Pseudomonas syringae pv. actinidiae Effector HopAU1 Interacts with Calcium-Sensing Receptor to Activate Plant Immunity
Abstract
:1. Introduction
2. Results
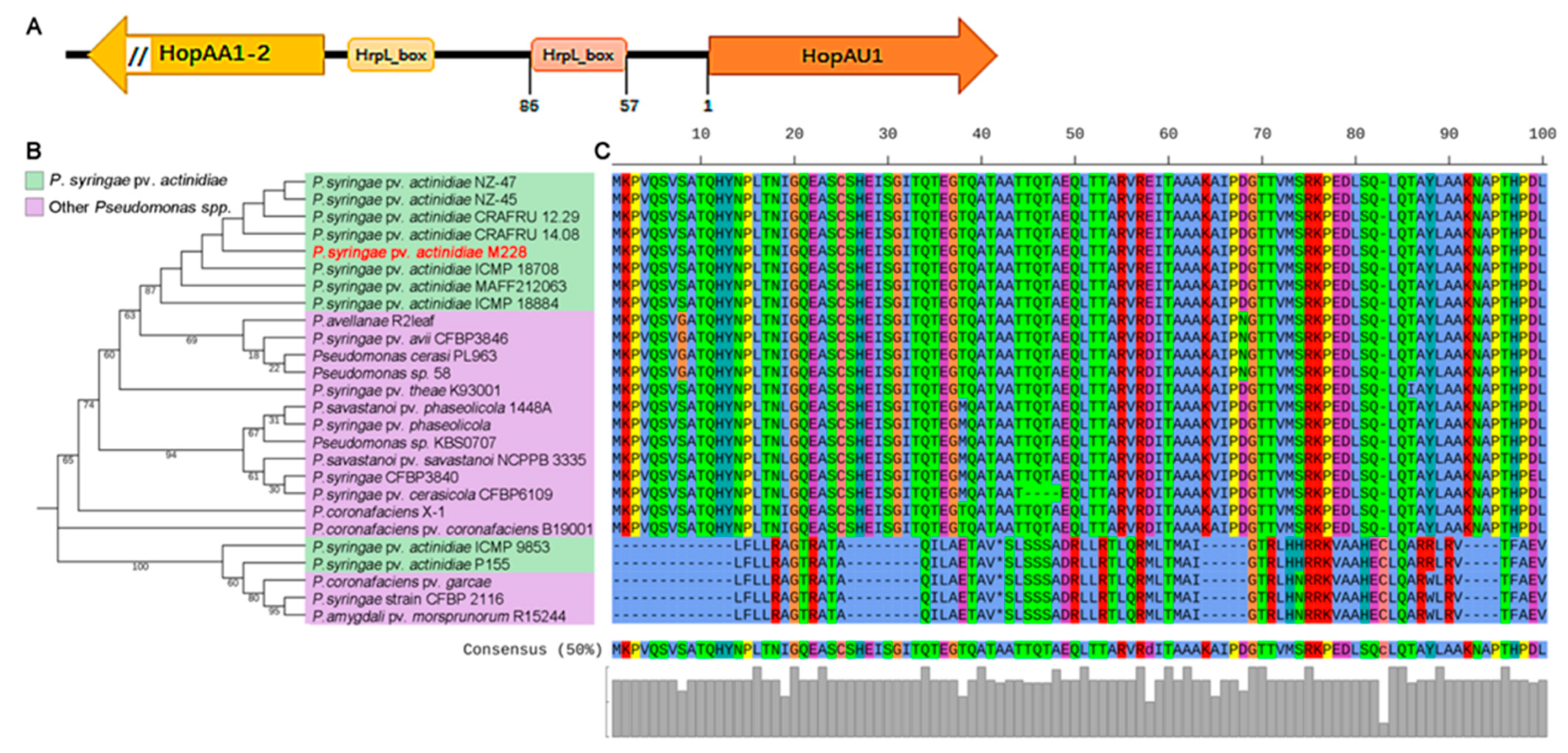
2.1. HopAU1 Is an Evolutionarily Conserved Effector in Pseudomonas
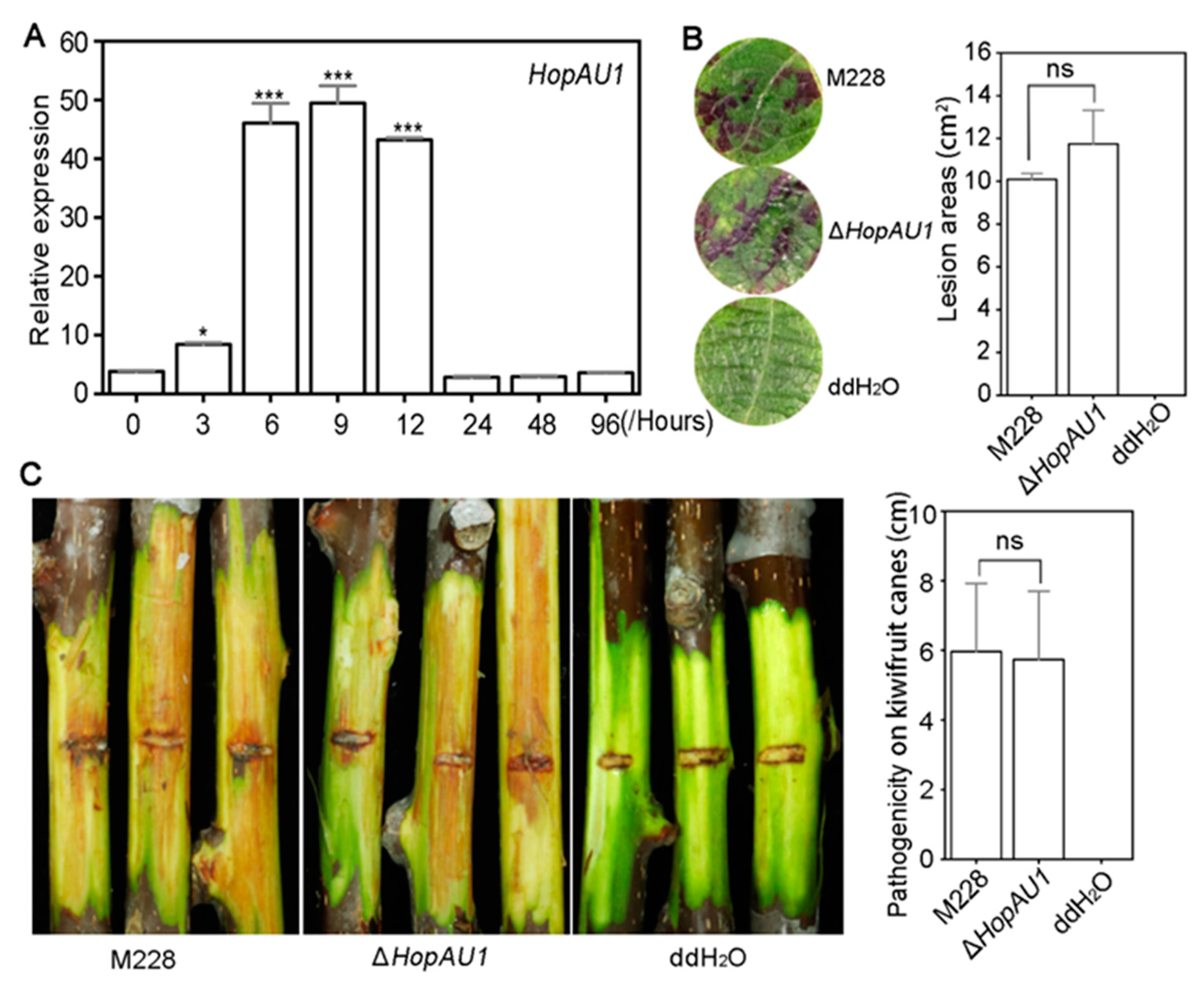
2.2. HopAU1 Makes No Contribution to Psa Virulence
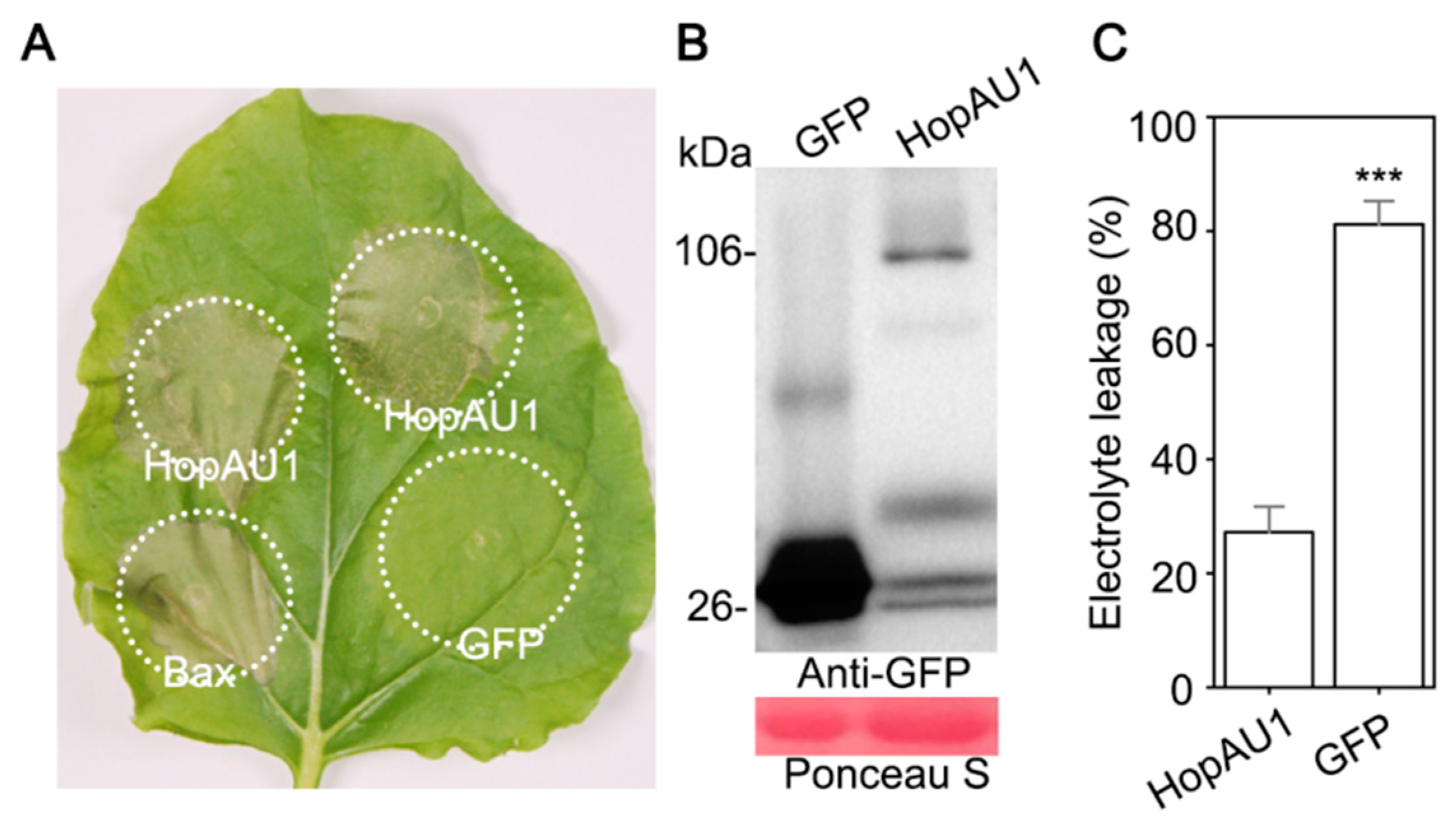
2.3. Transient Expression of HopAU1 Triggers Cell Death in N. benthamiana
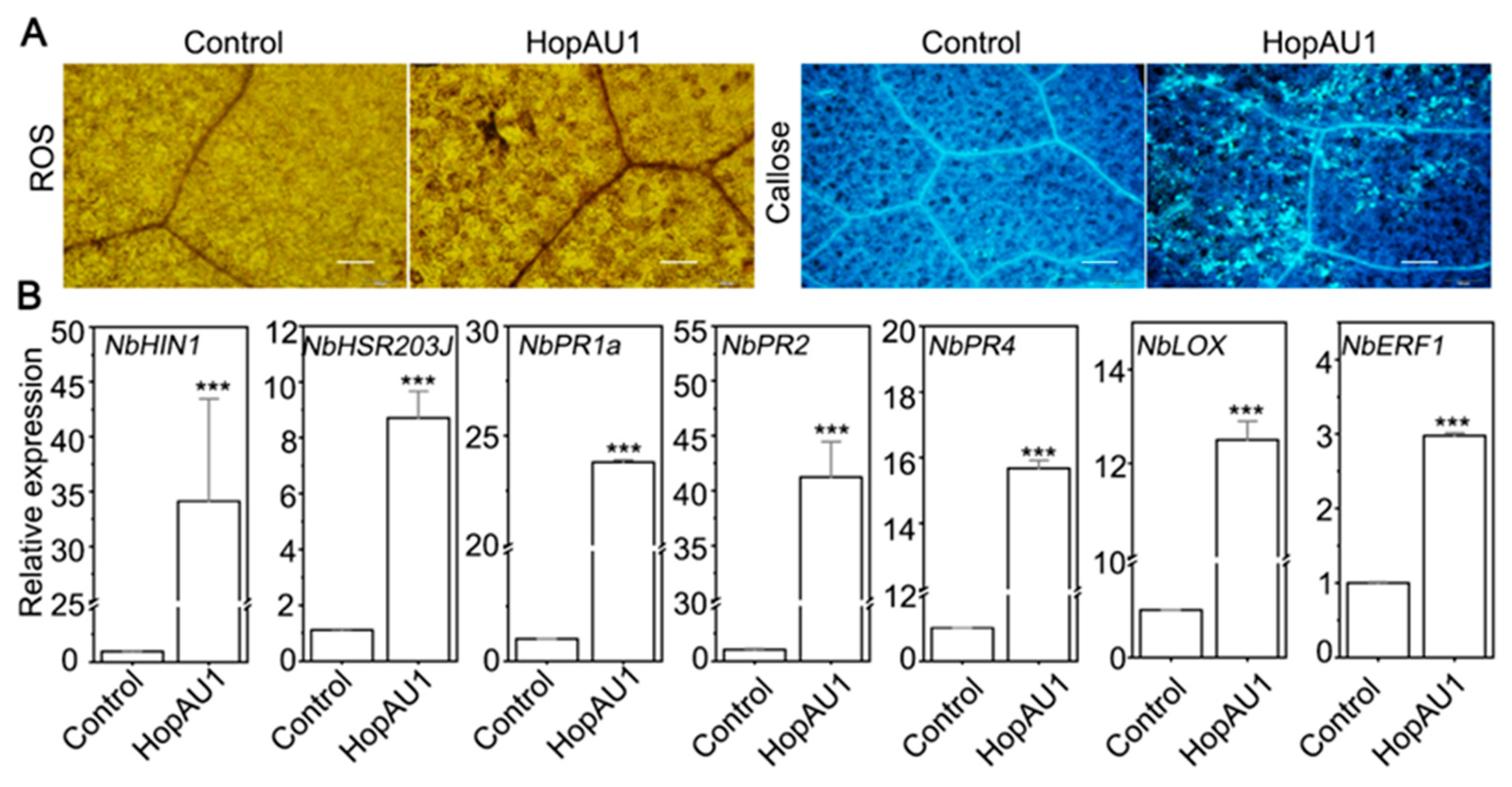
2.4. HopAU1 Induces Immune Responses in N. benthamiana
2.5. HopAU1 Interacts with Plant Calcium Sensing Receptor (CaS)
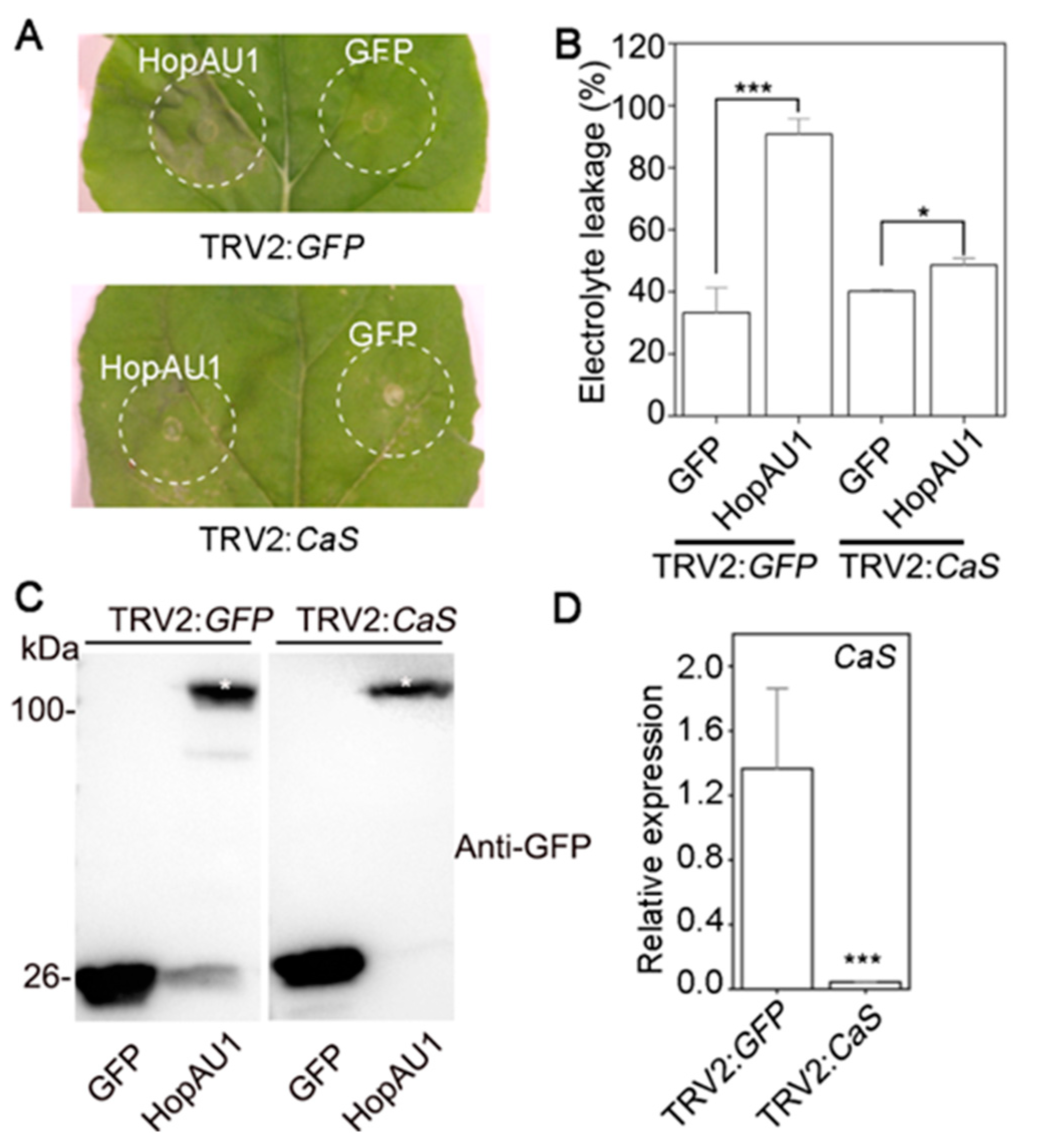
2.6. CaS Is Required for HopAU1-Triggered Cell Death in N. benthamiana
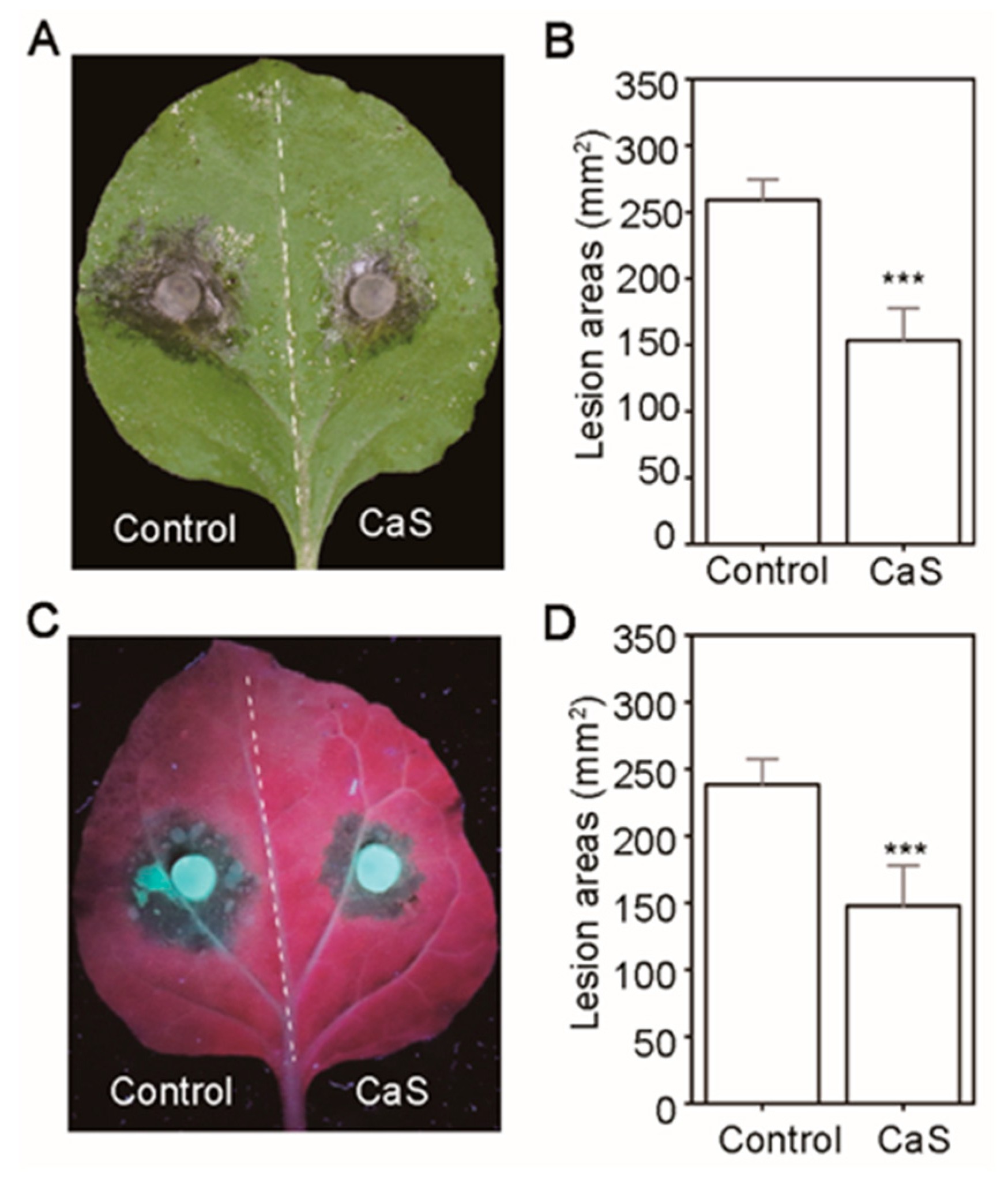
2.7. Overexpression of CaS Mediates N. benthamiana Resistance to Sclerotinia Sclerotiorum and Phytophthora Capsici
3. Discussion
4. Materials and Methods
4.1. Strains and the Growth of Plants
4.2. Plasmid Construction
4.3. Gene-Deletion and -Complement Mutants Generation
4.4. Bacterial Infiltrations for HR and Electrolyte Leakage Assays in N. benthamiana
4.5. RNA Isolation, cDNA Synthesis, and Expression Analysis
4.6. Confocal Microscopy and Co-Immunoprecipitation Assay (CoIP)
4.7. Callose and Reactive Oxygen Species (ROS) Staining
4.8. VIGS in N. benthamiana
4.9. Pathogenicity Tests
4.10. Accession Numbers and Bioinformatics Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, H.; Tsuda, K.; Parker, J.E. Effector-triggered immunity: From pathogen perception to robust defense. Annu. Rev. Plant Biol. 2015, 66, 487–511. [Google Scholar] [CrossRef] [PubMed]
- Breseghello, F.; Coelho, A.S.G. Traditional and modern plant breeding methods with examples in rice (Oryza sativa L.). J. Agric. Food Chem. 2013, 61, 8277–8286. [Google Scholar] [CrossRef]
- Hammond-Kosack, K.E.; Parker, J.E. Deciphering plant-pathogen communication: Fresh perspectives for molecular resistance breeding. Curr. Opin. Biotech. 2003, 14, 177–193. [Google Scholar] [CrossRef]
