Stem Cell-Derived β Cells: A Versatile Research Platform to Interrogate the Genetic Basis of β Cell Dysfunction
Abstract
1. Introduction
2. Models to Study the Genetic Basis of β Cell Dysfunction
2.1. Animal Models
2.2. Non-Human Cell Lines
2.3. Human Cell Lines
2.4. Human Islets
2.5. Human Stem Cell-Derived β Cells
3. Genetic Basis of β Cell Dysfunction
3.1. Monogenic Diabetes
- HNF1A
- HNF4A
- HNF1B
3.2. Type 2 Diabetes (T2D)
- CDKAL1
- KCNJ11
- KCNQ1
- SLC30A8
- TCF7L2
3.3. Type 1 Diabetes (T1D)
4. Classification of the Genetic Drivers of β Cell Dysfunction
4.1. Protein Coding Variants
4.2. Non-Coding Variants
4.2.1. Promoter
4.2.2. Enhancer
4.2.3. Three-Dimensional Chromatin Structure
4.2.4. Non-Coding RNA (ncRNA)
4.2.5. Other Non-Coding Variants
5. Classification of Processes Associated with β Cell Dysfunction
5.1. Pancreas Development
5.2. β Cell Mass and Adaptive Proliferation
5.3. β Cell Function
5.4. Response to Stress
6. Limitations
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Katsarou, A.; Gudbjörnsdottir, S.; Rawshani, A.; Dabelea, D.; Bonifacio, E.; Anderson, B.J.; Jacobsen, L.M.; Schatz, D.A.; Lernmark, Å. Type 1 diabetes mellitus. Nat. Publ. Group 2017, 3, 17016. [Google Scholar] [CrossRef]
- Chatterjee, S.; Khunti, K.; Davies, M.J. Type 2 diabetes. Lancet 2017, 389, 2239–2251. [Google Scholar] [CrossRef]
- Skoczek, D.; Dulak, J.; Kachamakova-Trojanowska, N. Maturity Onset Diabetes of the Young-New Approaches for Disease Modelling. Int. J. Mol. Sci. 2021, 22, 7553. [Google Scholar] [CrossRef]
- Beltrand, J.; Busiah, K.; Vaivre-Douret, L.; Fauret, A.L.; Berdugo, M.; Cavé, H.; Polak, M. Neonatal Diabetes Mellitus. Front. Pediatrics 2020, 8, 540718. [Google Scholar] [CrossRef] [PubMed]
- Katsanis, N. The continuum of causality in human genetic disorders. Genome Biol. 2016, 17, 233. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, J.K. Are rare variants responsible for susceptibility to complex diseases? Am. J. Hum. Genet. 2001, 69, 124–137. [Google Scholar] [CrossRef]
- Bodmer, W.; Bonilla, C. Common and rare variants in multifactorial susceptibility to common diseases. Nat. Genet. 2008, 40, 695–701. [Google Scholar] [CrossRef]
- Steinthorsdottir, V.; Thorleifsson, G.; Sulem, P.; Helgason, H.; Grarup, N.; Sigurdsson, A.; Helgadottir, H.T.; Johannsdottir, H.; Magnusson, O.T.; Gudjonsson, S.A.; et al. Identification of low-frequency and rare sequence variants associated with elevated or reduced risk of type 2 diabetes. Nat. Genet. 2014, 46, 294–298. [Google Scholar] [CrossRef]
- The UK10K Consortium; Walter, K.; Min, J.L.; Huang, J.; Crooks, L.; Memari, Y.; McCarthy, S.; Perry, J.R.B.; Xu, C.; Futema, M.; et al. The UK10K project identifies rare variants in health and disease. Nature 2015, 526, 82–90. [Google Scholar] [CrossRef]
- Mishra, R.; Chesi, A.; Cousminer, D.L.; Hawa, M.I.; Bradfield, J.P.; Hodge, K.M.; Guy, V.C.; Hakonarson, H.; Bone Mineral Density in Childhood Study; Mauricio, D.; et al. Relative contribution of type 1 and type 2 diabetes loci to the genetic etiology of adult-onset, non-insulin-requiring autoimmune diabetes. BMC Med. 2017, 15, 88. [Google Scholar] [CrossRef]
- The SIGMA Type 2 Diabetes Consortium; Estrada, K.; Aukrust, I.; Bjørkhaug, L.; Burtt, N.P.; Mercader, J.M.; García-Ortiz, H.; Huerta-Chagoya, A.; Moreno-Macías, H.; Walford, G.; et al. Association of a low-frequency variant in HNF1A with type 2 diabetes in a Latino population. JAMA 2014, 311, 2305–2314. [Google Scholar] [CrossRef]
- Wang, X.; Sterr, M.; Ansarullah; Burtscher, I.; Böttcher, A.; Beckenbauer, J.; Siehler, J.; Meitinger, T.; Häring, H.-U.; Staiger, H.; et al. Point mutations in the PDX1 transactivation domain impair human β-cell development and function. Mol. Metab. 2019, 24, 80–97. [Google Scholar] [CrossRef] [PubMed]
- Accili, D.; Talchai, S.C.; Kim-Muller, J.Y.; Cinti, F.; Ishida, E.; Ordelheide, A.M.; Kuo, T.; Fan, J.; Son, J. When β-cells fail: Lessons from dedifferentiation. Diabetes Obes. Metab. 2016, 18 (Suppl. 1), 117–122. [Google Scholar] [CrossRef] [PubMed]
- Son, J.; Ding, H.; Farb, T.B.; Efanov, A.M.; Sun, J.; Gore, J.L.; Syed, S.K.; Lei, Z.; Wang, Q.; Accili, D.; et al. BACH2 inhibition reverses β cell failure in type 2 diabetes models. J. Clin. Investig. 2021, 131, e153876. [Google Scholar] [CrossRef] [PubMed]
- Doria, A.; Patti, M.-E.; Kahn, C.R. The emerging genetic architecture of type 2 diabetes. Cell Metab. 2008, 8, 186–200. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, P.; Kyvik, K.O.; Vaag, A.; Beck-Nielsen, H. Heritability of type II (non-insulin-dependent) diabetes mellitus and abnormal glucose tolerance—A population-based twin study. Diabetologia 1999, 42, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Dendup, T.; Feng, X.; Clingan, S.; Astell-Burt, T. Environmental Risk Factors for Developing Type 2 Diabetes Mellitus: A Systematic Review. Int. J. Environ. Res. Public Health 2018, 15, 78. [Google Scholar] [CrossRef]
- Neuenschwander, M.; Ballon, A.; Weber, K.S.; Norat, T.; Aune, D.; Schwingshackl, L.; Schlesinger, S. Role of diet in type 2 diabetes incidence: Umbrella review of meta-analyses of prospective observational studies. BMJ 2019, 366, l2368. [Google Scholar] [CrossRef] [PubMed]
- Beulens, J.W.J.; Pinho, M.G.M.; Abreu, T.C.; den Braver, N.R.; Lam, T.M.; Huss, A.; Vlaanderen, J.; Sonnenschein, T.; Siddiqui, N.Z.; Yuan, Z.; et al. Environmental risk factors of type 2 diabetes—An exposome approach. Diabetologia 2021, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mering, J.; Minkowski, O. Diabetes mellitus nach Pankreasexstirpation. Arch. Exp. Pathol. Pharmakol. 1890, 5–6, 371–387. [Google Scholar] [CrossRef]
- Kleinert, M.; Clemmensen, C.; Hofmann, S.M.; Moore, M.C.; Renner, S.; Woods, S.C.; Huypens, P.; Beckers, J.; de Angelis, M.H.; Schürmann, A.; et al. Animal models of obesity and diabetes mellitus. Nat. Rev. Endocrinol. 2018, 14, 140–162. [Google Scholar] [CrossRef] [PubMed]
- Asahara, S.-I.; Etoh, H.; Inoue, H.; Teruyama, K.; Shibutani, Y.; Ihara, Y.; Kawada, Y.; Bartolomé, A.; Hashimoto, N.; Matsuda, T.; et al. Paternal allelic mutation at the Kcnq1 locus reduces pancreatic \beta-cell mass by epigenetic modification of Cdkn1c. Proc. Natl. Acad. Sci. USA 2015, 112, 8332–8337. [Google Scholar] [CrossRef]
- Walker, E.M.; Cha, J.; Tong, X.; Guo, M.; Liu, J.-H.; Yu, S.; Iacovazzo, D.; Mauvais-Jarvis, F.; Flanagan, S.E.; Korbonits, M.; et al. Sex-biased islet β cell dysfunction is caused by the MODY MAFA S64F variant by inducing premature aging and senescence in males. Cell Rep. 2021, 37, 109813. [Google Scholar] [CrossRef]
- Lloyd, K.C.K.; Robinson, P.N.; MacRae, C.A. Animal-based studies will be essential for precision medicine. Sci. Transl. Med. 2016, 8, 352ed312. [Google Scholar] [CrossRef]
- Skarnes, W.C.; Rosen, B.; West, A.P.; Koutsourakis, M.; Bushell, W.; Iyer, V.; Mujica, A.O.; Thomas, M.; Harrow, J.; Cox, T.; et al. A conditional knockout resource for the genome-wide study of mouse gene function. Nature 2011, 474, 337–342. [Google Scholar] [CrossRef] [PubMed]
- The International Mouse Knockout Consortium; Collins, F.S.; Rossant, J.; Wurst, W. A mouse for all reasons. Cell 2007, 128, 9–13. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, M.H.; Nicholson, G.; Selloum, M.; White, J.; Morgan, H.; Ramirez-Solis, R.; Sorg, T.; Wells, S.; Fuchs, H.; Fray, M.; et al. Analysis of mammalian gene function through broad-based phenotypic screens across a consortium of mouse clinics. Nat. Genet. 2015, 47, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Bennett, B.J.; Farber, C.R.; Orozco, L.; Kang, H.M.; Ghazalpour, A.; Siemers, N.; Neubauer, M.; Neuhaus, I.; Yordanova, R.; Guan, B.; et al. A high-resolution association mapping panel for the dissection of complex traits in mice. Genome Res. 2010, 20, 281–290. [Google Scholar] [CrossRef]
- Lusis, A.J.; Seldin, M.M.; Allayee, H.; Bennett, B.J.; Civelek, M.; Davis, R.C.; Eskin, E.; Farber, C.R.; Hui, S.; Mehrabian, M.; et al. The Hybrid Mouse Diversity Panel: A resource for systems genetics analyses of metabolic and cardiovascular traits. J. Lipid Res. 2016, 57, 925–942. [Google Scholar] [CrossRef]
- Parks, B.W.; Sallam, T.; Mehrabian, M.; Psychogios, N.; Hui, S.T.; Norheim, F.; Castellani, L.W.; Rau, C.D.; Pan, C.; Phun, J.; et al. Genetic architecture of insulin resistance in the mouse. Cell Metab. 2015, 21, 334–347. [Google Scholar] [CrossRef]
- Andreux, P.A.; Williams, E.G.; Koutnikova, H.; Houtkooper, R.H.; Champy, M.-F.; Henry, H.; Schoonjans, K.; Williams, R.W.; Auwerx, J. Systems genetics of metabolism: The use of the BXD murine reference panel for multiscalar integration of traits. Cell 2012, 150, 1287–1299. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Williams, E.G.; Dubuis, S.; Mottis, A.; Jovaisaite, V.; Houten, S.M.; Argmann, C.A.; Faridi, P.; Wolski, W.; Kutalik, Z.; et al. Multilayered genetic and omics dissection of mitochondrial activity in a mouse reference population. Cell 2014, 158, 1415–1430. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Ngo, D.; Psychogios, N.; Dejam, A.; Larson, M.G.; Vasan, R.S.; Ghorbani, A.; O’Sullivan, J.; Cheng, S.; Rhee, E.P.; et al. 2-Aminoadipic acid is a biomarker for diabetes risk. J. Clin. Investig. 2013, 123, 4309–4317. [Google Scholar] [CrossRef] [PubMed]
- Fogarty, M.P.; Cannon, M.E.; Vadlamudi, S.; Gaulton, K.J.; Mohlke, K.L. Identification of a regulatory variant that binds FOXA1 and FOXA2 at the CDC123/CAMK1D type 2 diabetes GWAS locus. PLoS Genet. 2014, 10, e1004633. [Google Scholar] [CrossRef] [PubMed]
- Xue, A.; Wu, Y.; Zhu, Z.; Zhang, F.; Kemper, K.E.; Zheng, Z.; Yengo, L.; Lloyd-Jones, L.R.; Sidorenko, J.; Wu, Y.; et al. Genome-wide association analyses identify 143 risk variants and putative regulatory mechanisms for type 2 diabetes. Nat. Commun. 2018, 9, 2941. [Google Scholar] [CrossRef]
- Wang, C.; Calcutt, M.W.; Ferguson, J.F. Knock-Out of DHTKD1 Alters Mitochondrial Respiration and Function, and May Represent a Novel Pathway in Cardiometabolic Disease Risk. Front. Endocrinol. 2021, 12, 710698. [Google Scholar] [CrossRef]
- Ridgway, W.M.; Peterson, L.B.; Todd, J.A.; Rainbow, D.B.; Healy, B.; Burren, O.S.; Wicker, L.S. Gene–Gene Interactions in the NOD Mouse Model of Type 1 Diabetes. In Advances in Immunology; Academic Press: Cambridge, MA, USA, 2008; Chapter 6; Volume 100, pp. 151–175. [Google Scholar]
- Adeyemo, A.A.; Zaghloul, N.A.; Chen, G.; Doumatey, A.P.; Leitch, C.C.; Hostelley, T.L.; Nesmith, J.E.; Zhou, J.; Bentley, A.R.; Shriner, D.; et al. ZRANB3 is an African-specific type 2 diabetes locus associated with beta-cell mass and insulin response. Nat. Commun. 2019, 10, 3195. [Google Scholar] [CrossRef]
- O’Hare, E.A.; Yerges-Armstrong, L.M.; Perry, J.A.; Shuldiner, A.R.; Zaghloul, N.A. Assignment of Functional Relevance to Genes at Type 2 Diabetes-Associated Loci Through Investigation of β-Cell Mass Deficits. Mol. Endocrinol. 2016, 30, 429–445. [Google Scholar] [CrossRef]
- Peiris, H.; Park, S.; Louis, S.; Gu, X.; Lam, J.Y.; Asplund, O.; Ippolito, G.C.; Bottino, R.; Groop, L.; Tucker, H.; et al. Discovering human diabetes-risk gene function with genetics and physiological assays. Nat. Commun. 2018, 9, 3855. [Google Scholar] [CrossRef]
- Skelin, M.; Rupnik, M.; Cencic, A. Pancreatic beta cell lines and their applications in diabetes mellitus research. ALTEX 2010, 27, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, J.I.; Araki, K.; Yamato, E.; Ikegami, H.; Asano, T.; Shibasaki, Y.; Oka, Y.; Yamamura, K.I. Establishment of a Pancreatic β Cell Line That Retains Glucose-Inducible Insulin Secretion: Special Reference to Expression of Glucose Transporter Isoforms. Endocrinology 1990, 127, 126–132. [Google Scholar] [CrossRef]
- Asfari, M.; Janjic, D.; Meda, P.; Li, G.; Halban, P.A.; Wollheim, C.B. Establishment of 2-mercaptoethanol-dependent differentiated insulin-secreting cell lines. Endocrinology 1992, 130, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Merglen, A.; Theander, S.; Rubi, B.; Chaffard, G.; Wollheim, C.B.; Maechler, P. Glucose sensitivity and metabolism-secretion coupling studied during two-year continuous culture in INS-1E insulinoma cells. Endocrinology 2004, 145, 667–678. [Google Scholar] [CrossRef]
- Burns, S.M.; Vetere, A.; Walpita, D.; Dančík, V.; Khodier, C.; Perez, J.; Clemons, P.A.; Wagner, B.K.; Altshuler, D. High-throughput luminescent reporter of insulin secretion for discovering regulators of pancreatic Beta-cell function. Cell Metab. 2015, 21, 126–137. [Google Scholar] [CrossRef]
