The Effect of Early Life Stress on Emotional Behaviors in GPR37KO Mice
Abstract
1. Introduction
2. Results
2.1. The Schematic of the Experimental Design
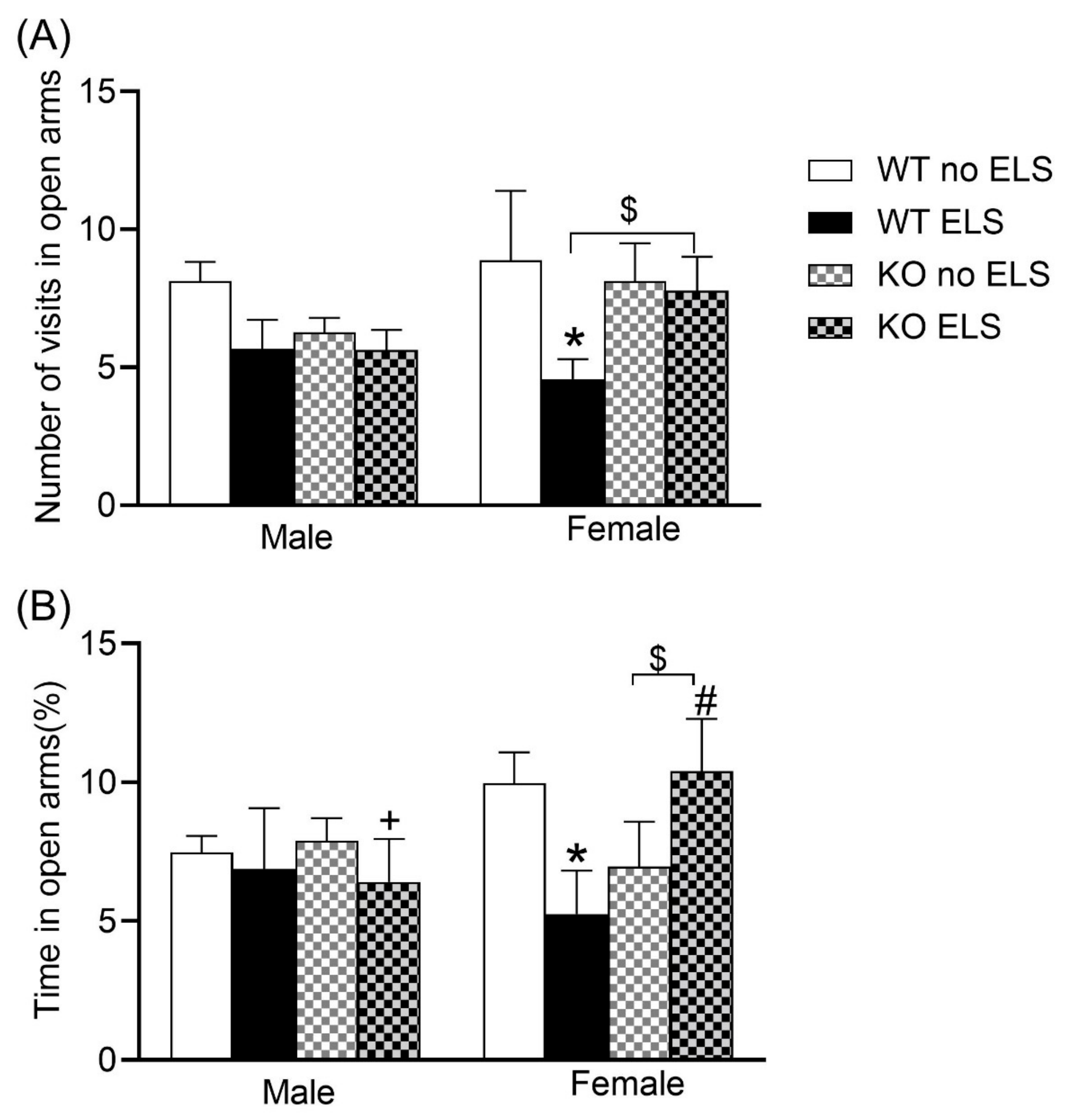
2.2. Effect of ELS on Anxiety-like Behaviors in Adult GPR37KO Mice
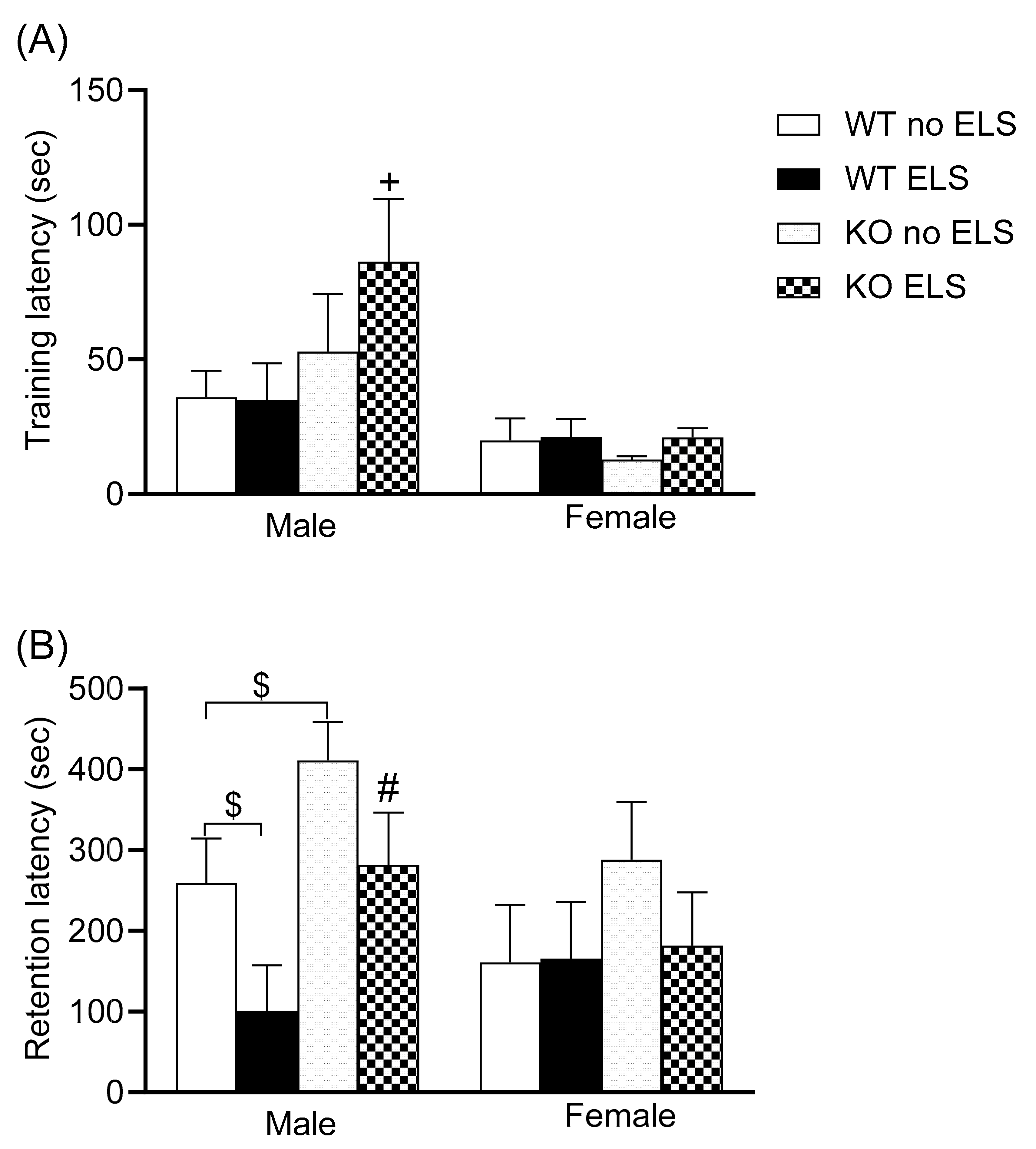
2.3. Effect of ELS on Emotional Memory Behavior in Adult GPR37KO Mice
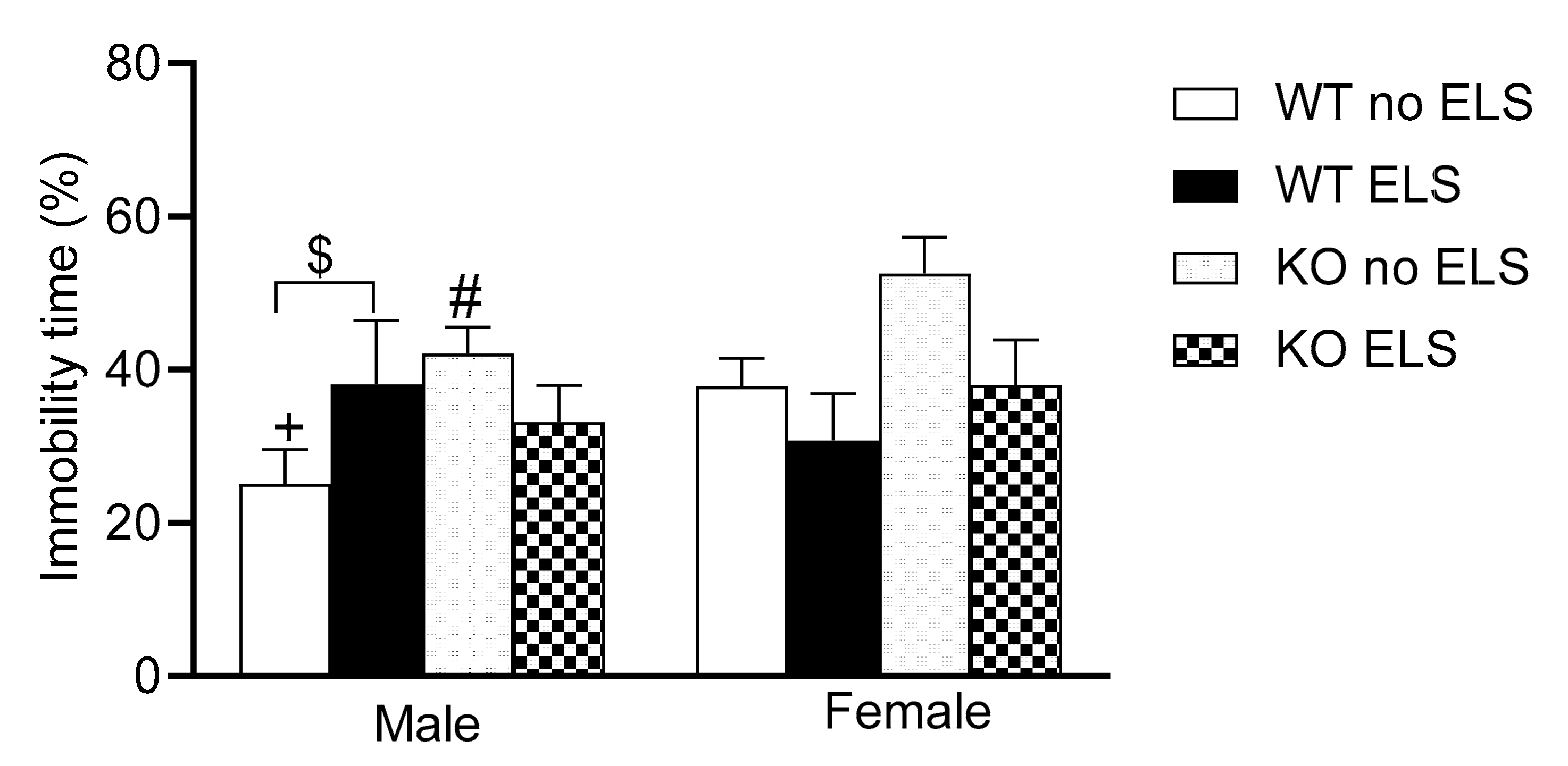
2.4. Effect of ELS on Depression-like Behavior in Adult GPR37KO Mice
2.5. Effect of ELS on Body Weight in GPR37KO Mice
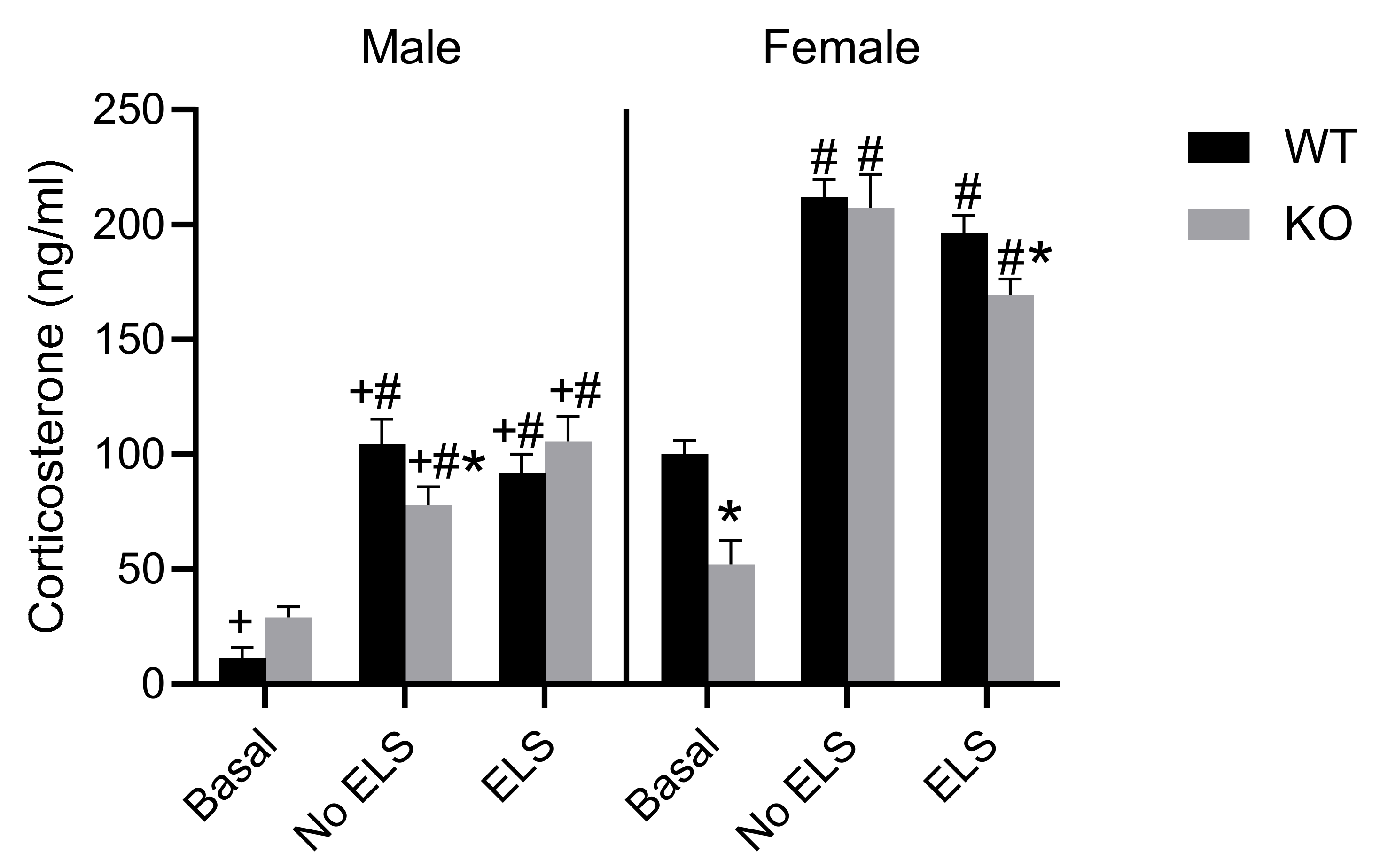
2.6. Effect of ELS on Corticosterone Level in Adult GPR37KO Mice
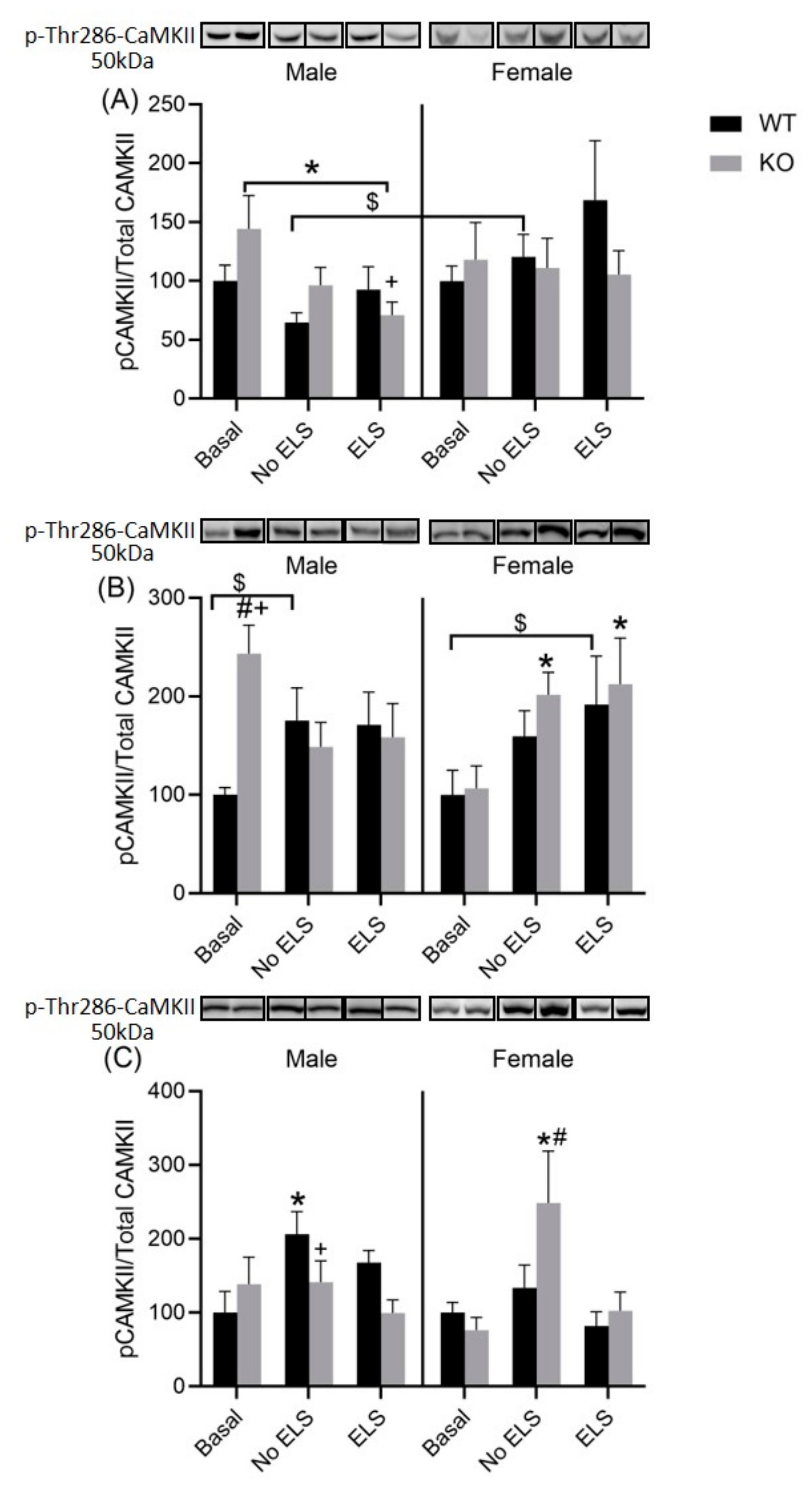
2.7. Effect of ELS on P-T286-CaMKII in DH, VH and Amygdala, in GPR37KO Mice
2.8. Effect of ELS on Total CaMKII in DH, VH and Amygdala, in GPR37KO Mice
3. Discussion
4. Materials and Methods
4.1. Subjects and Housing
4.2. Breeding and Genotype
4.3. General Experimental Plan
4.4. Limited Nesting Material
4.5. Behavioral Tests
4.5.1. Elevated Plus Maze
4.5.2. Light Dark Box
4.5.3. Passive Avoidance Task
4.5.4. Forced Swim Test
4.6. Brain Dissection
4.7. Western Blot
4.8. Corticosterone Assay
4.9. Statistical Analysis
5. Conclusions and Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marazziti, D.; Golini, E.; Gallo, A.; Lombardi, M.S.; Matteoni, R.; Tocchini-Valentini, G.P. Cloning of GPR37, a Gene Located on Chromosome 7 Encoding a Putative G-Protein-Coupled Peptide Receptor, from a Human Frontal Brain EST Library. Genomics 1997, 45, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Valdenaire, O.; Giller, T.; Breu, V.; Ardati, A.; Schweizer, A.; Richards, J.G. A new family of orphan G protein-coupled receptors predominantly expressed in the brain. FEBS Lett. 1998, 424, 193–196. [Google Scholar] [CrossRef]
- Marazziti, D.; Gallo, A.; Golini, E.; Matteoni, R.; Tocchini-Valentini, G.P. Molecular Cloning and Chromosomal Localization of the MouseGpr37Gene Encoding an Orphan G-Protein-Coupled Peptide Receptor Expressed in Brain and Testis. Genomics 1998, 53, 315–324. [Google Scholar] [CrossRef]