- Stavrinides, J.; McCann, H.C.; Guttman, D.S. Host-pathogen interplay and the evolution of bacterial effectors. Cell. Microbiol. 2008, 10, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Lindeberg, M.; Cunnac, S.; Collmer, A. The evolution of Pseudomonas syringae host specificity and type III effector repertoires. Mol. Plant Pathol. 2009, 10, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Seto, D.; Subramaniam, R.; Desveaux, D. Oh, the places they’ll go! A survey of phytopathogen effectors and their host targets. Plant J. 2018, 93, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Dillon, M.M.; Almeida, R.N.D.; Laflamme, B.; Martel, A.; Weir, B.S.; Desveaux, D.; Guttman, D.S. Molecular evolution of Pseudomonas syringae Type III secreted effector proteins. Front. Plant Sci. 2019, 10, 418. [Google Scholar] [CrossRef] [Green Version]
- Laflamme, B.; Dillon, M.M.; Martel, A.; Almeida, R.N.D.; Desveaux, D.; Guttman, D.S. The pan-genome effector-triggered immunity landscape of a host-pathogen interaction. Science 2020, 367, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Lin, N.C.; Martin, G.B. Two distinct Pseudomonas effector proteins interact with the Pto kinase and activate plant immunity. Cell 2002, 109, 589–598. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Caldwell, K.S.; Wroblewski, T.; Wright, M.E.; Michelmore, R.W. Proteolysis of a negative regulator of innate immunity is dependent on resistance genes in Tomato and Nicotiana benthamiana and induced by multiple bacterial effectors. Plant Cell 2009, 21, 2458–2472. [Google Scholar] [CrossRef] [Green Version]
- Cui, H.T.; Wang, Y.J.; Xue, L.; Chu, J.F.; Yan, C.Y.; Fu, J.H.; Chen, M.S.; Innes, R.W.; Zhou, J.M. Pseudomonas syringae effector protein AvrB perturbs Arabidopsis hormone signaling by activating MAP Kinase 4. Cell Host Microbe 2010, 7, 164–175. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.; Bourdais, G.; Yu, G.; Robatzek, S.; Coaker, G. Phosphorylation of the plant immune regulator RPM1-INTERACTING PROTEIN4 enhances plant plasma membrane H(+)-ATPase activity and inhibits flagellin-triggered immune responses in Arabidopsis. Plant Cell 2015, 27, 2042–2056. [Google Scholar] [CrossRef] [Green Version]
- Jiang, S.S.; Yao, J.; Ma, K.W.; Zhou, H.B.; Song, J.K.; He, S.Y.; Ma, W.B. Bacterial effector activates jasmonate signaling by directly targeting JAZ transcriptional repressors. PLoS Pathog. 2013, 9, e1003715. [Google Scholar] [CrossRef] [Green Version]
