Protective Effects of Meldonium in Experimental Models of Cardiovascular Complications with a Potential Application in COVID-19
Abstract
:1. Introduction
2. Results
2.1. Overall Animal Well-Being
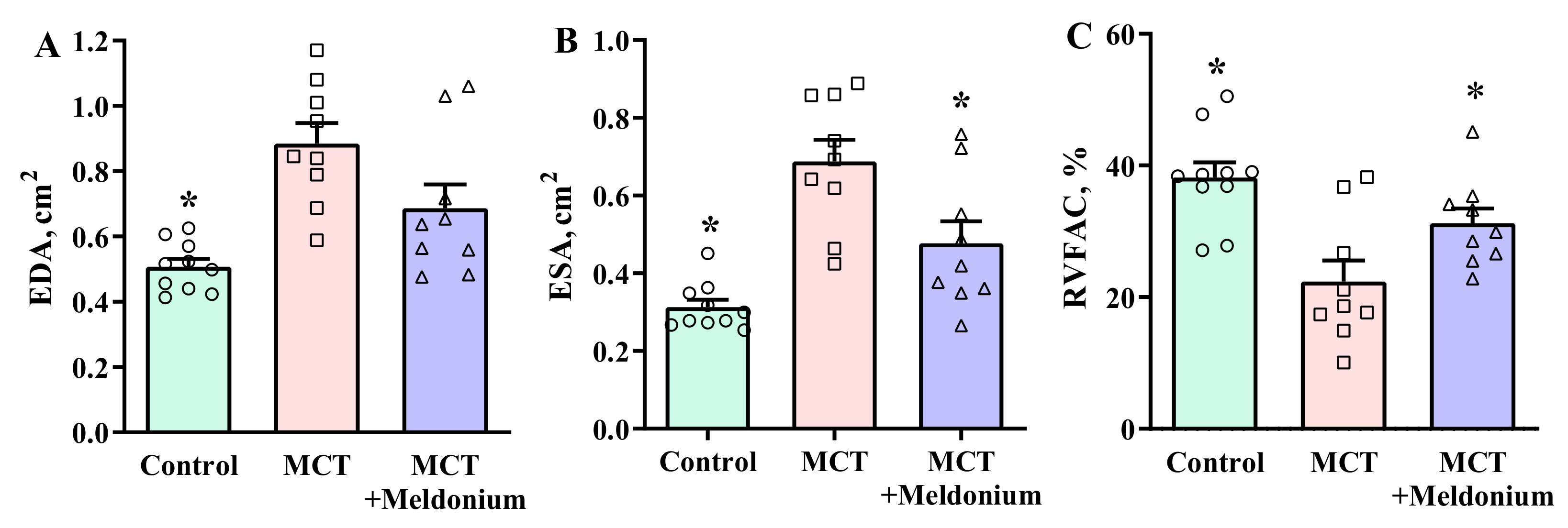
2.2. Effects of Meldonium on Ventricular Size and Function in the Right Ventricular Failure Model
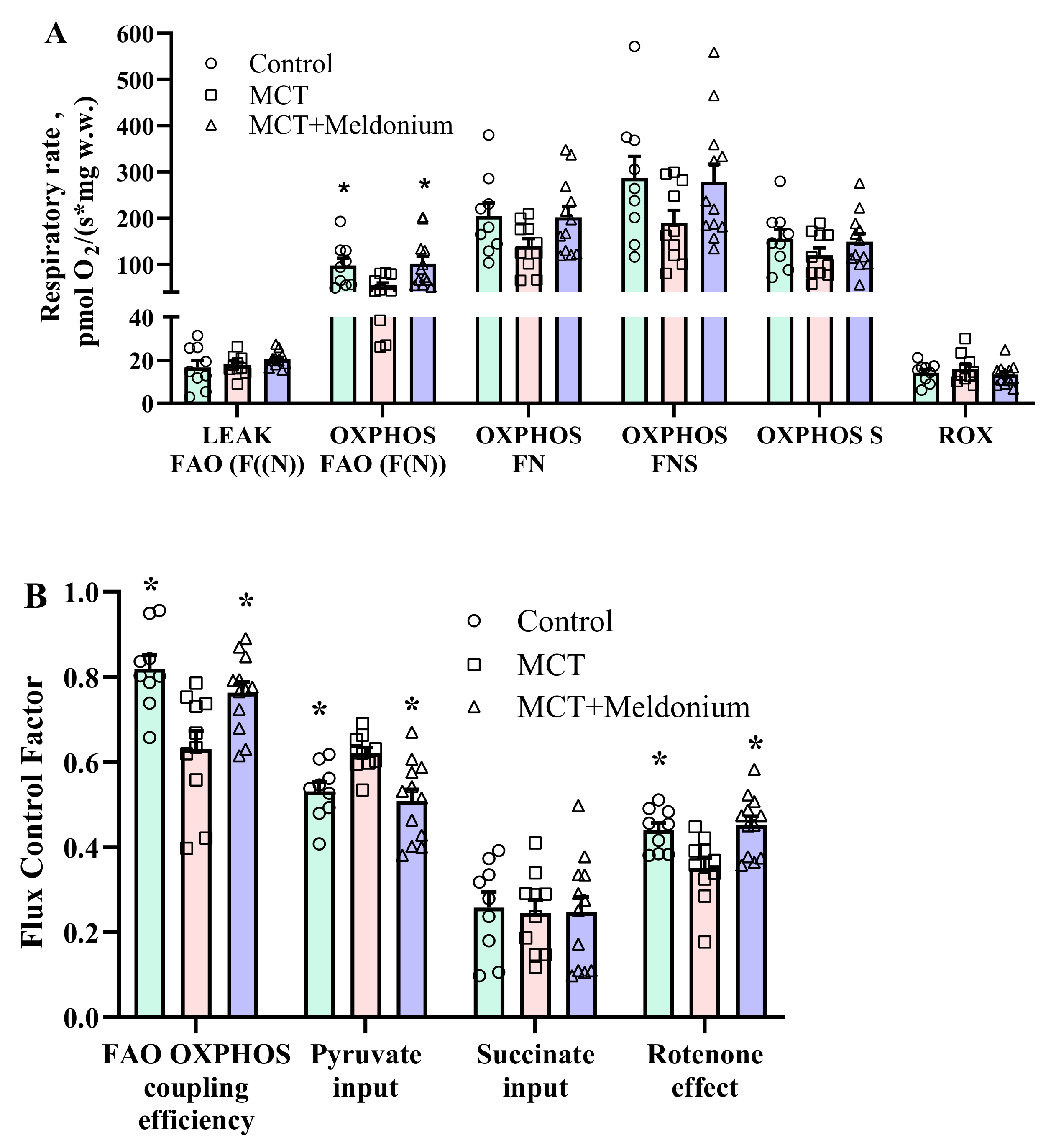
2.3. Effects of Meldonium on Mitochondrial Function in the Right Ventricular Failure Model
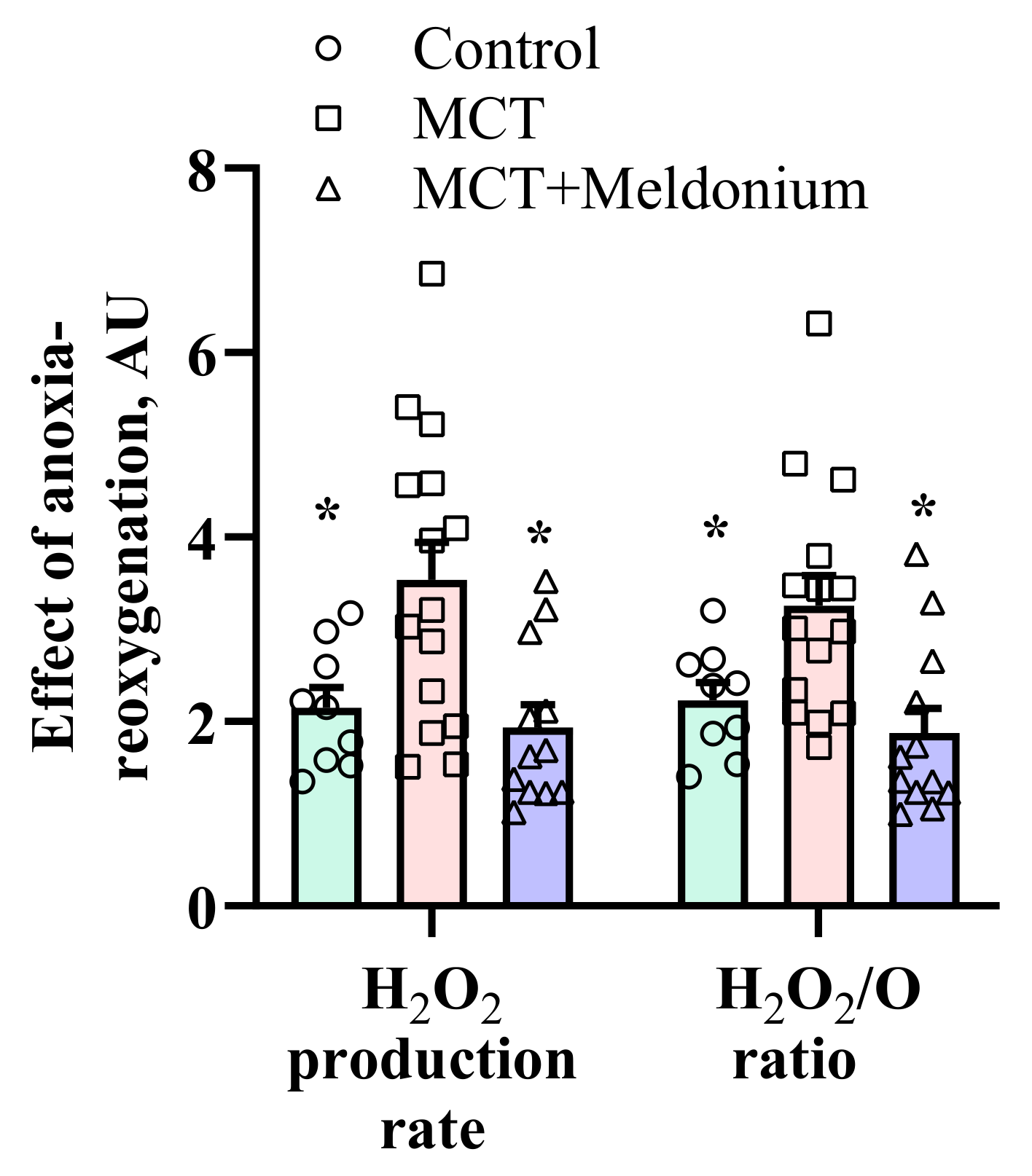
2.4. Effects of Meldonium on Mitochondrial H2O2 Production after Anoxia-Reoxygenation in the Right Ventricular Failure Model
2.5. The Effects of Meldonium on Lung Morphology, Endothelial Function and Blood Oxygen Saturation (SpO2) in a Right Ventricular Failure Model
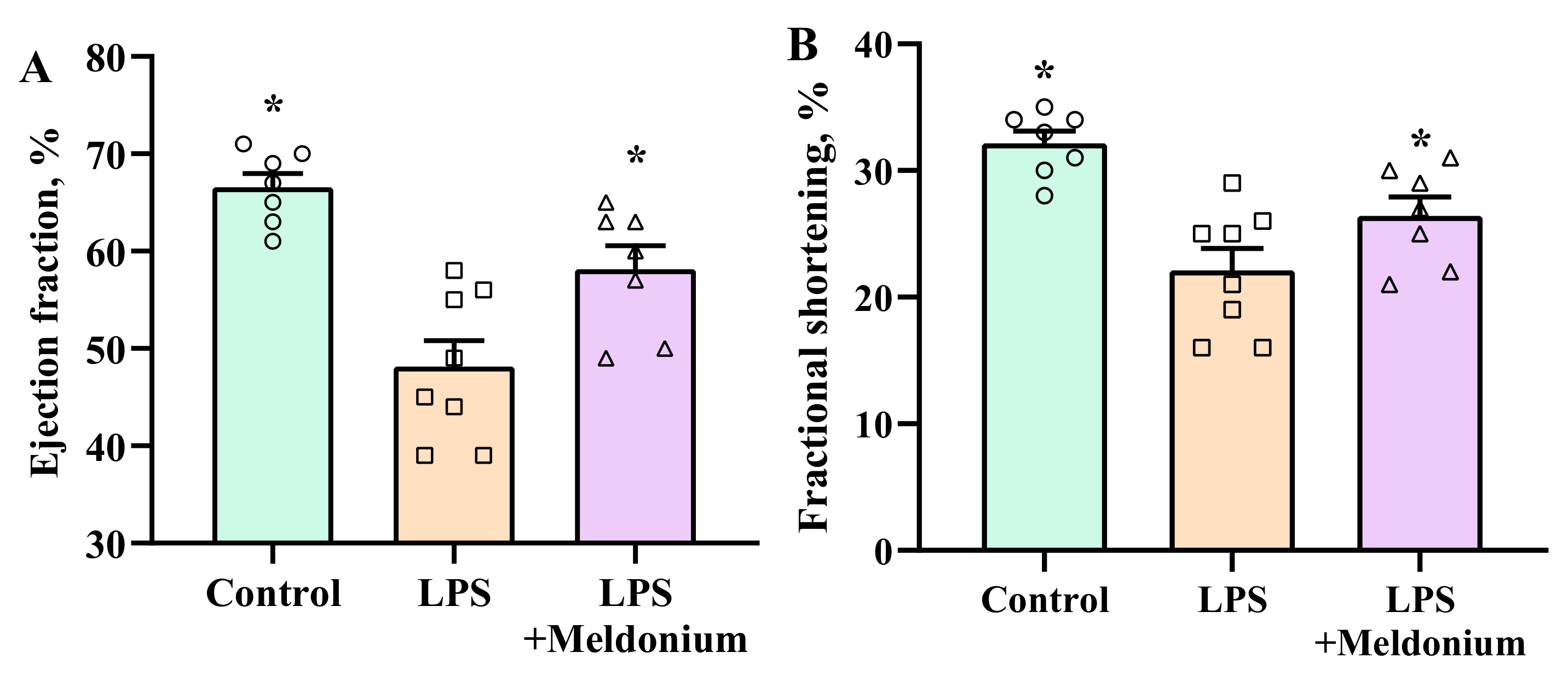
2.6. Effects of Meldonium on Inflammation-induced Left Ventricular Dysfunction
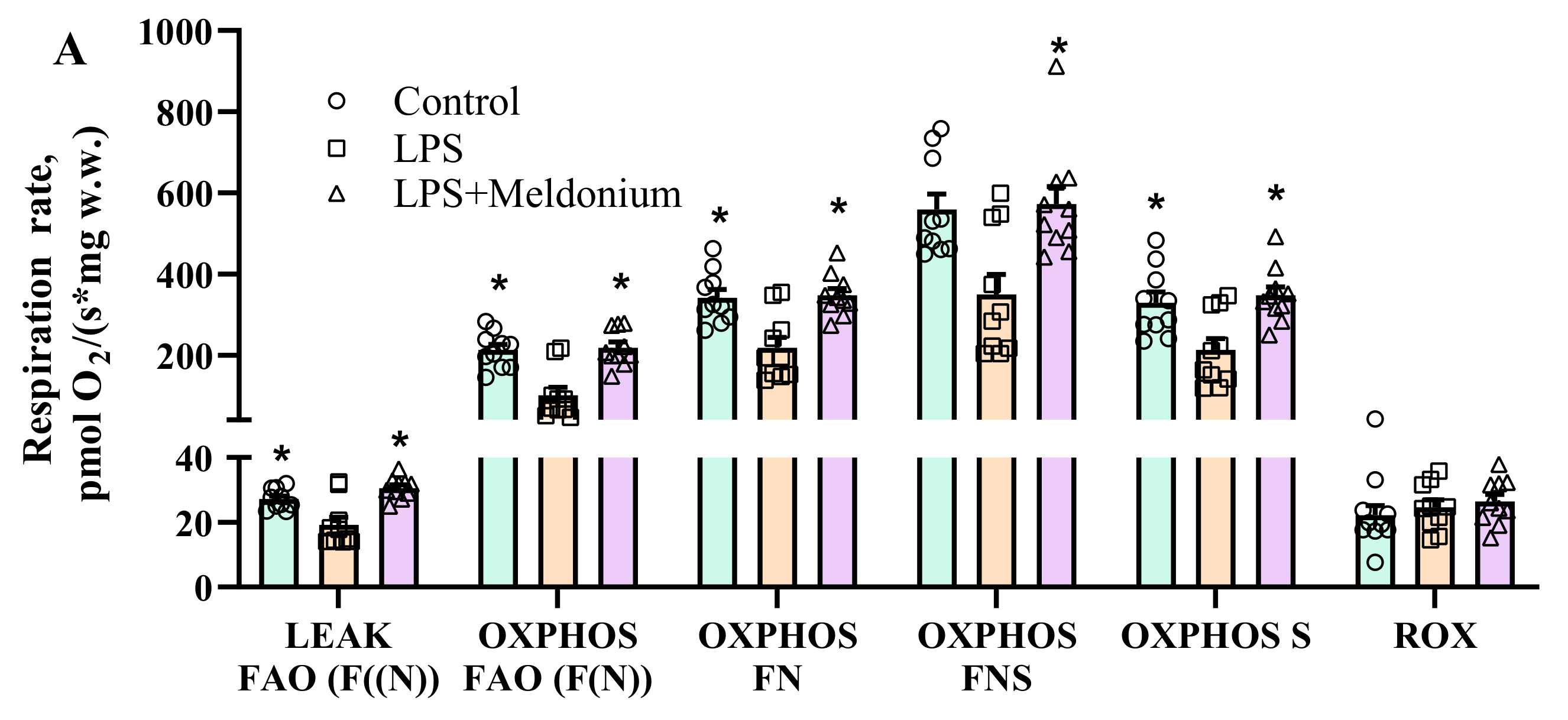
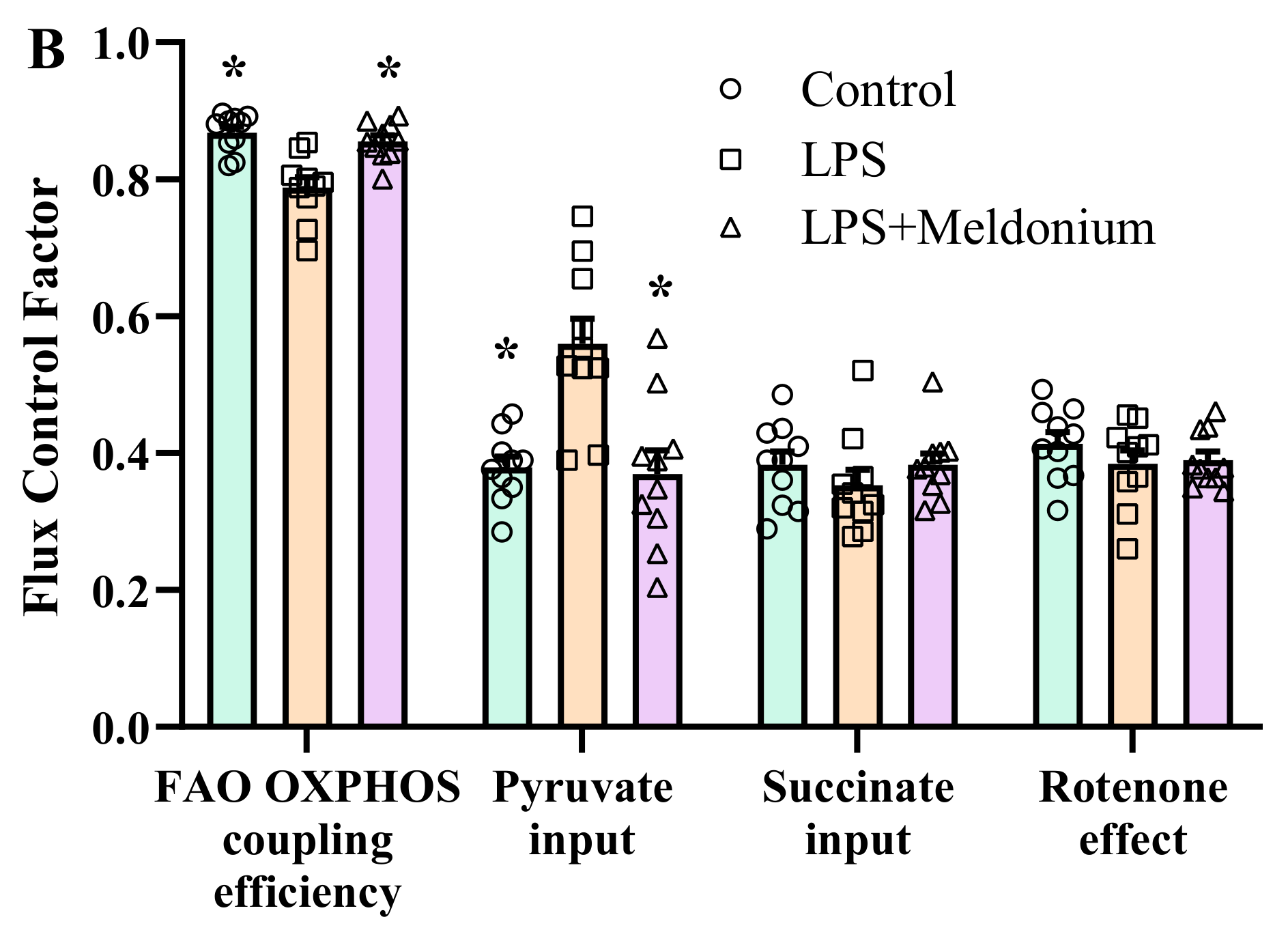
2.7. Effects of Meldonium on Mitochondrial Function in Inflammation-Induced Left Ventricular Dysfunction
3. Discussion
4. Materials and Methods
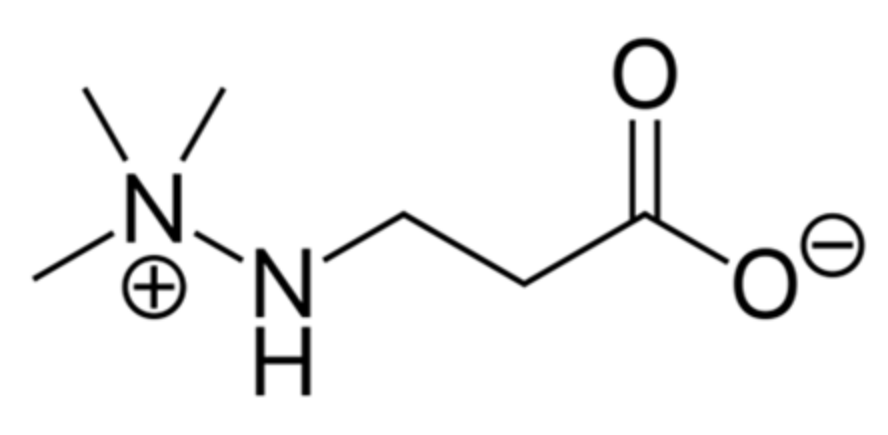
4.1. Materials
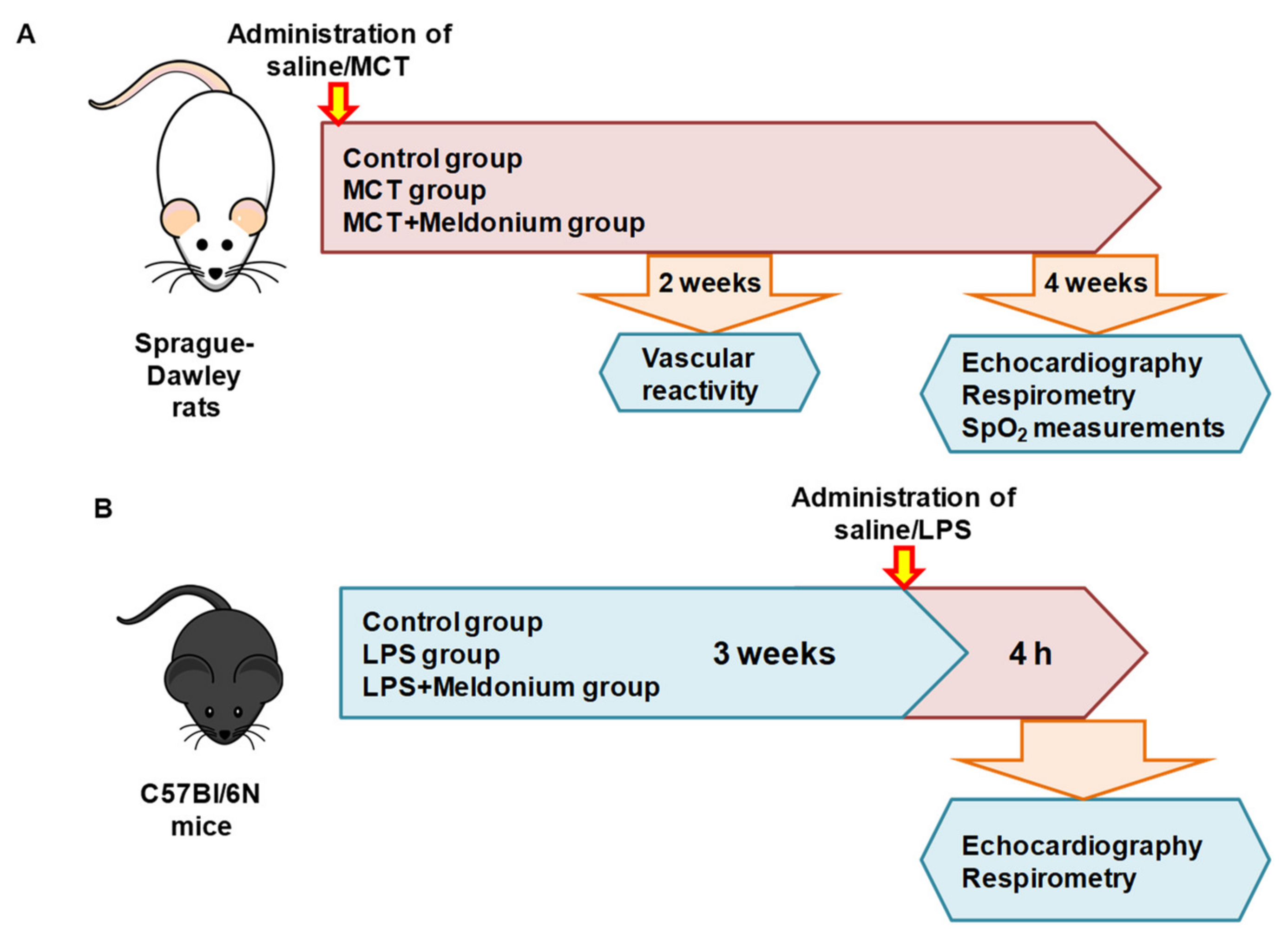
4.2. Animals
4.3. Pulmonary Hypertension and Right Ventricular Failure Model
4.4. Echocardiographic Assessment of Cardiac Function in Rats
4.5. Systolic Right Ventricular Pressure
4.6. Vascular Reactivity of Pulmonary Arteries
4.7. Blood Oxygen Saturation (SpO2)
4.8. Inflammation-Induced Left Ventricular Dysfunction Model
4.9. Echocardiographic Assessment of Left Ventricle Functioning in Mice
4.10. Mitochondrial Functionality Assessment
4.11. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACh | Acetylcholine |
| COVID-19 | coronavirus disease 2019 |
| EDA | end-diastolic area |
| EDV | end diastolic volume |
| ESA | end-systolic area |
| ESV | end systolic volume |
| FA | fatty acid |
| FAO | fatty acid oxidation |
| HR | heart rate |
| Il-1β | interleukin-1 beta |
| Il-6 | interleukin-6 |
| iNOS | inducible nitric oxide synthase |
| IVSd | interventricular septal thickness at end-diastole |
| IVSs | interventricular septal thickness at end-systole |
| LPS | lipopolysaccharide |
| LV | left ventricular |
| LVEF | left ventricular ejection fraction |
| LVIDd | left ventricular internal dimension at end-diastole |
| LVIDs | left ventricular internal dimension at end-systole |
| LVPWd | left ventricular posterior wall thickness at end-diastole |
| LVPWs | left ventricular posterior wall thickness at end-systole |
| MCT | monocrotaline |
| OXPHOS | oxidative phosphorylation-dependent respiration state |
| PGC1α | peroxisome proliferator-activated receptor-gamma coactivator 1α |