- Hill, J.A.; Szabat, M.; Hoesli, C.A.; Gage, B.K.; Yang, Y.H.C.; Williams, D.E.; Riedel, M.J.; Luciani, D.S.; Kalynyak, T.B.; Tsai, K.; et al. A multi-parameter, high-content, high-throughput screening platform to identify natural compounds that modulate insulin and Pdx1 expression. PLoS ONE 2010, 5, e12958. [Google Scholar] [CrossRef] [PubMed]
- Szabat, M.; Modi, H.; Ramracheya, R.; Girbinger, V.; Chan, F.; Lee, J.T.C.; Piske, M.; Kamal, S.; Carol Yang, Y.H.; Welling, A.; et al. High-content screening identifies a role for Na(+) channels in insulin production. R. Soc. Open Sci. 2015, 2, 150306. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.; Isaac, R.; Lavelin, I.; Hart, Y.; Volberg, T.; Shatz-Azoulay, H.; Geiger, B.; Zick, Y. An siRNA screen identifies transmembrane 7 superfamily member 3 (TM7SF3), a seven transmembrane orphan receptor, as an inhibitor of cytokine-induced death of pancreatic beta cells. Diabetologia 2011, 54, 2845–2855. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ku, G.M.; Pappalardo, Z.; Luo, C.C.; German, M.S.; McManus, M.T. An siRNA screen in pancreatic beta cells reveals a role for Gpr27 in insulin production. PLoS Genet. 2012, 8, e1002449. [Google Scholar] [CrossRef]
- Pappalardo, Z.; Gambhir Chopra, D.; Hennings, T.G.; Richards, H.; Choe, J.; Yang, K.; Baeyens, L.; Ang, K.; Chen, S.; Arkin, M.; et al. A Whole-Genome RNA Interference Screen Reveals a Role for Spry2 in Insulin Transcription and the Unfolded Protein Response. Diabetes 2017, 66, 1703–1712. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Weng, C.; Li, H.; Tao, R.; Mai, W.; Liu, X.; Lu, L.; Lai, S.; Duan, Q.; Alvarez, C.; et al. Single-Cell Heterogeneity Analysis and CRISPR Screen Identify Key β-Cell-Specific Disease Genes. Cell Rep. 2019, 26, 3132–3144.e7. [Google Scholar] [CrossRef] [PubMed]
- Cai, E.P.; Ishikawa, Y.; Zhang, W.; Leite, N.C.; Li, J.; Hou, S.; Kiaf, B.; Hollister-Lock, J.; Yilmaz, N.K.; Schiffer, C.A.; et al. Genome-scale in vivo CRISPR screen identifies RNLS as a target for beta cell protection in type 1 diabetes. Nat. Metab. 2020, 2, 934–945. [Google Scholar] [CrossRef]
- Lawlor, N.; Khetan, S.; Ucar, D.; Stitzel, M.L. Genomics of Islet (Dys)function and Type 2 Diabetes. Trends Genet. TIG 2017, 33, 244–255. [Google Scholar] [CrossRef]
- Scharfmann, R.; Staels, W.; Albagli, O. The supply chain of human pancreatic β cell lines. J. Clin. Investig. 2019, 129, 3511–3520. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Hansen, M.E.B.; Lo, Y.; Tishkoff, S.A. Going global by adapting local: A review of recent human adaptation. Science 2016, 354, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Benton, M.L.; Abraham, A.; LaBella, A.L.; Abbot, P.; Rokas, A.; Capra, J.A. The influence of evolutionary history on human health and disease. Nat. Rev. Genet. 2021, 22, 269–283. [Google Scholar] [CrossRef] [PubMed]
- Ravassard, P.; Hazhouz, Y.; Pechberty, S.; Bricout-Neveu, E.; Armanet, M.; Czernichow, P.; Scharfmann, R. A genetically engineered human pancreatic β cell line exhibiting glucose-inducible insulin secretion. J. Clin. Investig. 2011, 121, 3589–3597. [Google Scholar] [CrossRef]
- Scharfmann, R.; Didiesheim, M.; Richards, P.; Chandra, V.; Oshima, M.; Albagli, O. Mass production of functional human pancreatic β-cells: Why and how? Diabetes Obes. Metab. 2016, 18 (Suppl. 1), 128–136. [Google Scholar] [CrossRef]
- Hastoy, B.; Godazgar, M.; Clark, A.; Nylander, V.; Spiliotis, I.; van de Bunt, M.; Chibalina, M.V.; Barrett, A.; Burrows, C.; Tarasov, A.I.; et al. Electrophysiological properties of human beta-cell lines EndoC-βH1 and -βH2 conform with human beta-cells. Sci. Rep. 2018, 8, 16994. [Google Scholar] [CrossRef]
- Lawlor, N.; Marquez, E.J.; Orchard, P.; Narisu, N.; Shamim, M.S.; Thibodeau, A.; Varshney, A.; Kursawe, R.; Erdos, M.R.; Kanke, M.; et al. Multiomic Profiling Identifies cis-Regulatory Networks Underlying Human Pancreatic β Cell Identity and Function. Cell Rep. 2019, 26, 788–801.e6. [Google Scholar] [CrossRef]
- Tsonkova, V.G.; Sand, F.W.; Wolf, X.A.; Grunnet, L.G.; Kirstine Ringgaard, A.; Ingvorsen, C.; Winkel, L.; Kalisz, M.; Dalgaard, K.; Bruun, C.; et al. The EndoC-βH1 cell line is a valid model of human beta cells and applicable for screenings to identify novel drug target candidates. Mol. Metab. 2018, 8, 144–157. [Google Scholar] [CrossRef] [PubMed]
- Grotz, A.K.; Navarro-Guerrero, E.; Bevacqua, R.J.; Baronio, R.; Thomsen, S.K.; Nawaz, S.; Rajesh, V.; Wesolowska-Andersen, A.; Kim, S.K.; Ebner, D.; et al. A genome-wide CRISPR screen identifies regulators of beta cell function involved in type 2 diabetes risk. bioRxiv 2021. [Google Scholar] [CrossRef]
- Benaglio, P.; Zhu, H.; Okino, M.-L.; Yan, J.; Elgamal, R.; Nariai, N.; Beebe, E.; Korgaonkar, K.; Qiu, Y.; Donovan, M.; et al. Type 1 diabetes risk genes mediate pancreatic beta cell survival in response to proinflammatory cytokines. bioRxiv 2021. [Google Scholar] [CrossRef]
- Cardenas-Diaz, F.L.; Leavens, K.F.; Kishore, S.; Osorio-Quintero, C.; Chen, Y.-J.; Stanger, B.Z.; Wang, P.; French, D.; Gadue, P. A Dual Reporter EndoC-βH1 Human β-Cell Line for Efficient Quantification of Calcium Flux and Insulin Secretion. Endocrinology 2020, 161, bqaa005. [Google Scholar] [CrossRef]
- Scharfmann, R.; Pechberty, S.; Hazhouz, Y.; von Bülow, M.; Bricout-Neveu, E.; Grenier-Godard, M.; Guez, F.; Rachdi, L.; Lohmann, M.; Czernichow, P.; et al. Development of a conditionally immortalized human pancreatic β cell line. J. Clin. Investig. 2014, 124, 2087–2098. [Google Scholar] [CrossRef]
- Benazra, M.; Lecomte, M.-J.; Colace, C.; Müller, A.; Machado, C.; Pechberty, S.; Bricout-Neveu, E.; Grenier-Godard, M.; Solimena, M.; Scharfmann, R.; et al. A human beta cell line with drug inducible excision of immortalizing transgenes. Mol. Metab. 2015, 4, 916–925. [Google Scholar] [CrossRef]
- Nano, R.; Kerr-Conte, J.A.; Bosco, D.; Karlsson, M.; Lavallard, V.; Melzi, R.; Gmyr, V.; Mercalli, A.; Berney, T.; Pattou, F.; et al. Islets for Research: Nothing Is Perfect, but We Can Do Better. Diabetes 2019, 68, 1541–1543. [Google Scholar] [CrossRef]
- Morán, I.; Akerman, I.; van de Bunt, M.; Xie, R.; Benazra, M.; Nammo, T.; Arnes, L.; Nakić, N.; García-Hurtado, J.; Rodríguez-Seguí, S.; et al. Human β cell transcriptome analysis uncovers lncRNAs that are tissue-specific, dynamically regulated, and abnormally expressed in type 2 diabetes. Cell Metab. 2012, 16, 435–448. [Google Scholar] [CrossRef]
- Atla, G.; Bonàs-Guarch, S.; Cuenca, M.; Beucher, A.; García-Hurtado, J.; Morán, I.; Irimia, M.; Prasad, R.B.; Gloyn, A.L.; Marselli, L.; et al. Genetic regulation of RNA splicing in human pancreatic islets. bioRxiv 2021. [Google Scholar] [CrossRef]
- Pasquali, L.; Gaulton, K.J.; Rodríguez-Seguí, S.A.; Mularoni, L.; Miguel-Escalada, I.; Akerman, I.; Tena, J.J.; Morán, I.; Gómez-Marín, C.; van de Bunt, M.; et al. Pancreatic islet enhancer clusters enriched in type 2 diabetes risk-associated variants. Nat. Genet. 2014, 46, 136–143. [Google Scholar] [CrossRef]
- Stitzel, M.L.; Sethupathy, P.; Pearson, D.S.; Chines, P.S.; Song, L.; Erdos, M.R.; Welch, R.; Parker, S.C.J.; Boyle, A.P.; Scott, L.J.; et al. Global epigenomic analysis of primary human pancreatic islets provides insights into type 2 diabetes susceptibility loci. Cell Metab. 2010, 12, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Khetan, S.; Kursawe, R.; Youn, A.; Lawlor, N.; Jillette, A.; Marquez, E.J.; Ucar, D.; Stitzel, M.L. Type 2 Diabetes-Associated Genetic Variants Regulate Chromatin Accessibility in Human Islets. Diabetes 2018, 67, 2466–2477. [Google Scholar] [CrossRef] [PubMed]
- Thurner, M.; van de Bunt, M.; Torres, J.M.; Mahajan, A.; Nylander, V.; Bennett, A.J.; Gaulton, K.J.; Barrett, A.; Burrows, C.; Bell, C.G.; et al. Integration of human pancreatic islet genomic data refines regulatory mechanisms at Type 2 Diabetes susceptibility loci. eLife 2018, 7, e31977. [Google Scholar] [CrossRef] [PubMed]
- Miguel-Escalada, I.; Bonàs-Guarch, S.; Cebola, I.; Ponsa-Cobas, J.; Mendieta-Esteban, J.; Atla, G.; Javierre, B.M.; Rolando, D.M.Y.; Farabella, I.; Morgan, C.C.; et al. Human pancreatic islet three-dimensional chromatin architecture provides insights into the genetics of type 2 diabetes. Nature genetics 2019, 51, 1137–1148. [Google Scholar] [CrossRef] [PubMed]
- GTEx Consortium. Genetic effects on gene expression across human tissues. Nature 2017, 550, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Alonso, L.; Piron, A.; Morán, I.; Guindo-Martínez, M.; Bonàs-Guarch, S.; Atla, G.; Miguel-Escalada, I.; Royo, R.; Puiggròs, M.; Garcia-Hurtado, X.; et al. TIGER: The gene expression regulatory variation landscape of human pancreatic islets. Cell Rep. 2021, 37, 109807. [Google Scholar] [CrossRef] [PubMed]
- Viñuela, A.; Varshney, A.; van de Bunt, M.; Prasad, R.B.; Asplund, O.; Bennett, A.; Boehnke, M.; Brown, A.A.; Erdos, M.R.; Fadista, J.; et al. Genetic variant effects on gene expression in human pancreatic islets and their implications for T2D. Nat. Commun. 2020, 11, 4912. [Google Scholar] [CrossRef]
- Van de Bunt, M.; Manning Fox, J.E.; Dai, X.; Barrett, A.; Grey, C.; Li, L.; Bennett, A.J.; Johnson, P.R.; Rajotte, R.V.; Gaulton, K.J.; et al. Transcript Expression Data from Human Islets Links Regulatory Signals from Genome-Wide Association Studies for Type 2 Diabetes and Glycemic Traits to Their Downstream Effectors. PLoS Genet. 2015, 11, e1005694. [Google Scholar] [CrossRef]
- Fadista, J.; Vikman, P.; Laakso, E.O.; Mollet, I.G.; Esguerra, J.L.; Taneera, J.; Storm, P.; Osmark, P.; Ladenvall, C.; Prasad, R.B.; et al. Global genomic and transcriptomic analysis of human pancreatic islets reveals novel genes influencing glucose metabolism. Proc. Natl. Acad. Sci. USA 2014, 111, 13924–13929. [Google Scholar] [CrossRef] [PubMed]
- Baron, M.; Veres, A.; Wolock, S.L.; Faust, A.L.; Gaujoux, R.; Vetere, A.; Ryu, J.H.; Wagner, B.K.; Shen-Orr, S.S.; Klein, A.M.; et al. A Single-Cell Transcriptomic Map of the Human and Mouse Pancreas Reveals Inter- and Intra-cell Population Structure. Cell Syst. 2016, 3, 346–360.e4. [Google Scholar] [CrossRef]
- Li, J.; Klughammer, J.; Farlik, M.; Penz, T.; Spittler, A.; Barbieux, C.; Berishvili, E.; Bock, C.; Kubicek, S. Single-cell transcriptomes reveal characteristic features of human pancreatic islet cell types. EMBO Rep. 2016, 17, 178–187. [Google Scholar] [CrossRef]
- Muraro, M.J.; Dharmadhikari, G.; Grün, D.; Groen, N.; Dielen, T.; Jansen, E.; van Gurp, L.; Engelse, M.A.; Carlotti, F.; de Koning, E.J.P.; et al. A Single-Cell Transcriptome Atlas of the Human Pancreas. Cell Syst. 2016, 3, 385–394.e3. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Schug, J.; Won, K.-J.; Liu, C.; Naji, A.; Avrahami, D.; Golson, M.L.; Kaestner, K.H. Single-Cell Transcriptomics of the Human Endocrine Pancreas. Diabetes 2016, 65, 3028–3038. [Google Scholar] [CrossRef]
- Xin, Y.; Kim, J.; Okamoto, H.; Ni, M.; Wei, Y.; Adler, C.; Murphy, A.J.; Yancopoulos, G.D.; Lin, C.; Gromada, J. RNA Sequencing of Single Human Islet Cells Reveals Type 2 Diabetes Genes. Cell Metab. 2016, 24, 608–615. [Google Scholar] [CrossRef]
- Lawlor, N.; George, J.; Bolisetty, M.; Kursawe, R.; Sun, L.; Sivakamasundari, V.; Kycia, I.; Robson, P.; Stitzel, M.L. Single-cell transcriptomes identify human islet cell signatures and reveal cell-type-specific expression changes in type 2 diabetes. Genome Res. 2017, 27, 208–222. [Google Scholar] [CrossRef] [PubMed]
- Camunas-Soler, J.; Dai, X.-Q.; Hang, Y.; Bautista, A.; Lyon, J.; Suzuki, K.; Kim, S.K.; Quake, S.R.; MacDonald, P.E. Patch-Seq Links Single-Cell Transcriptomes to Human Islet Dysfunction in Diabetes. Cell Metab. 2020, 31, 1017–1031.e4. [Google Scholar] [CrossRef]
- Balboa, D.; Prasad, R.B.; Groop, L.; Otonkoski, T. Genome editing of human pancreatic beta cell models: Problems, possibilities and outlook. Diabetologia 2019, 62, 1329–1336. [Google Scholar] [CrossRef] [PubMed]
- Robitaille, K.; Rourke, J.L.; McBane, J.E.; Fu, A.; Baird, S.; Du, Q.; Kin, T.; Shapiro, A.M.J.; Screaton, R.A. High-throughput Functional Genomics Identifies Regulators of Primary Human Beta Cell Proliferation. J. Biol. Chem. 2016, 291, 4614–4625. [Google Scholar] [CrossRef]
- Wu, J.; Izpisua Belmonte, J.C. Stem Cells: A Renaissance in Human Biology Research. Cell 2016, 165, 1572–1585. [Google Scholar] [CrossRef]