- Gimenez-Ibanez, S.; Boter, M.; Fernandez-Barbero, G.; Chini, A.; Rathjen, J.P.; Solano, R. The bacterial effector HopX1 targets JAZ transcriptional repressors to activate jasmonate signaling and promote infection in Arabidopsis. PLoS Biol. 2014, 12, e1001792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Teixeira, P.J.P.L.; Biswas, S.; Finkel, O.M.; He, Y.J.; Salas-Gonzalez, I.; English, M.E.; Epple, P.; Mieczkowski, P.; Dangl, J.L. Pseudomonas syringae type III effector HopBB1 promotes host transcriptional repressor degradation to regulate phytohormone responses and virulence. Cell Host Microbe 2017, 21, 156–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sohn, K.H.; Saucet, S.B.; Clarke, C.R.; Vinatzer, B.A.; O’Brien, H.E.; Guttman, D.S.; Jones, J.D.G. HopAS1 recognition significantly contributes to Arabidopsis nonhost resistance to Pseudomonas syringae pathogens. New Phytol. 2012, 193, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Xu, N.; Bhandari, D.D.; Lapin, D.; Sun, X.; Luo, X.; Wang, Y.; Cao, J.; Wang, H.; Coaker, G.; et al. Bacterial effector targeting of a plant iron sensor facilitates iron acquisition and pathogen colonization. Plant Cell 2021, 19, 2015–2031. [Google Scholar] [CrossRef]
- Baltrus, D.A.; Nishimura, M.T.; Romanchuk, A.; Chang, J.H.; Mukhtar, M.S.; Cherkis, K.; Roach, J.; Grant, S.R.; Jones, C.D.; Dangl, J.L. Dynamic evolution of pathogenicity revealed by sequencing and comparative genomics of 19 Pseudomonas syringae isolates. PLoS Pathog. 2011, 7, e1002132. [Google Scholar] [CrossRef]
- Donati, I.; Cellini, A.; Sangiorgio, D.; Vanneste, J.L.; Scortichini, M.; Balestra, G.M.; Spinelli, F. Pseudomonas syringae pv. actinidiae: Ecology, infection dynamics and disease epidemiology. Microb. Ecol. 2020, 80, 81–102. [Google Scholar] [CrossRef]
- Scortichini, M.; Marcelletti, S.; Ferrante, P.; Petriccione, M.; Firrao, G. Pseudomonas syringae pv. actinidiae: A re-emerging, multi-faceted, pandemic pathogen. Mol. Plant Pathol. 2012, 13, 631–640. [Google Scholar] [PubMed]
- Tyson, J.L.; Curtis, C.L.; Manning, M.A.; Rees-George, J.; Snelgar, W.P.; Blattmann, P. Systemic movement of Pseudomonas syringae pv. actinidiae in kiwifruit vines in New Zealand. N. Z. Plant Prot. 2014, 67, 41–47. [Google Scholar] [CrossRef]
- Zhao, Z.B.; Gao, X.N.; Yang, D.H.; Huang, L.L.; Qin, H.Q.; Kang, Z.S.; Wang, N.N. Field detection of canker-causing bacteria on kiwifruit trees: Pseudomonas syringae pv. actinidiae is the major causal agent. Crop Prot. 2015, 75, 55–62. [Google Scholar] [CrossRef]