| PPARα | peroxisome proliferator-activated receptor α |
| ROS | reactive oxygen species |
| ROX | residual oxygen consumption |
| RV | right ventricular |
| RVFAC | right ventricular fractional area change |
| SNP | sodium nitroprusside |
| SpO2 | oxygen saturation |
| TNFα | tumor necrosis factor alpha |
References
- Park, J.F.; Banerjee, S.; Umar, S. In the eye of the storm: The right ventricle in COVID-19. Pulm. Circ. 2020, 10. [Google Scholar] [CrossRef]
- Szekely, Y.; Lichter, Y.; Taieb, P.; Banai, A.; Hochstadt, A.; Merdler, I.; Oz, A.G.; Rothschild, E.; Baruch, G.; Peri, Y.; et al. Spectrum of Cardiac Manifestations in COVID-19: A Systematic Echocardiographic Study. Circulation 2020, 142, 342–353. [Google Scholar] [CrossRef]
- Bieber, S.; Kraechan, A.; Hellmuth, J.C.; Muenchhoff, M.; Scherer, C.; Schroeder, I.; Irlbeck, M.; Kaeaeb, S.; Massberg, S.; Hausleiter, J.; et al. Left and right ventricular dysfunction in patients with COVID-19-associated myocardial injury. Infection 2021, 49, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Volodarskiy, A.; Sultana, R.; Pollie, M.P.; Yum, B.; Nambiar, L.; Tafreshi, R.; Mitlak, H.W.; RoyChoudhury, A.; Horn, E.M.; et al. Prognostic Utility of Right Ventricular Remodeling Over Conventional Risk Stratification in Patients with COVID-19. J. Am. Coll. Cardiol. 2020, 76, 1965–1977. [Google Scholar] [CrossRef]
- Faridi, K.F.; Hennessey, K.C.; Shah, N.; Soufer, A.; Wang, Y.; Sugeng, L.; Agarwal, V.; Sharma, R.; Sewanan, L.R.; Hur, D.J.; et al. Left Ventricular Systolic Function and Inpatient Mortality in Patients Hospitalized with Coronavirus Disease 2019 (COVID-19). J. Am. Soc. Echocardiogr. 2020, 33, 1414–1415. [Google Scholar] [CrossRef] [PubMed]
- Shahrbaf, M.A.; Tabary, M.; Khaheshi, I. The right ventricle in COVID-19 patients. Eur. Hear. J. 2021, 42, 559–560. [Google Scholar] [CrossRef] [PubMed]
- Fried, J.A.; Ramasubbu, K.; Bhatt, R.; Topkara, V.K.; Clerkin, K.J.; Horn, E.; Rabbani, L.; Brodie, D.; Jain, S.S.; Kirtane, A.J.; et al. The Variety of Cardiovascular Presentations of COVID-19. Circulation 2020, 141, 1930–1936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chitturi, K.R.; Thacker, S.; A Al-Saadi, M.; Kassi, M. Successful treatment of acute heart failure in COVID-19-induced cytokine storm with tocilizumab: A case report. Eur. Hear. J.-Case Rep. 2020, 4, 1–6. [Google Scholar] [CrossRef]
- Li, Y.; Li, H.; Zhu, S.; Xie, Y.; Wang, B.; He, L.; Zhang, D.; Zhang, Y.; Yuan, H.; Wu, C.; et al. Prognostic Value of Right Ventricular Longitudinal Strain in Patients With COVID-19. JACC Cardiovasc. Imaging 2020, 13, 2287–2299. [Google Scholar] [CrossRef]