- Leinartaité, L.; Svenningsson, P. Folding Underlies Bidirectional Role of GPR37/Pael-R in Parkinson Disease. Trends Pharmacol. Sci. 2017, 38, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y.; Soda, M.; Inoue, H.; Hattori, N.; Mizuno, Y.; Takahashi, R. An unfolded putative transmembrane polypeptide, which can lead to endoplasmic reticulum stress, is a substrate of Parkin. Cell 2001, 105, 891–902. [Google Scholar] [CrossRef]
- Lundius, E.G.; Vukojevic, V.; Hertz, E.; Stroth, N.; Cederlund, A.; Hiraiwa, M.; Terenius, L.; Svenningsson, P. GPR37 protein trafficking to the plasma membrane regulated by prosaposin and GM1 gangliosides promotes cell viability. J. Biol. Chem. 2014, 289, 4660–4673. [Google Scholar] [CrossRef] [PubMed]
- Dusonchet, J.; Bensadoun, J.C.; Schneider, B.L.; Aebischer, P. Targeted overexpression of the parkin substrate Pael-R in the nigrostriatal system of adult rats to model Parkinson’s disease. Neurobiol. Dis. 2009, 35, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Marazziti, D.; Di Pietro, C.; Mandillo, S.; Golini, E.; Matteoni, R.; Tocchini-Valentini, G.P. Absence of the GPR37/PAEL receptor impairs striatal Akt and ERK2 phosphorylation, ΔFosB expression, and conditioned place preference to amphetamine and cocaine. FASEB J. 2011, 25, 2071–2081. [Google Scholar] [CrossRef] [PubMed]
- Marazziti, D.; Golini, E.; Mandillo, S.; Magrelli, A.; Witke, W.; Matteoni, R.; Tocchini-Valentini, G.P. Altered dopamine signaling and MPTP resistance in mice lacking the Parkinson’s disease-associated GPR37/parkin-associated endothelin-like receptor. Proc. Natl. Acad. Sci. USA 2004, 101, 10189–10194. [Google Scholar] [CrossRef] [PubMed]
- Marazziti, D.; Mandillo, S.; Di Pietro, C.; Golini, E.; Matteoni, R.; Tocchini-Valentini, G.P. GPR37 associates with the dopamine transporter to modulate dopamine uptake and behavioral responses to dopaminergic drugs. Proc. Natl. Acad. Sci. USA 2007, 104, 9846–9851. [Google Scholar] [CrossRef]
- Zhang, X.; Mantas, I.; Fridjonsdottir, E.; Andrén, P.E.; Chergui, K.; Svenningsson, P. Deficits in Motor Performance, Neurotransmitters and Synaptic Plasticity in Elderly and Experimental Parkinsonian Mice Lacking GPR37. Front. Aging Neurosci. 2020, 12, 84. [Google Scholar] [CrossRef] [PubMed]