- Gao, X.N.; Huang, Q.L.; Zhao, Z.B.; Han, Q.M.; Ke, X.W.; Qin, H.Q.; Huang, L.L. Studies on the infection, colonization, and movement of Pseudomonas syringae pv. actinidiae in kiwifruit tissues using a GFPuv-Labeled strain. PLoS ONE 2016, 11, e0151169. [Google Scholar]
- Balestra, G.M.; Buriani, G.; Cellini, A.; Donati, I.; Mazzaglia, A.; Spinelli, F. First report of Pseudomonas syringae pv. actinidiae on kiwifruit pollen from Argentina. Plant Dis. 2018, 102, 237. [Google Scholar] [CrossRef]
- Vleeshouwers, V.G.A.A.; Oliver, R.P. Effectors as tools in disease resistance breeding against biotrophic, hemibiotrophic, and necrotrophic plant pathogens. Mol. Plant-Microbe Interact. 2014, 27, 196–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.; Jayaraman, J.; Segonzac, C.; Park, H.J.; Park, H.; Han, S.W.; Sohn, K.H. Pseudomonas syringae pv. actinidiae type III effectors localized at multiple cellular compartments activate or suppress innate immune responses in Nicotiana benthamiana. Front. Plant Sci. 2017, 8, 2157. [Google Scholar] [CrossRef]
- Jayaraman, J.; Yoon, M.; Applegate, E.R.; Stroud, E.A.; Templeton, M.D. AvrE1 and HopR1 from Pseudomonas syringae pv. actinidiae are additively required for full virulence on kiwifruit. Mol. Plant Pathol. 2020, 21, 1467–1480. [Google Scholar] [CrossRef]
- Badel, J.L.; Nomura, K.; Bandyopadhyay, S.; Shimizu, R.; Collmer, A.; He, S.Y. Pseudomonas syringae pv. tomato DC3000 HopPtoM (CEL ORF3) is important for lesion formation but not growth in tomato and is secreted and translocated by the Hrp type III secretion system in a chaperone-dependent manner. Mol. Microbiol. 2003, 49, 1239–1251. [Google Scholar] [CrossRef]
- Choi, S.; Prokchorchik, M.; Lee, H.; Gupta, R.; Lee, Y.; Chung, E.H.; Cho, B.; Kim, M.S.; Kim, S.T.; Sohn, K.H. Direct acetylation of a conserved threonine of RIN4 by the bacterial effector HopZ5 or AvrBsT activates RPM1-dependent immunity in Arabidopsis. Mol. Plant 2021, 14, 1951–1960. [Google Scholar] [CrossRef]
- Choi, S.; Ayaraman, J.; Sohn, K.H. Arabidopsis thaliana SOBER1 (Suppressor of Avrbst-Elicited Resistance 1) suppresses plant immunity triggered by multiple bacterial acetyltransferase effectors. New Phytol. 2018, 219, 324–335. [Google Scholar] [CrossRef] [Green Version]