- Fukui, M.; Cavalcante, J.L. Relation among Right Ventricular Dysfunction, Lung Damage, and Mortality in Patients with COVID-19. JACC Cardiovasc. Imaging 2020, 13, 1858–1859. [Google Scholar] [CrossRef] [PubMed]
- Jirak, P.; Larbig, R.; Shomanova, Z.; Fröb, E.J.; Dankl, D.; Torgersen, C.; Frank, N.; Mahringer, M.; Butkiene, D.; Haake, H.; et al. Myocardial injury in severe COVID-19 is similar to pneumonias of other origin: Results from a multicentre study. ESC Hear. Fail. 2021, 8, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Wang, S.; Ma, P.; Yang, B.; Si, D.; Liu, G.; Liu, L.; Ding, M.; Yang, W.; Li, J.; et al. Cardiac injury is associated with inflammation in geriatric COVID-19 patients. J. Clin. Lab. Anal. 2021, 35, e23654. [Google Scholar] [CrossRef] [PubMed]
- Santos-Ribeiro, D.; Ferreira, P.; Rocha, C.; Adão, R.; Leite-Moreira, A.F.; Brás-Silva, C. Pulmonary arterial hypertension: Basic knowledge for clinicians. Arch. Cardiovasc. Dis. 2016, 109, 550–561. [Google Scholar] [CrossRef] [PubMed]
- Dorfmüller, P.; Perros, F.; Balabanian, K.; Humbert, M. Inflammation in pulmonary arterial hypertension. Eur. Respir. J. 2003, 22, 358–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makrecka-Kuka, M.; Korzh, S.; Videja, M.; Vilskersts, R.; Sevostjanovs, E.; Zharkova-Malkova, O.; Arsenyan, P.; Kuka, J.; Dambrova, M.; Liepinsh, E. Inhibition of CPT2 exacerbates cardiac dysfunction and inflammation in experimental endotoxaemia. J. Cell. Mol. Med. 2020, 24, 11903–11911. [Google Scholar] [CrossRef] [PubMed]
- Koop, A.C.; Bossers, G.P.L.; Ploegstra, M.; Hagdorn, Q.A.J.; Berger, R.M.F.; Silljé, H.H.W.; Bartelds, B. Metabolic Remodeling in the Pressure-Loaded Right Ventricle: Shifts in Glucose and Fatty Acid Metabolism—A Systematic Review and Meta-Analysis. J. Am. Hear. Assoc. 2019, 8, e012086. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, S.; Vico, T.; Vanasco, V. Cardiac dysfunction, mitochondrial architecture, energy production, and inflammatory pathways: Interrelated aspects in endotoxemia and sepsis. Int. J. Biochem. Cell Biol. 2016, 81, 307–314. [Google Scholar] [CrossRef]
- Makrecka-Kuka, M.; Korzh, S.; Vilks, K.; Vilskersts, R.; Cirule, H.; Dambrova, M.; Liepinsh, E. Mitochondrial Function in the Kidney and Heart, but Not the Brain, is Mainly Altered in an Experimental Model of Endotoxaemia. Shock 2019, 52, e153–e162. [Google Scholar] [CrossRef]
- Sun, X.-Q.; Zhang, R.; Zhang, H.-D.; Yuan, P.; Wang, X.-J.; Zhao, Q.-H.; Wang, L.; Jiang, R.; Bogaard, H.J.; Jing, Z.-C. Reversal of right ventricular remodeling by dichloroacetate is related to inhibition of mitochondria-dependent apoptosis. Hypertens. Res. 2016, 39, 302–311. [Google Scholar] [CrossRef]
