A Fiber-Rich Diet and Radiation-Induced Injury in the Murine Intestinal Mucosa
Abstract
:1. Introduction
2. Results
2.1. Animal Health Status and Body Weight
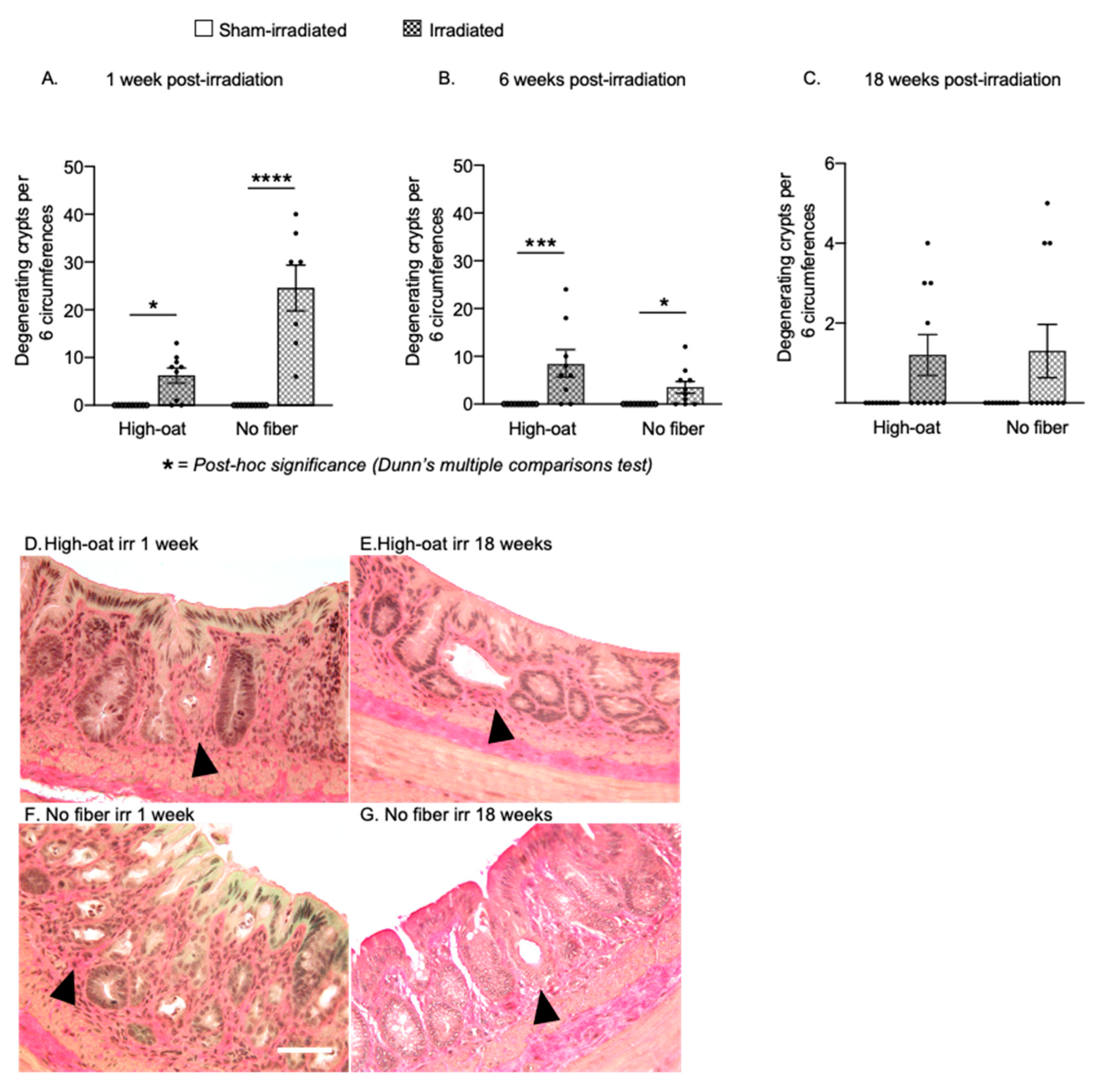
2.2. Pronounced Early Crypt Degeneration in Irradiated No-Fiber Animals
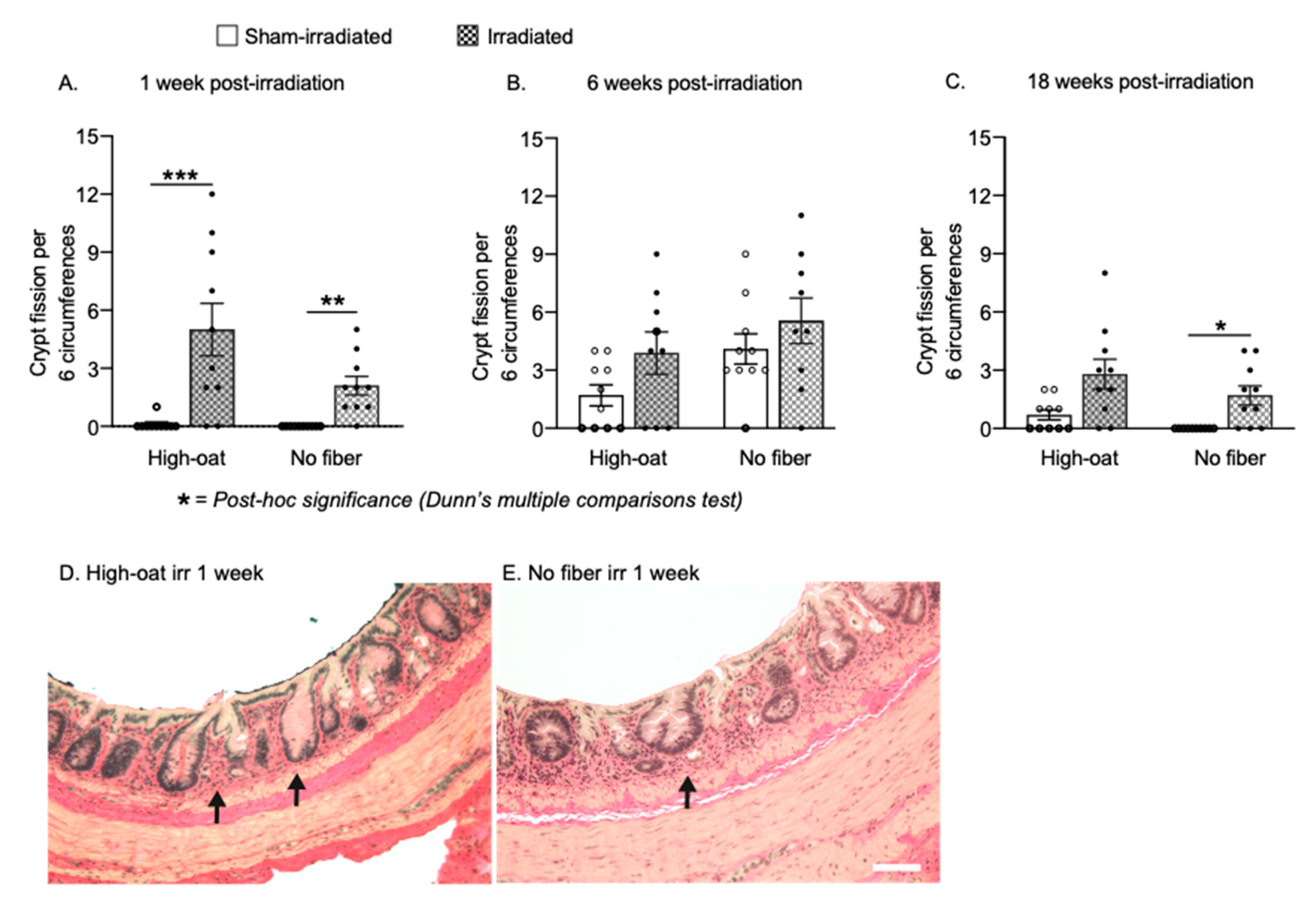
2.3. Increased Crypt Fission after Pelvic Irradiation
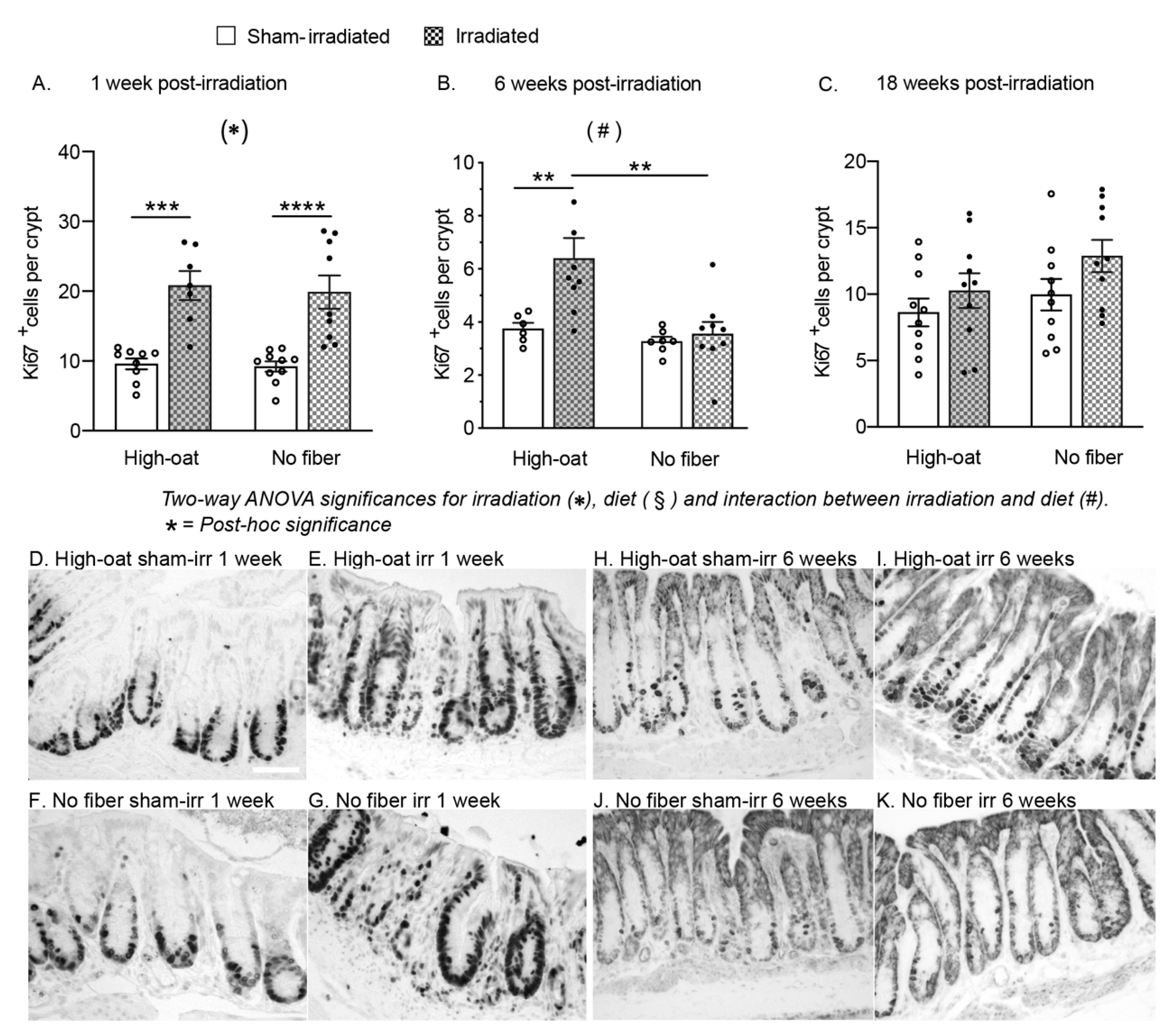
2.4. A Fiber-Rich Diet Promotes a Lasting Cell Proliferation Response in the Crypts
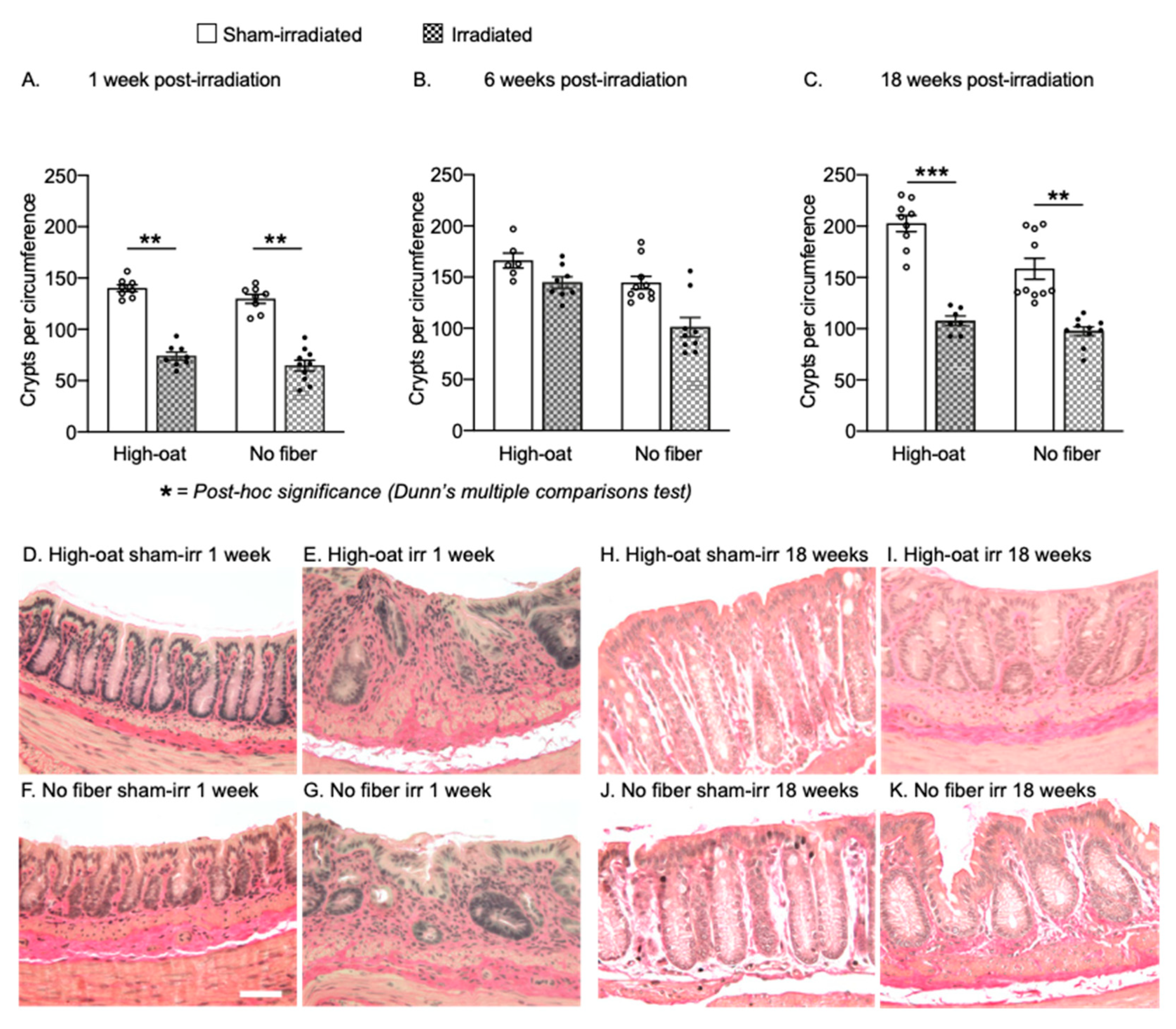
2.5. Dietary Fiber Does Not Protect against a Long-Lasting Reduction in Crypts after Irradiation
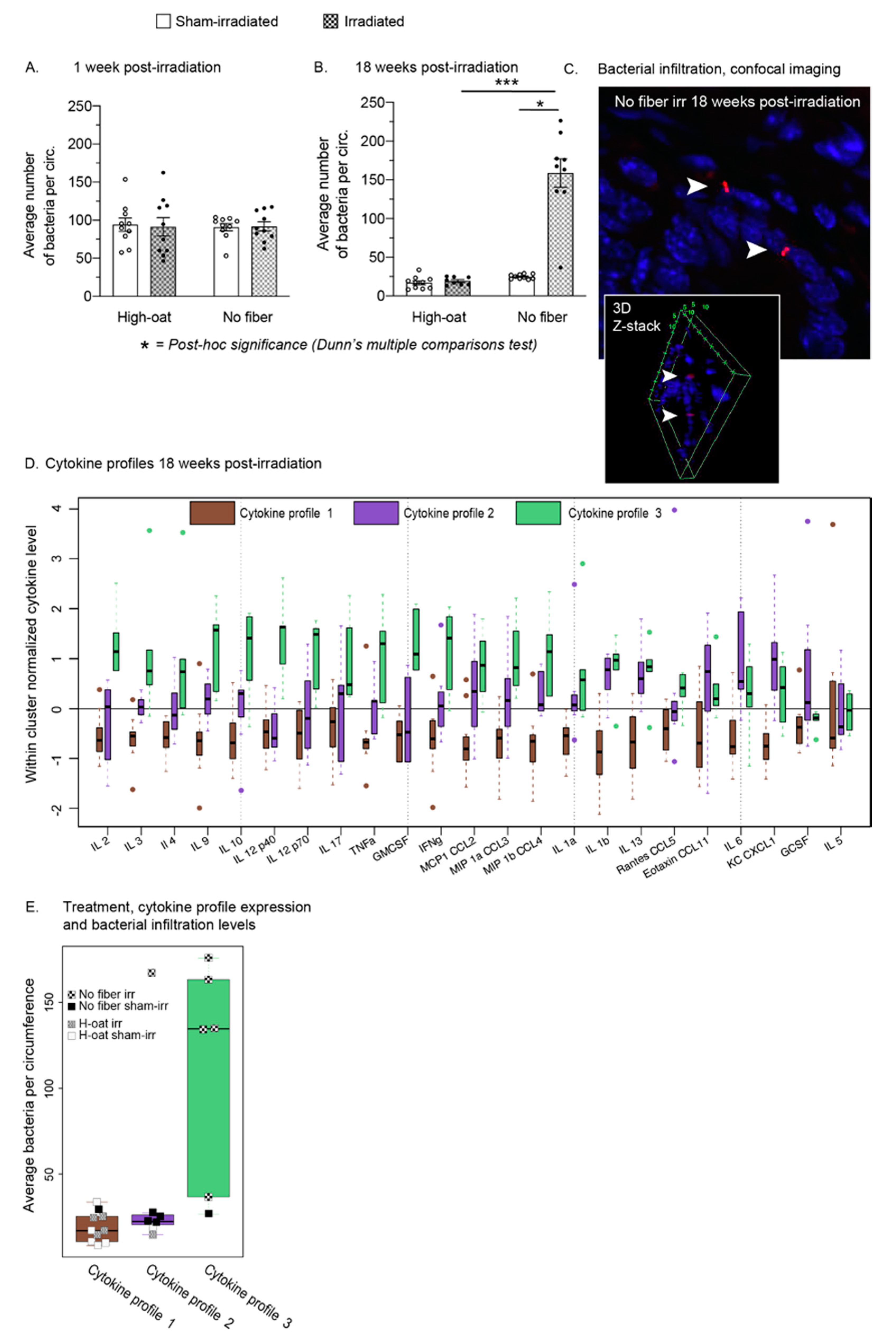
2.6. A Fiber-Rich Diet Completely Protects from Late Irradiation-Induced Bacterial Infiltration
2.7. Three Distinct Serum Cytokine Profiles 18 Weeks after Irradiation
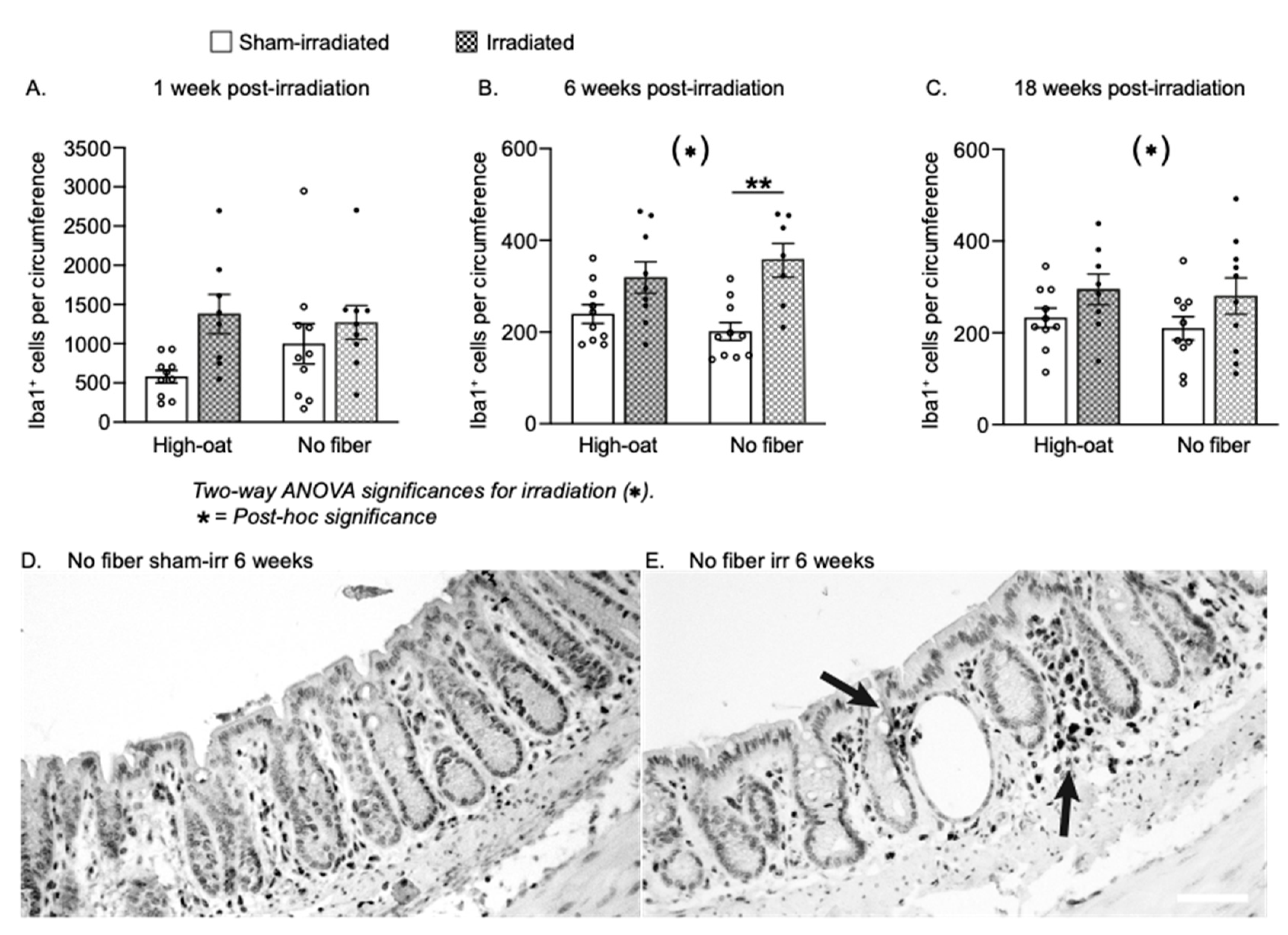
2.8. Inflammatory Activity
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experimental Design
4.3. Diets
4.4. Irradiation Procedure