- Jayaraman, J.; Choi, S.; Prokchorchik, M.; Choi, D.S.; Spiandore, A.; Rikkerink, E.H.; Templeton, M.D.; Segonzac, C.; Sohn, K.H. A bacterial acetyltransferase triggers immunity in Arabidopsis thaliana independent of hypersensitive response. Sci. Rep. 2017, 7, 3557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, M.; Rikkerink, E.H.A. Rpa1 mediates an immune response to avrRpm1Psa and confers resistance against Pseudomonas syringae pv. actinidiae. Plant J. 2019, 102, 688–702. [Google Scholar] [CrossRef] [PubMed]
- Pontier, D.; Godiard, L.; Marco, Y.; Roby, D. hsr203J, a tobacco gene whose activation is rapid, highly localized and specific for incompatible plant/pathogen interactions. Plant J. 1994, 5, 507–521. [Google Scholar] [CrossRef]
- Takahashi, Y.; Berberich, T.; Yamashita, K.; Uehara, Y.; Miyazaki, A.; Kusano, T. Identification of tobacco HIN1 and two closely related genes as spermine-responsive genes and their differential expression during the Tobacco mosaic virus -induced hypersensitive response and during leaf- and flower-senescence. Plant Mol. Biol. 2004, 54, 613–622. [Google Scholar] [CrossRef]
- Dean, J.D.; Goodwin, P.H.; Hsiang, T. Induction of glutathione S-transferase genes of Nicotiana benthamiana following infection by Colletotrichum destructivum and C. orbiculare and involvement of one in resistance. J. Exp. Bot. 2005, 56, 1525–1533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asai, S.; Yoshioka, H. Nitric oxide as a partner of reactive oxygen species participates in disease resistance to necrotrophic pathogen Botrytis Cinerea in Nicotiana benthamiana. Mol. Plant-Microbe Interact. 2009, 22, 619–629. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, P.A.; Stam, R.; Warbroek, T.; Bos, J.I.B. Mp10 and Mp42 from the aphid species Myzus persicae trigger plant defenses in Nicotiana benthamiana through different activities. Mol. Plant-Microbe Interact. 2014, 27, 30–39. [Google Scholar] [CrossRef] [Green Version]
- Pieterse, C.M.; Van der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S.C. Hormonal modulation of plant immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef] [Green Version]
