Novel Risk Associations between microRNA Polymorphisms and Gastric Cancer in a Chilean Population
Abstract
:1. Introduction
2. Results
2.1. Polymorphisms in miRNAs Associated with Gastric Cancer
2.2. Prediction of the Effect of Polymorphisms on the Secondary Structure of pre-miRNAs
2.3. Prediction of the Effect of the rs404337 Polymorphism on the Secondary Structure of the Mature miR-8084
2.4. Prediction of the Effect on the Secondary Structure of rs1553867776 and rs12416605 Polymorphisms in the Seed Region of miRs-4274-3p and -938
2.5. Predicted Targets of Novel miRNA Genes Containing Polymorphisms in Gastric Cancer
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Genotyping
4.3. Genotype Imputation
4.4. Association Analyses of Imputed Genotypes
4.5. Stratified Analyses
4.6. Prediction of the Biological Effect of Associated Variants
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cancer Today. Available online: https://gco.iarc.fr/today/home (accessed on 23 September 2021).
- Lin, S.J.; Gagnon-Bartsch, J.A.; Tan, I.B.; Earle, S.; Ruff, L.; Pettinger, K.; Ylstra, B.; van Grieken, N.; Rha, S.Y.; Chung, H.C.; et al. Signatures of tumour immunity distinguish Asian and non-Asian gastric adenocarcinomas. Gut 2015, 64, 1721–1731. [Google Scholar] [CrossRef]
- Ossandon, F.J.; Villarroel, C.; Aguayo, F.; Santibanez, E.; Oue, N.; Yasui, W.; Corvalán, A.H. In silico analysis of gastric carcinoma Serial Analysis of Gene Expression libraries reveals different profiles associated with ethnicity. Mol. Cancer 2008, 7, 22. [Google Scholar] [CrossRef] [Green Version]
