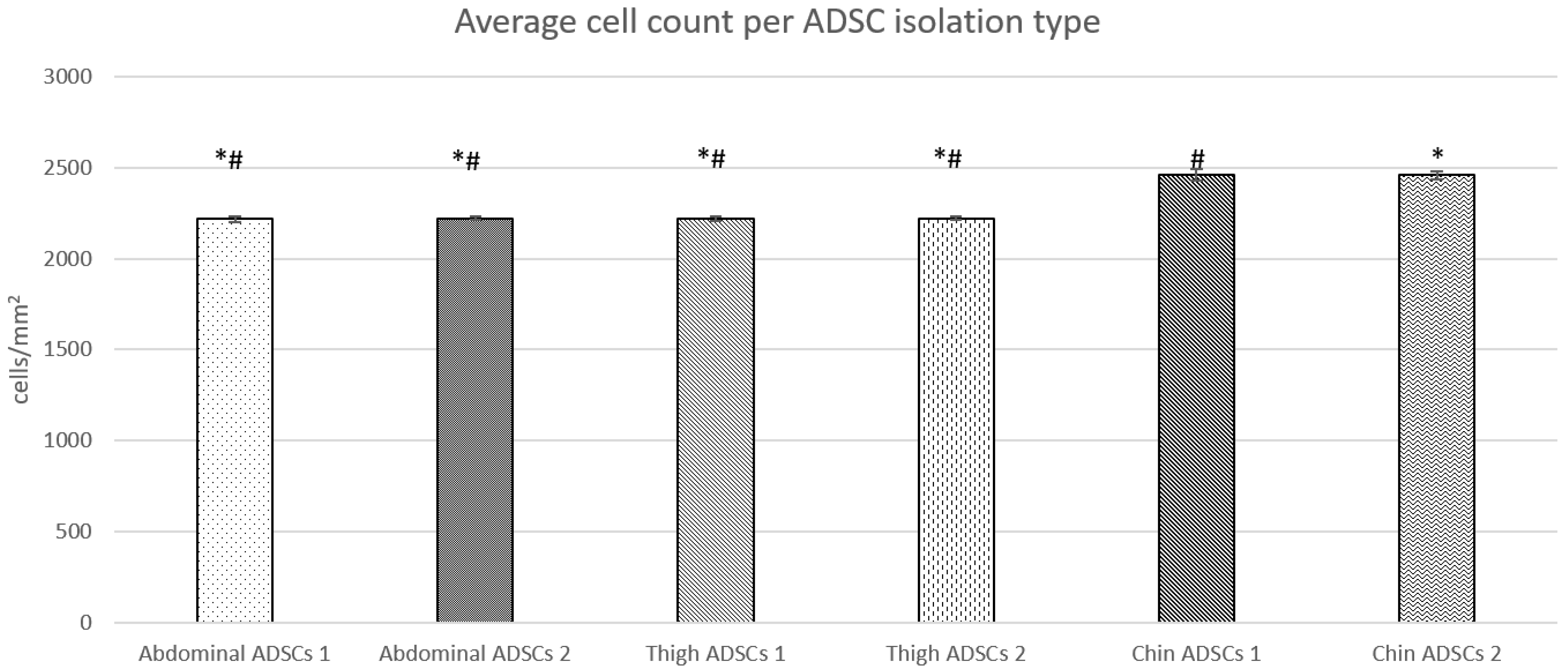
2.1. Live Cell Microscopy
2.2. Heatmap and Euclidean Clustering of Measured Cytokines
2.2.1. Cellular Cytokines
2.2.2. EV Cytokines
2.2.3. Secretion Cytokines
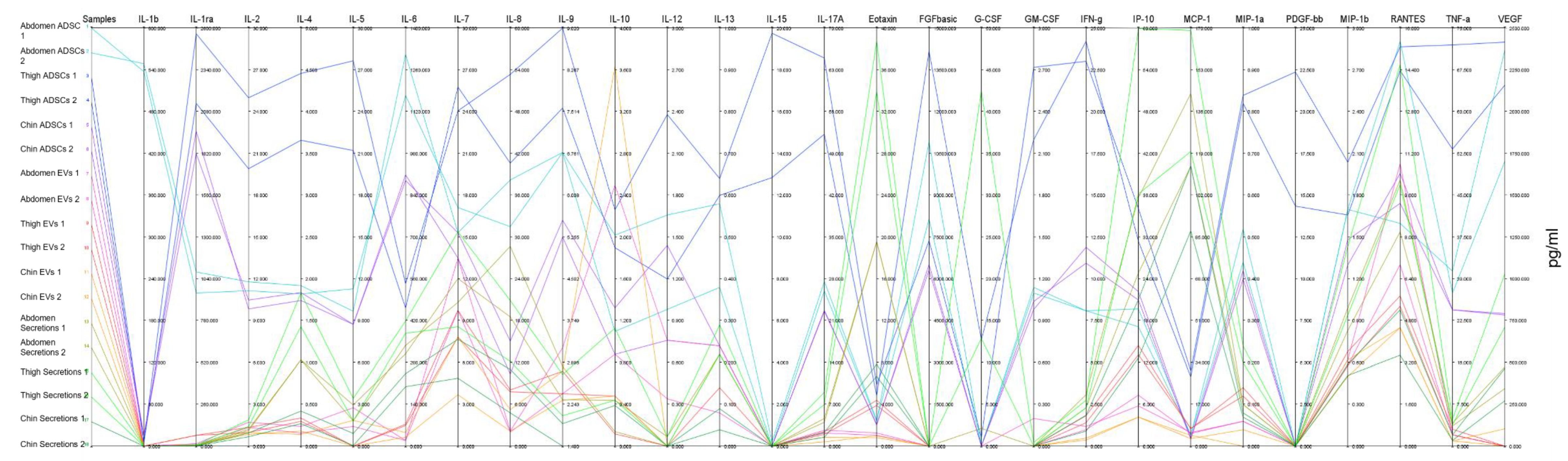
2.3. Parallel Coordinates
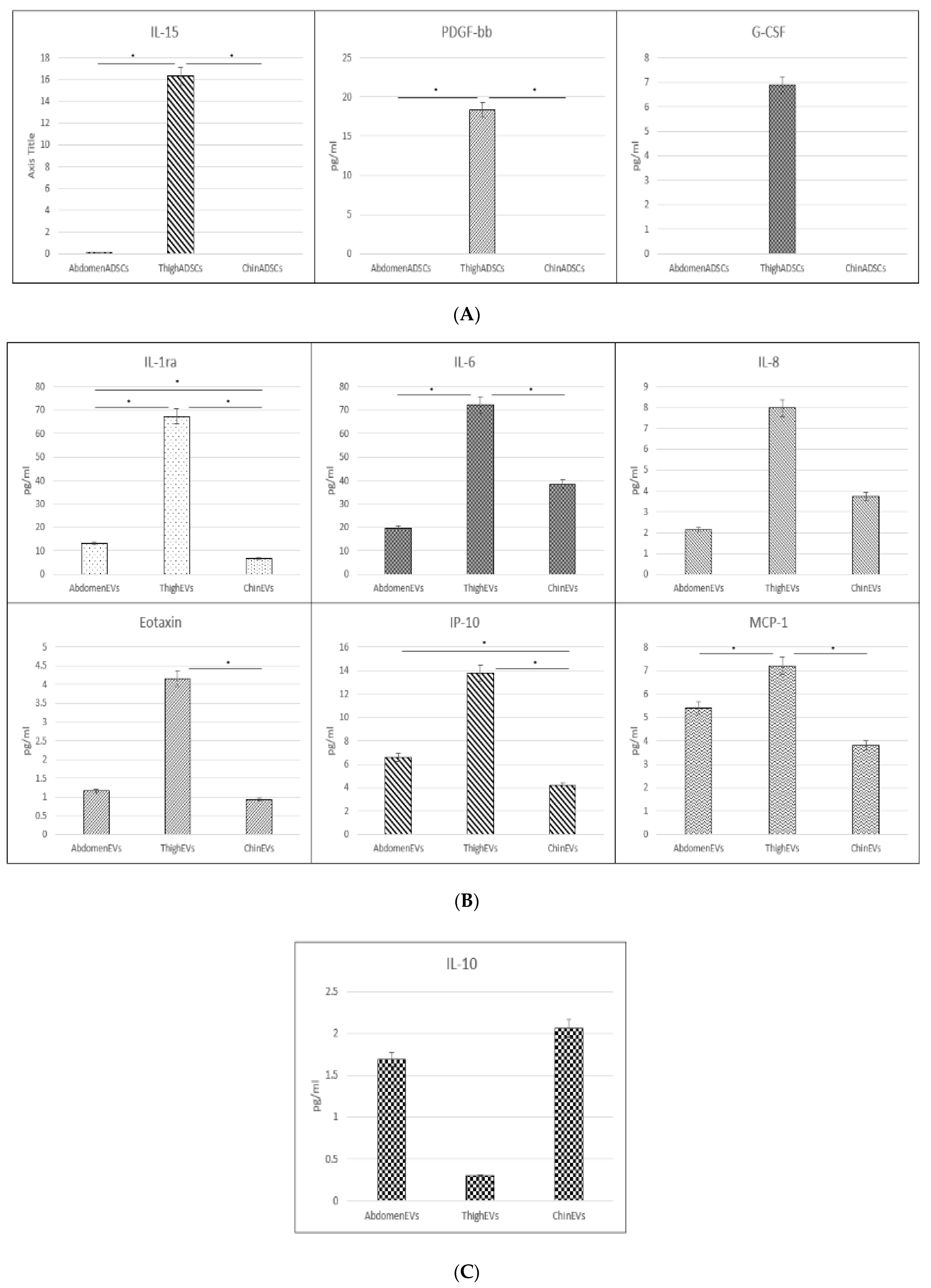
2.4. Critical Cytokine Content Comparison
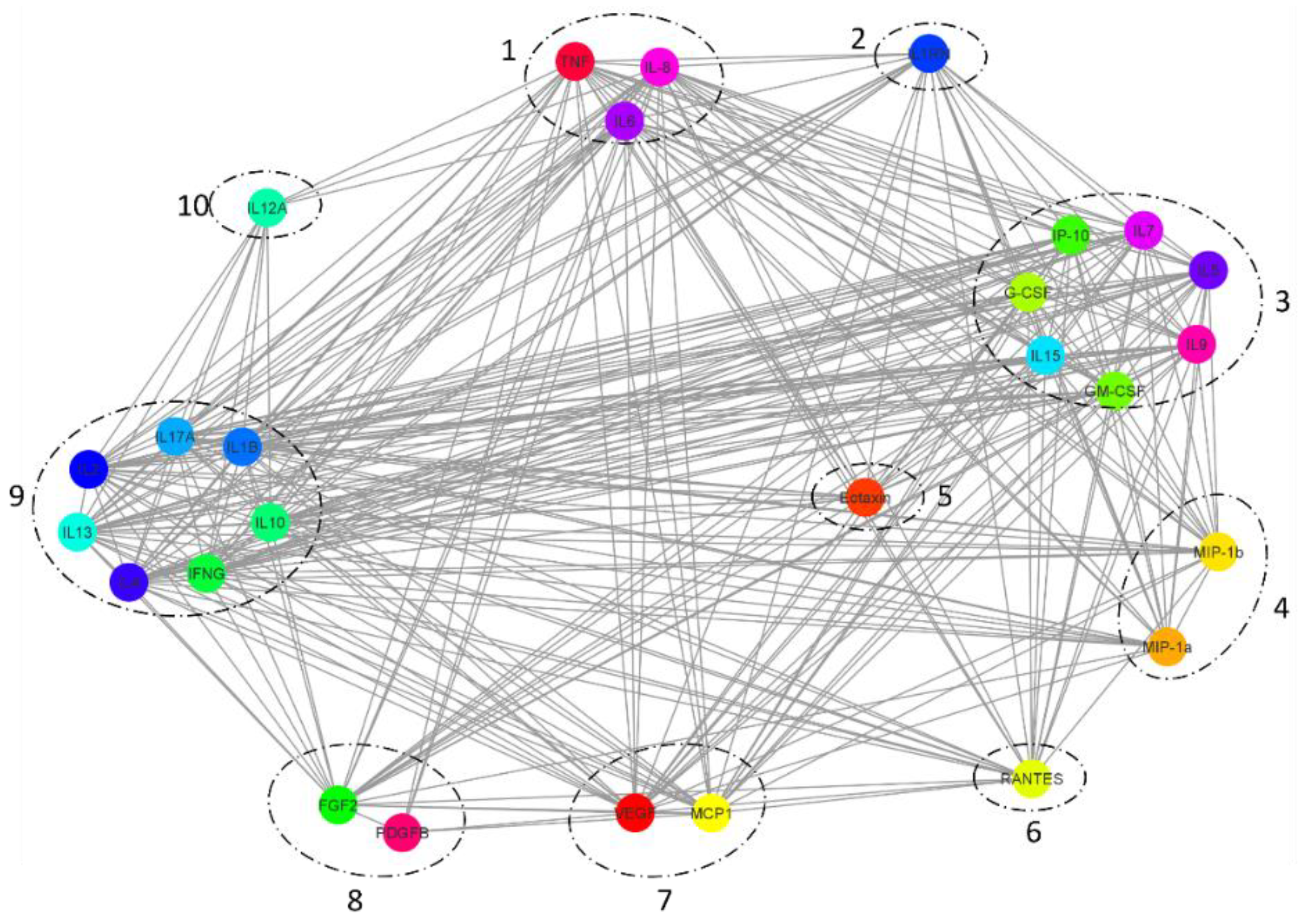
2.5. Cytokine Interaction Network Mapping
3. Discussion
3.1. TNF-Alpha, IL-6, and IL-8 Biological Effects in MSCs
3.2. Critical Cytokine Content Changes in Cells
3.3. Extracellular Vesicle Cytokine Content
4. Materials and Methods
4.1. Sample Collection
4.2. Cell Culture
4.3. Extracellular Vesicle Isolation
4.4. Secretion Isolation
4.5. Sample Preparation
4.6. Cytokine Assay
4.7. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, J.-M.; An, J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [Green Version]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef] [Green Version]
- Deverman, B.E.; Patterson, P.H. Cytokines and CNS development. Neuron 2009, 64, 61–78. [Google Scholar] [CrossRef] [Green Version]
- Aurora, A.B.; Olson, E.N. Immune modulation of stem cells and regeneration. Cell Stem Cell 2014, 15, 14–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caplan, A.I.; Bruder, S.P. Mesenchymal stem cells: Building blocks for molecular medicine in the 21st century. Trends Mol. Med. 2001, 7, 259–264. [Google Scholar] [CrossRef]
- Santos, J.; Hubert, T.; Milthorpe, B.K. Valproic Acid Promotes Early Neural Differentiation in Adult Mesenchymal Stem Cells Through Protein Signalling Pathways. Cells 2020, 9, 619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murakami, M.; Hayashi, Y.; Iohara, K.; Osako, Y.; Hirose, Y.; Nakashima, M. Trophic Effects and Regenerative Potential of Mobilized Mesenchymal Stem Cells from Bone Marrow and Adipose Tissue as Alternative Cell Sources for Pulp/Dentin Regeneration. Cell Transplant. 2015, 24, 1753–1765. [Google Scholar] [CrossRef] [PubMed]
- Kyurkchiev, D.; Bochev, I.; Ivanova-Todorova, E.; Mourdjeva, M.; Oreshkova, T.; Belemezova, K.; Kyurkchiev, S. Secretion of immunoregulatory cytokines by mesenchymal stem cells. World J. Stem Cells 2014, 6, 552–570. [Google Scholar] [CrossRef] [PubMed]
- Goshen, I.; Kreisel, T.; Ben-Menachem-Zidon, O.; Licht, T.; Weidenfeld, J.; Ben-Hur, T.; Yirmiya, R. Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol. Psychiatry 2008, 13, 717–728. [Google Scholar] [CrossRef] [Green Version]
