High-Dose Benzodiazepines Positively Modulate GABAA Receptors via a Flumazenil-Insensitive Mechanism
Abstract
1. Introduction
2. Results
2.1. Diazepam Exhibits Flumazenil-Insensitive Modulation of α1β2γ2, α2β2γ2, and α5β2γ2 GABAARs
2.2. Different BZD Ligands Exhibit Distinct Flumazenil-Insensitive Modulation of Different GABAARs
2.3. Pentylenetetrazol and Fa173 Abolish the Flumazenil-Insensitive Diazepam Effects
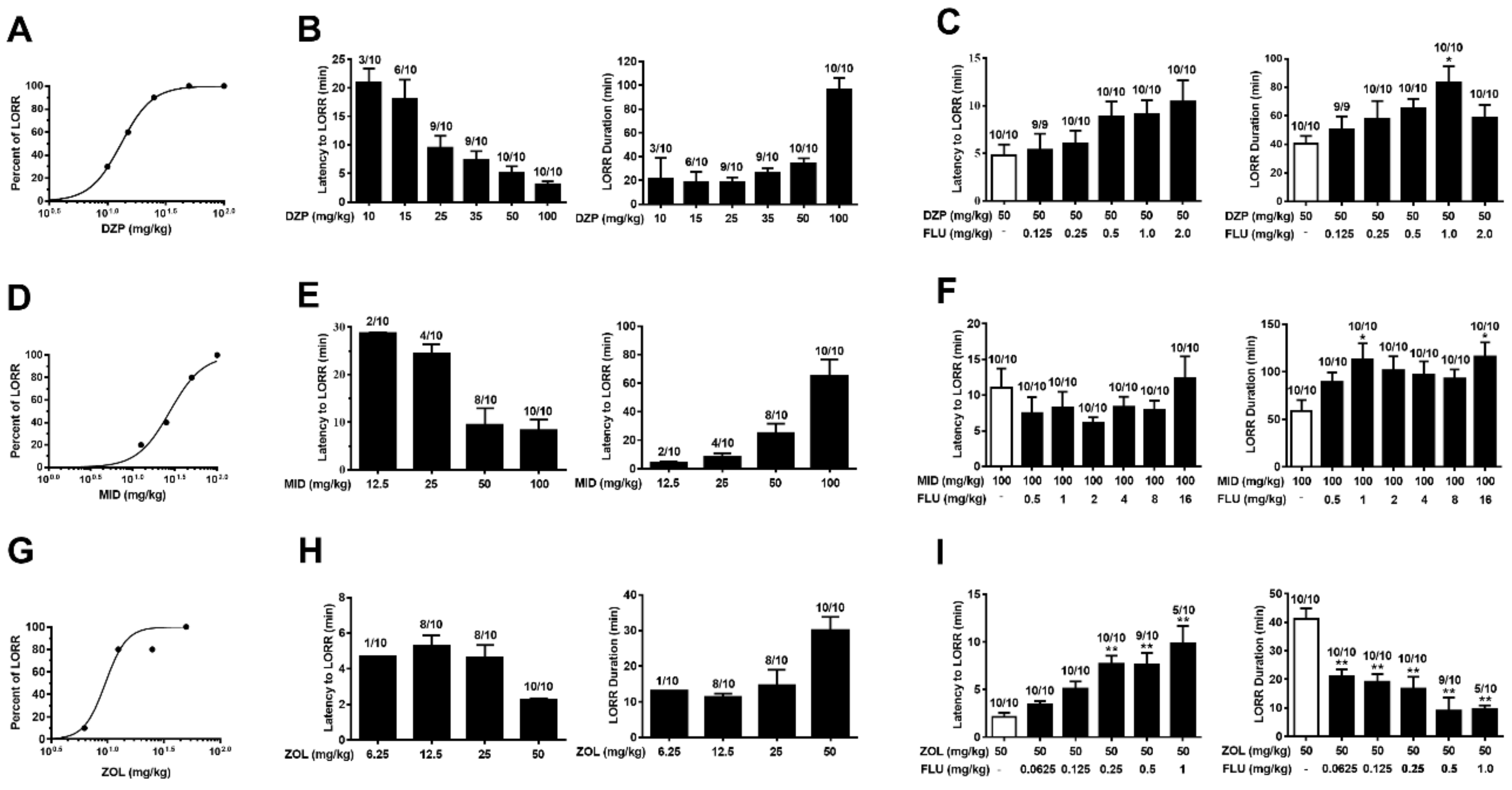
2.4. Anesthesia Induced by Diazepam and Midazolam, but Not Zolpidem, Is Resistant to Flumazenil
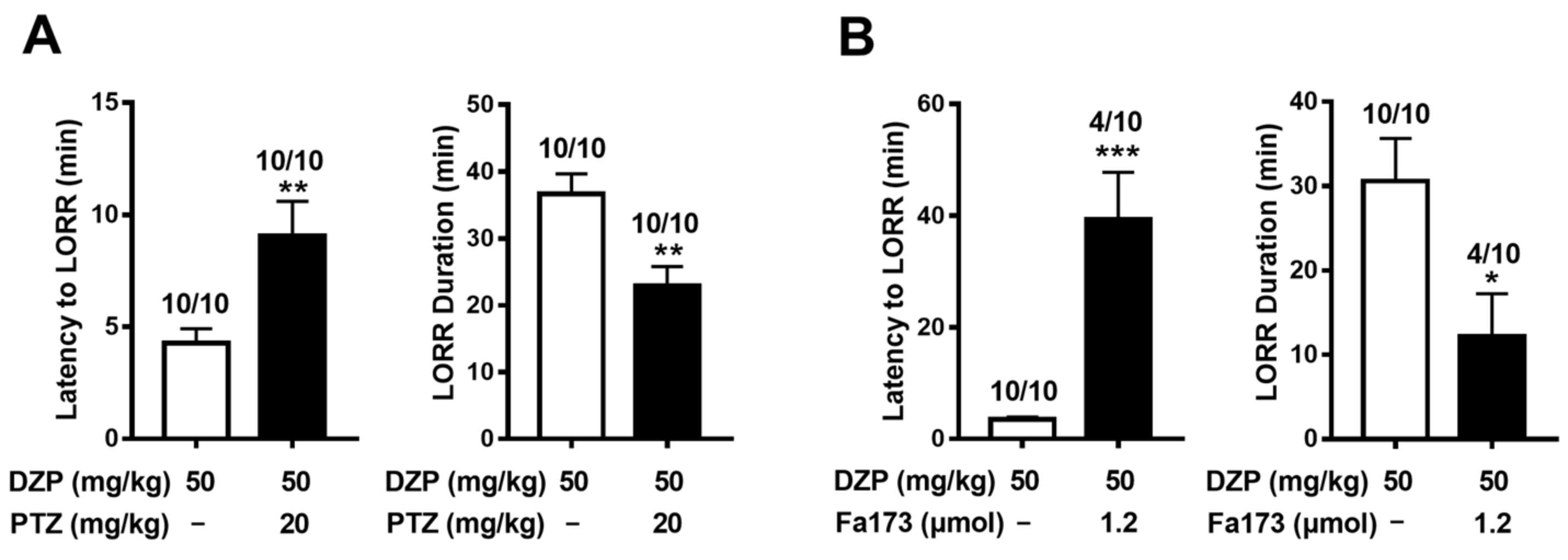
2.5. Diazepam-Induced Anesthesia Is Antagonized by Pentylenetetrazol and Fa173
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Expression of GABAA Receptors in Xenopus Oocytes
4.3. Two-Electrode Voltage Clamp Electrophysiology
4.4. Mouse Behavioral Test
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sieghart, W.; Sperk, G. Subunit composition, distribution and function of GABA(A) receptor subtypes. Curr. Top Med. Chem. 2002, 2, 795–816. [Google Scholar] [CrossRef]
- Baur, R.; Minier, F.; Sigel, E. A GABA(A) receptor of defined subunit composition and positioning: Concatenation of five subunits. FEBS Lett. 2006, 580, 1616–1620. [Google Scholar] [CrossRef] [PubMed]
- Farrant, M.; Nusser, Z. Variations on an inhibitory theme: Phasic and tonic activation of GABA(A) receptors. Nat. Rev. Neurosci. 2005, 6, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Jacob, T.C.; Moss, S.J.; Jurd, R. GABA(A) receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat. Rev. Neurosci. 2008, 9, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Olsen, R.W. GABA(A) receptor: Positive and negative allosteric modulators. Neuropharmacology 2018, 136, 10–22. [Google Scholar] [CrossRef]
- Sigel, E.; Ernst, M. The Benzodiazepine Binding Sites of GABA(A) Receptors. Trends Pharmacol. Sci. 2018, 39, 659–671. [Google Scholar] [CrossRef]
- Che Has, A.T.; Absalom, N.; van Nieuwenhuijzen, P.S.; Clarkson, A.N.; Ahring, P.K.; Chebib, M. Zolpidem is a potent stoichiometry-selective modulator of alpha1beta3 GABAA receptors: Evidence of a novel benzodiazepine site in the alpha1-alpha1 interface. Sci. Rep. 2016, 6, 28674. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, U.; Knoflach, F. Beyond classical benzodiazepines: Novel therapeutic potential of GABAA receptor subtypes. Nat. Rev. Drug Discov. 2011, 10, 685–697. [Google Scholar] [CrossRef]