- Bonequi, P.; Meneses-González, F.; Correa, P.; Rabkin, C.S.; Camargo, M.C. Risk factors for gastric cancer in Latin America: A meta-analysis. Cancer Causes Control CCC 2013, 24, 217–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russo, A.E.; Strong, V.E. Gastric Cancer Etiology and Management in Asia and the West. Annu. Rev. Med. 2019, 70, 353–367. [Google Scholar] [CrossRef] [PubMed]
- McLean, M.H.; El-Omar, E.M. Genetics of gastric cancer. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Tohidpour, A. CagA-mediated pathogenesis of Helicobacter pylori. Microb. Pathog. 2016, 93, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Landeros, N.; Santoro, P.M.; Carrasco-Avino, G.; Corvalan, A.H. Competing Endogenous RNA Networks in the Epithelial to Mesenchymal Transition in Diffuse-Type of Gastric Cancer. Cancers 2020, 12, 2741. [Google Scholar] [CrossRef] [PubMed]
- Slavin, T.P.; Weitzel, J.N.; Neuhausen, S.L.; Schrader, K.A.; Oliveira, C.; Karam, R. Genetics of gastric cancer: What do we know about the genetic risks? Transl. Gastroenterol. Hepatol. 2019, 4, 55. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. Metazoan MicroRNAs. Cell 2018, 173, 20–51. [Google Scholar] [CrossRef] [Green Version]
- Kozomara, A.; Birgaoanu, M.; Griffiths-Jones, S. miRBase: From microRNA sequences to function. Nucleic Acids Res. 2019, 47, D155–D162. [Google Scholar] [CrossRef]
- Roden, C.; Gaillard, J.; Kanoria, S.; Rennie, W.; Barish, S.; Cheng, J.; Pan, W.; Liu, J.; Cotsapas, C.; Ding, Y.; et al. Novel determinants of mammalian primary microRNA processing revealed by systematic evaluation of hairpin-containing transcripts and human genetic variation. Genome Res. 2017, 27, 374–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawahara, Y. Human diseases caused by germline and somatic abnormalities in microRNA and microRNA-related genes. Congenit. Anom. 2014, 54, 12–21. [Google Scholar] [CrossRef]