- Barnes, B.J.; Somerville, C.C. Modulating Cytokine Production via Select Packaging and Secretion from Extracellular Vesicles. Front. Immunol. 2020, 11, 1040. [Google Scholar] [CrossRef]
- Dalla, P.V.; Santos, J.; Milthorpe, B.K.; Padula, M.P. Selectively-Packaged Proteins in Breast Cancer Extracellular Vesicles Involved in Metastasis. Int. J. Mol. Sci. 2020, 21, 4990. [Google Scholar] [CrossRef] [PubMed]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Maas, S.L.N.; Breakefield, X.O.; Weaver, A.M. Extracellular Vesicles: Unique Intercellular Delivery Vehicles. Trends Cell Biol. 2017, 27, 172–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, J.; Milthorpe, B.K.; Herbert, B.R.; Padula, M.P. Proteomic Analysis of Human Adipose Derived Stem Cells during Small Molecule Chemical Stimulated Pre-neuronal Differentiation. Int. J. Stem Cells 2017, 10, 193–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, J.; Milthorpe, B.K.; Padula, M.P. Proteomic Analysis of Cyclic Ketamine Compounds Ability to Induce Neural Differentiation in Human Adult Mesenchymal Stem Cells. Int. J. Mol. Sci. 2019, 20, 523. [Google Scholar] [CrossRef] [Green Version]
- Fajardo, J.; Milthorpe, B.K.; Santos, J. Molecular Mechanisms Involved in Neural Substructure Development during Phosphodiesterase Inhibitor Treatment of Mesenchymal Stem Cells. Int. J. Mol. Sci. 2020, 21, 4867. [Google Scholar] [CrossRef]
- Mendelson, A.; Frenette, P.S. Hematopoietic stem cell niche maintenance during homeostasis and regeneration. Nat. Med. 2014, 20, 833–846. [Google Scholar] [CrossRef] [Green Version]
- Zakrzewski, W.; Dobrzyński, M.; Szymonowicz, M.; Rybak, Z. Stem cells: Past, present, and future. Stem Cell Res. Ther. 2019, 10, 68. [Google Scholar] [CrossRef]
- Gu, B.; Zhang, J.; Wang, W.; Mo, L.; Zhou, Y.; Chen, L.; Liu, Y.; Zhang, M. Global expression of cell surface proteins in embryonic stem cells. PLoS ONE 2010, 5, e15795. [Google Scholar] [CrossRef]
- Niehage, C.; Steenblock, C.; Pursche, T.; Bornhäuser, M.; Corbeil, D.; Hoflack, B. The cell surface proteome of human mesenchymal stromal cells. PLoS ONE 2011, 6, e20399. [Google Scholar] [CrossRef] [Green Version]
- Kristensen, D.M.; Kalisz, M.; Nielsen, J.H. Cytokine signalling in embryonic stem cells. Apmis 2005, 113, 756–772. [Google Scholar] [CrossRef] [PubMed]