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic stem cell lines derived from human blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Noggle, S.; Fung, H.-L.; Gore, A.; Martinez, H.; Satriani, K.C.; Prosser, R.; Oum, K.; Paull, D.; Druckenmiller, S.; Freeby, M.; et al. Human oocytes reprogram somatic cells to a pluripotent state. Nature 2011, 478, 70–75. [Google Scholar] [CrossRef]
- Wagers, A.J.; Weissman, I.L. Plasticity of adult stem cells. Cell 2004, 116, 639–648. [Google Scholar] [CrossRef]
- D’Amour, K.A.; Agulnick, A.D.; Eliazer, S.; Kelly, O.G.; Kroon, E.; Baetge, E.E. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat. Biotechnol. 2005, 23, 1534–1541. [Google Scholar] [CrossRef] [PubMed]
- D’Amour, K.A.; Bang, A.G.; Eliazer, S.; Kelly, O.G.; Agulnick, A.D.; Smart, N.G.; Moorman, M.A.; Kroon, E.; Carpenter, M.K.; Baetge, E.E. Production of pancreatic hormone–expressing endocrine cells from human embryonic stem cells. Nat. Biotechnol. 2006, 24, 1392–1401. [Google Scholar] [CrossRef] [PubMed]
- Kroon, E.; Martinson, L.A.; Kadoya, K.; Bang, A.G.; Kelly, O.G.; Eliazer, S.; Young, H.; Richardson, M.; Smart, N.G.; Cunningham, J.; et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat. Biotechnol. 2008, 26, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Rezania, A.; Bruin, J.E.; Riedel, M.J.; Mojibian, M.; Asadi, A.; Xu, J.; Gauvin, R.; Narayan, K.; Karanu, F.; O’Neil, J.J.; et al. Maturation of human embryonic stem cell-derived pancreatic progenitors into functional islets capable of treating pre-existing diabetes in mice. Diabetes 2012, 61, 2016–2029. [Google Scholar] [CrossRef] [PubMed]
- Bruin, J.E.; Asadi, A.; Fox, J.K.; Erener, S.; Rezania, A.; Kieffer, T.J. Accelerated Maturation of Human Stem Cell-Derived Pancreatic Progenitor Cells into Insulin-Secreting Cells in Immunodeficient Rats Relative to Mice. Stem Cell Rep. 2015, 5, 1081–1096. [Google Scholar] [CrossRef] [PubMed]
- Ramzy, A.; Thompson, D.M.; Ward-Hartstonge, K.A.; Ivison, S.; Cook, L.; Garcia, R.V.; Loyal, J.; Kim, P.T.W.; Warnock, G.L.; Levings, M.K.; et al. Implanted pluripotent stem-cell-derived pancreatic endoderm cells secrete glucose-responsive C-peptide in patients with type 1 diabetes. Stem Cell 2021, 28, 2047–2061.e5. [Google Scholar] [CrossRef]
- Shapiro, A.M.J.; Thompson, D.; Donner, T.W.; Bellin, M.D.; Hsueh, W.; Pettus, J.; Wilensky, J.; Daniels, M.; Wang, R.M.; Brandon, E.P.; et al. Insulin expression and C-peptide in type 1 diabetes subjects implanted with stem cell-derived pancreatic endoderm cells in an encapsulation device. Cell Rep. Med. 2021, 2, 100466. [Google Scholar] [CrossRef]
- Rezania, A.; Bruin, J.E.; Arora, P.; Rubin, A.; Batushansky, I.; Asadi, A.; O’Dwyer, S.; Quiskamp, N.; Mojibian, M.; Albrecht, T.; et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat. Biotechnol. 2014, 32, 1121–1133. [Google Scholar] [CrossRef]
- Pagliuca, F.W.; Millman, J.R.; Gürtler, M.; Segel, M.; Van Dervort, A.; Ryu, J.H.; Peterson, Q.P.; Greiner, D.; Melton, D.A. Generation of functional human pancreatic β cells in vitro. Cell 2014, 159, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Millman, J.R.; Xie, C.; Van Dervort, A.; Gürtler, M.; Pagliuca, F.W.; Melton, D.A. Generation of stem cell-derived β-cells from patients with type 1 diabetes. Nat. Commun. 2016, 7, 11463. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Russ, H.A.; Wang, X.; Zhang, M.; Ma, T.; Xu, T.; Tang, S.; Hebrok, M.; Ding, S. Human pancreatic beta-like cells converted from fibroblasts. Nat. Commun. 2016, 7, 10080. [Google Scholar] [CrossRef] [PubMed]
- Sui, L.; Danzl, N.; Campbell, S.R.; Viola, R.; Williams, D.; Xing, Y.; Wang, Y.; Phillips, N.; Poffenberger, G.; Johannesson, B.; et al. Beta Cell Replacement in Mice Using Human Type 1 Diabetes Nuclear Transfer Embryonic Stem Cells. Diabetes 2017, 67, 26–35. [Google Scholar] [CrossRef]
- Nair, G.G.; Liu, J.S.; Russ, H.A.; Tran, S.; Saxton, M.S.; Chen, R.; Juang, C.; Li, M.-l.; Nguyen, V.Q.; Giacometti, S.; et al. Recapitulating endocrine cell clustering in culture promotes maturation of human stem-cell-derived β cells. Nat. Cell Biol. 2019, 21, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Velazco-Cruz, L.; Song, J.; Maxwell, K.G.; Goedegebuure, M.M.; Augsornworawat, P.; Hogrebe, N.J.; Millman, J.R. Acquisition of Dynamic Function in Human Stem Cell-Derived β Cells. Stem Cell Rep. 2019, 12, 351–365. [Google Scholar] [CrossRef]
- Grün, D.; Muraro, M.J.; Boisset, J.-C.; Wiebrands, K.; Lyubimova, A.; Dharmadhikari, G.; van den Born, M.; van Es, J.; Jansen, E.; Clevers, H.; et al. De Novo Prediction of Stem Cell Identity using Single-Cell Transcriptome Data. Cell Stem Cell 2016, 19, 266–277. [Google Scholar] [CrossRef]
- Docherty, F.M.; Riemondy, K.A.; Castro-Gutierrez, R.; Dwulet, J.M.; Shilleh, A.H.; Hansen, M.S.; Williams, S.P.M.; Armitage, L.H.; Santostefano, K.E.; Wallet, M.A.; et al. ENTPD3 Marks Mature Stem Cell Derived Beta Cells Formed by Self-Aggregation in Vitro. Diabetes 2021, 70, 2554–2567. [Google Scholar] [CrossRef]
- Veres, A.; Faust, A.L.; Bushnell, H.L.; Engquist, E.N.; Kenty, J.H.-R.; Harb, G.; Poh, Y.-C.; Sintov, E.; Gürtler, M.; Pagliuca, F.W.; et al. Charting cellular identity during human in vitro β-cell differentiation. Nature 2019, 569, 368–373. [Google Scholar] [CrossRef]
- Augsornworawat, P.; Maxwell, K.G.; Velazco-Cruz, L.; Millman, J.R. Single-Cell Transcriptome Profiling Reveals β Cell Maturation in Stem Cell-Derived Islets after Transplantation. Cell Rep. 2020, 32, 108067. [Google Scholar] [CrossRef]
- Balboa, D.; Barsby, T.; Lithovius, V.; Saarimäki-Vire, J.; Omar-Hmeadi, M.; Dyachok, O.; Montaser, H.; Lund, P.-E.; Yang, M.; Ibrahim, H.; et al. Functional, metabolic and transcriptional maturation of stem cell derived beta cells. bioRxiv 2021. [Google Scholar] [CrossRef]
- Micallef, S.J.; Li, X.; Schiesser, J.V.; Hirst, C.E.; Yu, Q.C.; Lim, S.M.; Nostro, M.C.; Elliott, D.A.; Sarangi, F.; Harrison, L.C.; et al. INS(GFP/w) human embryonic stem cells facilitate isolation of in vitro derived insulin-producing cells. Diabetologia 2012, 55, 694–706. [Google Scholar] [CrossRef] [PubMed]
- Gerace, D.; Boulanger, K.R.; Hyoje-Ryu Kenty, J.; Melton, D.A. Generation of a heterozygous GAPDH-Luciferase human ESC line (HVRDe008-A-1) for in vivo monitoring of stem cells and their differentiated progeny. Stem Cell Res. 2021, 53, 102371. [Google Scholar] [CrossRef] [PubMed]
- Ihry, R.J.; Worringer, K.A.; Salick, M.R.; Frias, E.; Ho, D.; Theriault, K.; Kommineni, S.; Chen, J.; Sondey, M.; Ye, C.; et al. p53 inhibits CRISPR–Cas9 engineering in human pluripotent stem cells. Nat. Med. 2018, 24, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Li, Q.V.; Lee, K.; Rosen, B.P.; González, F.; Soh, C.-L.; Huangfu, D. Genome Editing of Lineage Determinants in Human Pluripotent Stem Cells Reveals Mechanisms of Pancreatic Development and Diabetes. Cell Stem Cell 2016, 18, 755–768. [Google Scholar] [CrossRef]
- Ashcroft, F.M.; Rorsman, P. Diabetes mellitus and the β cell: The last ten years. Cell 2012, 148, 1160–1171. [Google Scholar] [CrossRef] [PubMed]
- Docherty, L.E.; Kabwama, S.; Lehmann, A.; Hawke, E.; Harrison, L.; Flanagan, S.E.; Ellard, S.; Hattersley, A.T.; Shield, J.P.H.; Ennis, S.; et al. Clinical presentation of 6q24 transient neonatal diabetes mellitus (6q24 TNDM) and genotype-phenotype correlation in an international cohort of patients. Diabetologia 2013, 56, 758–762. [Google Scholar] [CrossRef] [PubMed]
- Babenko, A.P.; Polak, M.; Cavé, H.; Busiah, K.; Czernichow, P.; Scharfmann, R.; Bryan, J.; Aguilar-Bryan, L.; Vaxillaire, M.; Froguel, P. Activating mutations in the ABCC8 gene in neonatal diabetes mellitus. N. Engl. J. Med. 2006, 355, 456–466. [Google Scholar] [CrossRef]
- Gloyn, A.L.; Pearson, E.R.; Antcliff, J.F.; Proks, P.; Bruining, G.J.; Slingerland, A.S.; Howard, N.; Srinivasan, S.; Silva, J.M.C.L.; Molnes, J.; et al. Activating mutations in the gene encoding the ATP-sensitive potassium-channel subunit Kir6.2 and permanent neonatal diabetes. N. Engl. J. Med. 2004, 350, 1838–1849. [Google Scholar] [CrossRef]
- Støy, J.; Edghill, E.L.; Flanagan, S.E.; Ye, H.; Paz, V.P.; Pluzhnikov, A.; Below, J.E.; Hayes, M.G.; Cox, N.J.; Lipkind, G.M.; et al. Insulin gene mutations as a cause of permanent neonatal diabetes. Proc. Natl. Acad. Sci. USA 2007, 104, 15040–15044. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, M.; Kayo, T.; Ikeda, T.; Koizumi, A. A novel locus, Mody4, distal to D7Mit189 on chromosome 7 determines early-onset NIDDM in nonobese C57BL/6 (Akita) mutant mice. Diabetes 1997, 46, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Herbach, N.; Rathkolb, B.; Kemter, E.; Pichl, L.; Klaften, M.; de Angelis, M.H.; Halban, P.A.; Wolf, E.; Aigner, B.; Wanke, R. Dominant-negative effects of a novel mutated Ins2 allele causes early-onset diabetes and severe beta-cell loss in Munich Ins2C95S mutant mice. Diabetes 2007, 56, 1268–1276. [Google Scholar] [CrossRef] [PubMed]
- Allen, H.L.; Flanagan, S.E.; Shaw-Smith, C.; De Franco, E.; Akerman, I.; Caswell, R.; The International Pancreatic Agenesis Consortium; Ferrer, J.; Hattersley, A.T.; Ellard, S. GATA6 haploinsufficiency causes pancreatic agenesis in humans. Nat. Genet. 2011, 44, 20–22. [Google Scholar] [CrossRef]
- Shaw-Smith, C.; De Franco, E.; Lango Allen, H.; Batlle, M.; Flanagan, S.E.; Borowiec, M.; Taplin, C.E.; van Alfen-van der Velden, J.; Cruz-Rojo, J.; Perez de Nanclares, G.; et al. GATA4 mutations are a cause of neonatal and childhood-onset diabetes. Diabetes 2014, 63, 2888–2894. [Google Scholar] [CrossRef] [PubMed]
- Sellick, G.S.; Barker, K.T.; Stolte-Dijkstra, I.; Fleischmann, C.; Coleman, R.J.; Garrett, C.; Gloyn, A.L.; Edghill, E.L.; Hattersley, A.T.; Wellauer, P.K.; et al. Mutations in PTF1A cause pancreatic and cerebellar agenesis. Nat. Genet. 2004, 36, 1301–1305. [Google Scholar] [CrossRef]
- De Franco, E.; Owens, N.D.L.; Montaser, H.; Wakeling, M.N.; Saarimäki-Vire, J.; Ibrahim, H.; Triantou, A.; Balboa, D.; Caswell, R.C.; Johnson, M.B.; et al. Primate-specific ZNF808 is essential for pancreatic development in humans. medRxiv 2021. [Google Scholar] [CrossRef]
- Kleinberger, J.W.; Copeland, K.C.; Gandica, R.G.; Haymond, M.W.; Levitsky, L.L.; Linder, B.; Shuldiner, A.R.; Tollefsen, S.; White, N.H.; Pollin, T.I. Monogenic diabetes in overweight and obese youth diagnosed with type 2 diabetes: The TODAY clinical trial. Nat. Publ. Group 2018, 20, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Kleinberger, J.W.; Pollin, T.I. Undiagnosed MODY: Time for Action. Curr. Diabetes Rep. 2015, 15, 110. [Google Scholar] [CrossRef]
- Froguel, P.; Vaxillaire, M.; Sun, F.; Velho, G.; Zouali, H.; Butel, M.O.; Lesage, S.; Vionnet, N.; Clément, K.; Fougerousse, F. Close linkage of glucokinase locus on chromosome 7p to early-onset non-insulin-dependent diabetes mellitus. Nature 1992, 356, 162–164. [Google Scholar] [CrossRef]
- Colclough, K.; Bellanne-Chantelot, C.; Saint-Martin, C.; Flanagan, S.E.; Ellard, S. Mutations in the genes encoding the transcription factors hepatocyte nuclear factor 1 alpha and 4 alpha in maturity-onset diabetes of the young and hyperinsulinemic hypoglycemia. Hum. Mutat. 2013, 34, 669–685. [Google Scholar] [CrossRef] [PubMed]
- Bellanne-Chantelot, C.; Carette, C.; Riveline, J.-P.; Valéro, R.; Gautier, J.-F.; Larger, E.; Reznik, Y.; Ducluzeau, P.-H.; Sola, A.; Hartemann-Heurtier, A.; et al. The type and the position of HNF1A mutation modulate age at diagnosis of diabetes in patients with maturity-onset diabetes of the young (MODY)-3. Diabetes 2008, 57, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Bellanne-Chantelot, C.; Chauveau, D.; Gautier, J.-F.; Dubois-Laforgue, D.; Clauin, S.; Beaufils, S.; Wilhelm, J.-M.; Boitard, C.; Noël, L.-H.; Velho, G.; et al. Clinical spectrum associated with hepatocyte nuclear factor-1beta mutations. Ann. Intern. Med. 2004, 140, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Aarthy, R.; Aston-Mourney, K.; Mikocka-Walus, A.; Radha, V.; Amutha, A.; Anjana, R.M.; Unnikrishnan, R.; Mohan, V. Clinical features, complications and treatment of rarer forms of maturity-onset diabetes of the young (MODY)—A review. J. Diabetes Its Complicat. 2021, 35, 107640. [Google Scholar] [CrossRef] [PubMed]
- Philippi, A.; Heller, S.; Costa, I.G.; Senée, V.; Breunig, M.; Li, Z.; Kwon, G.; Russell, R.; Illing, A.; Lin, Q.; et al. Mutations and variants of ONECUT1 in diabetes. Nat. Med. 2021, 27, 1928–1940. [Google Scholar] [CrossRef]
- Urano, F. Wolfram Syndrome: Diagnosis, Management, and Treatment. Curr. Diabetes Rep. 2016, 16, 6. [Google Scholar] [CrossRef]