- Dragomir, M.P.; Kopetz, S.; Ajani, J.A.; Calin, G.A. Non-coding RNAs in GI cancers: From cancer hallmarks to clinical utility. Gut 2020, 69, 748–763. [Google Scholar] [CrossRef]
- Park, J.H.; Jeong, G.H.; Lee, K.S.; Lee, K.H.; Suh, J.S.; Eisenhut, M.; van der Vliet, H.J.; Kronbichler, A.; Stubbs, B.; Solmi, M.; et al. Genetic variations in MicroRNA genes and cancer risk: A field synopsis and meta-analysis. Eur. J. Clin. Investig. 2020, 50, e13203. [Google Scholar] [CrossRef] [PubMed]
- Rong, G.; Zhu, Y.; Tang, W.; Qiu, H.; Zhang, S. The correlation of microRNA-499 rs3746444 T>C locus with the susceptibility of gastric cancer: From a case-control study to a meta-analysis. Biosci. Rep. 2021, 41, BSR20203461. [Google Scholar] [CrossRef]
- Yan, W.; Gao, X.; Zhang, S. Association of miR-196a2 rs11614913 and miR-499 rs3746444 polymorphisms with cancer risk: A meta-analysis. Oncotarget 2017, 8, 114344–114359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, R.; Lopes, L.; Saeed, A. Association between the Functional miR-146a SNP rs2910164 and Risk of Digestive System Cancer: Updated Meta-analysis. Anticancer Res. 2020, 40, 1495–1502. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, Q.; Wang, F. Association Between miR-149 Gene rs2292832 Polymorphism and Risk of Gastric Cancer. Arch. Med. Res. 2018, 49, 270–277. [Google Scholar] [CrossRef]
- Hashemi, M.; Moazeni-Roodi, A.; Bahari, G.; Taheri, M.; Ghavami, S. Association between miR-34b/c rs4938723 polymorphism and risk of cancer: An updated meta-analysis of 27 case-control studies. J. Cell Biochem. 2019, 120, 3306–3314. [Google Scholar] [CrossRef]