- Vanneste, J.L. The Scientific, Economic, and Social Impacts of the New Zealand Outbreak of Bacterial Canker of Kiwifruit (Pseudomonas syringae pv. actinidiae). Annu. Rev. Phytopathol. 2017, 55, 377–399. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, J.; Gao, X.; Zhang, D.; Zhang, J.; Wen, J.; Qin, H.; Guo, M.; Huang, L. Comparative genomics reveal pathogenicity-related loci in Pseudomonas syringae pv. actinidiae biovar 3. Mol. Plant Pathol. 2019, 20, 923–942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, R.; Liu, P.; Jia, B.; Xue, S.Z.; Wang, X.J.; Hu, J.Y.; Al Shoffe, Y.; Gallipoli, L.; Mazzaglia, A.; Balestra, G.M.; et al. Genetic diversity of Pseudomonas syringae pv. actinidiae strains from different geographic regions in China. Phytopathology 2019, 109, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Sawada, H.; Fujikawa, T. Genetic diversity of Pseudomonas syringae pv. actinidiae, pathogen of kiwifruit bacterial canker. Plant Pathol. 2019, 68, 1235–1248. [Google Scholar] [CrossRef]
- Tzelepis, G.; Dolfors, F.; Holmquist, L.; Dixelius, C. Plant mitochondria and chloroplasts are targeted by the Rhizoctonia solani RsCRP1 effector. Biochem. Biophys. Res. Commun. 2021, 544, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Jelenska, J.; Yao, N.; Vinatzer, B.A.; Wright, C.M.; Brodsky, J.L.; Greenberg, J.T. A J domain virulence effector of Pseudomonas syringae remodels host chloroplasts and suppresses defenses. Curr. Biol. 2007, 17, 499–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Herva, J.J.; Gonzalez-Melendi, P.; Cuartas-Lanza, R.; Antunez-Lamas, M.; Rio-Alvarez, I.; Li, Z.; Lopez-Torrejon, G.; Diaz, I.; del Pozo, J.C.; Chakravarthy, S.; et al. A bacterial cysteine protease effector protein interferes with photosynthesis to suppress plant innate immune responses. Cell. Microbiol. 2012, 14, 669–681. [Google Scholar] [CrossRef] [Green Version]
- Tang, L.; Yang, G.; Ma, M.; Liu, X.; Li, B.; Xie, J.; Fu, Y.; Chen, T.; Yu, Y.; Chen, W.; et al. An effector of a necrotrophic fungal pathogen targets the calcium-sensing receptor in chloroplasts to inhibit host resistance. Mol. Plant Pathol. 2020, 21, 686–701. [Google Scholar] [CrossRef] [Green Version]
- Nomura, H.; Komori, T.; Uemura, S.; Kanda, Y.; Shimotani, K.; Nakai, K.; Furuichi, T.; Takebayashi, K.; Sugimoto, T.; Sano, S.; et al. Chloroplast-mediated activation of plant immune signalling in Arabidopsis. Nat. Commun. 2012, 3, 926. [Google Scholar] [CrossRef] [Green Version]
- Han, S.; Tang, R.; Anderson, L.K.; Woerner, T.E.; Pei, Z.M. A cell surface receptor mediates extracellular Ca(2+) sensing in guard cells. Nature 2003, 425, 196–200. [Google Scholar] [CrossRef]