- Fowler, E.D.; Hauton, D.; Boyle, J.; Egginton, S.; Steele, D.S.; White, E. Energy Metabolism in the Failing Right Ventricle: Limitations of Oxygen Delivery and the Creatine Kinase System. Int. J. Mol. Sci. 2019, 20, 1805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Zhu, Y.; Xiao, J.; Tian, Y.; Ma, M.; Li, X.; Li, L.; Zhang, P.; Li, M.; Wang, J.; et al. Maresin conjugates in tissue regeneration 1 prevents lipopolysaccharide-induced cardiac dysfunction through improvement of mitochondrial biogenesis and function. Biochem. Pharmacol. 2020, 177, 114005. [Google Scholar] [CrossRef]
- Thomas, T.; Stefanoni, D.; Dzieciatkowska, M.; Issaian, A.; Nemkov, T.; Hill, R.C.; Francis, R.O.; Hudson, K.E.; Buehler, P.W.; Zimring, J.C.; et al. Evidence of Structural Protein Damage and Membrane Lipid Remodeling in Red Blood Cells from COVID-19 Patients. J. Proteome Res. 2020, 19, 4455–4469. [Google Scholar] [CrossRef]
- Thomas, T.; Stefanoni, D.; Reisz, J.A.; Nemkov, T.; Bertolone, L.; Francis, R.O.; Hudson, K.E.; Zimring, J.C.; Hansen, K.C.; Hod, E.A.; et al. COVID-19 infection alters kynurenine and fatty acid metabolism, correlating with IL-6 levels and renal status. JCI Insight 2020, 5, e140327. [Google Scholar] [CrossRef]
- Mubarik, S.; Liu, X.; Eshak, E.S.; Liu, K.; Liu, Q.; Wang, F.; Shi, F.; Wen, H.; Bai, J.; Yu, C.; et al. The Association of Hypertension with the Severity of and Mortality from the COVID-19 in the Early Stage of the Epidemic in Wuhan, China: A Multicenter Retrospective Cohort Study. Front. Med. 2021, 8, 623608. [Google Scholar] [CrossRef]
- Sardu, C.; Gargiulo, G.; Esposito, G.; Paolisso, G.; Marfella, R. Impact of diabetes mellitus on clinical outcomes in patients affected by Covid-19. Cardiovasc. Diabetol. 2020, 19, 1–4. [Google Scholar] [CrossRef]
- Dambrova, M.; Makrecka-Kuka, M.; Vilskersts, R.; Makarova, E.; Kuka, J.; Liepinsh, E. Pharmacological effects of meldonium: Biochemical mechanisms and biomarkers of cardiometabolic activity. Pharmacol. Res. 2016, 113, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Liepinsh, E.; Skapare, E.; Kuka, J.; Makrecka, M.; Cirule, H.; Vavers, E.; Sevostjanovs, E.; Grinberga, S.; Pugovics, O.; Dambrova, M. Activated peroxisomal fatty acid metabolism improves cardiac recovery in ischemia-reperfusion. Naunyn-Schmiedebergs Arch. Exp. Pathol. Pharmakol. 2013, 386, 541–550. [Google Scholar] [CrossRef]
- Liepinsh, E.; Vilskersts, R.; Skapare, E.; Svalbe, B.; Kuka, J.; Cirule, H.; Pugovics, O.; Kalvinsh, I.; Dambrova, M. Mildronate decreases carnitine availability and up-regulates glucose uptake and related gene expression in the mouse heart. Life Sci. 2008, 83, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Đurašević, S.; Stojković, M.; Sopta, J.; Pavlović, S.; Borković-Mitić, S.; Ivanović, A.; Jasnić, N.; Tosti, T.; Đurović, S.; Đorđević, J.; et al. The effects of meldonium on the acute ischemia/reperfusion liver injury in rats. Sci. Rep. 2021, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- E Statsenko, M.; Shilina, N.; Turkina, S.V. Use of meldonium in the combination treatment of patients with heart failure in the early postinfarction period. Ter. arkhiv 2014, 86, 30–35. [Google Scholar]