- Mandillo, S.; Golini, E.; Marazziti, D.; Di Pietro, C.; Matteoni, R.; Tocchini-Valentini, G.P. Mice lacking the Parkinson’s related GPR37/PAEL receptor show non-motor behavioral phenotypes: Age and gender effect. Genes Brain Behav. 2013, 12, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Alavi, M.S.; Karimi, G.; Roohbakhsh, A. The role of orphan G protein-coupled receptors in the pathophysiology of multiple sclerosis: A review. Life Sci. 2019, 224, 33–40. [Google Scholar] [CrossRef]
- Alavi, M.S.; Shamsizadeh, A.; Azhdari-Zarmehri, H.; Roohbakhsh, A. Orphan G protein-coupled receptors: The role in CNS disorders. Biomed. Pharmacother. 2018, 98, 222–232. [Google Scholar] [CrossRef]
- Tomita, H.; Ziegler, M.E.; Kim, H.B.; Evans, S.J.; Choudary, P.V.; Li, J.Z.; Meng, F.; Dai, M.; Myers, R.M.; Neal, C.R.; et al. G protein-linked signaling pathways in bipolar and major depressive disorders. Front. Genet. 2013, 4, 297. [Google Scholar] [CrossRef]
- Chaudhuri, K.R.; Martinez-Martin, P.; Brown, R.G.; Sethi, K.; Stocchi, F.; Odin, P.; Ondo, W.; Abe, K.; MacPhee, G.; MacMahon, D.; et al. The metric properties of a novel non-motor symptoms scale for Parkinson’s disease: Results from an international pilot study. Mov. Disord. 2007, 22, 1901–1911. [Google Scholar] [CrossRef]
- Menza, M.A.; Robertson-Hoffman, D.E.; Bonapace, A.S. Parkinson’s disease and anxiety: Comorbidity with depression. Biol. Psychiatry 1993, 34, 465–470. [Google Scholar] [CrossRef]
- Pontone, G.M.; Dissanayka, N.; Apostolova, L.; Brown, R.G.; Dobkin, R.; Dujardin, K.; Friedman, J.H.; Leentjens, A.F.G.; Lenze, E.J.; Marsh, L.; et al. Report from a multidisciplinary meeting on anxiety as a non-motor manifestation of Parkinson’s disease. NPJ Parkinsons Dis. 2019, 5, 30. [Google Scholar] [CrossRef]