- Montaser, H.; Patel, K.A.; Balboa, D.; Ibrahim, H.; Lithovius, V.; Näätänen, A.; Chandra, V.; Demir, K.; Acar, S.; Ben-Omran, T.; et al. Loss of MANF Causes Childhood-Onset Syndromic Diabetes Due to Increased Endoplasmic Reticulum Stress. Diabetes 2021, 70, 1006–1018. [Google Scholar] [CrossRef] [PubMed]
- Campuzano, V.; Montermini, L.; Moltò, M.D.; Pianese, L.; Cossée, M.; Cavalcanti, F.; Monros, E.; Rodius, F.; Duclos, F.; Monticelli, A.; et al. Friedreich’s ataxia: Autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science 1996, 271, 1423–1427. [Google Scholar] [CrossRef] [PubMed]
- Delépine, M.; Nicolino, M.; Barrett, T.; Golamaully, M.; Lathrop, G.M.; Julier, C. EIF2AK3, encoding translation initiation factor 2-alpha kinase 3, is mutated in patients with Wolcott-Rallison syndrome. Nat. Genet. 2000, 25, 406–409. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, S.; Sawatani, T.; Van Mulders, A.; De Leu, N.; Heremans, Y.; Heimberg, H.; Cnop, M.; Staels, W. Towards a Functional Cure for Diabetes Using Stem Cell-Derived Beta Cells: Are We There Yet? Cells 2021, 10, 191. [Google Scholar] [CrossRef]
- Kyttälä, A.; Moraghebi, R.; Valensisi, C.; Kettunen, J.; Andrus, C.; Pasumarthy, K.K.; Nakanishi, M.; Nishimura, K.; Ohtaka, M.; Weltner, J.; et al. Genetic Variability Overrides the Impact of Parental Cell Type and Determines iPSC Differentiation Potential. Stem Cell Rep. 2016, 6, 200–212. [Google Scholar] [CrossRef]
- Griscelli, F.; Ezanno, H.; Soubeyrand, M.; Feraud, O.; Oudrhiri, N.; Bonnefond, A.; Turhan, A.G.; Froguel, P.; Bennaceur-Griscelli, A. Generation of an induced pluripotent stem cell (iPSC) line from a patient with maturity-onset diabetes of the young type 3 (MODY3) carrying a hepatocyte nuclear factor 1-alpha (HNF1A) mutation. Stem Cell Res. 2018, 29, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Teo, A.K.K.; Windmueller, R.; Johansson, B.B.; Dirice, E.; Njølstad, P.R.; Tjora, E.; Raeder, H.; Kulkarni, R.N. Derivation of human induced pluripotent stem cells from patients with maturity onset diabetes of the young. J. Biol. Chem. 2013, 288, 5353–5356. [Google Scholar] [CrossRef] [PubMed]
- Yabe, S.G.; Nishida, J.; Fukuda, S.; Takeda, F.; Nasiro, K.; Yasuda, K.; Iwasaki, N.; Okochi, H. Expression of mutant mRNA and protein in pancreatic cells derived from MODY3- iPS cells. PLoS ONE 2019, 14, e0217110. [Google Scholar] [CrossRef]
- González, B.J.; Zhao, H.; Niu, J.; Williams, D.J.; Lee, J.; Goulbourne, C.N.; Xing, Y.; Wang, Y.; Oberholzer, J.; Chen, X.; et al. Human stem cell model of HNF1A deficiency shows uncoupled insulin to C-peptide secretion with accumulation of abnormal insulin granules. bioRxiv 2021. [Google Scholar] [CrossRef]
- Kachamakova-Trojanowska, N.; Stepniewski, J.; Dulak, J. Human iPSCs-Derived Endothelial Cells with Mutation in HNF1A as a Model of Maturity-Onset Diabetes of the Young. Cells 2019, 8, 1440. [Google Scholar] [CrossRef] [PubMed]
- Cardenas-Diaz, F.L.; Osorio-Quintero, C.; Diaz-Miranda, M.A.; Kishore, S.; Leavens, K.; Jobaliya, C.; Stanescu, D.; Ortiz-Gonzalez, X.; Yoon, C.; Chen, C.S.; et al. Modeling Monogenic Diabetes using Human ESCs Reveals Developmental and Metabolic Deficiencies Caused by Mutations in HNF1A. Cell Stem Cell 2019, 25, 273–289.e5. [Google Scholar] [CrossRef] [PubMed]
- Braverman-Gross, C.; Nudel, N.; Ronen, D.; Beer, N.L.; McCarthy, M.I.; Benvenisty, N. Derivation and molecular characterization of pancreatic differentiated MODY1-iPSCs. Stem Cell Res. 2018, 31, 16–26. [Google Scholar] [CrossRef]
- Ng, N.H.J.; Jasmen, J.B.; Lim, C.S.; Lau, H.H.; Krishnan, V.G.; Kadiwala, J.; Kulkarni, R.N.; Raeder, H.; Vallier, L.; Hoon, S.; et al. HNF4A Haploinsufficiency in MODY1 Abrogates Liver and Pancreas Differentiation from Patient-Derived Induced Pluripotent Stem Cells. Iscience 2019, 16, 192–205. [Google Scholar] [CrossRef]
- Vethe, H.; Bjørlykke, Y.; Ghila, L.M.; Paulo, J.A.; Scholz, H.; Gygi, S.P.; Chera, S.; Raeder, H. Probing the missing mature β-cell proteomic landscape in differentiating patient iPSC-derived cells. Sci. Rep. 2017, 7, 4780. [Google Scholar] [CrossRef] [PubMed]
- Haumaitre, C.; Barbacci, E.; Jenny, M.; Ott, M.O.; Gradwohl, G.; Cereghini, S. Lack of TCF2/vHNF1 in mice leads to pancreas agenesis. Proc. Natl. Acad. Sci. USA 2005, 102, 1490–1495. [Google Scholar] [CrossRef] [PubMed]
- Yabe, S.G.; Iwasaki, N.; Yasuda, K.; Hamazaki, T.S.; Konno, M.; Fukuda, S.; Takeda, F.; Kasuga, M.; Okochi, H. Establishment of maturity-onset diabetes of the young-induced pluripotent stem cells from a Japanese patient. J. Diabetes Investig. 2015, 6, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Teo, A.K.K.; Lau, H.H.; Valdez, I.A.; Dirice, E.; Tjora, E.; Raeder, H.; Kulkarni, R.N. Early Developmental Perturbations in a Human Stem Cell Model of MODY5/HNF1B Pancreatic Hypoplasia. Stem Cell Rep. 2016, 6, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, S.; Burtscher, I.; Sterr, M.; Hieronimus, A.; Machicao, F.; Staiger, H.; Häring, H.-U.; Lederer, G.; Meitinger, T.; et al. Generation of a human induced pluripotent stem cell (iPSC) line from a patient carrying a P33T mutation in the PDX1 gene. Stem Cell Res. 2016, 17, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Griscelli, F.; Feraud, O.; Ernault, T.; Oudrihri, N.; Turhan, A.G.; Bonnefond, A.; Froguel, P.; Bennaceur-Griscelli, A. Generation of an induced pluripotent stem cell (iPSC) line from a patient with maturity-onset diabetes of the young type 13 (MODY13) with a the potassium inwardly-rectifying channel, subfamily J, member 11 (KCNJ11) mutation. Stem Cell Res. 2017, 23, 178–181. [Google Scholar] [CrossRef]
- Balboa, D.; Saarimäki-Vire, J.; Borshagovski, D.; Survila, M.; Lindholm, P.; Galli, E.; Eurola, S.; Ustinov, J.; Grym, H.; Huopio, H.; et al. Insulin mutations impair beta-cell development in a patient-derived iPSC model of neonatal diabetes. eLife 2018, 7, e38519. [Google Scholar] [CrossRef]
- Ma, S.; Viola, R.; Sui, L.; Cherubini, V.; Barbetti, F.; Egli, D. β Cell Replacement after Gene Editing of a Neonatal Diabetes-Causing Mutation at the Insulin Locus. Stem Cell Rep. 2018, 11, 1407–1415. [Google Scholar] [CrossRef]
- De Franco, E.; Lytrivi, M.; Ibrahim, H.; Montaser, H.; Wakeling, M.N.; Fantuzzi, F.; Patel, K.; Demarez, C.; Cai, Y.; Igoillo-Esteve, M.; et al. YIPF5 mutations cause neonatal diabetes and microcephaly through endoplasmic reticulum stress. J. Clin. Investig. 2020, 130, 6338–6353. [Google Scholar] [CrossRef]
- Tiyaboonchai, A.; Cardenas-Diaz, F.L.; Ying, L.; Maguire, J.A.; Sim, X.; Jobaliya, C.; Gagne, A.L.; Kishore, S.; Stanescu, D.E.; Hughes, N.; et al. GATA6 Plays an Important Role in the Induction of Human Definitive Endoderm, Development of the Pancreas, and Functionality of Pancreatic β Cells. Stem Cell Rep. 2017, 8, 589–604. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Hua, H.; Foo, K.; Martinez, H.; Watanabe, K.; Zimmer, M.; Kahler, D.J.; Freeby, M.; Chung, W.; LeDuc, C.; et al. β-cell dysfunction due to increased ER stress in a stem cell model of Wolfram syndrome. Diabetes 2014, 63, 923–933. [Google Scholar] [CrossRef]
- Maxwell, K.G.; Augsornworawat, P.; Velazco-Cruz, L.; Kim, M.H.; Asada, R.; Hogrebe, N.J.; Morikawa, S.; Urano, F.; Millman, J.R. Gene-edited human stem cell-derived β cells from a patient with monogenic diabetes reverse preexisting diabetes in mice. Sci. Transl. Med. 2020, 12, eaax9106. [Google Scholar] [CrossRef]
- Cosentino, C.; Toivonen, S.; Diaz Villamil, E.; Atta, M.; Ravanat, J.-L.; Demine, S.; Schiavo, A.A.; Pachera, N.; Deglasse, J.-P.; Jonas, J.-C.; et al. Pancreatic β-cell tRNA hypomethylation and fragmentation link TRMT10A deficiency with diabetes. Nucleic Acids Res. 2018, 46, 10302–10318. [Google Scholar] [CrossRef]
- Elsayed, A.K.; Younis, I.; Ali, G.; Hussain, K.; Abdelalim, E.M. Aberrant development of pancreatic beta cells derived from human iPSCs with FOXA2 deficiency. Cell Death Dis. 2021, 12, 103. [Google Scholar] [CrossRef]
- Saarimäki-Vire, J.; Balboa, D.; Russell, M.A.; Saarikettu, J.; Kinnunen, M.; Keskitalo, S.; Malhi, A.; Valensisi, C.; Andrus, C.; Eurola, S.; et al. An Activating STAT3 Mutation Causes Neonatal Diabetes through Premature Induction of Pancreatic Differentiation. Cell Rep. 2017, 19, 281–294. [Google Scholar] [CrossRef]
- Lithovius, V.; Saarimäki-Vire, J.; Balboa, D.; Ibrahim, H.; Montaser, H.; Barsby, T.; Otonkoski, T. SUR1-mutant iPS cell-derived islets recapitulate the pathophysiology of congenital hyperinsulinism. Diabetologia 2021, 64, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Lorberbaum, D.S.; Docherty, F.M.; Sussel, L. Animal Models of Pancreas Development, Developmental Disorders, and Disease. Adv. Exp. Med. Biol. 2020, 1236, 65–85. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.-D.; Lee, K.; Yang, D.; Amin, S.; Verma, N.; Li, Q.V.; Zhu, Z.; Soh, C.-L.; Kumar, R.; Evans, T.; et al. Genome Editing in hPSCs Reveals GATA6 Haploinsufficiency and a Genetic Interaction with GATA4 in Human Pancreatic Development. Cell Stem Cell 2017, 20, 675–688.e6. [Google Scholar] [CrossRef] [PubMed]
- Romer, A.I.; Singer, R.A.; Sui, L.; Egli, D.; Sussel, L. Murine Perinatal β-Cell Proliferation and the Differentiation of Human Stem Cell-Derived Insulin-Expressing Cells Require NEUROD1. Diabetes 2019, 68, 2259–2271. [Google Scholar] [CrossRef] [PubMed]
- Amin, S.; Cook, B.; Zhou, T.; Ghazizadeh, Z.; Lis, R.; Zhang, T.; Khalaj, M.; Crespo, M.; Perera, M.; Xiang, J.Z.; et al. Discovery of a drug candidate for GLIS3-associated diabetes. Nat. Commun. 2018, 9, 2681. [Google Scholar] [CrossRef] [PubMed]
- Murtaugh, L.C.; Stanger, B.Z.; Kwan, K.M.; Melton, D.A. Notch signaling controls multiple steps of pancreatic differentiation. Proc. Natl. Acad. Sci. USA 2003, 100, 14920–14925. [Google Scholar] [CrossRef]
- Gradwohl, G.; Dierich, A.; LeMeur, M.; Guillemot, F. neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc. Natl. Acad. Sci. USA 2000, 97, 1607–1611. [Google Scholar] [CrossRef]
- Rubio-Cabezas, O.; Jensen, J.N.; Hodgson, M.I.; Codner, E.; Ellard, S.; Serup, P.; Hattersley, A.T. Permanent Neonatal Diabetes and Enteric Anendocrinosis Associated With Biallelic Mutations in NEUROG3. Diabetes 2011, 60, 1349–1353. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Cabezas, O.; Codner, E.; Flanagan, S.E.; Gómez, J.L.; Ellard, S.; Hattersley, A.T. Neurogenin 3 is important but not essential for pancreatic islet development in humans. Diabetologia 2014, 57, 2421–2424. [Google Scholar] [CrossRef] [PubMed]
- McGrath, P.S.; Watson, C.L.; Ingram, C.; Helmrath, M.A.; Wells, J.M. The Basic Helix-Loop-Helix Transcription Factor NEUROG3 Is Required for Development of the Human Endocrine Pancreas. Diabetes 2015, 64, 2497–2505. [Google Scholar] [CrossRef]
- Inagaki, N.; Tsuura, Y.; Namba, N.; Masuda, K.; Gonoi, T.; Horie, M.; Seino, Y.; Mizuta, M.; Seino, S. Cloning and functional characterization of a novel ATP-sensitive potassium channel ubiquitously expressed in rat tissues, including pancreatic islets, pituitary, skeletal muscle, and heart. J. Biol. Chem. 1995, 270, 5691–5694. [Google Scholar] [CrossRef]
- Pearson, E.R.; Flechtner, I.; Njølstad, P.R.; Malecki, M.T.; Flanagan, S.E.; Larkin, B.; Ashcroft, F.M.; Klimes, I.; Codner, E.; Iotova, V.; et al. Switching from insulin to oral sulfonylureas in patients with diabetes due to Kir6.2 mutations. N. Engl. J. Med. 2006, 355, 467–477. [Google Scholar] [CrossRef]
- Mansour Aly, D.; Dwivedi, O.P.; Prasad, R.B.; Karajamaki, A.; Hjort, R.; Thangam, M.; Akerlund, M.; Mahajan, A.; Udler, M.S.; Florez, J.C.; et al. Genome-wide association analyses highlight etiological differences underlying newly defined subtypes of diabetes. Nat. Genet. 2021, 53, 1534–1542. [Google Scholar] [CrossRef]
- Vujkovic, M.; Keaton, J.M.; Lynch, J.A.; Miller, D.R.; Zhou, J.; Tcheandjieu, C.; Huffman, J.E.; Assimes, T.L.; Lorenz, K.; Zhu, X.; et al. Discovery of 318 new risk loci for type 2 diabetes and related vascular outcomes among 1.4 million participants in a multi-ancestry meta-analysis. Nat. Genet. 2020, 52, 680–691. [Google Scholar] [CrossRef]
- Spracklen, C.N.; Horikoshi, M.; Kim, Y.J.; Lin, K.; Bragg, F.; Moon, S.; Suzuki, K.; Tam, C.H.T.; Tabara, Y.; Kwak, S.-H.; et al. Identification of type 2 diabetes loci in 433,540 East Asian individuals. Nature 2020, 582, 240–245. [Google Scholar] [CrossRef]