- E Statsenko, M.; Belenkova, S.V.; E Sporova, O.; Shilina, N. The use of mildronate in combined therapy of postinfarction chronic heart failure in patients with type 2 diabetes mellitus. Klin. Med. 2007, 85, 39–42. [Google Scholar]
- Dzerve, V. MILSS I Study Group A Dose-Dependent Improvement in Exercise Tolerance in Patients with Stable Angina Treated With Mildronate: A Clinical Trial “MILSS I”. Medicina 2011, 47, 78. [Google Scholar] [CrossRef]
- Bhat, L.; Hawkinson, J.; Cantillon, M.; Reddy, D.G.; Bhat, S.R.; Laurent, C.-E.; Bouchard, A.; Biernat, M.; Salvail, D. Evaluation of the effects of RP5063, a novel, multimodal, serotonin receptor modulator, as single-agent therapy and co-administrated with sildenafil, bosentan, and treprostinil in a monocrotaline-induced pulmonary arterial hypertension rat model. Eur. J. Pharmacol. 2018, 827, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Tavares-Silva, M.; Alaa, M.; Leite, S.; Oliveira-Pinto, J.; Lopes, L.; Leite-Moreira, A.; Lourenço, A.P. Dose–Response Head-to-Head Comparison of Inodilators Dobutamine, Milrinone, and Levosimendan in Chronic Experimental Pulmonary Hypertension. J. Cardiovasc. Pharmacol. Ther. 2017, 22, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Arroyo, J.; Mizuno, S.; Szczepanek, K.; Van Tassell, B.; Natarajan, R.; dos Remedios, C.G.; Drake, J.I.; Farkas, L.; Kraskauskas, D.; Wijesinghe, D.S.; et al. Metabolic Gene Remodeling and Mitochondrial Dysfunction in Failing Right Ventricular Hypertrophy Secondary to Pulmonary Arterial Hypertension. Circ. Hear. Fail. 2013, 6, 136–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nouws, J.; Brinke, H.T.; Nijtmans, L.G.; Houten, S.M. ACAD9, a complex I assembly factor with a moonlighting function in fatty acid oxidation deficiencies. Hum. Mol. Genet. 2014, 23, 1311–1319. [Google Scholar] [CrossRef] [Green Version]
- Liepinsh, E.; Makrecka-Kuka, M.; Volska, K.; Kuka, J.; Makarova, E.; Antone, U.; Sevostjanovs, E.; Vilskersts, R.; Strods, A.; Tars, K.; et al. Long-chain acylcarnitines determine ischaemia/reperfusion-induced damage in heart mitochondria. Biochem. J. 2016, 473, 1191–1202. [Google Scholar] [CrossRef] [PubMed]
- Rafikov, R.; Sun, X.; Rafikova, O.; Meadows, M.L.; Desai, A.; Khalpey, Z.; Yuan, J.X.-J.; Fineman, J.R.; Black, S.M. Complex I dysfunction underlies the glycolytic switch in pulmonary hypertensive smooth muscle cells. Redox Biol. 2015, 6, 278–286. [Google Scholar] [CrossRef] [Green Version]