- Lopes, J.P.; Morató, X.; Souza, C.; Pinhal, C.; Machado, N.J.; Canas, P.M.; Silva, H.B.; Stagljar, I.; Gandía, J.; Fernández-Dueñas, V.; et al. The role of parkinson’s disease-associated receptor GPR37 in the hippocampus: Functional interplay with the adenosinergic system. J. Neurochem. 2015, 134, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Heim, C.; Nemeroff, C.B. The role of childhood trauma in the neurobiology of mood and anxiety disorders: Preclinical and clinical studies. Biol. Psychiatry 2001, 49, 1023–1039. [Google Scholar] [CrossRef]
- Rice, C.J.; Sandman, C.A.; Lenjavi, M.R.; Baram, T.Z. A novel mouse model for acute and long-lasting consequences of early life stress. Endocrinology 2008, 149, 4892–4900. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, M.C.; Ogawa, S. Long-Lasting Consequences of Neonatal Maternal Separation on Social Behaviors in Ovariectomized Female Mice. PLoS ONE 2012, 7, e33028. [Google Scholar] [CrossRef]
- Morató, X.; Garcia-Esparcia, P.; Argerich, J.; Llorens, F.; Zerr, I.; Paslawski, W.; Borràs, E.; Sabidó, E.; Petäjä-Repo, U.E.; Fernández-Dueñas, V.; et al. Ecto-GPR37: A potential biomarker for Parkinson’s disease. Transl. Neurodegener. 2021, 10, 8. [Google Scholar] [CrossRef]
- Picconi, B.; Gardoni, F.; Centonze, D.; Mauceri, D.; Cenci, M.A.; Bernardi, G.; Calabresi, P.; Di Luca, M. Abnormal Ca2+-calmodulin-dependent protein kinase II function mediates synaptic and motor deficits in experimental parkinsonism. J. Neurosci. 2004, 24, 5283–5291. [Google Scholar] [CrossRef] [PubMed]
- Brydges, N.M.; Best, C.; Thomas, K.L. Female HPA axis displays heightened sensitivity to pre-pubertal stress. Stress 2020, 23, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Burtscher, J.; Copin, J.-C.; Rodrigues, J.; Thangaraj, S.K.; Chiki, A.; de Suduiraut, M.-I.G.; Sandi, C.; Lashuel, H.A. Chronic corticosterone enhancement aggravates alpha-synuclein brain spreading pathology and substantia nigra neurodegeneration in mice. bioRxiv 2018, 469999. [Google Scholar] [CrossRef]