- Robb, L. Cytokine receptors and hematopoietic differentiation. Oncogene 2007, 26, 6715–6723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stillwell, W. Membrane Transport. In An Introduction to Biological Membranes; Elsevier: Amsterdam, The Netherlands, 2016; pp. 423–451. [Google Scholar] [CrossRef]
- Burbidge, K.; Zwikelmaier, V.; Cook, B.; Long, M.M.; Balva, B.; Lonigro, M.; Ispas, G.; Rademacher, D.J.; Campbell, E.M. Cargo and cell-specific differences in extracellular vesicle populations identified by multiplexed immunofluorescent analysis. J. Extracell. Vesicles 2020, 9, 1789326. [Google Scholar] [CrossRef]
- Idriss, H.T.; Naismith, J.H. TNF alpha and the TNF receptor superfamily: Structure-function relationship(s). Microsc. Res. Tech. 2000, 50, 184–195. [Google Scholar] [CrossRef]
- Kern, P.A.; Ranganathan, S.; Li, C.; Wood, L.; Ranganathan, G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E745–E751. [Google Scholar] [CrossRef]
- Wang, X.; Lin, Y. Tumor necrosis factor and cancer, buddies or foes? Acta Pharmacol. Sin. 2008, 29, 1275–1288. [Google Scholar] [CrossRef] [Green Version]
- Ghali, O.; Chauveau, C.; Hardouin, P.; Broux, O.; Devedjian, J.C. TNF-alpha’s effects on proliferation and apoptosis in human mesenchymal stem cells depend on RUNX2 expression. J. Bone Miner. Res. 2010, 25, 1616–1626. [Google Scholar] [CrossRef]
- Widera, D.; Mikenberg, I.; Elvers, M.; Kaltschmidt, C.; Kaltschmidt, B. Tumor necrosis factor alpha triggers proliferation of adult neural stem cells via IKK/NF-kappaB signaling. BMC Neurosci. 2006, 7, 64. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Li, Z.; Hou, Y.; Fang, W. Potential mechanisms underlying the Runx2 induced osteogenesis of bone marrow mesenchymal stem cells. Am. J. Transl. Res. 2015, 7, 2527–2535. [Google Scholar]
- Stein, G.S.; Lian, J.B.; Wijnen, A.J.v.; Stein, J.L.; Montecino, M.; Javed, A.; Zaidi, S.K.; Young, D.W.; Choi, J.-Y.; Pockwinse, S.M. Runx2 control of organization, assembly and activity of the regulatory machinery for skeletal gene expression. Oncogene 2004, 23, 4315–4329. [Google Scholar] [CrossRef] [Green Version]
- Sabio, G.; Davis, R.J. TNF and MAP kinase signalling pathways. Semin. Immunol. 2014, 26, 237–245. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, J.P.; Ríos, S.; Fernández, M.; Santibañez, J.F. Differential activation of ERK1,2 MAP kinase signaling pathway in mesenchymal stem cell from control and osteoporotic postmenopausal women. J. Cell. Biochem. 2004, 92, 745–754. [Google Scholar] [CrossRef]