- Tang, R.H.; Han, S.; Zheng, H.; Cook, C.W.; Choi, C.S.; Woerner, T.E.; Jackson, R.B.; Pei, Z.M. Coupling diurnal cytosolic Ca2+ oscillations to the CAS-IP3 pathway in Arabidopsis. Science 2007, 315, 1423–1426. [Google Scholar] [CrossRef] [Green Version]
- Weinl, S.; Held, K.; Schlucking, K.; Steinhorst, L.; Kuhlgert, S.; Hippler, M.; Kudla, J. A plastid protein crucial for Ca2+-regulated stomatal responses. New Phytol. 2008, 179, 675–686. [Google Scholar] [CrossRef]
- Huang, S.S.; Chen, J.; Dong, X.J.; Patton, J.; Pei, Z.M.; Zheng, H.L. Calcium and calcium receptor CAS promote Arabidopsis thaliana de-etiolation. Physiol. Plantarum. 2012, 144, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.H.; Yi, X.Q.; Han, A.D.; Liu, T.W.; Chen, J.; Wu, F.H.; Dong, X.J.; He, J.X.; Pei, Z.M.; Zheng, H.L. Calcium-sensing receptor regulates stomatal closure through hydrogen peroxide and nitric oxide in response to extracellular calcium in Arabidopsis. J. Exp. Bot. 2012, 63, 177–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medina-Puche, L.; Kim, C.; Lozano-Duran, R.; Dogra, V. Protocol for evaluating protein relocalization from the plasma membrane to chloroplasts. STAR Protoc. 2021, 14, 100816. [Google Scholar] [CrossRef]
- Medina-Puche, L.; Tan, H.; Dogra, V.; Wu, M.; Rosas-Diaz, T.; Wang, L.; Ding, X.; Zhang, D.; Fu, X.; Kim, C.; et al. A defense pathway linking plasma membrane and chloroplasts and co-opted by pathogens. Cell 2020, 182, 1109–1124 e25. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Kang, L.; Anand, A.; Lazarovits, G.; Mysore, K.S. Monitoring in planta bacterial infection at both cellular and whole-plant levels using the green fluorescent protein variant GFPuv. New Phytol. 2007, 174, 212–223. [Google Scholar] [CrossRef]
- Kvitko, B.H.; Collmer, A. Construction of Pseudomonas syringae pv. tomato DC3000 mutant and polymutant strains. Methods Mol. Biol. 2011, 712, 109–128. [Google Scholar]
- Yu, X.; Feng, B.; He, P.; Shan, L. From chaos to harmony: Responses and signaling upon microbial pattern recognition. Annu. Rev. Phytopathol. 2017, 55, 109–137. [Google Scholar] [CrossRef]