- Weng, Y.; Wang, D.; Bai, R. Associated of rs895819 with risk of stomach neoplasms. Gene 2020, 726, 144173. [Google Scholar] [CrossRef]
- Moazeni-Roodi, A.; Ghavami, S.; Hashemi, M. Lack of Association between miR-605 rs2043556 Polymorphism and Overall Cancer Risk: A Meta-analysis of Case-control Studies. MicroRNA 2019, 8, 94–100. [Google Scholar] [CrossRef]
- Moazeni-Roodi, A.; Ghavami, S.; Hashemi, M. Association between miR-423 rs6505162 Polymorphism and Susceptibility to Cancer. Arch. Med. Res. 2019, 50, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Torruella-Loran, I.; Ramirez Viña, M.K.; Zapata-Contreras, D.; Muñoz, X.; Garcia-Ramallo, E.; Bonet, C.; Gonzalez, C.A.; Sala, N.; Espinosa-Parrilla, Y. rs12416605:C>T in MIR938 associates with gastric cancer through affecting the regulation of the CXCL12 chemokine gene. Mol. Genet. Genom. Med. 2019, 7, e832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Bae, W.J.; Ahn, J.M.; Heo, J.H.; Kim, K.M.; Choi, K.W.; Sung, C.O.; Lee, D. MicroRNA signatures associated with lymph node metastasis in intramucosal gastric cancer. Mod. Pathol. 2020, 34, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Liu, D.; Li, Z.; Tian, N.; Han, Z.; Wang, G.; Fu, Y.; Guo, Z.; Zhu, Z.; Du, C.; et al. HOXB1 Is a Tumor Suppressor Gene Regulated by miR-3175 in Glioma. PLoS ONE 2015, 10, e0142387. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Qing, X.Y.; Zhou, Q.; Li, H.D.; Hu, Z.Y. Silencing of microRNA-3175 represses cell proliferation and invasion in prostate cancer by targeting the potential tumor-suppressor SCN4B. Kaohsiung. J. Med. Sci. 2021, 37, 20–26. [Google Scholar] [CrossRef]