- Rehman, K.; Akash, M.S.H.; Liaqat, A.; Kamal, S.; Qadir, M.I.; Rasul, A. Role of Interleukin-6 in Development of Insulin Resistance and Type 2 Diabetes Mellitus. Crit. Rev. Eukaryot. Gene Expr. 2017, 27, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Dorronsoro, A.; Lang, V.; Ferrin, I.; Fernández-Rueda, J.; Zabaleta, L.; Pérez-Ruiz, E.; Sepúlveda, P.; Trigueros, C. Intracellular role of IL-6 in mesenchymal stromal cell immunosuppression and proliferation. Sci. Rep. 2020, 10, 21853. [Google Scholar] [CrossRef] [PubMed]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta 2011, 1813, 878–888. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; van Boxel-Dezaire, A.H.; Cheon, H.; Yang, J.; Stark, G.R. STAT3 activation in response to IL-6 is prolonged by the binding of IL-6 receptor to EGF receptor. Proc. Natl. Acad. Sci. USA 2013, 110, 16975–16980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnold, P.; Lückstädt, W.; Li, W.; Boll, I.; Lokau, J.; Garbers, C.; Lucius, R.; Rose-John, S.; Becker-Pauly, C. Joint Reconstituted Signaling of the IL-6 Receptor via Extracellular Vesicles. Cells 2020, 9, 1307. [Google Scholar] [CrossRef]
- Pricola, K.L.; Kuhn, N.Z.; Haleem-Smith, H.; Song, Y.; Tuan, R.S. Interleukin-6 maintains bone marrow-derived mesenchymal stem cell stemness by an ERK1/2-dependent mechanism. J. Cell. Biochem. 2009, 108, 577–588. [Google Scholar] [CrossRef] [Green Version]
- Hou, Y.; Ryu, C.H.; Jun, J.A.; Kim, S.M.; Jeong, C.H.; Jeun, S.S. IL-8 enhances the angiogenic potential of human bone marrow mesenchymal stem cells by increasing vascular endothelial growth factor. Cell Biol. Int. 2014, 38, 1050–1059. [Google Scholar] [CrossRef]
- Chen, G.; Shi, X.; Sun, C.; Li, M.; Zhou, Q.; Zhang, C.; Huang, J.; Qiu, Y.; Wen, X.; Zhang, Y.; et al. VEGF-Mediated Proliferation of Human Adipose Tissue-Derived Stem Cells. PLoS ONE 2013, 8, e73673. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; He, Y.R.; Liu, S.J.; Hu, L.; Liang, L.C.; Liu, D.L.; Liu, L.; Zhu, Z.Q. Enhanced Effect of IL-1β-Activated Adipose-Derived MSCs (ADMSCs) on Repair of Intestinal Ischemia-Reperfusion Injury via COX-2-PGE2 Signaling. Stem Cells Int. 2020, 2020, 2803747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sullivan, C.B.; Porter, R.M.; Evans, C.H.; Ritter, T.; Shaw, G.; Barry, F.; Murphy, J.M. TNFα and IL-1β influence the differentiation and migration of murine MSCs independently of the NF-κB pathway. Stem Cell Res. Ther. 2014, 5, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrell, C.R.; Markovic, B.S.; Fellabaum, C.; Arsenijevic, N.; Djonov, V.; Volarevic, V. The role of Interleukin 1 receptor antagonist in mesenchymal stem cell-based tissue repair and regeneration. BioFactors 2020, 46, 263–275. [Google Scholar] [CrossRef]
- Raica, M.; Cimpean, A.M. Platelet-Derived Growth Factor (PDGF)/PDGF Receptors (PDGFR) Axis as Target for Antitumor and Antiangiogenic Therapy. Pharmaceuticals 2010, 3, 572–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostman, A.; Thyberg, J.; Westermark, B.; Heldin, C.H. PDGF-AA and PDGF-BB biosynthesis: Proprotein processing in the Golgi complex and lysosomal degradation of PDGF-BB retained intracellularly. J. Cell Biol. 1992, 118, 509–519. [Google Scholar] [CrossRef] [Green Version]
- Blažević, T.; Schwaiberger, A.V.; Schreiner, C.E.; Schachner, D.; Schaible, A.M.; Grojer, C.S.; Atanasov, A.G.; Werz, O.; Dirsch, V.M.; Heiss, E.H. 12/15-Lipoxygenase Contributes to Platelet-derived Growth Factor-induced Activation of Signal Transducer and Activator of Transcription 3. J. Biol. Chem. 2013, 288, 35592–35603. [Google Scholar] [CrossRef] [Green Version]
- Symes, K.; Mercola, M. Embryonic mesoderm cells spread in response to platelet-derived growth factor and signaling by phosphatidylinositol 3-kinase. Proc. Natl. Acad. Sci. USA 1996, 93, 9641–9644. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Nicola, D.; Valle-Argos, B.; Pallas-Bazarra, N.; Nieto-Sampedro, M. Interleukin-15 regulates proliferation and self-renewal of adult neural stem cells. Mol. Biol. Cell 2011, 22, 1960–1970. [Google Scholar] [CrossRef]
- Cui, G.; Hara, T.; Simmons, S.; Wagatsuma, K.; Abe, A.; Miyachi, H.; Kitano, S.; Ishii, M.; Tani-ichi, S.; Ikuta, K. Characterization of the IL-15 niche in primary and secondary lymphoid organs in vivo. Proc. Natl. Acad. Sci. USA 2014, 111, 1915–1920. [Google Scholar] [CrossRef] [Green Version]
- Avila-Portillo, L.M.; Aristizabal, F.; Riveros, A.; Abba, M.C.; Correa, D. Modulation of Adipose-Derived Mesenchymal Stem/Stromal Cell Transcriptome by G-CSF Stimulation. Stem Cells Int. 2020, 2020, 5045124. [Google Scholar] [CrossRef]