- Nguyen, K.; Kanamori, K.; Shin, C.S.; Hamid, A.; Lutfy, K. The impact of sex on changes in plasma corticosterone and cotinine levels induced by nicotine in c57bl/6j mice. Brain Sci. 2020, 10, 705. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.D.; Labermaier, C.; Holsboer, F.; Wurst, W.; Deussing, J.M.; Müller, M.B.; Schmidt, M.V. Early-life stress-induced anxiety-related behavior in adult mice partially requires forebrain corticotropin-releasing hormone receptor 1. Eur. J. Neurosci. 2012, 36, 2360–2367. [Google Scholar] [CrossRef]
- Molendijk, M.L.; de Kloet, E.R. Forced swim stressor: Trends in usage and mechanistic consideration. Eur. J. Neurosci. 2021. [Google Scholar] [CrossRef]
- Gorman-Sandler, E.; Hollis, F. The forced swim test: Giving up on behavioral despair (Commentary on Molendijk & de Kloet, 2021). Eur. J. Neurosci. 2021. [Google Scholar] [CrossRef]
- Cervo, L.; Mennini, T.; Rozio, M.; Ekalle-Soppo, C.B.; Canetta, A.; Burbassi, S.; Guiso, G.; Pirona, L.; Riva, A.; Morazzoni, P.; et al. Potential antidepressant properties of IDN 5491 (hyperforin-trimethoxybenzoate), a semisynthetic ester of hyperforin. Eur. Neuropsychopharmacol. 2005, 15, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Zimmerberg, B.; Farley, M.J. Sex differences in anxiety behavior in rats: Role of gonadal hormones. Physiol. Behav. 1993, 54, 1119–1124. [Google Scholar] [CrossRef]
- Romeo, R.D.; Mueller, A.; Sisti, H.M.; Ogawa, S.; McEwen, B.S.; Brake, W.G. Anxiety and fear behaviors in adult male and female C57BL/6 mice are modulated by maternal separation. Horm. Behav. 2003, 43, 561–567. [Google Scholar] [CrossRef]
- Palanza, P. Animal models of anxiety and depression: How are females different? Neurosci. Biobehav. Rev. 2001, 25, 219–233. [Google Scholar] [CrossRef]
- Marques, A.A.; Cesar, M.; Bevilaqua, N.; Pinto Da Fonseca, A.M.; Nardi, A.E.; Thuret, S.; Dias, G.P. Gender Differences in the Neurobiology of Anxiety: Focus on Adult Hippocampal Neurogenesis. Neural Plast. 2016, 2016, 5026713. [Google Scholar] [CrossRef] [PubMed]
- McDowell, K.; Chesselet, M.F. Animal models of the non-motor features of Parkinson’s disease. Neurobiol. Dis. 2012, 46, 597–606. [Google Scholar] [CrossRef]