- Wüst, R.C.; De Vries, H.J.; Wintjes, L.T.; Rodenburg, R.J.; Niessen, H.; Stienen, G.J. Mitochondrial complex I dysfunction and altered NAD(P)H kinetics in rat myocardium in cardiac right ventricular hypertrophy and failure. Cardiovasc. Res. 2016, 111, 362–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fillmore, N.; Mori, J.; Lopaschuk, G.D. Mitochondrial fatty acid oxidation alterations in heart failure, ischaemic heart disease and diabetic cardiomyopathy. Br. J. Pharmacol. 2014, 171, 2080–2090. [Google Scholar] [CrossRef] [Green Version]
- Dong, Z.; Zhao, P.; Xu, M.; Zhang, C.; Guo, W.; Chen, H.; Tian, J.; Wei, H.; Lu, R.; Cao, T. Astragaloside IV alleviates heart failure via activating PPARα to switch glycolysis to fatty acid β-oxidation. Sci. Rep. 2017, 7, 2691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piao, L.; Marsboom, G.; Archer, S.L. Mitochondrial metabolic adaptation in right ventricular hypertrophy and failure. J. Mol. Med. 2010, 88, 1011–1020. [Google Scholar] [CrossRef] [Green Version]
- Dambrova, M.; Zuurbier, C.J.; Borutaite, V.; Liepinsh, E.; Makrecka-Kuka, M. Energy substrate metabolism and mitochondrial oxidative stress in cardiac ischemia/reperfusion injury. Free Radic. Biol. Med. 2021, 165, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Marin, W.; Marin, D.; Ao, X.; Liu, Y. Mitochondria as a therapeutic target for cardiac ischemia-reperfusion injury (Review). Int. J. Mol. Med. 2020, 47, 485–499. [Google Scholar] [CrossRef] [PubMed]
- Roshdy, A.; Zaher, S.; Fayed, H.; Coghlan, J.G. COVID-19 and the Heart: A Systematic Review of Cardiac Autopsies. Front. Cardiovasc. Med. 2020, 7, 626975. [Google Scholar] [CrossRef] [PubMed]
- Kuka, J.; Vilskersts, R.; Cirule, H.; Makrecka, M.; Pugovics, O.; Kalvinsh, I.; Dambrova, M.; Liepinsh, E. The Cardioprotective Effect of Mildronate is Diminished after Co-Treatment With l-Carnitine. J. Cardiovasc. Pharmacol. Ther. 2012, 17, 215–222. [Google Scholar] [CrossRef]
- Konstam, M.A.; Kiernan, M.S.; Bernstein, D.; Bozkurt, B.; Jacob, M.; Kapur, N.K.; Kociol, R.D.; Lewis, E.F.; Mehra, M.R.; Pagani, F.D.; et al. Evaluation and Management of Right-Sided Heart Failure: A Scientific Statement From the American Heart Association. Circulation 2018, 137, e578–e622. [Google Scholar] [CrossRef] [PubMed]
- Rabin, O.; Uiba, V.; Miroshnikova, Y.; Zabelin, M.; Samoylov, A.; Karkischenko, V.; Semyonov, S.; Astrelina, T.; Razinkin, S. Meldonium long-term excretion period and pharmacokinetics in blood and urine of healthy athlete volunteers. Drug Test. Anal. 2019, 11, 554–566. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.; Cuthill, I.C.; Emerson, M.; Altman, D.G.; NC3Rs Reporting Guidelines Working Group. Animal research: Reporting in vivo experiments: The ARRIVE guidelines. Br. J. Pharmacol. 2010, 160, 1577–1579. [Google Scholar] [CrossRef]