- Boehme, S.A.; Sullivan, S.K.; Crowe, P.D.; Santos, M.; Conlon, P.J.; Sriramarao, P.; Bacon, K.B. Activation of Mitogen-Activated Protein Kinase Regulates Eotaxin-Induced Eosinophil Migration. J. Immunol. 1999, 163, 1611. [Google Scholar] [PubMed]
- Werle, M.; Schmal, U.; Hanna, K.; Kreuzer, J. MCP-1 induces activation of MAP-kinases ERK, JNK and p38 MAPK in human endothelial cells. Cardiovasc. Res. 2002, 56, 284–292. [Google Scholar] [CrossRef] [Green Version]
- Viedt, C.; Dechend, R.; Fei, J.; Hansch, G.M.; Kreuzer, J.; Orth, S.R. MCP-1 induces inflammatory activation of human tubular epithelial cells: Involvement of the transcription factors, nuclear factor-kappaB and activating protein-1. J. Am. Soc. Nephrol. 2002, 13, 1534–1547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iyer, S.S.; Cheng, G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit Rev. Immunol. 2012, 32, 23–63. [Google Scholar] [CrossRef] [Green Version]
- Wilbers, R.H.P.; van Raaij, D.R.; Westerhof, L.B.; Bakker, J.; Smant, G.; Schots, A. Re-evaluation of IL-10 signaling reveals novel insights on the contribution of the intracellular domain of the IL-10R2 chain. PLoS ONE 2017, 12, e0186317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirano, T.; Ishihara, K.; Hibi, M. Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene 2000, 19, 2548–2556. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]


| Name | Full Name | Accession | Gene | Biological Annotation |
|---|---|---|---|---|
| IL1b | Interleukin 1, beta | P01584 | IL1B | Potent proinflammatory cytokine |
| IL1rn | Interleukin-1 receptor antagonist protein | P18510 | IL1RN | Inhibits the activity of interleukin-1 |
| IL2 | Interleukin-2 | P60568 | IL2 | T-cell growth factor |
| IL4 | Interleukin-4 | P05112 | IL4 | Lymphocyte stimulatory factor 1 |
| IL5 | Interleukin-5 | P05113 | IL5 | Eosinophil differentiation factor |
| IL6 | Interleukin-6 | P05231 | IL6 | B-cell stimulatory factor 2 |
| IL7 | Interleukin-7 | P13232 | IL7 | Hematopoietic growth factor capable of stimulating the proliferation of lymphoid progenitors |
| IL-8 | Interleukin-8 | Q9UCS0 | CXCL8 | Monocyte-derived neutrophil chemotactic factor; IL-8 is a chemotactic factor that attracts neutrophils, basophils, and T-cells, but not monocytes |
| IL9 | Interleukin-9 | P15248 | IL9 | T-cell growth factor P40 |