- Taylor, T.N.; Greene, J.G.; Miller, G.W. Behavioral phenotyping of mouse models of Parkinson’s disease. Behav. Brain Res. 2010, 211, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mitra, R.; Sapolsky, R.M. Acute corticosterone treatment is sufficient to induce anxiety and amygdaloid dendritic hypertrophy. Proc. Natl. Acad. Sci. USA 2008, 105, 5573–5578. [Google Scholar] [CrossRef] [PubMed]
- Knight, D.C.; Smith, C.N.; Cheng, D.T.; Stein, E.A.; Helmstetter, F.J. Amygdala and hippocampal activity during acquisition and extinction of human fear conditioning. Cogn. Affect. Behav. Neurosci. 2004, 4, 317–325. [Google Scholar] [CrossRef]
- Izquierdo, I.; Cammarota, M.; Da Silva, W.C.; Bevilaqua, L.R.M.; Rossato, J.I.; Bonini, J.S.; Mello, P.; Benetti, F.; Costa, J.C.; Medina, J.H. The evidence for hippocampal long-term potentiation as a basis of memory for simple tasks. An. Acad. Bras. Cienc. 2008, 80, 115–127. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bannerman, D.M.; Deacon, R.M.J.; Offen, S.; Friswell, J.; Grubb, M.; Rawlins, J.N.P. Double dissociation of function within the hippocampus: Spatial memory and hyponeophagia. Behav. Neurosci. 2002, 116, 884–901. [Google Scholar] [CrossRef]
- Bannerman, D.M.; Rawlins, J.N.P.; McHugh, S.B.; Deacon, R.M.J.; Yee, B.K.; Bast, T.; Zhang, W.N.; Pothuizen, H.H.J.; Feldon, J. Regional dissociations within the hippocampus—memory and anxiety. Neurosci. Biobehav. Rev. 2004, 28, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Segal, M.; Richter-Levin, G.; Maggio, N. Stress-induced dynamic routing of hippocampal connectivity: A hypothesis. Hippocampus 2010, 20, 1332–1338. [Google Scholar] [CrossRef] [PubMed]
- Kjelstrup, K.G.; Tuvnes, F.A.; Steffenach, H.-A.; Murison, R.; Moser, E.I.; Moser, M.-B. Reduced fear expression after lesions of the ventral hippocampus. Proc. Natl. Acad. Sci. USA 2002, 99, 10825–10830. [Google Scholar] [CrossRef]
- Moriguchi, S.; Shioda, N.; Han, F.; Yeh, J.Z.; Narahashi, T.; Fukunaga, K. Galantamine enhancement of long-term potentiation is mediated by calcium/calmodulin-dependent protein kinase II and protein kinase C activation. Hippocampus 2009, 19, 844–854. [Google Scholar] [CrossRef] [PubMed]