- Masiulis, S.; Desai, R.; Uchański, T.; Martin, I.S.; Laverty, D.; Karia, D.; Aricescu, A.R. GABA(A) receptor signalling mechanisms revealed by structural pharmacology. Nature 2019, 565, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Middendorp, S.; Maldifassi, M.C.; Baur, R.; Sigel, E. Positive modulation of synaptic and extrasynaptic GABAA receptors by an antagonist of the high affinity benzodiazepine binding site. Neuropharmacology 2019, 95, 459–467. [Google Scholar] [CrossRef]
- Kim, J.J.; Gharpure, A.; Teng, J.; Zhuang, Y.; Howard, R.J.; Zhu, S.; Noviello, C.M.; Walsh, R.M., Jr.; Lindahl, E.; Hibbs, R.E. Shared structural mechanisms of general anaesthetics and benzodiazepines. Nature 2020, 585, 303–308. [Google Scholar] [CrossRef]
- Drexler, B.; Zinser, S.; Hentschke, H.; Antkowiak, B. Diazepam Decreases Action Potential Firing of Neocortical Neurons via Two Distinct Mechanisms. Anesth. Analg. 2010, 111, 1394–1399. [Google Scholar] [CrossRef]
- Zhu, S.; Noviello, C.M.; Teng, J.; Walsh, R.M., Jr.; Kim, J.J.; Hibbs, R.E. Structure of a human synaptic GABAA receptor. Nature 2018, 559, 67–72. [Google Scholar] [CrossRef]
- Chua, H.C.; Chebib, M. GABA(A) receptors and the diversity in their structure and pharmacology. Adv. Pharmacol. 2017, 79, 1–34. [Google Scholar] [PubMed]
- Walters, R.J.; Hadley, S.H.; Morris, K.D.; Amin, J. Benzodiazepines act on GABAA receptors via two distinct and separable mechanisms. Nat. Neurosci. 2000, 3, 1274–1281. [Google Scholar] [CrossRef]
- Sieghart, W. Allosteric modulation of GABAA receptors via multiple drug-binding sites. Adv. Pharmacol. 2014, 72, 53–96. [Google Scholar] [CrossRef]
- Baur, R.; Tan, K.R.; Lüscher, B.P.; Gonthier, A.; Goeldner, M.; Sigel, E. Covalent modification of GABAA receptor isoforms by a diazepam analogue provides evidence for a novel benzodiaz-epine binding site that prevents modulation by these drugs. J. Neurochem. 2008, 106, 2353–2363. [Google Scholar] [CrossRef] [PubMed]
- Wongsamitkul, N.; Maldifassi, M.C.; Simeone, X.; Baur, R.; Ernst, M.; Sigel, E. α subunits in GABAA receptors are dispensable for GABA and diazepam action. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.J.; Cao, Y.Q.; Li, Y.L.; Yu, G.; Su, R.B. Flumazenil-Insensitive Benzodiazepine Effects in Recombinant αβ and Neuronal GABA(A) Receptors. Brain Sci. 2020, 10, 150. [Google Scholar] [CrossRef]