4.5. Collection and Processing of Tissue
4.6. Immunohistochemistry and Histochemistry
4.7. Fluorescence-In Situ Hybridization to Detect Bacteria
4.8. Serum Cytokine Analysis
4.9. Assessment of Crypt Fission and Crypt Degeneration
4.10. Crypt Number
4.11. Quantification of Proliferating Cells
4.12. Quantification of Colonic Macrophages
4.13. Quantification of Bacteria
4.14. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Andreyev, H.J.; Wotherspoon, A.; Denham, J.W.; Hauer-Jensen, M. “Pelvic radiation disease”: New understanding and new solutions for a new disease in the era of cancer survivorship. Scand. J. Gastroenterol. 2011, 46, 389–397. [Google Scholar] [CrossRef]
- Steineck, G.; Skokic, V.; Sjöberg, F.; Bull, C.; Alevronta, E.; Dunberger, G.; Bergmark, K.; Wilderäng, U.; Oh, J.H.; Deasy, J.O.; et al. Identifying radiation-induced survivorship syndromes affecting bowel health in a cohort of gynecological cancer survivors. PLoS ONE 2017, 12, e0171461. [Google Scholar] [CrossRef] [PubMed]
- Hur, W.; Yoon, S.K. Molecular Pathogenesis of Radiation-Induced Cell Toxicity in Stem Cells. Int. J. Mol. Sci. 2017, 18, 2749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potten, C.S.; Grant, H.K. The relationship between ionizing radiation-induced apoptosis and stem cells in the small and large intestine. Br. J. Cancer 1998, 78, 993–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francois, A.; Milliat, F.; Guipaud, O.; Benderitter, M. Inflammation and immunity in radiation damage to the gut mucosa. BioMed Res. Int. 2013, 2013, 123241. [Google Scholar] [CrossRef] [Green Version]
- Wedlake, L.; Shaw, C.; McNair, H.; Lalji, A.; Mohammed, K.; Klopper, T.; Allan, L.; Tait, D.; Hawkins, M.; Somaiah, N.; et al. Randomized controlled trial of dietary fiber for the prevention of radiation-induced gastrointestinal toxicity during pelvic radiotherapy. Am. J. Clin. Nutr. 2017, 106, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Brotherton, C.S.; Taylor, A.G.; Bourguignon, C.; Anderson, J.G. A high-fiber diet may improve bowel function and health-related quality of life in patients with Crohn disease. Gastroenterol. Nurs. 2014, 37, 206–216. [Google Scholar] [CrossRef] [Green Version]