- Song, J.; Bai, Z.; Zhang, Z. MicroRNAs are implicated in the initiation and progression of gastric cancer. Chin. Med. J. 2014, 127, 554–559. [Google Scholar] [PubMed]
- Ma, G.; Li, Q.; Dai, W.; Yang, X.; Sang, A. Prognostic Implications of miR-302a/b/c/d in Human Gastric Cancer. Pathol. Oncol. Res. 2017, 23, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.F.; Xu, Q.; He, C.Y.; Li, Y.; Liu, J.W.; Deng, N.; Sun, L.P.; Yuan, Y. Association of Polymorphisms in three pri-miRNAs that Target Pepsinogen C with the Risk and Prognosis of Gastric Cancer. Sci. Rep. 2017, 7, 39528. [Google Scholar] [CrossRef] [Green Version]
- Zhan, Y.; Liang, X.; Li, L.; Wang, B.; Ding, F.; Li, Y.; Wang, X.; Zhan, Q.; Liu, Z. MicroRNA-548j functions as a metastasis promoter in human breast cancer by targeting Tensin1. Mol. Oncol. 2016, 10, 838–849. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Ma, H.; Gao, C.; Lv, Y.; Chen, X.; Xu, R.; Sun, M.; Liu, X.; Lu, X.; Pei, X.; et al. Tumor-promoting properties of miR-8084 in breast cancer through enhancing proliferation, suppressing apoptosis and inducing epithelial-mesenchymal transition. J. Transl. Med. 2018, 16, 38. [Google Scholar] [CrossRef] [Green Version]
- Chomczynski, P.; Sacchi, N. The single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction: Twenty-something years on. Nat. Protoc. 2006, 1, 581–585. [Google Scholar] [CrossRef]
- Anderson, C.A.; Pettersson, F.H.; Clarke, G.M.; Cardon, L.R.; Morris, A.P.; Zondervan, K.T. Data quality control in genetic case-control association studies. Nat. Protoc. 2010, 5, 1564–1573. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.C.; Chow, C.C.; Tellier, L.C.; Vattikuti, S.; Purcell, S.M.; Lee, J.J. Second-generation PLINK: Rising to the challenge of larger and richer datasets. Gigascience 2015, 4, 7. [Google Scholar] [CrossRef]