- Golpayegani, A.; Nodehi, R.N.; Rezaei, F.; Alimohammadi, M.; Douraghi, M. Real-time polymerase chain reaction assays for rapid detection and virulence evaluation of the environmental Pseudomonas aeruginosa isolates. Mol. Biol. Rep. 2019, 46, 4049–4061. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Lyu, Q.; Han, S.; Xu, X.; Walcott, R.R.; Li, J.; Luo, L. Evaluation of suitable reference genes for normalization of quantitative reverse transcription PCR analyses in Clavibacter michiganensis. MicrobiologyOpen 2019, 8, e928. [Google Scholar] [CrossRef] [Green Version]
- Sainsbury, F.; Lomonossoff, G.P. Extremely high-level and rapid transient protein production in plants without the use of viral replication. Plant Physiol. 2008, 148, 1212–1218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C-T method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.J.; Yin, Z.Y.; Li, Z.P.; Wu, Y.X.; Huang, L.L. A small cysteine-rich protein from two kingdoms of microbes is recognized as a novel pathogen-associated molecular pattern. New Phytol. 2019, 222, 995–1011. [Google Scholar] [CrossRef]
- Qi, M.; Link, T.I.; Muller, M.; Hirschburger, D.; Pudake, R.N.; Pedley, K.F.; Braun, E.; Voegele, R.T.; Baum, T.J.; Whitham, S.A. A small cysteine-rich protein from the Asian soybean rust fungus, Phakopsora pachyrhizi, suppresses plant immunity. PLoS Pathog. 2016, 12, e1005827. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Zhou, M.; Liu, W.; Nie, J.; Huang, L. Pseudomonas syringae pv. actinidiae Effector HopAU1 Interacts with Calcium-Sensing Receptor to Activate Plant Immunity. Int. J. Mol. Sci. 2022, 23, 508. https://doi.org/10.3390/ijms23010508
Zhang J, Zhou M, Liu W, Nie J, Huang L. Pseudomonas syringae pv. actinidiae Effector HopAU1 Interacts with Calcium-Sensing Receptor to Activate Plant Immunity. International Journal of Molecular Sciences. 2022; 23(1):508. https://doi.org/10.3390/ijms23010508
Chicago/Turabian StyleZhang, Jinlong, Mingxia Zhou, Wei Liu, Jiajun Nie, and Lili Huang. 2022. "Pseudomonas syringae pv. actinidiae Effector HopAU1 Interacts with Calcium-Sensing Receptor to Activate Plant Immunity" International Journal of Molecular Sciences 23, no. 1: 508. https://doi.org/10.3390/ijms23010508
APA StyleZhang, J., Zhou, M., Liu, W., Nie, J., & Huang, L. (2022). Pseudomonas syringae pv. actinidiae Effector HopAU1 Interacts with Calcium-Sensing Receptor to Activate Plant Immunity. International Journal of Molecular Sciences, 23(1), 508. https://doi.org/10.3390/ijms23010508