- Mahajan, A.; Taliun, D.; Thurner, M.; Robertson, N.R.; Torres, J.M.; Rayner, N.W.; Payne, A.J.; Steinthorsdottir, V.; Scott, R.A.; Grarup, N.; et al. Fine-mapping type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenome maps. Nat. Genet. 2018, 50, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Rasheed, A.; Tikkanen, E.; Lee, J.-J.; Butterworth, A.S.; Howson, J.M.M.; Assimes, T.L.; Chowdhury, R.; Orho-Melander, M.; Damrauer, S.; et al. Identification of new susceptibility loci for type 2 diabetes and shared etiological pathways with coronary heart disease. Nat. Genet. 2017, 49, 1450–1457. [Google Scholar] [CrossRef]
- Morris, A.P.; Voight, B.F.; Teslovich, T.M.; Ferreira, T.; Segrè, A.V.; Steinthorsdottir, V.; Strawbridge, R.J.; Khan, H.; Grallert, H.; Mahajan, A.; et al. Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nat. Genet. 2012, 44, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, J.; Langenberg, C.; Prokopenko, I.; Saxena, R.; Soranzo, N.; Jackson, A.U.; Wheeler, E.; Glazer, N.L.; Bouatia-Naji, N.; Gloyn, A.L.; et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat. Genet. 2010, 42, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Voight, B.F.; Scott, L.J.; Steinthorsdottir, V.; Morris, A.P.; Dina, C.; Welch, R.P.; Zeggini, E.; Huth, C.; Aulchenko, Y.S.; Thorleifsson, G.; et al. Twelve type 2 diabetes susceptibility loci identified through large-scale association analysis. Nat. Genet. 2010, 42, 579–589. [Google Scholar] [CrossRef]
- Zeggini, E.; Scott, L.J.; Saxena, R.; Voight, B.F.; Marchini, J.L.; Hu, T.; de Bakker, P.I.W.; Abecasis, G.R.; Almgren, P.; Andersen, G.; et al. Meta-analysis of genome-wide association data and large-scale replication identifies additional susceptibility loci for type 2 diabetes. Nat. Genet. 2008, 40, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.J.; Mohlke, K.L.; Bonnycastle, L.L.; Willer, C.J.; Li, Y.; Duren, W.L.; Erdos, M.R.; Stringham, H.M.; Chines, P.S.; Jackson, A.U.; et al. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science 2007, 316, 1341–1345. [Google Scholar] [CrossRef] [PubMed]
- Sladek, R.; Rocheleau, G.; Rung, J.; Dina, C.; Shen, L.; Serre, D.; Boutin, P.; Vincent, D.; Belisle, A.; Hadjadj, S.; et al. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature 2007, 445, 881–885. [Google Scholar] [CrossRef] [PubMed]
- Downie, C.G.; Dimos, S.F.; Bien, S.A.; Hu, Y.; Darst, B.F.; Polfus, L.M.; Wang, Y.; Wojcik, G.L.; Tao, R.; Raffield, L.M.; et al. Multi-ethnic GWAS and fine-mapping of glycaemic traits identify novel loci in the PAGE Study. Diabetologia 2021, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Huyghe, J.R.; Jackson, A.U.; Fogarty, M.P.; Buchkovich, M.L.; Stančáková, A.; Stringham, H.M.; Sim, X.; Yang, L.; Fuchsberger, C.; Cederberg, H.; et al. Exome array analysis identifies new loci and low-frequency variants influencing insulin processing and secretion. Nat. Genet. 2013, 45, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.; Kullo, I.J. Methods for the selection of tagging SNPs: A comparison of tagging efficiency and performance. Eur. J. Hum. Genet. EJHG 2007, 15, 228–236. [Google Scholar] [CrossRef]
- Slatkin, M. Linkage disequilibrium—Understanding the evolutionary past and mapping the medical future. Nat. Rev. Genet. 2008, 9, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Edwards, S.L.; Beesley, J.; French, J.D.; Dunning, A.M. Beyond GWASs: Illuminating the dark road from association to function. Am. J. Hum. Genet. 2013, 93, 779–797. [Google Scholar] [CrossRef] [PubMed]
- Corradin, O.; Saiakhova, A.; Akhtar-Zaidi, B.; Myeroff, L.; Willis, J.; Cowper-Sal, R.; Lupien, M.; Markowitz, S.; Scacheri, P.C. Combinatorial effects of multiple enhancer variants in linkage disequilibrium dictate levels of gene expression to confer susceptibility to common traits. Genome Res. 2014, 24, 1–13. [Google Scholar] [CrossRef]
- Schaid, D.J.; Chen, W.; Larson, N.B. From genome-wide associations to candidate causal variants by statistical fine-mapping. Nat. Rev. Genet. 2018, 19, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Gloudemans, M.J.; Rao, A.S.; Ingelsson, E.; Montgomery, S.B. Abundant associations with gene expression complicate GWAS follow-up. Nat. Genet. 2019, 51, 768–769. [Google Scholar] [CrossRef] [PubMed]
- Ongen, H.; Brown, A.A.; Delaneau, O.; Panousis, N.I.; Nica, A.C.; GTEx Consortium; Dermitzakis, E.T. Estimating the causal tissues for complex traits and diseases. Nat. Genet. 2017, 49, 1676–1683. [Google Scholar] [CrossRef]
- Mizuno, A.; Okada, Y. Biological characterization of expression quantitative trait loci (eQTLs) showing tissue-specific opposite directional effects. Eur. J. Hum. Genet. EJHG 2019, 27, 1745–1756. [Google Scholar] [CrossRef]
- Fu, J.; Wolfs, M.G.M.; Deelen, P.; Westra, H.-J.; Fehrmann, R.S.N.; Te Meerman, G.J.; Buurman, W.A.; Rensen, S.S.M.; Groen, H.J.M.; Weersma, R.K.; et al. Unraveling the regulatory mechanisms underlying tissue-dependent genetic variation of gene expression. PLoS Genet. 2012, 8, e1002431. [Google Scholar] [CrossRef]
- Porcu, E.; Sadler, M.C.; Lepik, K.; Auwerx, C.; Wood, A.R.; Weihs, A.; Sleiman, M.S.B.; Ribeiro, D.M.; Bandinelli, S.; Tanaka, T.; et al. Differentially expressed genes reflect disease-induced rather than disease-causing changes in the transcriptome. Nat. Commun. 2021, 12, 5647. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, Y.I.; Pritchard, J.K. Trans Effects on Gene Expression Can Drive Omnigenic Inheritance. Cell 2019, 177, 1022–1034.e6. [Google Scholar] [CrossRef]
- Crouch, D.J.M.; Bodmer, W.F. Polygenic inheritance, GWAS, polygenic risk scores, and the search for functional variants. Proc. Natl. Acad. Sci. USA 2020, 117, 18924–18933. [Google Scholar] [CrossRef]
- Udler, M.S.; McCarthy, M.I.; Florez, J.C.; Mahajan, A. Genetic Risk Scores for Diabetes Diagnosis and Precision Medicine. Endocr. Rev. 2019, 40, 1500–1520. [Google Scholar] [CrossRef]
- Ritchie, S.C.; Lambert, S.A.; Arnold, M.; Teo, S.M.; Lim, S.; Scepanovic, P.; Marten, J.; Zahid, S.; Chaffin, M.; Liu, Y.; et al. Integrative analysis of the plasma proteome and polygenic risk of cardiometabolic diseases. Nat. Metab. 2021, 3, 1476–1483. [Google Scholar] [CrossRef]
- Kim, D.S.; Gloyn, A.L.; Knowles, J.W. Genetics of Type 2 Diabetes: Opportunities for Precision Medicine: JACC Focus Seminar. J. Am. Coll. Cardiol. 2021, 78, 496–512. [Google Scholar] [CrossRef]
- Wei, F.-Y.; Suzuki, T.; Watanabe, S.; Kimura, S.; Kaitsuka, T.; Fujimura, A.; Matsui, H.; Atta, M.; Michiue, H.; Fontecave, M.; et al. Deficit of tRNA(Lys) modification by Cdkal1 causes the development of type 2 diabetes in mice. J. Clin. Investig. 2011, 121, 3598–3608. [Google Scholar] [CrossRef]
- Zhou, B.; Wei, F.-Y.; Kanai, N.; Fujimura, A.; Kaitsuka, T.; Tomizawa, K. Identification of a splicing variant that regulates type 2 diabetes risk factor CDKAL1 level by a coding-independent mechanism in human. Hum. Mol. Genet. 2014, 23, 4639–4650. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Guo, M.; Zhou, T.; Tan, L.; Chong, C.N.; Zhang, T.; Dong, X.; Xiang, J.Z.; Yu, A.S.; Yue, L.; et al. An Isogenic Human ESC Platform for Functional Evaluation of Genome-wide-Association-Study-Identified Diabetes Genes and Drug Discovery. Cell Stem Cell 2016, 19, 326–340. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Zhang, T.; Dong, X.; Xiang, J.Z.; Lei, M.; Evans, T.; Graumann, J.; Chen, S. Using hESCs to Probe the Interaction of the Diabetes-Associated Genes CDKAL1 and MT1E. Cell Rep. 2017, 19, 1512–1521. [Google Scholar] [CrossRef]
- Thomas, P.; Ye, Y.; Lightner, E. Mutation of the pancreatic islet inward rectifier Kir6.2 also leads to familial persistent hyperinsulinemic hypoglycemia of infancy. Hum. Mol. Genet. 1996, 5, 1809–1812. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, Y.; Inoue, H.; Keshavarz, P.; Miyawaki, K.; Yamaguchi, Y.; Moritani, M.; Kunika, K.; Nakamura, N.; Yoshikawa, T.; Yasui, N.; et al. SNPs in the KCNJ11-ABCC8 gene locus are associated with type 2 diabetes and blood pressure levels in the Japanese population. J. Hum. Genet. 2007, 52, 781–793. [Google Scholar] [CrossRef]
- Haghvirdizadeh, P.; Mohamed, Z.; Abdullah, N.A.; Haghvirdizadeh, P.; Haerian, M.S.; Haerian, B.S. KCNJ11: Genetic Polymorphisms and Risk of Diabetes Mellitus. J. Diabetes Res. 2015, 2015, 908152. [Google Scholar] [CrossRef] [PubMed]
- Remedi, M.S.; Rocheleau, J.V.; Tong, A.; Patton, B.L.; McDaniel, M.L.; Piston, D.W.; Koster, J.C.; Nichols, C.G. Hyperinsulinism in mice with heterozygous loss of K(ATP) channels. Diabetologia 2006, 49, 2368–2378. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, Y.-H.; Xu, S.-J.; Bendahhou, S.; Wang, X.-L.; Wang, Y.; Xu, W.-Y.; Jin, H.-W.; Sun, H.; Su, X.-Y.; Zhuang, Q.-N.; et al. KCNQ1 gain-of-function mutation in familial atrial fibrillation. Science 2003, 299, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Abbott, G.W. Biology of the KCNQ1 potassium channel. New J. Sci. 2014, 2014, 237431. [Google Scholar] [CrossRef]
- Unoki, H.; Takahashi, A.; Kawaguchi, T.; Hara, K.; Horikoshi, M.; Andersen, G.; Ng, D.P.K.; Holmkvist, J.; Borch-Johnsen, K.; Jørgensen, T.; et al. SNPs in KCNQ1 are associated with susceptibility to type 2 diabetes in East Asian and European populations. Nat. Genet. 2008, 40, 1098–1102. [Google Scholar] [CrossRef]
- Yu, X.-X.; Liao, M.-Q.; Zeng, Y.-F.; Gao, X.-P.; Liu, Y.-H.; Sun, W.; Zhu, S.; Zeng, F.-F.; Ye, Y.-B. Associations of KCNQ1 Polymorphisms with the Risk of Type 2 Diabetes Mellitus: An Updated Meta-Analysis with Trial Sequential Analysis. J. Diabetes Res. 2020, 2020, 7145139. [Google Scholar] [CrossRef]
- Kong, A.; Steinthorsdottir, V.; Masson, G.; Thorleifsson, G.; Sulem, P.; Besenbacher, S.; Jonasdottir, A.; Sigurdsson, A.; Kristinsson, K.T.; Jonasdottir, A.; et al. Parental origin of sequence variants associated with complex diseases. Nature 2009, 462, 868–874. [Google Scholar] [CrossRef]
- Zhou, Z.; Gong, M.; Pande, A.; Lisewski, U.; Röpke, T.; Purfürst, B.; Liang, L.; Jia, S.; Frühler, S.; Margineanu, A.; et al. A missense KCNQ1 Mutation Impairs Insulin Secretion in Neonatal Diabetes. bioRxiv 2021. [Google Scholar] [CrossRef]
- Chiou, J.; Zeng, C.; Cheng, Z.; Han, J.Y.; Schlichting, M.; Miller, M.; Mendez, R.; Huang, S.; Wang, J.; Sui, Y.; et al. Single-cell chromatin accessibility identifies pancreatic islet cell type- and state-specific regulatory programs of diabetes risk. Nat. Genet. 2021, 53, 455–466. [Google Scholar] [CrossRef]
- Chimienti, F.; Devergnas, S.; Favier, A.; Seve, M. Identification and cloning of a beta-cell-specific zinc transporter, ZnT-8, localized into insulin secretory granules. Diabetes 2004, 53, 2330–2337. [Google Scholar] [CrossRef]
- Flannick, J.; Thorleifsson, G.; Beer, N.L.; Jacobs, S.B.R.; Grarup, N.; Burtt, N.P.; Mahajan, A.; Fuchsberger, C.; Atzmon, G.; Benediktsson, R.; et al. Loss-of-function mutations in SLC30A8 protect against type 2 diabetes. Nat. Genet. 2014, 46, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Lemaire, K.; Ravier, M.A.; Schraenen, A.; Creemers, J.W.M.; Van de Plas, R.; Granvik, M.; Van Lommel, L.; Waelkens, E.; Chimienti, F.; Rutter, G.A.; et al. Insulin crystallization depends on zinc transporter ZnT8 expression, but is not required for normal glucose homeostasis in mice. Proc. Natl. Acad. Sci. USA 2009, 106, 14872–14877. [Google Scholar] [CrossRef] [PubMed]
- Nicolson, T.J.; Bellomo, E.A.; Wijesekara, N.; Loder, M.K.; Baldwin, J.M.; Gyulkhandanyan, A.V.; Koshkin, V.; Tarasov, A.I.; Carzaniga, R.; Kronenberger, K.; et al. Insulin storage and glucose homeostasis in mice null for the granule zinc transporter ZnT8 and studies of the type 2 diabetes-associated variants. Diabetes 2009, 58, 2070–2083. [Google Scholar] [CrossRef]
- Syring, K.E.; Bosma, K.J.; Goleva, S.B.; Singh, K.; Oeser, J.K.; Lopez, C.A.; Skaar, E.P.; McGuinness, O.P.; Davis, L.K.; Powell, D.R.; et al. Potential positive and negative consequences of ZnT8 inhibition. J. Endocrinol. 2020, 246, 189–205. [Google Scholar] [CrossRef]
- Dwivedi, O.P.; Lehtovirta, M.; Hastoy, B.; Chandra, V.; Krentz, N.A.J.; Kleiner, S.; Jain, D.; Richard, A.-M.; Abaitua, F.; Beer, N.L.; et al. Loss of ZnT8 function protects against diabetes by enhanced insulin secretion. Nat. Genet. 2019, 51, 1596–1606. [Google Scholar] [CrossRef]
- Li, W.; Ma, Q.; Xiao, Y.-N.; Li, S.; Wang, M.; Yang, Z.; Xiao, T.; Xu, M.; Zhang, T.; Hu, R.; et al. ZnT8 Loss-of-Function Accelerates Functional Maturation of hESC-Derived β Cells and Resists Metabolic Stress Induced Cell Death in Diabetes. Nat. Portf. 2021. [Google Scholar] [CrossRef]
- Jin, T.; Liu, L. The Wnt signaling pathway effector TCF7L2 and type 2 diabetes mellitus. Mol. Endocrinol. 2008, 22, 2383–2392. [Google Scholar] [CrossRef] [PubMed]