- McCrary, M.R.; Jiang, M.Q.; Giddens, M.M.; Zhang, J.Y.; Owino, S.; Wei, Z.Z.; Zhong, W.; Gu, X.; Xin, H.; Hall, R.A.; et al. Protective effects of GPR37 via regulation of inflammation and multiple cell death pathways after ischemic stroke in mice. FASEB J. 2019, 33, 10680–10691. [Google Scholar] [CrossRef]
- Thu Nguyen, T.; Dammer, E.B.; Owino, S.A.; Giddens, M.M.; Madaras, N.S.; Duong, D.M.; Seyfried, N.T.; Hall, R.A. Quantitative Proteomics Reveal an Altered Pattern of Protein Expression in Brain Tissue from Mice Lacking GPR37 and GPR37L1. J. Proteome Res. 2020, 19, 744–755. [Google Scholar] [CrossRef] [PubMed]
- Pellow, S.; File, S.E. Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: A novel test of anxiety in the rat. Pharmacol. Biochem. Behav. 1986, 24, 525–529. [Google Scholar] [CrossRef]
- Crawley, J.N. Neuropharmacologic specificity of a simple animal model for the behavioral actions of benzodiazepines. Pharmacol. Biochem. Behav. 1981, 15, 695–699. [Google Scholar] [CrossRef]
- Eriksson, T.M.; Alvarsson, A.; Stan, T.L.; Zhang, X.; Hascup, K.N.; Hascup, E.R.; Kehr, J.; Gerhardt, G.A.; Warner-Schmidt, J.; Arango-Lievano, M.; et al. Bidirectional regulation of emotional memory by 5-HT1B receptors involves hippocampal p11. Mol. Psychiatry 2013, 18, 1096–1105. [Google Scholar] [CrossRef] [PubMed]
- Guzzetti, S.; Calcagno, E.; Canetta, A.; Sacchetti, G.; Fracasso, C.; Caccia, S.; Cervo, L.; Invernizzi, R.W. Strain differences in paroxetine-induced reduction of immobility time in the forced swimming test in mice: Role of serotonin. Eur. J. Pharmacol. 2008, 594, 117–124. [Google Scholar] [CrossRef]
- Lazarevic, V.; Yang, Y.; Ivanova, D.; Fejtova, A.; Svenningsson, P. Riluzole attenuates the efficacy of glutamatergic transmission by interfering with the size of the readily releasable neurotransmitter pool. Neuropharmacology 2018, 143, 38–48. [Google Scholar] [CrossRef] [PubMed]



| Structure | Gender | Group | Total CaMKII |
|---|---|---|---|
| DH | Male | WT Basal | 100 ± 5.3 |
| KO Basal | 115.2 ± 9.6 | ||
| WT no ELS | 124.8 ± 15.9 | ||
| KO no ELS | 99.2 ± 5.0 | ||
| WT ELS | 111.1 ± 12.3 | ||
| KO ELS | 110.3 ± 8.7 | ||
| Female | WT Basal | 100 ± 5.7 | |