- Delaneau, O.; Zagury, J.F.; Robinson, M.R.; Marchini, J.L.; Dermitzakis, E.T. Accurate, scalable and integrative haplotype estimation. Nat. Commun. 2019, 10, 5436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, D.; Eishi, Y.; Ohkusa, T.; Ishige, I.; Suzuki, T.; Minami, J.; Yamada, T.; Takizawa, T.; Koike, M. Gastric mucosal density of Helicobacter pylori estimated by real-time PCR compared with results of urea breath test and histological grading. J. Med. Microbiol. 2002, 51, 305–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez-Hormazabal, P.; Pelaez, D.; Musleh, M.; Bustamante, M.; Stambuk, J.; Pisano, R.; Valladares, H.; Lanzarini, E.; Chiong, H.; Suazo, J.; et al. NOD1 rs2075820 (p.E266K) polymorphism is associated with gastric cancer among individuals infected with cagPAI-positive H. pylori. Biol. Res. 2021, 54, 13. [Google Scholar] [CrossRef]
- Vejnar, C.E.; Zdobnov, E.M. MiRmap: Comprehensive prediction of microRNA target repression strength. Nucleic Acids Res. 2012, 40, 11673–11683. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]
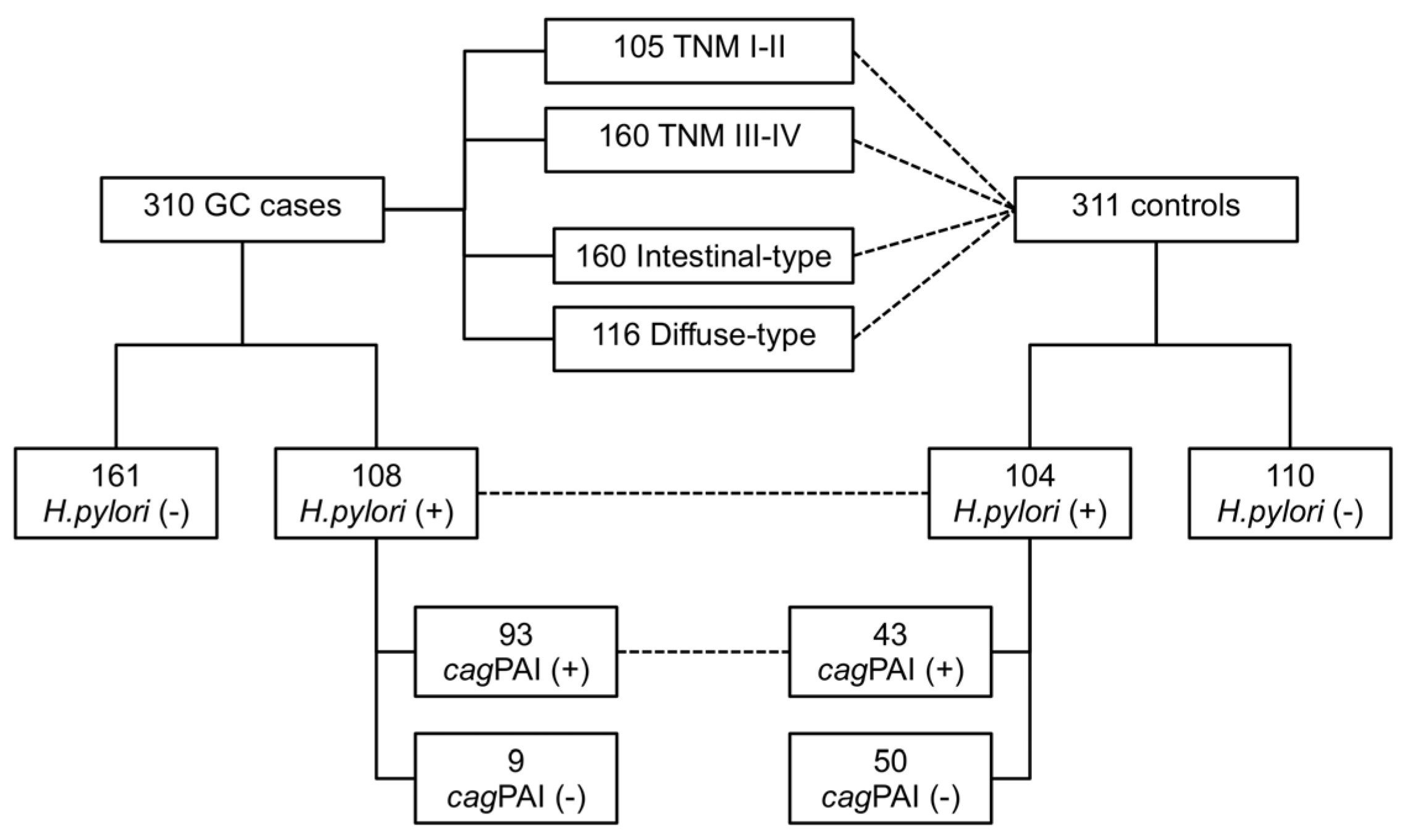
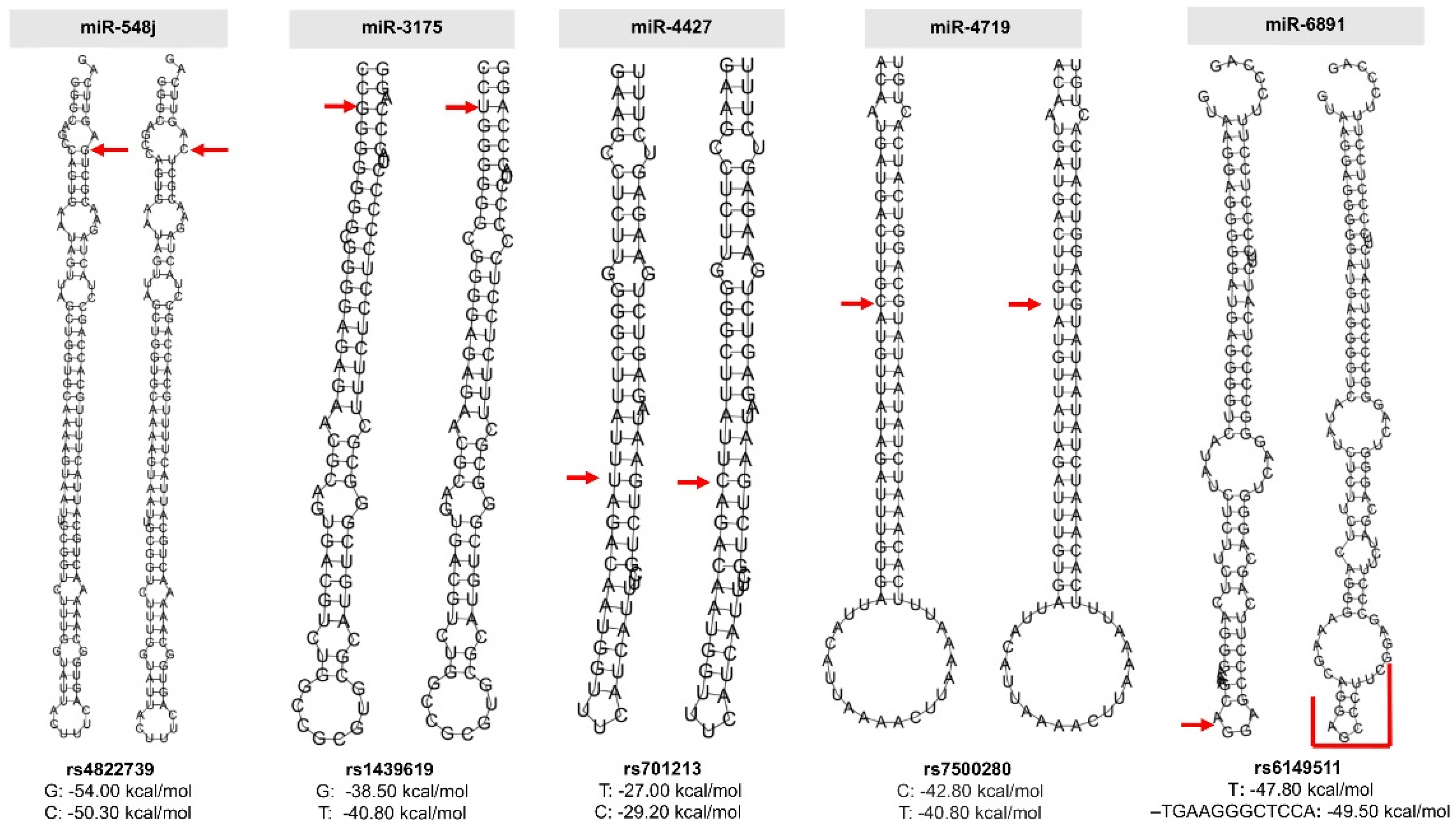
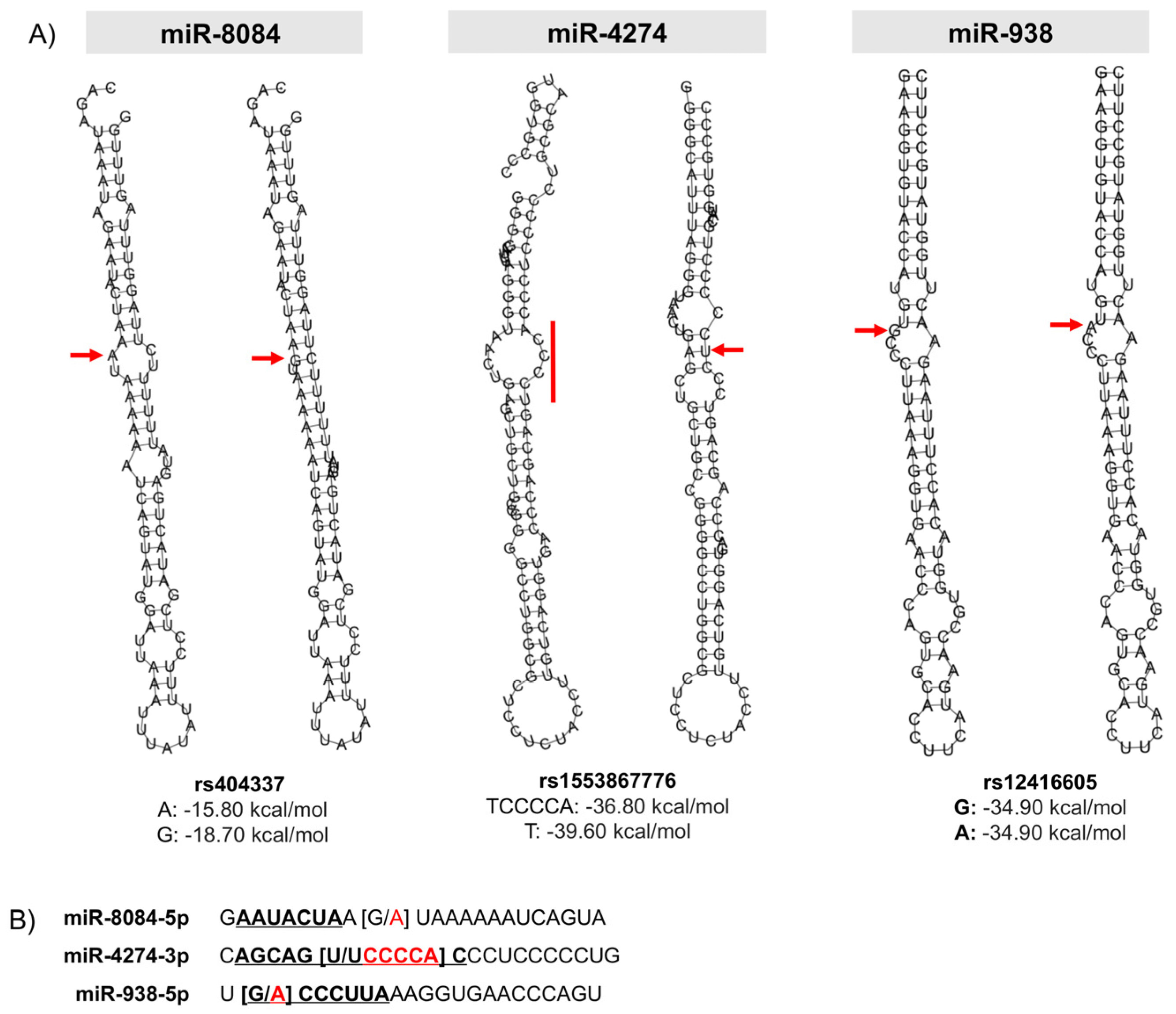
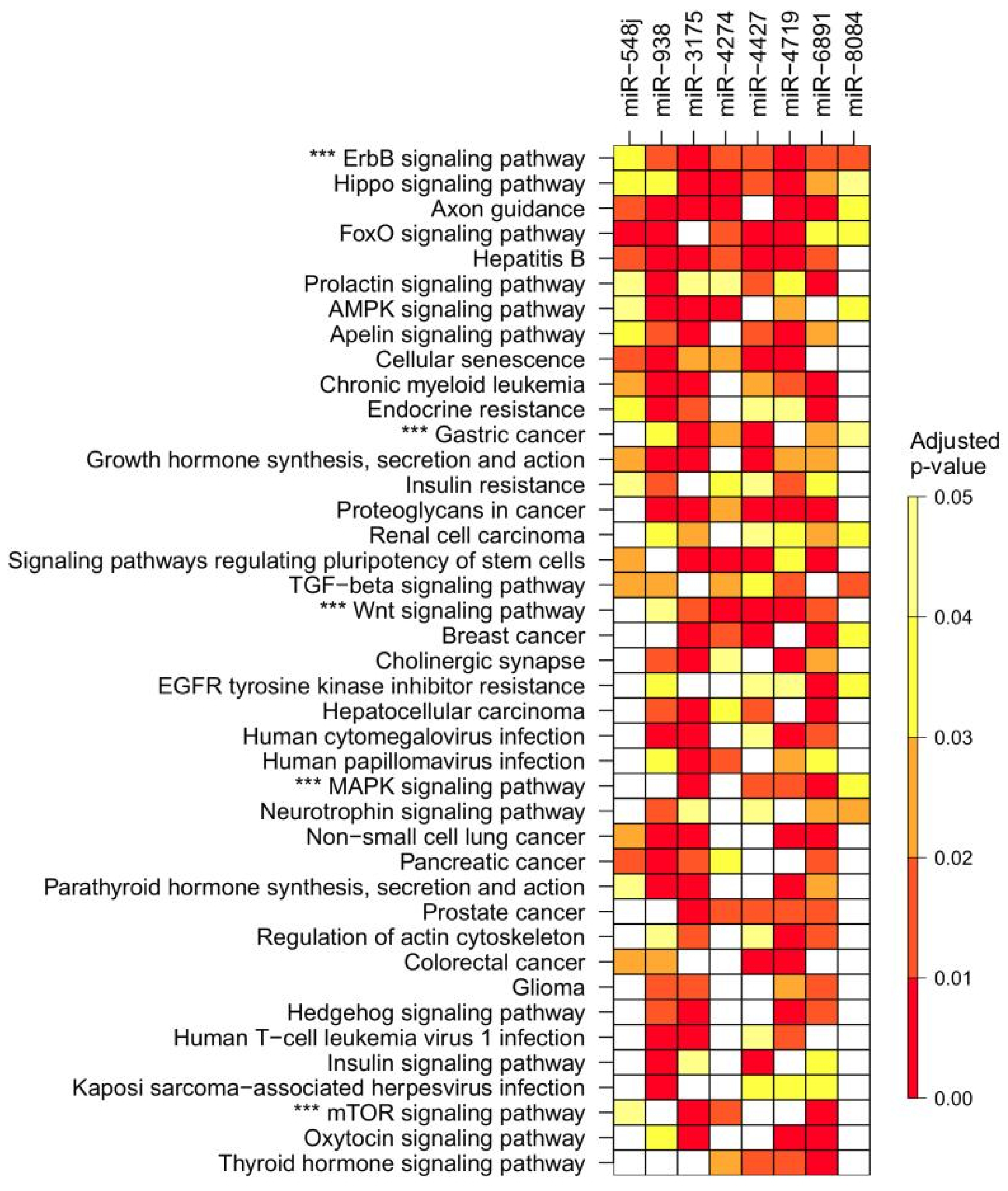
| Variable | Gastric Cancer | Controls | p-Value |
|---|---|---|---|
| Female | 108 (34.8%) | 124 (39.9%) | |
| Male | 202 (65.2%) | 187 (60.1%) | 0.225 1 |
| Age (SD) | 64.1 (12.1) | 51.1 (15.6) | <0.001 2 |
| Lauren’s classification | |||
| Intestinal | 160 (51.6%) | - | |