- Cao, Y.; Yan, H.; Yu, G.; Su, R. Flumazenil-insensitive benzodiazepine binding sites in GABAA receptors contribute to benzodiazepine-induced immo-bility in zebrafish larvae. Life Sci. 2019, 239, 117033. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, S.P.; Karim, N.; Mewett, K.N.; Chebib, M.; Johnston, G.A.; Hanrahan, J.R. Flavan-3-ol esters: New agents for exploring modulatory sites on GABA(A) receptors. Br. J. Pharmacol. 2012, 165, 965–977. [Google Scholar] [CrossRef]
- Gunja, N. The Clinical and Forensic Toxicology of Z-drugs. J. Med. Toxicol. 2013, 9, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Baird, E.S.; Hailey, D.M. Delayed recovery from a sedative: Correlation of the plasma levels of diazepam with clinical effects after oral and intravenous administration. Br. J. Anaesth. 1972, 44, 803–808. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Klockowski, P.M.; Levy, G. Kinetics of drug action in disease states. XXIV. Pharmacodynamics of diazepam and its active me-tabolites in rats. J. Pharmacol. Exp. Ther. 1988, 244, 912–918. [Google Scholar] [PubMed]
- Ramerstorfer, J.; Furtmüller, R.; Sarto-Jackson, I.; Varagic, Z.; Sieghart, W.; Ernst, M. The GABAA receptor α+β- interface: A novel target for subtype selective drugs. J. Neurosci. 2011, 31, 870–877. [Google Scholar] [CrossRef]
- McGrath, M.; Hoyt, H.; Pence, A.; Forman, S.A.; Raines, D.E. Selective actions of benzodiazepines at the transmembrane anaesthetic binding sites of the GABAA receptor: In vitro and in vivo studies. Br. J. Pharmacol. 2021, 178, P4842–P4858. [Google Scholar] [CrossRef] [PubMed]
- Akk, G.; Steinbach, J.H. Structural studies of the actions of anesthetic drugs on the γ-aminobutyric acid type A receptor. Anesthesiology 2011, 115, 1338–1348. [Google Scholar] [CrossRef]
- McGrath, M.; Hoyt, H.; Pence, A.; Jayakar, S.S.; Zhou, X.; Forman, S.A.; Raines, D.E. Competitive Antagonism of Etomidate Action by Diazepam: In Vitro GABAA Receptor and In Vivo Zebrafish Studies. Anesthesiology 2020, 133, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Bonetti, E.P.; Pieri, L.; Cumin, R.; Schaffner, R.; Pieri, M.; Gamzu, E.R.; Müller, R.K.; Haefely, W. Benzodiazepine antagonist Ro 15-1788: Neurological and behavioral effects. Psychopharmacology 1982, 78, 8–18. [Google Scholar] [CrossRef]