| IL10 | Interleukin-10 | P22301 | IL10 | Cytokine synthesis inhibitory factor |
| IL12 | Interleukin-12 | P29459 | IL12A | Cytotoxic lymphocyte maturation factor 35 kDa subunit |
| IL13 | Interleukin-13 | P35225 | IL13 | Inhibits inflammatory cytokine production |
| IL15 | Interleukin-15 | P40933 | IL15 | Cytokine that stimulates the proliferation of T-lymphocytes |
| IL17A | Interleukin-17A | Q16552 | IL17A | Cytotoxic T-lymphocyte-associated antigen 8 |
| Eotaxin | Chemokine (C-C motif) ligand 11 | P51671 | CCL11 | In response to the presence of allergens |
| FGF-basic | Fibroblast growth factor 2 (basic) | Q9UCS5 | FGF2 | Plays an important role in the regulation of cell survival, cell division, angiogenesis, cell differentiation, and cell migration. |
| G-CSF | Granulocyte colony stimulating factor 3 | P09919 | CSF3 | Granulocyte/macrophage colony-stimulating factor |
| GM-CSF | Granulocyte-macrophage colony stimulating factor 2 | P04141 | CSF2 | Cytokine that stimulates growth and differentiation |
| IFN-γ | Immune interferon gamma | P01579 | IFNG | Produced by lymphocytes activated by specific antigens or mitogens |
| IP-10 | 10 kDa interferon gamma-induced protein | P02778 | CXCL10 | Chemotactic for monocytes and T-lymphocytes |
| MCP1 | Monocyte chemotactic and activating factor | P13500 | CCL2 | Chemotactic factor that attracts monocytes and basophils but not neutrophils or eosinophils |
| MIP-1a | Macrophage inflammatory protein 1-alpha | P10147 | CCL3 | Monokine with inflammatory and chemokinetic properties |
| MIP-1b | Monocyte adherence-induced protein 5-alpha | Q8NHW4 | CCL4L1 | Chemokine that induces chemotaxis of cells expressing CCR5 or CCR1 |
| PDGFB | Platelet-derived growth factor beta polypeptide | P01127 | PDGFB | Growth factor that plays an essential role in the regulation of embryonic development, cell proliferation, cell migration, survival, and chemotaxis |
| RANTES | Eosinophil chemotactic cytokine | P13501 | CCL5 | Chemoattractant for blood monocytes, memory T helper cells, and eosinophils; causes the release of histamine from basophils and activates eosinophils |
| TNF-α | Tumour necrosis factor ligand superfamily member 2 | P01375 | TNF | Cytokine that binds to TNFRSF1A/TNFR1 and TNFRSF1B/TNFBR |
| VEGF | Vascular endothelial growth factor A | Q9H1W9 | VEGFA | Growth factor active in angiogenesis, vasculogenesis, and endothelial cell growth |