- Nyman, M.; Nguyen, T.D.; Wikman, O.; Hjortswang, H.; Hallert, C. Oat Bran Increased Fecal Butyrate and Prevented Gastrointestinal Symptoms in Patients with Quiescent Ulcerative Colitis—Randomized Controlled Trial. Crohn’s Colitis 360 2020, 2, otaa005. [Google Scholar] [CrossRef]
- Sureban, S.M.; May, R.; Qu, D.; Chandrakesan, P.; Weygant, N.; Ali, N.; Lightfoot, S.A.; Ding, K.; Umar, S.; Schlosser, M.J.; et al. Dietary Pectin Increases Intestinal Crypt Stem Cell Survival following Radiation Injury. PLoS ONE 2015, 10, e0135561. [Google Scholar] [CrossRef] [Green Version]
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A.; et al. A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell 2016, 167, 1339–1353.e1. [Google Scholar] [CrossRef] [Green Version]
- Bach Knudsen, K.E.; Laerke, H.N.; Hedemann, M.S.; Nielsen, T.S.; Ingerslev, A.K.; Gundelund Nielsen, D.S.; Theil, P.K.; Purup, S.; Hald, S.; Schioldan, A.G.; et al. Impact of Diet-Modulated Butyrate Production on Intestinal Barrier Function and Inflammation. Nutrients 2018, 10, 1499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCullogh, J.S.; Ratcliffe, B.; Mandir, N.; Carr, K.E.; Goodlad, R.A. Dietary fibre and intestinal microflora: Effects on intestinal morphometry and crypt branching. Gut 1998, 42, 799–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sengupta, S.; Tang, C.L.; Wong, C.S.; Tjandra, J.J.; Gibson, P.R. Colonic epithelial atrophy induced by a fibre-free diet in rats is reversed by minimal amounts of luminal butyrate, but only in the short term. ANZ J. Surg. 2002, 72, 871–876. [Google Scholar] [CrossRef]
- Ahlin, R.; Sjoberg, F.; Bull, C.; Steineck, G.; Hedelin, M. [Differing dietary advice are given to gynaecological and prostate cancer patients receiving radiotherapy in Sweden]. Lakartidningen 2018, 115. Available online: https://europepmc.org/article/med/30325475 (accessed on 20 December 2021).