- Çelik, T.; Deniz, G.; Uzbay, I.T.; Palaoğlu, Ö.; Ayhan, I.H. The effects of flumazenil on two way active avoidance and locomotor activity in diazepam-treated rats. Eur. Neuropsychopharmacol. 1999, 9, 45–50. [Google Scholar] [CrossRef]
- Auta, J.; Costa, E.; Davis, J.; Guidotti, A. Imidazenil: An antagonist of the sedative but not the anticonvulsant action of diazepam. Neuropharmacology 2005, 49, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Maldifassi, M.C.; Baur, R.; Pierce, D.; Nourmahnad, A.; Forman, S.A.; Sigel, E. Novel positive allosteric modulators of GABAA receptors with anesthetic activity. Sci. Rep. 2016, 6, 25943. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, R.-G.; Peng, P.; Zheng, J.-Q.; Zhang, Y.-L.; Wen, L.; Wei, X.-L.; Ma, X.-Y. The glycine hinge of transmembrane segment 2 modulates the subcellular localization and gating properties in TREK channels. Biochem. Biophys. Res. Commun. 2017, 490, 1125–1131. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, N.; Lian, J.; Cao, Y.; Muheyati, A.; Yuan, S.; Ma, Y.; Zhang, S.; Yu, G.; Su, R. High-Dose Benzodiazepines Positively Modulate GABAA Receptors via a Flumazenil-Insensitive Mechanism. Int. J. Mol. Sci. 2022, 23, 42. https://doi.org/10.3390/ijms23010042
Wang N, Lian J, Cao Y, Muheyati A, Yuan S, Ma Y, Zhang S, Yu G, Su R. High-Dose Benzodiazepines Positively Modulate GABAA Receptors via a Flumazenil-Insensitive Mechanism. International Journal of Molecular Sciences. 2022; 23(1):42. https://doi.org/10.3390/ijms23010042
Chicago/Turabian StyleWang, Na, Jingjing Lian, Yanqing Cao, Alai Muheyati, Shanshan Yuan, Yujie Ma, Shuzhuo Zhang, Gang Yu, and Ruibin Su. 2022. "High-Dose Benzodiazepines Positively Modulate GABAA Receptors via a Flumazenil-Insensitive Mechanism" International Journal of Molecular Sciences 23, no. 1: 42. https://doi.org/10.3390/ijms23010042
APA StyleWang, N., Lian, J., Cao, Y., Muheyati, A., Yuan, S., Ma, Y., Zhang, S., Yu, G., & Su, R. (2022). High-Dose Benzodiazepines Positively Modulate GABAA Receptors via a Flumazenil-Insensitive Mechanism. International Journal of Molecular Sciences, 23(1), 42. https://doi.org/10.3390/ijms23010042