- Del Bosque-Plata, L.; Martínez-Martínez, E.; Espinoza-Camacho, M.Á.; Gragnoli, C. The Role of TCF7L2 in Type 2 Diabetes. Diabetes 2021, 70, 1220–1228. [Google Scholar] [CrossRef] [PubMed]
- Gaulton, K.J.; Nammo, T.; Pasquali, L.; Simon, J.M.; Giresi, P.G.; Fogarty, M.P.; Panhuis, T.M.; Mieczkowski, P.; Secchi, A.; Bosco, D.; et al. A map of open chromatin in human pancreatic islets. Nat. Genet. 2010, 42, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Lyssenko, V.; Lupi, R.; Marchetti, P.; Del Guerra, S.; Orho-Melander, M.; Almgren, P.; Sjögren, M.; Ling, C.; Eriksson, K.-F.; Lethagen, A.-L.; et al. Mechanisms by which common variants in the TCF7L2 gene increase risk of type 2 diabetes. J. Clin. Investig. 2007, 117, 2155–2163. [Google Scholar] [CrossRef]
- Da Silva Xavier, G.; Loder, M.K.; McDonald, A.; Tarasov, A.I.; Carzaniga, R.; Kronenberger, K.; Barg, S.; Rutter, G.A. TCF7L2 regulates late events in insulin secretion from pancreatic islet beta-cells. Diabetes 2009, 58, 894–905. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.K.; Mondragon, A.; Chen, L.; McGinty, J.A.; French, P.M.; Ferrer, J.; Thorens, B.; Hodson, D.J.; Rutter, G.A.; da Silva Xavier, G. Selective disruption of Tcf7l2 in the pancreatic β cell impairs secretory function and lowers β cell mass. Hum. Mol. Genet. 2015, 24, 1390–1399. [Google Scholar] [CrossRef] [PubMed]
- Bailey, K.A.; Savic, D.; Zielinski, M.; Park, S.-Y.; Wang, L.-J.; Witkowski, P.; Brady, M.; Hara, M.; Bell, G.I.; Nobrega, M.A. Evidence of non-pancreatic beta cell-dependent roles of Tcf7l2 in the regulation of glucose metabolism in mice. Hum. Mol. Genet. 2015, 24, 1646–1654. [Google Scholar] [CrossRef] [PubMed]
- Weng, C.; Xi, J.; Li, H.; Cui, J.; Gu, A.; Lai, S.; Leskov, K.; Ke, L.; Jin, F.; Li, Y. Single-cell lineage analysis reveals extensive multimodal transcriptional control during directed beta-cell differentiation. Nat. Metab. 2020, 2, 1443–1458. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, J.P.; D’Antonio-Chronowska, A.; Fujita, K.; Salgado, B.M.; Matsui, H.; Arthur, T.D.; iPSCORE Consortium; Donovan, M.K.R.; D’Antonio, M.; Frazer, K.A. Regulatory variants active in iPSC-derived pancreatic progenitor cells are associated with Type 2 Diabetes in adults. bioRxiv 2021. [Google Scholar] [CrossRef]
- Geusz, R.J.; Wang, A.; Chiou, J.; Lancman, J.J.; Wetton, N.; Kefalopoulou, S.; Wang, J.; Qiu, Y.; Yan, J.; Aylward, A.; et al. Pancreatic progenitor epigenome maps prioritize type 2 diabetes risk genes with roles in development. eLife 2021, 10, e59067. [Google Scholar] [CrossRef]
- Heller, S.; Li, Z.; Lin, Q.; Geusz, R.; Breunig, M.; Hohwieler, M.; Zhang, X.; Nair, G.G.; Seufferlein, T.; Hebrok, M.; et al. Transcriptional changes and the role of ONECUT1 in hPSC pancreatic differentiation. Commun. Biol. 2021, 4, 1298. [Google Scholar] [CrossRef]
- Geusz, R.J.; Wang, A.; Lam, D.K.; Vinckier, N.K.; Alysandratos, K.-D.; Roberts, D.A.; Wang, J.; Kefalopoulou, S.; Ramirez, A.; Qiu, Y.; et al. Sequence logic at enhancers governs a dual mechanism of endodermal organ fate induction by FOXA pioneer factors. Nat. Commun. 2021, 12, 6636. [Google Scholar] [CrossRef]
- Lee, K.; Cho, H.; Rickert, R.W.; Li, Q.V.; Pulecio, J.; Leslie, C.S.; Huangfu, D. FOXA2 Is Required for Enhancer Priming during Pancreatic Differentiation. Cell Rep. 2019, 28, 382–393.e7. [Google Scholar] [CrossRef]
- Redondo, M.J.; Steck, A.K.; Pugliese, A. Genetics of type 1 diabetes. Pediatric Diabetes 2018, 19, 346–353. [Google Scholar] [CrossRef]
- Onengut-Gumuscu, S.; Chen, W.-M.; Burren, O.; Cooper, N.J.; Quinlan, A.R.; Mychaleckyj, J.C.; Farber, E.; Bonnie, J.K.; Szpak, M.; Schofield, E.; et al. Fine mapping of type 1 diabetes susceptibility loci and evidence for colocalization of causal variants with lymphoid gene enhancers. Nat. Genet. 2015, 47, 381–386. [Google Scholar] [CrossRef]
- Barrett, J.C.; Clayton, D.G.; Concannon, P.; Akolkar, B.; Cooper, J.D.; Erlich, H.A.; Julier, C.; Morahan, G.; Nerup, J.; Nierras, C.; et al. Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat. Genet. 2009, 41, 703–707. [Google Scholar] [CrossRef]
- Bradfield, J.P.; Qu, H.-Q.; Wang, K.; Zhang, H.; Sleiman, P.M.; Kim, C.E.; Mentch, F.D.; Qiu, H.; Glessner, J.T.; Thomas, K.A.; et al. A genome-wide meta-analysis of six type 1 diabetes cohorts identifies multiple associated loci. PLoS Genet. 2011, 7, e1002293. [Google Scholar] [CrossRef]
- Chiou, J.; Geusz, R.J.; Okino, M.-L.; Han, J.Y.; Miller, M.; Melton, R.; Beebe, E.; Benaglio, P.; Huang, S.; Korgaonkar, K.; et al. Interpreting type 1 diabetes risk with genetics and single-cell epigenomics. Nature 2021, 594, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Noble, J.A.; Valdes, A.M. Genetics of the HLA region in the prediction of type 1 diabetes. Curr. Diabetes Rep. 2011, 11, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Chelala, C.; Duchatelet, S.; Joffret, M.-L.; Bergholdt, R.; Dubois-Laforgue, D.; Ghandil, P.; Pociot, F.; Caillat-Zucman, S.; Timsit, J.; Julier, C. PTPN22 R620W functional variant in type 1 diabetes and autoimmunity related traits. Diabetes 2007, 56, 522–526. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ge, Y.; Onengut-Gumuscu, S.; Quinlan, A.R.; Mackey, A.J.; Wright, J.A.; Buckner, J.H.; Habib, T.; Rich, S.S.; Concannon, P. Targeted Deep Sequencing in Multiple-Affected Sibships of European Ancestry Identifies Rare Deleterious Variants in PTPN22 That Confer Risk for Type 1 Diabetes. Diabetes 2016, 65, 794–802. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nejentsev, S.; Walker, N.; Riches, D.; Egholm, M.; Todd, J.A. Rare variants of IFIH1, a gene implicated in antiviral responses, protect against type 1 diabetes. Science 2009, 324, 387–389. [Google Scholar] [CrossRef] [PubMed]
- Wallet, M.A.; Santostefano, K.E.; Terada, N.; Brusko, T.M. Isogenic Cellular Systems Model the Impact of Genetic Risk Variants in the Pathogenesis of Type 1 Diabetes. Front. Endocrinol. 2017, 8, 276. [Google Scholar] [CrossRef]
- Eizirik, D.L.; Szymczak, F.; Alvelos, M.I.; Martin, F. From Pancreatic β-Cell Gene Networks to Novel Therapies for Type 1 Diabetes. Diabetes 2021, 70, 1915–1925. [Google Scholar] [CrossRef]
- Joshi, K.; Cameron, F.; Tiwari, S.; Mannering, S.I.; Elefanty, A.G.; Stanley, E.G. Modeling Type 1 Diabetes Using Pluripotent Stem Cell Technology. Front. Endocrinol. 2021, 12, 635662. [Google Scholar] [CrossRef]
- Demine, S.; Schiavo, A.A.; Marín-Cañas, S.; Marchetti, P.; Cnop, M.; Eizirik, D.L. Pro-inflammatory cytokines induce cell death, inflammatory responses, and endoplasmic reticulum stress in human iPSC-derived beta cells. Stem Cell Res. Ther. 2019, 11, 7. [Google Scholar] [CrossRef]
- Hosokawa, Y.; Toyoda, T.; Fukui, K.; Baden, M.Y.; Funato, M.; Kondo, Y.; Sudo, T.; Iwahashi, H.; Kishida, M.; Okada, C.; et al. Insulin-producing cells derived from ‘induced pluripotent stem cells’ of patients with fulminant type 1 diabetes: Vulnerability to cytokine insults and increased expression of apoptosis-related genes. J. Diabetes Investig. 2017, 9, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Joshi, K.; Elso, C.; Motazedian, A.; Labonne, T.; Schiesser, J.V.; Cameron, F.; Mannering, S.I.; Elefanty, A.G.; Stanley, E.G. Induced pluripotent stem cell macrophages present antigen to proinsulin-specific T cell receptors from donor-matched islet-infiltrating T cells in type 1 diabetes. Diabetologia 2019, 62, 2245–2251. [Google Scholar] [CrossRef]
- Leite, N.C.; Sintov, E.; Meissner, T.B.; Brehm, M.A.; Greiner, D.L.; Harlan, D.M.; Melton, D.A. Modeling Type 1 Diabetes In Vitro Using Human Pluripotent Stem Cells. Cell Rep. 2020, 32, 107894. [Google Scholar] [CrossRef]
- Armitage, L.H.; Stimpson, S.E.; Santostefano, K.E.; Sui, L.; Ogundare, S.; Newby, B.N.; Castro-Gutierrez, R.; Huber, M.K.; Taylor, J.P.; Sharma, P.; et al. Use of Induced Pluripotent Stem Cells to Build Isogenic Systems and Investigate Type 1 Diabetes. Front. Endocrinol. 2021, 12, 737276. [Google Scholar] [CrossRef]
- Maxwell, K.G.; Millman, J.R. Applications of iPSC-derived beta cells from patients with diabetes. Cell Rep. Med. 2021, 2, 100238. [Google Scholar] [CrossRef]
- Turner, M.; Leslie, S.; Martin, N.G.; Peschanski, M.; Rao, M.; Taylor, C.J.; Trounson, A.; Turner, D.; Yamanaka, S.; Wilmut, I. Toward the development of a global induced pluripotent stem cell library. Cell Stem Cell 2013, 13, 382–384. [Google Scholar] [CrossRef] [PubMed]
- Deuse, T.; Hu, X.; Agbor-Enoh, S.; Koch, M.; Spitzer, M.H.; Gravina, A.; Alawi, M.; Marishta, A.; Peters, B.; Kosaloglu-Yalcin, Z.; et al. De novo mutations in mitochondrial DNA of iPSCs produce immunogenic neoepitopes in mice and humans. Nat. Biotechnol. 2019, 37, 1137–1144. [Google Scholar] [CrossRef]
- Yoshihara, E.; O’Connor, C.; Gasser, E.; Wei, Z.; Oh, T.G.; Tseng, T.W.; Wang, D.; Cayabyab, F.; Dai, Y.; Yu, R.T.; et al. Immune-evasive human islet-like organoids ameliorate diabetes. Nature 2020, 586, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.A.; Kettunen, J.; Laakso, M.; Stančáková, A.; Laver, T.W.; Colclough, K.; Johnson, M.B.; Abramowicz, M.; Groop, L.; Miettinen, P.J.; et al. Heterozygous RFX6 protein truncating variants are associated with MODY with reduced penetrance. Nat. Commun. 2017, 8, 888. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Cabezas, O.; Díaz González, F.; Aragonés, A.; Argente, J.; Campos-Barros, A. Permanent neonatal diabetes caused by a homozygous nonsense mutation in the glucokinase gene. Pediatric Diabetes 2008, 9, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, A.; Wessel, J.; Willems, S.M.; Zhao, W.; Robertson, N.R.; Chu, A.Y.; Gan, W.; Kitajima, H.; Taliun, D.; Rayner, N.W.; et al. Refining the accuracy of validated target identification through coding variant fine-mapping in type 2 diabetes. Nat. Genet. 2018, 50, 559–571. [Google Scholar] [CrossRef] [PubMed]
- Flannick, J. The Contribution of Low-Frequency and Rare Coding Variation to Susceptibility to Type 2 Diabetes. Curr. Diabetes Rep. 2019, 19, 25. [Google Scholar] [CrossRef] [PubMed]
- Gusev, A.; Lee, S.H.; Trynka, G.; Finucane, H.; Vilhjálmsson, B.J.; Xu, H.; Zang, C.; Ripke, S.; Bulik-Sullivan, B.; Stahl, E.; et al. Partitioning heritability of regulatory and cell-type-specific variants across 11 common diseases. Am. J. Hum. Genet. 2014, 95, 535–552. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Lupski, J.R. Non-coding genetic variants in human disease. Hum. Mol. Genet. 2015, 24, R102–R110. [Google Scholar] [CrossRef]
- Gasperíková, D.; Tribble, N.D.; Staník, J.; Hucková, M.; Misovicová, N.; van de Bunt, M.; Valentínová, L.; Barrow, B.A.; Barák, L.; Dobránsky, R.; et al. Identification of a novel beta-cell glucokinase (GCK) promoter mutation (-71G>C) that modulates GCK gene expression through loss of allele-specific Sp1 binding causing mild fasting hyperglycemia in humans. Diabetes 2009, 58, 1929–1935. [Google Scholar] [CrossRef]
- Kishore, S.; De Franco, E.; Cardenas-Diaz, F.L.; Letourneau-Freiberg, L.R.; Sanyoura, M.; Osorio-Quintero, C.; French, D.L.; Greeley, S.A.W.; Hattersley, A.T.; Gadue, P. A Non-Coding Disease Modifier of Pancreatic Agenesis Identified by Genetic Correction in a Patient-Derived iPSC Line. Cell Stem Cell 2020, 27, 137–146.e6. [Google Scholar] [CrossRef]
- Weedon, M.N.; Cebola, I.; Patch, A.-M.; Flanagan, S.E.; De Franco, E.; Caswell, R.; Rodríguez-Seguí, S.A.; Shaw-Smith, C.; Cho, C.H.-H.; Allen, H.L.; et al. Recessive mutations in a distal PTF1A enhancer cause isolated pancreatic agenesis. Nat. Genet. 2014, 46, 61–64. [Google Scholar] [CrossRef]
- Stitzel, M.L.; Kycia, I.; Kursawe, R.; Ucar, D. Transcriptional Regulation of the Pancreatic Islet: Implications for Islet Function. Curr. Diabetes Rep. 2015, 15, 66. [Google Scholar] [CrossRef][Green Version]
- Zhang, K.; Hocker, J.D.; Miller, M.; Hou, X.; Chiou, J.; Poirion, O.B.; Qiu, Y.; Li, Y.E.; Gaulton, K.J.; Wang, A.; et al. A single-cell atlas of chromatin accessibility in the human genome. Cell 2021, 184, 5985–6001.e19. [Google Scholar] [CrossRef]
- Varshney, A.; VanRenterghem, H.; Orchard, P.; Boyle, A.P.; Stitzel, M.L.; Ucar, D.; Parker, S.C.J. Cell Specificity of Human Regulatory Annotations and Their Genetic Effects on Gene Expression. Genetics 2019, 211, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Gragnoli, C.; Lindner, T.; Cockburn, B.N.; Kaisaki, P.J.; Gragnoli, F.; Marozzi, G.; Bell, G.I. Maturity-onset diabetes of the young due to a mutation in the hepatocyte nuclear factor-4 alpha binding site in the promoter of the hepatocyte nuclear factor-1 alpha gene. Diabetes 1997, 46, 1648–1651. [Google Scholar] [CrossRef] [PubMed]