- Bull, C.; Malipatlolla, D.; Kalm, M.; Sjoberg, F.; Alevronta, E.; Grander, R.; Sultanian, P.; Persson, L.; Bostrom, M.; Eriksson, Y.; et al. A novel mouse model of radiation-induced cancer survivorship diseases of the gut. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 313, G456–G466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, P.; Malipatlolla, D.K.; Devarakonda, S.; Bull, C.; Rascón, A.; Nyman, M.; Stringer, A.; Tremaroli, V.; Steineck, G.; Sjöberg, F. Dietary Oat Bran Reduces Systemic Inflammation in Mice Subjected to Pelvic Irradiation. Nutrients 2020, 12, 2172. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.; Lee, J.; Park, S.; Kim, J.S.; Shim, S.; Lee, S.B.; Han, S.H.; Myung, H.; Kim, H.; Jang, W.S.; et al. Baicalein Mitigates Radiation-Induced Enteritis by Improving Endothelial Dysfunction. Front. Pharmacol. 2019, 10, 892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bensemmane, L.; Squiban, C.; Demarquay, C.; Mathieu, N.; Benderitter, M.; Le Guen, B.; Milliat, F.; Linard, C. The stromal vascular fraction mitigates radiation-induced gastrointestinal syndrome in mice. Stem Cell Res. Ther. 2021, 12, 309. [Google Scholar] [CrossRef]
- Schroeder, B.O.; Birchenough, G.M.H.; Stahlman, M.; Arike, L.; Johansson, M.E.V.; Hansson, G.C.; Backhed, F. Bifidobacteria or Fiber Protects against Diet-Induced Microbiota-Mediated Colonic Mucus Deterioration. Cell Host Microbe 2018, 23, 27–40.e7. [Google Scholar] [CrossRef] [Green Version]
- Eftychi, C.; Schwarzer, R.; Vlantis, K.; Wachsmuth, L.; Basic, M.; Wagle, P.; Neurath, M.F.; Becker, C.; Bleich, A.; Pasparakis, M. Temporally Distinct Functions of the Cytokines IL-12 and IL-23 Drive Chronic Colon Inflammation in Response to Intestinal Barrier Impairment. Immunity 2019, 51, 367–380.e4. [Google Scholar] [CrossRef]
- Khanna, R.; Afif, W. Ustekinumab for Ulcerative Colitis. Gastroenterology 2021, 160, 2184–2186. [Google Scholar] [CrossRef] [PubMed]
- Nizzoli, G.; Burrello, C.; Cribiu, F.M.; Lovati, G.; Ercoli, G.; Botti, F.; Trombetta, E.; Porretti, L.; Todoerti, K.; Neri, A.; et al. Pathogenicity of In Vivo Generated Intestinal Th17 Lymphocytes is IFNgamma Dependent. J. Crohns Colitis 2018, 12, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Chewning, J.H.; Weaver, C.T. Development and survival of Th17 cells within the intestines: The influence of microbiome- and diet-derived signals. J. Immunol. 2014, 193, 4769–4777. [Google Scholar] [CrossRef] [Green Version]
- Malipatlolla, D.K.; Patel, P.; Sjoberg, F.; Devarakonda, S.; Kalm, M.; Angenete, E.; Lindskog, E.B.; Grander, R.; Persson, L.; Stringer, A.; et al. Long-term mucosal injury and repair in a murine model of pelvic radiotherapy. Sci. Rep. 2019, 9, 13803. [Google Scholar] [CrossRef] [Green Version]
- Nagai, T.; Ishizuka, S.; Hara, H.; Aoyama, Y. Dietary sugar beet fiber prevents the increase in aberrant crypt foci induced by gamma-irradiation in the colorectum of rats treated with an immunosuppressant. J. Nutr. 2000, 130, 1682–1687. [Google Scholar] [CrossRef] [Green Version]
- Nowacki, M.R. Cell proliferation in colonic crypts of germ-free and conventional mice—Preliminary report. Folia Histochem. Cytobiol. 1993, 31, 77–81. [Google Scholar]
- Alam, M.; Midtvedt, T.; Uribe, A. Differential cell kinetics in the ileum and colon of germfree rats. Scand. J. Gastroenterol. 1994, 29, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Gerassy-Vainberg, S.; Blatt, A.; Danin-Poleg, Y.; Gershovich, K.; Sabo, E.; Nevelsky, A.; Daniel, S.; Dahan, A.; Ziv, O.; Dheer, R.; et al. Radiation induces proinflammatory dysbiosis: Transmission of inflammatory susceptibility by host cytokine induction. Gut 2018, 67, 97–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cairnie, A.B.; Millen, B.H. Fission of crypts in the small intestine of the irradiated mouse. Cell Tissue Kinet. 1975, 8, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Berlanga-Acosta, J.; Playford, R.J.; Mandir, N.; Goodlad, R.A. Gastrointestinal cell proliferation and crypt fission are separate but complementary means of increasing tissue mass following infusion of epidermal growth factor in rats. Gut 2001, 48, 803–807. [Google Scholar] [CrossRef] [Green Version]
- Bruens, L.; Ellenbroek, S.I.J.; van Rheenen, J.; Snippert, H.J. In Vivo Imaging Reveals Existence of Crypt Fission and Fusion in Adult Mouse Intestine. Gastroenterology 2017, 153, 674–677.e3. [Google Scholar] [CrossRef] [Green Version]
- Baker, A.M.; Gabbutt, C.; Williams, M.J.; Cereser, B.; Jawad, N.; Rodriguez-Justo, M.; Jansen, M.; Barnes, C.P.; Simons, B.D.; McDonald, S.A.; et al. Crypt fusion as a homeostatic mechanism in the human colon. Gut 2019, 68, 1986–1993. [Google Scholar] [CrossRef] [Green Version]