| Diffuse | 116 (37.4%) | - | |
| Mixed | 31 (10.0%) | - | |
| Not available | 3 (1.0%) | - | |
| TNM | |||
| I | 55 (17.7%) | - | |
| II | 50 (16.1%) | - | |
| III | 148 (47.7%) | - | |
| IV | 12 (3.9%) | - | |
| Not available | 45 (14.5%) | - | |
| H.pylori status | |||
| Positive | 108 (40.1%) | 104 (48.6%) | |
| Negative | 161 (59.9%) | 110 (51.4%) | 0.077 1 |
| cagPAI status | |||
| Positive | 93 (86.1%) | 43 (41.3%) | |
| Negative | 9 (8.3%) | 50 (48.1%) | <0.001 1 |
| Not available | 6 (5.6%) | 11 (10.6%) |
| rsID (Gene Name) | Tested Allele | R2 | Allelic Frequency (Cases/Controls) | AMR | OR 1 | p-Value 1 | OR 2 | p-Value 2 | OR 3 | p-Value 3 |
|---|---|---|---|---|---|---|---|---|---|---|
| All Gastric Cancer cases | ||||||||||
| rs701213 T>C (miR-4427) | C | 0.44 | 0.367/0.412 | 0.39 | 0.65 | 0.009 | 0.71 | 0.067 | 0.68 | 0.0212 |
| rs4822739 C>G (miR-548j) | G | 0.95 | 0.156/0.101 | 0.11 | 1.60 | 0.009 | 1.87 | 0.002 | 1.54 | 0.0153 |
| Intestinal-type Gastric Cancer | ||||||||||
| rs12416605 C>T(miR-938) | T | 0.96 | 0.202/0.279 | 0.22 | 0.64 | 0.008 | 0.68 | 0.041 | 0.65 | 0.0109 |
| rs1553867776 T>TCCCCA (miR-4274) | TCCCCA | 0.79 | 0.91/0.855 | 0.90 | 2.08 | 0.005 | 1.95 | 0.018 | 1.98 | 0.0083 |
| TNM I-II stage | ||||||||||
| rs1439619 T>G (miR-3175) | G | 0.91 | 0.520/0.623 | 0.52 | 0.59 | 0.002 | 0.62 | 0.014 | 0.63 | 0.0059 |
| rs4822739 C>G (miR-548j) | G | 0.95 | 0.179/0.111 | 0.11 | 1.90 | 0.006 | 1.98 | 0.008 | 1.81 | 0.0094 |
| H. pylori-infected subjects | ||||||||||
| rs1439619 T>G (miR-3175) | G | 0.91 | 0.558/0.672 | 0.52 | 0.52 | 0.006 | 0.52 | 0.015 | 0.61 | 0.0229 |
| rs6149511 T>TGAAGGGCTCCA(miR-6891) | TGAAGGGCTCCA | 0.67 | 0.461/0.353 | 0.48 | 2.02 | 0.008 | 2.17 | 0.012 | 1.86 | 0.0130 |