| Group | Cytokines in Group | Number of Cytokines in Group | Interaction Description | Affiliates/Non-Affiliates |
|---|---|---|---|---|
| 1 | TNF, IL-8 and IL-6 | 3 | Interacts with all groups | Groups 1–10 |
| 2 | IL1rn | 1 | Does not interact with | Group 8 Group 10 |
| 3 | IL-5, IL-7, IL-9, IL-15, GM-CSF, G-CSF and IP-10 | 7 | Does not interact with | Group 10 PDGFB from group 8 |
| 4 | MIP-1a and MIP-1b | 2 | Does not interact with | Group 2 Group 5 Group 8 Group10 |
| 5 | Eotaxin | 1 | Does not interact with | Group 4 Group 6 Group 7 Group 10 PDGF from group 8 |
| 6 | Rantes | 1 | Does not interact with | Group 10 MIP-1a from group 4PDGF from group 8 |
| 7 | MCP1 and VEGF | 2 | Does not interact with | Group 5 Group 10 |
| 8 | FGF2 | 2 | Does not interact with | Group 2 Group 10 |
| PDGF | Does not interact with | Group 2 Group 3 Group 4 Group 5 Group 9 Group 10 | ||
| 9 | IL-2, IL-17A, IL-1B, IL-10, IFN-γ, IL4, and IL13 | 7 | Does not interact with | PDGF from group 8 |
| 10 | IL-12A | 1 | Interacts only with | Group 1 Group 9 Group 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, J.; Dalla, P.V.; Milthorpe, B.K. A Molecular Analysis of Cytokine Content across Extracellular Vesicles, Secretions, and Intracellular Space from Different Site-Specific Adipose-Derived Stem Cells. Int. J. Mol. Sci. 2022, 23, 397. https://doi.org/10.3390/ijms23010397
Santos J, Dalla PV, Milthorpe BK. A Molecular Analysis of Cytokine Content across Extracellular Vesicles, Secretions, and Intracellular Space from Different Site-Specific Adipose-Derived Stem Cells. International Journal of Molecular Sciences. 2022; 23(1):397. https://doi.org/10.3390/ijms23010397
Chicago/Turabian StyleSantos, Jerran, Penelope V. Dalla, and Bruce K. Milthorpe. 2022. "A Molecular Analysis of Cytokine Content across Extracellular Vesicles, Secretions, and Intracellular Space from Different Site-Specific Adipose-Derived Stem Cells" International Journal of Molecular Sciences 23, no. 1: 397. https://doi.org/10.3390/ijms23010397
APA StyleSantos, J., Dalla, P. V., & Milthorpe, B. K. (2022). A Molecular Analysis of Cytokine Content across Extracellular Vesicles, Secretions, and Intracellular Space from Different Site-Specific Adipose-Derived Stem Cells. International Journal of Molecular Sciences, 23(1), 397. https://doi.org/10.3390/ijms23010397