- Bain, C.C.; Bravo-Blas, A.; Scott, C.L.; Perdiguero, E.G.; Geissmann, F.; Henri, S.; Malissen, B.; Osborne, L.C.; Artis, D.; Mowat, A.M. Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat. Immunol. 2014, 15, 929–937. [Google Scholar] [CrossRef] [Green Version]
- Saha, S.; Aranda, E.; Hayakawa, Y.; Bhanja, P.; Atay, S.; Brodin, N.P.; Li, J.; Asfaha, S.; Liu, L.; Tailor, Y.; et al. Macrophage-derived extracellular vesicle-packaged WNTs rescue intestinal stem cells and enhance survival after radiation injury. Nat. Commun. 2016, 7, 13096. [Google Scholar] [CrossRef]
- Cai, W.B.; Roberts, S.A.; Potten, C.S. The number of clonogenic cells in crypts in three regions of murine large intestine. Int. J. Radiat. Biol. 1997, 71, 573–579. [Google Scholar] [PubMed]
- Potten, C.S. Radiation, the ideal cytotoxic agent for studying the cell biology of tissues such as the small intestine. Radiat. Res. 2004, 161, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Steineck, G.; Bull, C.; Kalm, M.; Sjoberg, F.; Alevronta, E.; Malipatlolla, D.K.; Bergmark, K.; Jeppsson, B.; Wilderang, U.; Bjork-Eriksson, T. Radiation physiology—Evidence for a higher biological effect of 24 Gy in four fractions as compared to three. Acta Oncol. 2017, 56, 1240–1243. [Google Scholar] [CrossRef] [Green Version]
- Johansson, M.E.; Hansson, G.C. Preservation of mucus in histological sections, immunostaining of mucins in fixed tissue, and localization of bacteria with FISH. Methods Mol. Biol. 2012, 842, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Kohler, C. Allograft inflammatory factor-1/Ionized calcium-binding adapter molecule 1 is specifically expressed by most subpopulations of macrophages and spermatids in testis. Cell Tissue Res. 2007, 330, 291–302. [Google Scholar] [CrossRef]
- Chen, Z.W.; Ahren, B.; Ostenson, C.G.; Cintra, A.; Bergman, T.; Moller, C.; Fuxe, K.; Mutt, V.; Jornvall, H.; Efendic, S. Identification, isolation, and characterization of daintain (allograft inflammatory factor 1), a macrophage polypeptide with effects on insulin secretion and abundantly present in the pancreas of prediabetic BB rats. Proc. Natl. Acad. Sci. USA 1997, 94, 13879–13884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mouton, P.R.; Phoulady, H.A.; Goldgof, D.; Hall, L.O.; Gordon, M.; Morgan, D. Unbiased estimation of cell number using the automatic optical fractionator. J. Chem. Neuroanat. 2017, 80, A1–A8. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| (A) | ||
| Nutritional information per 100 g of powder | ||
| Energy (average) | 280 kcal | |
| Moisture | 8–9 g | |
| Protein | 23 g | |
| Carbohydrate total -of which sugars | 9 g 6 g | |
| Fat total | 5 g | |
| Dietary Fiber total -of which β-glucan | 52 g 28 g | |
| (B) | ||
| Diet composition (%) | High oat * 15% fiber | No fiber *0% fiber |
| Bioprocessed oat bran | 28.8 * | 0 |
| Corn starch | 4.7 | 33.5 |
| Basal diet mixture | 66.5 | 66.5 |
| Total | 100 | 100 |
| (C) | ||
| Basal diet mixture (g/100 g High oat or No fiber diet) | ||
| Casein | 13.3 | |
| DL-Methionine | 0.2 | |
| Corn starch | 25.0 | |
| Maltodextrin | 8.7 | |
| Sucrose | 10.6 | |
| Olive oil | 4.7 | |
| Vitamin mixture | 1.0 | |
| Choline bitartrate | 0.2 | |
| TBHQ # | 0.001 | |
| Mineral mixture | 1.3 | |
| Calcium phosphate | 1.1 | |
| Calcium carbonate | 0.4 | |
| Total weight (g) | 66.5 | |
| 1 week Post-Irradiation | High-oat | No-fiber | |||||||||||
| Sham-irr | Irr | Sham-irr | Irr | ||||||||||
| Parameter | Figure | Mean | ± SEM | N | Mean | ± SEM | N | Mean | ± SEM | N | Mean | ± SEM | N |
| Degenerating crypts | 2A | 0 | 0 | 10 | 6.2 | 1.6 | 9 | 0 | 0 | 10 | 24.6 | 4.8 | 7 |
| Crypt fisson | 3A | 0.1 | 0.1 | 10 | 5 | 1.4 | 10 | 0 | 0 | 10 | 2.1 | 0.5 | 10 |
| ki67+ cells per crypt | 4A | 9.6 | 0.8 | 9 | 20.8 | 2.1 | 7 | 9.2 | 0.7 | 10 | 19.9 | 2.4 | 9 |
| Crypts/circumference | 5A | 140.1 | 3.3 | 8 | 74 | 3.8 | 8 | 129.7 | 4.4 | 8 | 64.7 | 5.2 | 10 |
| Bacteria/circumference | 6A | 94 | 8.8 | 10 | 91 | 12 | 10 | 91 | 4.8 | 10 | 92 | 6.1 | 10 |
| Iba1+ cells/circumference | 7A | 579.9 | 79.5 | 10 | 1378 | 250.7 | 8 | 999.6 | 256.5 | 10 | 1271 | 215.4 | 9 |
| 6 weeks Post-irradiation | High-oat | No-fiber | |||||||||||
| Sham-irr | Irr | Sham-irr | Irr | ||||||||||
| Parameter | Figure | Mean | ± SEM | N | Mean | ± SEM | N | Mean | ± SEM | N | Mean | ± SEM | N |
| Degenerating crypts | 2B | 0.0 | 0.0 | 10 | 8.3 | 2.7 | 9 | 0.0 | 0.0 | 10 | 3.5 | 1.2 | 10 |
| Crypt fisson | 3B | 1.7 | 0.5 | 10 | 3.9 | 1.1 | 9 | 4.1 | 0.8 | 10 | 5.6 | 1.2 | 9 |
| ki67+ cells per crypt | 4B | 3.7 | 0.2 | 6 | 6.4 | 0.8 | 9 | 3.3 | 0.2 | 7 | 3.6 | 0.4 | 9 |
| Crypts/circumference | 5B | 166.2 | 7.4 | 6 | 144.7 | 5.7 | 8 | 144.5 | 6.3 | 10 | 101.0 | 9.5 | 9 |
| Iba1+ cells/circumference | 7B | 239.2 | 20.7 | 10 | 318.8 | 34.3 | 9 | 201.2 | 19.7 | 10 | 356.5 | 36.9 | 7 |
| 18 weeks Post-irradiation | High-oat | No-fiber | |||||||||||
| Sham-irr | Irr | Sham-irr | Irr | ||||||||||