| rs404337 G>A (miR-8084) | A | 0.80 | 0.888/0.820 | 0.83 | 2.56 | 0.009 | 2.91 | 0.009 | 1.97 | 0.0415 |
| H. pylori cagPAI-positive subjects | ||||||||||
| rs1439619 T>G (miR-3175) | G | 0.91 | 0.538/0.688 | 0.52 | 0.44 | 0.009 | 0.48 | 0.034 | 0.52 | 0.0204 |
| rs7500280 T>C (miR-4719) | C | 0.80 | 0.569/0.406 | 0.59 | 2.25 | 0.009 | 2.56 | 0.011 | 2.25 | 0.0074 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Landeros, N.; Corvalan, A.H.; Musleh, M.; Quiñones, L.A.; Varela, N.M.; Gonzalez-Hormazabal, P. Novel Risk Associations between microRNA Polymorphisms and Gastric Cancer in a Chilean Population. Int. J. Mol. Sci. 2022, 23, 467. https://doi.org/10.3390/ijms23010467
Landeros N, Corvalan AH, Musleh M, Quiñones LA, Varela NM, Gonzalez-Hormazabal P. Novel Risk Associations between microRNA Polymorphisms and Gastric Cancer in a Chilean Population. International Journal of Molecular Sciences. 2022; 23(1):467. https://doi.org/10.3390/ijms23010467
Chicago/Turabian StyleLanderos, Natalia, Alejandro H. Corvalan, Maher Musleh, Luis A. Quiñones, Nelson M. Varela, and Patricio Gonzalez-Hormazabal. 2022. "Novel Risk Associations between microRNA Polymorphisms and Gastric Cancer in a Chilean Population" International Journal of Molecular Sciences 23, no. 1: 467. https://doi.org/10.3390/ijms23010467
APA StyleLanderos, N., Corvalan, A. H., Musleh, M., Quiñones, L. A., Varela, N. M., & Gonzalez-Hormazabal, P. (2022). Novel Risk Associations between microRNA Polymorphisms and Gastric Cancer in a Chilean Population. International Journal of Molecular Sciences, 23(1), 467. https://doi.org/10.3390/ijms23010467