- McGrath, J.; Drummond, G.B.; McLachlan, E.; Kilkenny, C.; Wainwright, C. Guidelines for reporting experiments involving animals: The ARRIVE guidelines. Br. J. Pharmacol. 2010, 160, 1573–1576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Videja, M.; Vilskersts, R.; Korzh, S.; Cirule, H.; Sevostjanovs, E.; Dambrova, M.; Makrecka-Kuka, M. Microbiota-Derived Metabolite Trimethylamine N-Oxide Protects Mitochondrial Energy Metabolism and Cardiac Functionality in a Rat Model of Right Ventricle Heart Failure. Front. Cell Dev. Biol. 2021, 8, 1808. [Google Scholar] [CrossRef] [PubMed]
- Liepinsh, E.; Makrecka-Kuka, M.; Kuka, J.; Vilskersts, R.; Makarova, E.; Cirule, H.; Loza, E.; Lola, D.; Grinberga, S.; Pugovics, O.; et al. Inhibition of L-carnitine biosynthesis and transport by methyl-γ-butyrobetaine decreases fatty acid oxidation and protects against myocardial infarction. Br. J. Pharmacol. 2015, 172, 1319–1332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakazawa, H.; Hori, M.; Ozaki, H.; Karaki, H. Mechanisms underlying the impairment of endothelium-dependent relaxation in the pulmonary artery of monocrotaline-induced pulmonary hypertensive rats. Br. J. Pharmacol. 1999, 128, 1098–1104. [Google Scholar] [CrossRef]
- Mathew, R.; Zeballos, G.A.; Tun, H.; Gewitz, M.H. Role of nitric oxide and endothelin-1 in monocrotaline-induced pulmonary hypertension in rats. Cardiovasc. Res. 1995, 30, 739–746. [Google Scholar] [CrossRef]
- Guillon, A.; Translational Research Committee of the French Intensive Care Society (Société de Réanimation de Langue Française); Preau, S.; Aboab, J.; Azabou, E.; Jung, B.; Silva, S.; Textoris, J.; Uhel, F.; Vodovar, D.; et al. Preclinical septic shock research: Why we need an animal ICU. Ann. Intensiv. Care 2019, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Allen, M.E.; Pennington, E.R.; Perry, J.B.; Dadoo, S.; Makrecka-Kuka, M.; Dambrova, M.; Moukdar, F.; Patel, H.D.; Han, X.; Kidd, G.; et al. The cardiolipin-binding peptide elamipretide mitigates fragmentation of cristae networks following cardiac ischemia reperfusion in rats. Commun. Biol. 2020, 3, 1–12. [Google Scholar] [CrossRef]
- Perry, J.B.; Davis, G.N.; Allen, M.E.; Makrecka-Kuka, M.; Dambrova, M.; Grange, R.W.; Shaikh, S.R.; Brown, D.A. Cardioprotective effects of idebenone do not involve ROS scavenging: Evidence for mitochondrial complex I bypass in ischemia/reperfusion injury. J. Mol. Cell. Cardiol. 2019, 135, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Makrecka-Kuka, M.; Krumschnabel, G.; Gnaiger, E. High-Resolution Respirometry for Simultaneous Measurement of Oxygen and Hydrogen Peroxide Fluxes in Permeabilized Cells, Tissue Homogenate and Isolated Mitochondria. Biomolecules 2015, 5, 1319–1338. [Google Scholar] [CrossRef] [Green Version]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef] [Green Version]