| Parameter | Figure | Mean | ± SEM | N | Mean | ± SEM | N | Mean | ± SEM | N | Mean | ± SEM | N |
| Bodyweight (grams) | 1C | 34.2 | 0.5 | 10 | 32.5 | 0.5 | 10 | 41.1 | 1.9 | 10 | 41.8 | 1.1 | 10 |
| Degenerating crypts | 2C | 0.0 | 0.0 | 10 | 1.2 | 0.5 | 10 | 0.0 | 0.0 | 10 | 1.3 | 0.7 | 10 |
| Crypt fisson | 3C | 0.7 | 0.3 | 10 | 2.8 | 0.8 | 10 | 0.0 | 0.0 | 10 | 1.7 | 0.5 | 10 |
| ki67+ cells per crypt | 4C | 8.6 | 1.0 | 10 | 10.3 | 1.3 | 10 | 9.9 | 1.2 | 10 | 12.9 | 1.2 | 10 |
| Crypts/circumference | 5C | 202.6 | 7.8 | 9 | 107.6 | 4.7 | 7 | 158.5 | 10.3 | 10 | 97.6 | 4.3 | 10 |
| Bacteria/circumference | 6B | 17 | 2.5 | 10 | 19 | 1.8 | 8 | 25 | 0.94 | 10 | 159 | 18 | 9 |
| Iba1+ cells/circumference | 7C | 232.5 | 21.3 | 10 | 294.8 | 33.6 | 8 | 209.7 | 25.7 | 10 | 280.2 | 39.5 | 10 |
| 1 week post-irradiation | Dunn’s post-hoc | Dunn’s post-hoc | ||||||
| H-oat irr vs. sham-irr | No fiber irr vs. sham-irr | H-oat irr vs. No fiber-irr | H-oat sham-irr vs. No fiber sham-irr | |||||
| Parameter | Figure | p value, Kruskal-Wallis test | p-value | p-value | p-value | p-value | ||
| Degenerating crypts | 2A | <0.0001 **** | 0.0191 * | <0.0001 **** | 0.2644 | >0.9999 | ||
| Crypt fisson | 3A | <0.0001 **** | 0.001 *** | 0.0034 ** | >0.9999 | >0.9999 | ||
| Crypts/circumference | 5A | <0.0001 **** | 0.0025 ** | 0.0017 ** | >0.9999 | >0.9999 | ||
| Bacteria/circumference | 6A | 0.9953 | N/A | N/A | N/A | N/A | ||
| p-value, Two-way ANOVA | Bonferroni’s post-hoc | Bonferroni’s post-hoc | ||||||
| Parameter | Figure | Irradiation | Diet | Interaction | p-value | p-value | p-value | p-value |
| Ki67+ cells per crypt | 4A | <0.0001 **** | 0.6883 | 0.8585 | 0.0002 *** | 0.0001 **** | >0.9999 | >0.9999 |
| Iba1+cells/circumference | 7A | 0.6054 | 0.1290 | 0.5243 | N/A | N/A | N/A | N/A |
| 6 weeks post-irradiation | Dunn’s post-hoc | Dunn’s post-hoc | ||||||
| H-oat irr vs. sham-irr | No fiber irr vs. sham-irr | H-oat irr vs. No fiber-irr | H-oat sham-irr vs. No fiber sham-irr | |||||
| Parameter | Figure | p value, Kruskal-Wallis test | p-value | p-value | p-value | p-value | ||
| Degenerating crypts | 2B | <0.0001 **** | 0.0008 *** | 0.0205 * | >0.9999 | >0.9999 | ||
| Crypt fisson | 3B | 0.0545 | N/A | N/A | N/A | N/A | ||
| Crypts/circumference | 5B | 0.0022 ** | 0.5057 | 0.1155 | 0.0771 | 0.2537 | ||
| p-value, Two-way ANOVA | Bonferroni’s post-hoc | Bonferroni’s post-hoc | ||||||
| Parameter | Figure | Irradiation | Diet | Interaction | p-value | p-value | p-value | p-value |
| Ki67+ cells per crypt | 4B | 0.0117 * | 0.0049 ** | 0.0378 * | 0.0088 ** | >0.9999 | 0.0015 ** | >0.9999 |
| Iba1+ cells/circumference | 7B | 0.0002 *** | 0.9951 | 0.1800 | 0.1700 | 0.0022 ** | >0.9999 | >0.9999 |
| 18 weeks post-irradiation | Dunn’s post-hoc | Dunn’s post-hoc | ||||||
| H-oat irr vs. sham-irr | No fiber irr vs. sham-irr | H-oat irr vs. No fiber-irr | H-oat sham-irr vs. No fiber sham-irr | |||||
| Parameter | Figure | p value, Kruskal-Wallis test | p-value | p-value | p-value | p-value | ||
| Degenerating crypts | 2C | 0.0408 * | 0.1209 | 0.2414 | >0.9999 | >0.9999 | ||
| Crypt fisson | 3C | 0.0011 ** | 0.1212 | 0.0157 * | >0.9999 | 0.4556 | ||
| Crypts/circumference | 5C | <0.0001 **** | 0.0008 *** | 0.0025 ** | >0.9999 | 0.6512 | ||
| Bacteria/circumference | 6B | <0.0001 **** | >0.9999 | 0.0451 * | 0.0005 *** | 0.1234 | ||
| p-value, Two-way ANOVA | Bonferroni’s post-hoc | Bonferroni’s post-hoc | ||||||
| Parameter | Figure | Irradiation | Diet | Interaction | p-value | p-value | p-value | p-value |
| Body weight (grams) | 1C | 0.6889 | <0.0001 **** | 0.3026 | >0.9999 | >0.9999 | <0.0001 **** | 0.0006 *** |
| ki67+ cells per crypt | 4C | 0.0641 | 0.1077 | 0.5966 | N/A | N/A | N/A | N/A |
| Iba1+ cells/circumference | 7C | 0.0386 * | 0.5489 | 0.8944 | 0.6982 | 0.4198 | >0.9999 | >0.9999 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malipatlolla, D.K.; Devarakonda, S.; Patel, P.; Sjöberg, F.; Rascón, A.; Grandér, R.; Skokic, V.; Kalm, M.; Danial, J.; Mehdin, E.; et al. A Fiber-Rich Diet and Radiation-Induced Injury in the Murine Intestinal Mucosa. Int. J. Mol. Sci. 2022, 23, 439. https://doi.org/10.3390/ijms23010439
Malipatlolla DK, Devarakonda S, Patel P, Sjöberg F, Rascón A, Grandér R, Skokic V, Kalm M, Danial J, Mehdin E, et al. A Fiber-Rich Diet and Radiation-Induced Injury in the Murine Intestinal Mucosa. International Journal of Molecular Sciences. 2022; 23(1):439. https://doi.org/10.3390/ijms23010439
Chicago/Turabian StyleMalipatlolla, Dilip Kumar, Sravani Devarakonda, Piyush Patel, Fei Sjöberg, Ana Rascón, Rita Grandér, Viktor Skokic, Marie Kalm, Jolie Danial, Eva Mehdin, and et al. 2022. "A Fiber-Rich Diet and Radiation-Induced Injury in the Murine Intestinal Mucosa" International Journal of Molecular Sciences 23, no. 1: 439. https://doi.org/10.3390/ijms23010439