| KO Basal | 87.8 ± 11.4 | ||
| WT no ELS | 91.0 ± 9.1 | ||
| KO no ELS | 94.7 ± 5.3 | ||
| WT ELS | 84.1 ± 14.1 | ||
| KO ELS | 89.4 ± 7.2 | ||
| VH | Male | WT Basal | 100 ± 11.3 |
| KO Basal | 95.1 ± 16.1 | ||
| WT no ELS | 90.4 ± 8.8 | ||
| KO no ELS | 86.8 ± 6.1 | ||
| WT ELS | 68.0 ± 8.2 | ||
| KO ELS | 82.2 ± 9.2 | ||
| Female | WT Basal | 100 ± 15.5 | |
| KO Basal | 87.3 ± 4.6 | ||
| WT no ELS | 81.6 ± 12.1 | ||
| KO no ELS | 97.8 ± 10.4 | ||
| WT ELS | 90.3 ± 11.1 | ||
| KO ELS | 92.0 ± 9.3 | ||
| Amy | Male | WT Basal | 100 ± 13.6 |
| KO Basal | 121.1 ± 26.8 | ||
| WT no ELS | 90.4 ± 20.7 | ||
| KO no ELS | 81.5 ± 10.5 | ||
| WT ELS | 76.2 ± 12.4 | ||
| KO ELS | 101.6 ± 21.5 | ||
| Female | WT Basal | 100 ± 9.5 | |
| KO Basal | 107.6 ± 20.4 | ||
| WT no ELS | 144.7 ± 22.9 | ||
| KO no ELS | 132.6 ± 14.9 | ||
| WT ELS | 127.8 ± 19.7 | ||
| KO ELS | 135.7 ± 23.1 |
| Primer | Sequence, 5′–3′ |
|---|---|
| GPR37 mutant, forward | GGGTGGGATTAGATAAATGCCTGCTCT |
| GPR37 WT, forward | AACGGGTCTGCAGATGACTGGGTTC |
| Common, reverse | GGCCAAGAGAGAATTGGAGATCGTC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veenit, V.; Zhang, X.; Ambrosini, A.; Sousa, V.; Svenningsson, P. The Effect of Early Life Stress on Emotional Behaviors in GPR37KO Mice. Int. J. Mol. Sci. 2022, 23, 410. https://doi.org/10.3390/ijms23010410
Veenit V, Zhang X, Ambrosini A, Sousa V, Svenningsson P. The Effect of Early Life Stress on Emotional Behaviors in GPR37KO Mice. International Journal of Molecular Sciences. 2022; 23(1):410. https://doi.org/10.3390/ijms23010410
Chicago/Turabian StyleVeenit, Vandana, Xiaoqun Zhang, Antonio Ambrosini, Vasco Sousa, and Per Svenningsson. 2022. "The Effect of Early Life Stress on Emotional Behaviors in GPR37KO Mice" International Journal of Molecular Sciences 23, no. 1: 410. https://doi.org/10.3390/ijms23010410
APA StyleVeenit, V., Zhang, X., Ambrosini, A., Sousa, V., & Svenningsson, P. (2022). The Effect of Early Life Stress on Emotional Behaviors in GPR37KO Mice. International Journal of Molecular Sciences, 23(1), 410. https://doi.org/10.3390/ijms23010410