| Control | MCT | MCT + Meldonium | |
|---|---|---|---|
| RV systolic pressure, mmHg | 19 ± 1 * | 52 ± 5 | 41 ± 4 |
| Right ventricle-to-body mass index, mg/g | 0.50 ± 0.01 * | 1.13 ± 0.06 | 0.88 ± 0.08 * |
| Fulton index, g/g | 0.27 ± 0.01 * | 0.53 ± 0.03 | 0.43 ± 0.04 * |
| Left ventricle-to-body mass index, mg/g | 1.8 ± 0.1 | 2.0 ± 0.1 | 2.0 ± 0.1 |
| Control | LPS | LPS + Meldonium | |
|---|---|---|---|
| IVSs, mm | 1.1 ± 0.03 * | 1.0 ± 0.03 | 1.1 ± 0.02 * |
| IVSd, mm | 0.6 ± 0.02 | 0.5 ± 0.01 | 0.6 ± 0.02 |
| LVPWs, mm | 1.1 ± 0.04 * | 0.8 ± 0.03 | 1.0 ± 0.04 # |
| LVPWd, mm | 0.6 ± 0.04 | 0.5 ± 0.03 | 0.5 ± 0.02 |
| LVIDs, mm | 3.2 ± 0.1 * | 3.6 ± 0.1 | 3.2 ± 0.1 * |
| LVIDd, mm | 4.6 ± 0.1 | 4.5 ± 0.1 | 4.3 ± 0.1 |
| ESV, mL | 0.09 ± 0.01 * | 0.11 ± 0.01 | 0.09 ± 0.01 * |
| EDV, mL | 0.25 ± 0.02 | 0.23 ± 0.02 | 0.21 ± 0.01 |
| HR, bpm | 453 ± 27 | 501 ± 11 | 477 ± 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilskersts, R.; Kigitovica, D.; Korzh, S.; Videja, M.; Vilks, K.; Cirule, H.; Skride, A.; Makrecka-Kuka, M.; Liepinsh, E.; Dambrova, M. Protective Effects of Meldonium in Experimental Models of Cardiovascular Complications with a Potential Application in COVID-19. Int. J. Mol. Sci. 2022, 23, 45. https://doi.org/10.3390/ijms23010045
Vilskersts R, Kigitovica D, Korzh S, Videja M, Vilks K, Cirule H, Skride A, Makrecka-Kuka M, Liepinsh E, Dambrova M. Protective Effects of Meldonium in Experimental Models of Cardiovascular Complications with a Potential Application in COVID-19. International Journal of Molecular Sciences. 2022; 23(1):45. https://doi.org/10.3390/ijms23010045
Chicago/Turabian StyleVilskersts, Reinis, Dana Kigitovica, Stanislava Korzh, Melita Videja, Karlis Vilks, Helena Cirule, Andris Skride, Marina Makrecka-Kuka, Edgars Liepinsh, and Maija Dambrova. 2022. "Protective Effects of Meldonium in Experimental Models of Cardiovascular Complications with a Potential Application in COVID-19" International Journal of Molecular Sciences 23, no. 1: 45. https://doi.org/10.3390/ijms23010045
APA StyleVilskersts, R., Kigitovica, D., Korzh, S., Videja, M., Vilks, K., Cirule, H., Skride, A., Makrecka-Kuka, M., Liepinsh, E., & Dambrova, M. (2022). Protective Effects of Meldonium in Experimental Models of Cardiovascular Complications with a Potential Application in COVID-19. International Journal of Molecular Sciences, 23(1), 45. https://doi.org/10.3390/ijms23010045