- Yoshiuchi, I.; Yamagata, K.; Yang, Q.; Iwahashi, H.; Okita, K.; Yamamoto, K.; Oue, T.; Imagawa, A.; Hamaguchi, T.; Yamasaki, T.; et al. Three new mutations in the hepatocyte nuclear factor-1alpha gene in Japanese subjects with diabetes mellitus: Clinical features and functional characterization. Diabetologia 1999, 42, 621–626. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Godart, F.; Bellanné-Chantelot, C.; Clauin, S.; Gragnoli, C.; Abderrahmani, A.; Blanché, H.; Boutin, P.; Chèvre, J.C.; Froguel, P.; Bailleul, B. Identification of seven novel nucleotide variants in the hepatocyte nuclear factor-1alpha (TCF1) promoter region in MODY patients. Hum. Mutat. 2000, 15, 173–180. [Google Scholar] [CrossRef]
- Radha, V.; Ek, J.; Anuradha, S.; Hansen, T.; Pedersen, O.; Mohan, V. Identification of novel variants in the hepatocyte nuclear factor-1alpha gene in South Indian patients with maturity onset diabetes of young. J. Clin. Endocrinol. Metab. 2009, 94, 1959–1965. [Google Scholar] [CrossRef]
- Wirsing, A.; Johnstone, K.A.; Harries, L.W.; Ellard, S.; Ryffel, G.U.; Stanik, J.; Gasperikova, D.; Klimes, I.; Murphy, R. Novel monogenic diabetes mutations in the P2 promoter of the HNF4A gene are associated with impaired function in vitro. Diabet. Med. J. Br. Diabet. Assoc. 2010, 27, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Galán, M.; García-Herrero, C.-M.; Azriel, S.; Gargallo, M.; Durán, M.; Gorgojo, J.-J.; Andía, V.-M.; Navas, M.-A. Differential effects of HNF-1α mutations associated with familial young-onset diabetes on target gene regulation. Mol. Med. 2011, 17, 256–265. [Google Scholar] [CrossRef]
- Komazec, J.; Ristivojevic, B.; Zukic, B.; Zdravkovic, V.; Karan-Djurasevic, T.; Pavlovic, S.; Ugrin, M. Analysis of the promoter regions of disease-causing genes in maturity-onset diabetes of the young patients. Mol. Biol. Rep. 2020, 47, 6759–6768. [Google Scholar] [CrossRef]
- Garin, I.; Edghill, E.L.; Akerman, I.; Rubio-Cabezas, O.; Rica, I.; Locke, J.M.; Maestro, M.A.; Alshaikh, A.; Bundak, R.; del Castillo, G.; et al. Recessive mutations in the INS gene result in neonatal diabetes through reduced insulin biosynthesis. Proc. Natl. Acad. Sci. USA 2010, 107, 3105–3110. [Google Scholar] [CrossRef]
- Akerman, I.; Maestro, M.A.; De Franco, E.; Grau, V.; Flanagan, S.; García-Hurtado, J.; Mittler, G.; Ravassard, P.; Piemonti, L.; Ellard, S.; et al. Neonatal diabetes mutations disrupt a chromatin pioneering function that activates the human insulin gene. Cell Rep. 2021, 35, 108981. [Google Scholar] [CrossRef] [PubMed]
- Kulzer, J.R.; Stitzel, M.L.; Morken, M.A.; Huyghe, J.R.; Fuchsberger, C.; Kuusisto, J.; Laakso, M.; Boehnke, M.; Collins, F.S.; Mohlke, K.L. A common functional regulatory variant at a type 2 diabetes locus upregulates ARAP1 expression in the pancreatic beta cell. Am. J. Hum. Genet. 2014, 94, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Bouatia-Naji, N.; Bonnefond, A.; Baerenwald, D.A.; Marchand, M.; Bugliani, M.; Marchetti, P.; Pattou, F.; Printz, R.L.; Flemming, B.P.; Umunakwe, O.C.; et al. Genetic and functional assessment of the role of the rs13431652-A and rs573225-A alleles in the G6PC2 promoter that are strongly associated with elevated fasting glucose levels. Diabetes 2010, 59, 2662–2671. [Google Scholar] [CrossRef]
- Baerenwald, D.A.; Bonnefond, A.; Bouatia-Naji, N.; Flemming, B.P.; Umunakwe, O.C.; Oeser, J.K.; Pound, L.D.; Conley, N.L.; Cauchi, S.; Lobbens, S.; et al. Multiple functional polymorphisms in the G6PC2 gene contribute to the association with higher fasting plasma glucose levels. Diabetologia 2013, 56, 1306–1316. [Google Scholar] [CrossRef]
- Fløyel, T.; Brorsson, C.; Nielsen, L.B.; Miani, M.; Bang-Berthelsen, C.H.; Friedrichsen, M.; Overgaard, A.J.; Berchtold, L.A.; Wiberg, A.; Poulsen, P.; et al. CTSH regulates β-cell function and disease progression in newly diagnosed type 1 diabetes patients. Proc. Natl. Acad. Sci. USA 2014, 111, 10305–10310. [Google Scholar] [CrossRef] [PubMed]
- Andersson, R.; Gebhard, C.; Miguel-Escalada, I.; Hoof, I.; Bornholdt, J.; Boyd, M.; Chen, Y.; Zhao, X.; Schmidl, C.; Suzuki, T.; et al. An atlas of active enhancers across human cell types and tissues. Nature 2014, 507, 455–461. [Google Scholar] [CrossRef]
- Ackermann, A.M.; Wang, Z.; Schug, J.; Naji, A.; Kaestner, K.H. Integration of ATAC-seq and RNA-seq identifies human alpha cell and beta cell signature genes. Mol. Metab. 2016, 5, 233–244. [Google Scholar] [CrossRef]
- Rai, V.; Quang, D.X.; Erdos, M.R.; Cusanovich, D.A.; Daza, R.M.; Narisu, N.; Zou, L.S.; Didion, J.P.; Guan, Y.; Shendure, J.; et al. Single-cell ATAC-Seq in human pancreatic islets and deep learning upscaling of rare cells reveals cell-specific type 2 diabetes regulatory signatures. Mol. Metab. 2020, 32, 109–121. [Google Scholar] [CrossRef]
- Gaulton, K.J.; Ferreira, T.; Lee, Y.; Raimondo, A.; Mägi, R.; Reschen, M.E.; Mahajan, A.; Locke, A.; Rayner, N.W.; Robertson, N.; et al. Genetic fine mapping and genomic annotation defines causal mechanisms at type 2 diabetes susceptibility loci. Nat. Genet. 2015, 47, 1415–1425. [Google Scholar] [CrossRef]
- Hodson, D.J.; Mitchell, R.K.; Marselli, L.; Pullen, T.J.; Gimeno Brias, S.; Semplici, F.; Everett, K.L.; Cooper, D.M.F.; Bugliani, M.; Marchetti, P.; et al. ADCY5 couples glucose to insulin secretion in human islets. Diabetes 2014, 63, 3009–3021. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.; Kraakman, M.J.; Damle, M.; Gill, R.; Lazar, M.A.; Accili, D. Identification of C2CD4A as a human diabetes susceptibility gene with a role in β cell insulin secretion. Proc. Natl. Acad. Sci. USA 2019, 116, 20033–20042. [Google Scholar] [CrossRef] [PubMed]
- Soleimanpour, S.A.; Gupta, A.; Bakay, M.; Ferrari, A.M.; Groff, D.N.; Fadista, J.; Spruce, L.A.; Kushner, J.A.; Groop, L.; Seeholzer, S.H.; et al. The diabetes susceptibility gene Clec16a regulates mitophagy. Cell 2014, 157, 1577–1590. [Google Scholar] [CrossRef] [PubMed]
- Gingerich, M.A.; Sidarala, V.; Soleimanpour, S.A. Clarifying the function of genes at the chromosome 16p13 locus in type 1 diabetes: CLEC16A and DEXI. Genes Immun. 2020, 21, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Krivega, I.; Dean, A. Enhancer and promoter interactions-long distance calls. Curr. Opin. Genet. Dev. 2012, 22, 79–85. [Google Scholar] [CrossRef]
- Sanyal, A.; Lajoie, B.R.; Jain, G.; Dekker, J. The long-range interaction landscape of gene promoters. Nature 2012, 489, 109–113. [Google Scholar] [CrossRef]
- Matharu, N.; Ahituv, N. Minor Loops in Major Folds: Enhancer-Promoter Looping, Chromatin Restructuring, and Their Association with Transcriptional Regulation and Disease. PLoS Genet. 2015, 11, e1005640. [Google Scholar] [CrossRef]
- Hanssen, L.L.P.; Kassouf, M.T.; Oudelaar, A.M.; Biggs, D.; Preece, C.; Downes, D.J.; Gosden, M.; Sharpe, J.A.; Sloane-Stanley, J.A.; Hughes, J.R.; et al. Tissue-specific CTCF-cohesin-mediated chromatin architecture delimits enhancer interactions and function in vivo. Nat. Cell Biol. 2017, 19, 952–961. [Google Scholar] [CrossRef] [PubMed]
- Grubert, F.; Srivas, R.; Spacek, D.V.; Kasowski, M.; Ruiz-Velasco, M.; Sinnott-Armstrong, N.; Greenside, P.; Narasimha, A.; Liu, Q.; Geller, B.; et al. Landscape of cohesin-mediated chromatin loops in the human genome. Nature 2020, 583, 737–743. [Google Scholar] [CrossRef] [PubMed]
- McCord, R.P.; Kaplan, N.; Giorgetti, L. Chromosome Conformation Capture and Beyond: Toward an Integrative View of Chromosome Structure and Function. Mol. Cell 2020, 77, 688–708. [Google Scholar] [CrossRef]
- Greenwald, W.W.; Chiou, J.; Yan, J.; Qiu, Y.; Dai, N.; Wang, A.; Nariai, N.; Aylward, A.; Han, J.Y.; Kadakia, N.; et al. Pancreatic islet chromatin accessibility and conformation reveals distal enhancer networks of type 2 diabetes risk. Nat. Commun. 2019, 10, 2078. [Google Scholar] [CrossRef]
- Chun, S.; Gao, L.; May, C.L.; Pippin, J.A.; Boehm, K.; Lee, M.; Liu, C.; Pahl, M.C.; Golson, M.L.; Naji, A.; et al. The three-dimensional chromatin structure of the major human pancreatic cell types reveals lineage-specific regulatory architecture of T2D risk. bioRxiv 2021. [Google Scholar] [CrossRef]
- Jian, X.; Felsenfeld, G. Large parental differences in chromatin organization in pancreatic beta cell line explaining diabetes susceptibility effects. Nat. Commun. 2021, 12, 4338. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Cebola, I.; Carrat, G.; Jiang, S.; Nawaz, S.; Khamis, A.; Canouil, M.; Froguel, P.; Schulte, A.; Solimena, M.; et al. Chromatin 3D interaction analysis of the STARD10 locus unveils FCHSD2 as a regulator of insulin secretion. Cell Rep. 2021, 34, 108703. [Google Scholar] [CrossRef]
- Carrat, G.R.; Hu, M.; Nguyen-Tu, M.-S.; Chabosseau, P.; Gaulton, K.J.; van de Bunt, M.; Siddiq, A.; Falchi, M.; Thurner, M.; Canouil, M.; et al. Decreased STARD10 Expression Is Associated with Defective Insulin Secretion in Humans and Mice. Am. J. Hum. Genet. 2017, 100, 238–256. [Google Scholar] [CrossRef] [PubMed]
- Arnes, L.; Sussel, L. Epigenetic modifications and long noncoding RNAs influence pancreas development and function. Trends Genet. TIG 2015, 31, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Pullen, T.J.; Rutter, G.A. Roles of lncRNAs in pancreatic beta cell identity and diabetes susceptibility. Front. Genet. 2014, 5, 193. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Sanchez, A.; Rutter, G.A.; Latreille, M. MiRNAs in β-Cell Development, Identity, and Disease. Front. Genet. 2016, 7, 226. [Google Scholar] [CrossRef]
- Van de Bunt, M.; Gaulton, K.J.; Parts, L.; Morán, I.; Johnson, P.R.; Lindgren, C.M.; Ferrer, J.; Gloyn, A.L.; McCarthy, M.I. The miRNA profile of human pancreatic islets and beta-cells and relationship to type 2 diabetes pathogenesis. PLoS ONE 2013, 8, e55272. [Google Scholar] [CrossRef]
- Broadbent, H.M.; Peden, J.F.; Lorkowski, S.; Goel, A.; Ongen, H.; Green, F.; Clarke, R.; Collins, R.; Franzosi, M.G.; Tognoni, G.; et al. Susceptibility to coronary artery disease and diabetes is encoded by distinct, tightly linked SNPs in the ANRIL locus on chromosome 9p. Hum. Mol. Genet. 2008, 17, 806–814. [Google Scholar] [CrossRef]
- Travers, M.E.; Mackay, D.J.G.; Dekker Nitert, M.; Morris, A.P.; Lindgren, C.M.; Berry, A.; Johnson, P.R.; Hanley, N.; Groop, L.C.; McCarthy, M.I.; et al. Insights into the molecular mechanism for type 2 diabetes susceptibility at the KCNQ1 locus from temporal changes in imprinting status in human islets. Diabetes 2013, 62, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Ye, Q.; Xu, K.; Cheng, J.; Gao, Y.; Li, Q.; Du, J.; Shi, H.; Zhou, L. Single-nucleotide polymorphisms inside microRNA target sites influence the susceptibility to type 2 diabetes. J. Hum. Genet. 2013, 58, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Griesemer, D.; Xue, J.R.; Reilly, S.K.; Ulirsch, J.C.; Kukreja, K.; Davis, J.R.; Kanai, M.; Yang, D.K.; Butts, J.C.; Guney, M.H.; et al. Genome-wide functional screen of 3′UTR variants uncovers causal variants for human disease and evolution. Cell 2021, 184, 5247–5260.e9. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.I.; van de Geijn, B.; Raj, A.; Knowles, D.A.; Petti, A.A.; Golan, D.; Gilad, Y.; Pritchard, J.K. RNA splicing is a primary link between genetic variation and disease. Science 2016, 352, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Harries, L.W.; Sloman, M.J.; Sellers, E.A.C.; Hattersley, A.T.; Ellard, S. Diabetes susceptibility in the Canadian Oji-Cree population is moderated by abnormal mRNA processing of HNF1A G319S transcripts. Diabetes 2008, 57, 1978–1982. [Google Scholar] [CrossRef]
- Dayeh, T.; Volkov, P.; Salö, S.; Hall, E.; Nilsson, E.; Olsson, A.H.; Kirkpatrick, C.L.; Wollheim, C.B.; Eliasson, L.; Rönn, T.; et al. Genome-wide DNA methylation analysis of human pancreatic islets from type 2 diabetic and non-diabetic donors identifies candidate genes that influence insulin secretion. PLoS Genet. 2014, 10, e1004160. [Google Scholar] [CrossRef] [PubMed]
- Volkov, P.; Bacos, K.; Ofori, J.K.; Esguerra, J.L.S.; Eliasson, L.; Rönn, T.; Ling, C. Whole-Genome Bisulfite Sequencing of Human Pancreatic Islets Reveals Novel Differentially Methylated Regions in Type 2 Diabetes Pathogenesis. Diabetes 2017, 66, 1074–1085. [Google Scholar] [CrossRef]
- Dayeh, T.A.; Olsson, A.H.; Volkov, P.; Almgren, P.; Rönn, T.; Ling, C. Identification of CpG-SNPs associated with type 2 diabetes and differential DNA methylation in human pancreatic islets. Diabetologia 2013, 56, 1036–1046. [Google Scholar] [CrossRef]
- Liu, R.-K.; Lin, X.; Wang, Z.; Greenbaum, J.; Qiu, C.; Zeng, C.-P.; Zhu, Y.-Y.; Shen, J.; Deng, H.-W. Identification of novel functional CpG-SNPs associated with Type 2 diabetes and birth weight. Aging 2021, 13, 10619–10658. [Google Scholar] [CrossRef]
- Li, J.; Wu, X.; Zhou, Y.; Lee, M.; Guo, L.; Han, W.; Mo, W.; Cao, W.-M.; Sun, D.; Xie, R.; et al. Decoding the dynamic DNA methylation and hydroxymethylation landscapes in endodermal lineage intermediates during pancreatic differentiation of hESC. Nucleic Acids Res. 2018, 46, 2883–2900. [Google Scholar] [CrossRef]
- Li, J.; Wu, X.; Lee, M.; Ke, J.; Lan, Q.; Li, J.; Huang, Y.; Sun, D.-Q.; Xie, R. TET1 dioxygenase is required for FOXA2-associated chromatin remodeling in pancreatic beta-cell differentiation. bioRxiv 2021. [Google Scholar] [CrossRef]
- Boo, S.H.; Kim, Y.K. The emerging role of RNA modifications in the regulation of mRNA stability. Exp. Mol. Med. 2020, 52, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Luo, K.; Zou, Z.; Qiu, M.; Tian, J.; Sieh, L.; Shi, H.; Zou, Y.; Wang, G.; Morrison, J.; et al. Genetic analyses support the contribution of mRNA N6-methyladenosine (m6A) modification to human disease heritability. Nat. Genet. 2020, 52, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Lin, W.; Yi, J.; Zhao, Z. Exploring the Epigenetic Regulatory Role of m6A-Associated SNPs in Type 2 Diabetes Pathogenesis. Pharmacogenomics Pers. Med. 2021, 14, 1369–1378. [Google Scholar] [CrossRef] [PubMed]
- Hegele, R.A.; Cao, H.; Harris, S.B.; Hanley, A.J.; Zinman, B. The hepatic nuclear factor-1alpha G319S variant is associated with early-onset type 2 diabetes in Canadian Oji-Cree. J. Clin. Endocrinol. Metab. 1999, 84, 1077–1082. [Google Scholar] [CrossRef][Green Version]
- Weir, G.C.; Bonner-Weir, S. Five stages of evolving beta-cell dysfunction during progression to diabetes. Diabetes 2004, 53 (Suppl. 3), S16–S21. [Google Scholar] [CrossRef]
- Kendall, D.M.; Sutherland, D.E.; Najarian, J.S.; Goetz, F.C.; Robertson, R.P. Effects of hemipancreatectomy on insulin secretion and glucose tolerance in healthy humans. N. Engl. J. Med. 1990, 322, 898–903. [Google Scholar] [CrossRef] [PubMed]
- Rahier, J.; Guiot, Y.; Goebbels, R.M.; Sempoux, C.; Henquin, J.C. Pancreatic β-cell mass in European subjects with type 2 diabetes. Diabetes Obes. Metab. 2008, 10, 32–42. [Google Scholar] [CrossRef]
- Saisho, Y.; Butler, A.E.; Manesso, E.; Elashoff, D.; Rizza, R.A.; Butler, P.C. β-cell mass and turnover in humans: Effects of obesity and aging. Diabetes Care 2013, 36, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Stanger, B.Z.; Tanaka, A.J.; Melton, D.A. Organ size is limited by the number of embryonic progenitor cells in the pancreas but not the liver. Nature 2007, 445, 886–891. [Google Scholar] [CrossRef]
- Yang, K.; Lee, M.; Jones, P.A.; Liu, S.S.; Zhou, A.; Xu, J.; Sreekanth, V.; Wu, J.L.Y.; Vo, L.; Lee, E.A.; et al. A 3D culture platform enables development of zinc-binding prodrugs for targeted proliferation of β cells. Sci. Adv. 2020, 6, eabc3207. [Google Scholar] [CrossRef]
- Oakie, A.; Nostro, M.C. Harnessing Proliferation for the Expansion of Stem Cell-Derived Pancreatic Cells: Advantages and Limitations. Front. Endocrinol. 2021, 12, 636182. [Google Scholar] [CrossRef] [PubMed]
- Rosado-Olivieri, E.A.; Anderson, K.; Kenty, J.H.; Melton, D.A. YAP inhibition enhances the differentiation of functional stem cell-derived insulin-producing β cells. Nat. Commun. 2019, 10, 1464. [Google Scholar] [CrossRef] [PubMed]
- Sui, L.; Xin, Y.; Du, Q.; Georgieva, D.; Diedenhofen, G.; Haataja, L.; Su, Q.; Zuccaro, M.V.; Kim, J.; Fu, J.; et al. Reduced replication fork speed promotes pancreatic endocrine differentiation and controls graft size. JCI Insight 2021, 6, e141553. [Google Scholar] [CrossRef] [PubMed]
- Bartolomé, A.; Zhu, C.; Sussel, L.; Pajvani, U.B. Notch signaling dynamically regulates adult β cell proliferation and maturity. J. Clin. Investig. 2019, 129, 268–280. [Google Scholar] [CrossRef]
- Rosado-Olivieri, E.A.; Aigha, I.I.; Kenty, J.H.; Melton, D.A. Identification of a LIF-Responsive, Replication-Competent Subpopulation of Human β Cells. Cell Metab. 2020, 31, 327–338.e6. [Google Scholar] [CrossRef]
- Davis, J.C.; Alves, T.C.; Helman, A.; Chen, J.C.; Kenty, J.H.; Cardone, R.L.; Liu, D.R.; Kibbey, R.G.; Melton, D.A. Glucose Response by Stem Cell-Derived β Cells In Vitro Is Inhibited by a Bottleneck in Glycolysis. Cell Rep. 2020, 31, 107623. [Google Scholar] [CrossRef]
- Wang, C.; Guan, Y.; Yang, J. Cytokines in the Progression of Pancreatic β-Cell Dysfunction. Int. J. Endocrinol. 2010, 2010, 515136. [Google Scholar] [CrossRef]
- Gerber, P.A.; Rutter, G.A. The Role of Oxidative Stress and Hypoxia in Pancreatic Beta-Cell Dysfunction in Diabetes Mellitus. Antioxid. Redox Signal. 2017, 26, 501–518. [Google Scholar] [CrossRef]
- Cnop, M.; Toivonen, S.; Igoillo-Esteve, M.; Salpea, P. Endoplasmic reticulum stress and eIF2α phosphorylation: The Achilles heel of pancreatic β cells. Mol. Metab. 2017, 6, 1024–1039. [Google Scholar] [CrossRef]
- Hussain, M.A.; Akalestou, E.; Song, W.-J. Inter-organ communication and regulation of beta cell function. Diabetologia 2016, 59, 659–667. [Google Scholar] [CrossRef]
- Cai, Q.; Brissova, M.; Reinert, R.B.; Pan, F.C.; Brahmachary, P.; Jeansson, M.; Shostak, A.; Radhika, A.; Poffenberger, G.; Quaggin, S.E.; et al. Enhanced expression of VEGF-A in β cells increases endothelial cell number but impairs islet morphogenesis and β cell proliferation. Dev. Biol. 2012, 367, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Banaei-Bouchareb, L.; Gouon-Evans, V.; Samara-Boustani, D.; Castellotti, M.C.; Czernichow, P.; Pollard, J.W.; Polak, M. Insulin cell mass is altered in Csf1op/Csf1op macrophage-deficient mice. J. Leukoc. Biol. 2004, 76, 359–367. [Google Scholar] [CrossRef]
- Fernandez-Twinn, D.S.; Hjort, L.; Novakovic, B.; Ozanne, S.E.; Saffery, R. Intrauterine programming of obesity and type 2 diabetes. Diabetologia 2019, 62, 1789–1801. [Google Scholar] [CrossRef]
- Remacle, C.; Dumortier, O.; Bol, V.; Goosse, K.; Romanus, P.; Theys, N.; Bouckenooghe, T.; Reusens, B. Intrauterine programming of the endocrine pancreas. Diabetes Obes. Metab. 2007, 9 (Suppl. 2), 196–209. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.C.-H.; Lee, K.F.; Yeung, W.S.B.; Lee, Y.L. Human embryonic stem cells as an in vitro model for studying developmental origins of type 2 diabetes. World J. Stem Cells 2020, 12, 761–775. [Google Scholar] [CrossRef] [PubMed]
- Santostefano, K.E.; Hamazaki, T.; Biel, N.M.; Jin, S.; Umezawa, A.; Terada, N. A practical guide to induced pluripotent stem cell research using patient samples. Lab. Investig. J. Tech. Methods Pathol. 2015, 95, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Lamm, N.; Ben-David, U.; Golan-Lev, T.; Storchová, Z.; Benvenisty, N.; Kerem, B. Genomic Instability in Human Pluripotent Stem Cells Arises from Replicative Stress and Chromosome Condensation Defects. Cell Stem Cell 2016, 18, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.-H.; Gao, X.; DeKeyser, J.-M.; Fetterman, K.A.; Pinheiro, E.A.; Weddle, C.J.; Fonoudi, H.; Orman, M.V.; Romero-Tejeda, M.; Jouni, M.; et al. Negligible-Cost and Weekend-Free Chemically Defined Human iPSC Culture. Stem Cell Rep. 2020, 14, 256–270. [Google Scholar] [CrossRef] [PubMed]
- King, N.M.P.; Perrin, J. Ethical issues in stem cell research and therapy. Stem Cell Res. Ther. 2014, 5, 85. [Google Scholar] [CrossRef]
- Yamanaka, S. Pluripotent Stem Cell-Based Cell Therapy—Promise and Challenges. Cell Stem Cell 2020, 27, 523–531. [Google Scholar] [CrossRef]
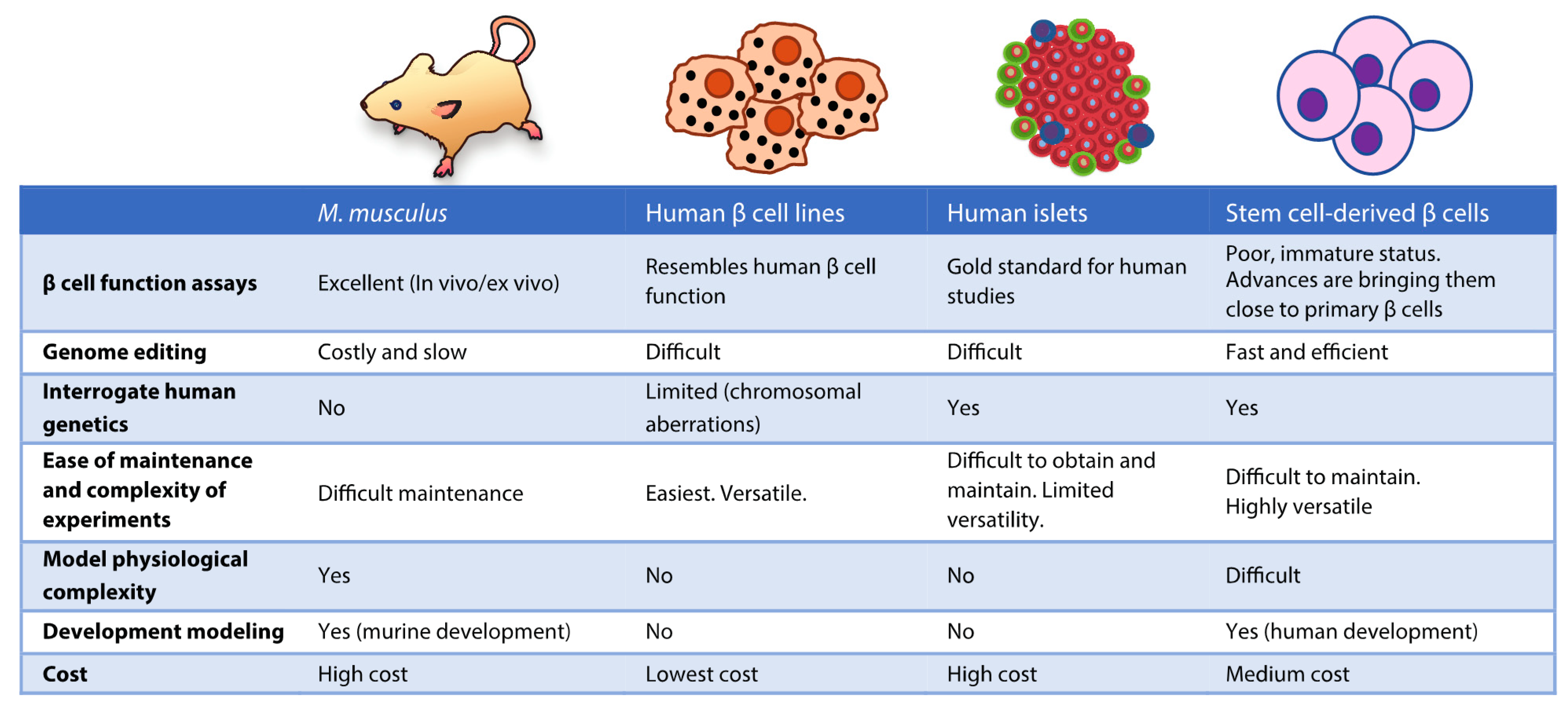
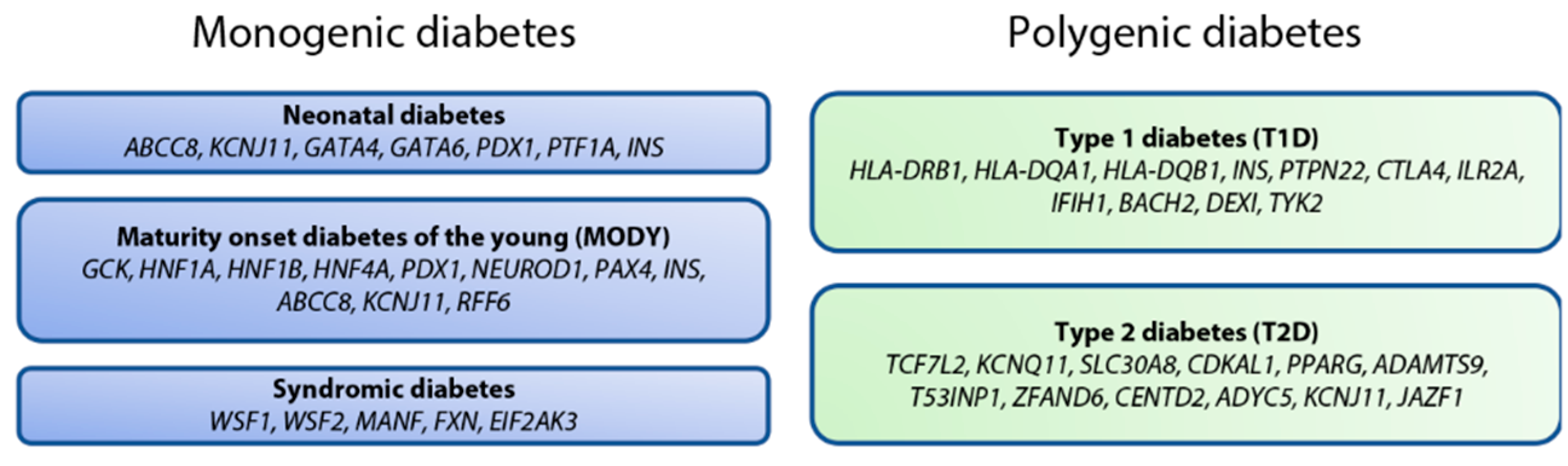
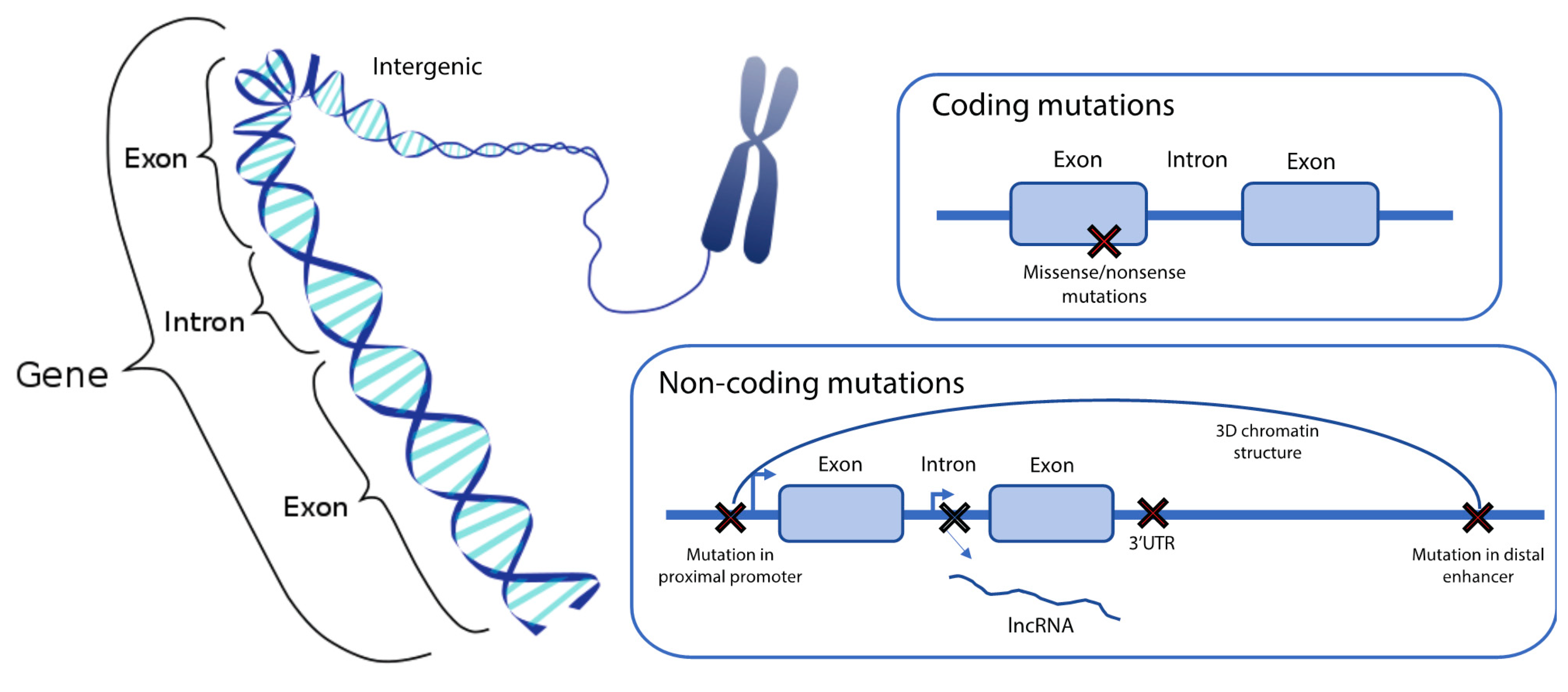
| Genetic Variant | Cell Lines | Phenotype | References |
|---|---|---|---|
| ABCC8 V187D | Patient-derived iPSC and isogenic controls | Increased insulin secretion in low glucose, increased proliferation | [166] |
| ARX −/Y | hESC | No α cells and differentiation shifted toward δ cells | [117] |
| CACNA1A −/− | hESC | Normal differentiation, reduced intracellular Ca2+ levels, impaired GSIS | [146] |
| CDKAL1 −/− | hESC | Normal differentiation, impaired GSIS, susceptibility to ER stress | [208,209] |
| FOXA1 −/− | hESC | Dispensable for PP generation | [239] |
| FOXA2 +/−, −/− | Patient-derived iPSC and healthy controls, hESC | Impaired generation of PPs | [164,239,240] |
| GATA4 +/− | hESC | GATA4 dosage influences the phenotype in GATA6 +/− | [168] |
| GATA6 −/−, R456C, Val204fs | hESC | Impaired endoderm differentiation (−/−) or formation of PP stage (+/−) | [168] |
| GLIS3 −/− | hESC | Impaired differentiation of PPs and Eps, cell death | [170] |
| HES1 −/− | hESC | Accelerated endocrine differentiation | [117] |
| HNF1 +/−, −/−, Pro291fs, R200Q | hESC and patient-derived iPSC with isogenic controls | Differentiation biased toward α cells, impaired GSIS, altered insulin:C-peptide stoichiometry | [145,146,148] |
| HNF1B R177*, S148L | Patient-derived iPSC and family controls | Hypomorphic variants, decreased PAX6 expression | [153,154] |
| HNF4A Ile271fs, Q268* | Patient-derived iPSC and family controls | Altered differentiation with decreased HNF1A and PDX1 expression | [149,150,151] |
| INS C96R, INSM1I/M1I | Patient-derived iPSC and isogenic controls | C96R: ER stress, reduced functionINSM1I/M1I: total absence of insulin | [157,158] |
| KCNJ11 −/− | hESC | Normal differentiation, impaired GSIS | [208] |
| KCNQ1 −/−, R397W | hESC | −/−: Normal differentiation, impaired GSIS R397W: hypersecretion of insulin, followed by cell death and functional demise | [208,219] |
| KCNQ1 ΔEnhancer | hESC | Decreased INS and CDKN1C expression | [220] |
| NEUROD1 −/− | hESC | Impaired endocrine differentiation | [169] |
| NEUROG3 −/−, +/− | hESC | Abolished/highly impaired generation of endocrine cells | [117,175] |
| NEUROG3-gain-of-function | hESC | Accelerated endocrine differentiation | [117] |
| NOTCH1-gain-of-function (N1ICD) | hESC | Abolished endocrine differentiation | [117] |
| ONECUT1 −/−, E231*, E231D | Patient-derived iPSC and hESC | Altered NKX6.1 and NKX6.2 during PP stage | [136] |
| PAX4 −/− | hESC | Differentiation biased toward α cells | [146] |
| PDX1 −/−, P33T, C18 | Patient-derived iPSC and healthy control. hESCs | Reduced differentiation efficiency.−/−: abolished differentiation | [13] |
| RFX6 −/− | hESC | Abolished endocrine differentiation | [117] |
| SLC30A8 −/−, R138*, Ser38fs | hESC | Hypomorphic variants, improved GSIS | [226,227] |
| STAT3 K392R (activating mutation) | Patient-derived iPSC and isogenic controls | Premature differentiation, biased toward α cells, upregulation of NEUROG3 and targets | [165] |
| SYT13 −/− | hESC | Normal differentiation, impaired GSIS | [146] |
| TCF7L2 ΔEnhancer | hESC | Improved differentiation of EPs | [235] |
| TRMT10A R127*, E27* | Patient-derived iPSC and healthy controls | Oxidative stress and apoptosis | [163] |
| WFS1 various mutations | Patient-derived iPSC and healthy or isogenic controls | Normal differentiation, ER stress, decreased insulin content | [161,162] |
| YIPF5 −/− and I98S | hESC and patient-derived iPSC with isogenic controls | Proinsulin retention in the ER, susceptible to ER stress-induced death | [159] |
| ZNF808 −/− | hESC | Inappropriate specification of cell fate, loss of pancreatic identity | [128] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartolomé, A. Stem Cell-Derived β Cells: A Versatile Research Platform to Interrogate the Genetic Basis of β Cell Dysfunction. Int. J. Mol. Sci. 2022, 23, 501. https://doi.org/10.3390/ijms23010501
Bartolomé A. Stem Cell-Derived β Cells: A Versatile Research Platform to Interrogate the Genetic Basis of β Cell Dysfunction. International Journal of Molecular Sciences. 2022; 23(1):501. https://doi.org/10.3390/ijms23010501
Chicago/Turabian StyleBartolomé, Alberto. 2022. "Stem Cell-Derived β Cells: A Versatile Research Platform to Interrogate the Genetic Basis of β Cell Dysfunction" International Journal of Molecular Sciences 23, no. 1: 501. https://doi.org/10.3390/ijms23010501
APA StyleBartolomé, A. (2022). Stem Cell-Derived β Cells: A Versatile Research Platform to Interrogate the Genetic Basis of β Cell Dysfunction. International Journal of Molecular Sciences, 23(1), 501. https://doi.org/10.3390/ijms23010501






