Apolipoprotein CIII Reduction Protects White Adipose Tissues against Obesity-Induced Inflammation and Insulin Resistance in Mice
Abstract
:1. Introduction
2. Results
2.1. ApoCIII Reduction Improves Metabolic Status in HFD-Fed Mice
2.2. Lowering apoCIII Reduces Inflammation and Size of Adipocytes in WAT
2.3. Reduction in apoCIII Increased Expression of Genes Related to Thermogenesis and Improved Function of SAT
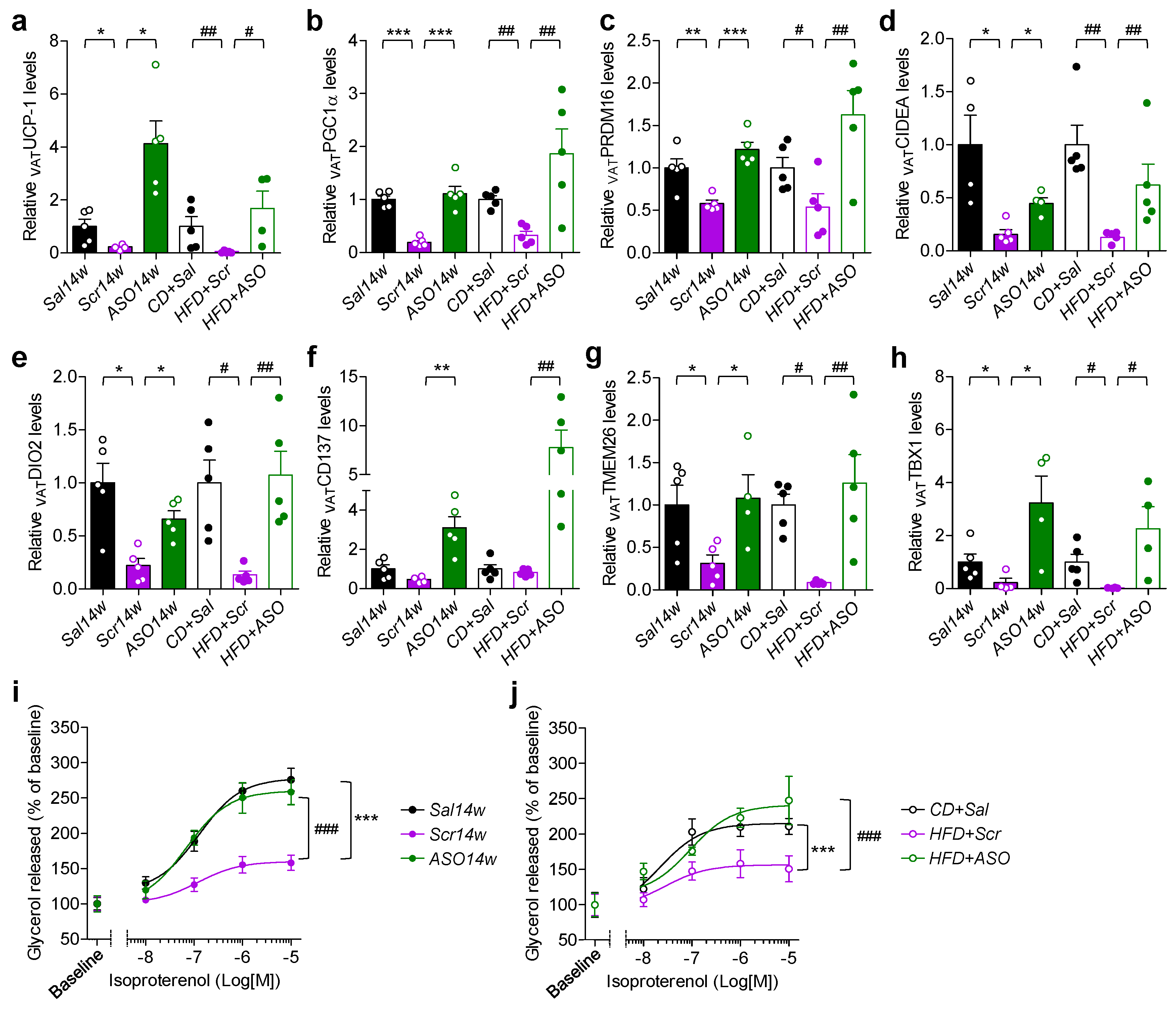
2.4. Reduced Levels of apoCIII and VAT Function
2.5. ApoCIII Reduction during HFD Maintains and Restores Insulin Sensitivity in WAT
3. Discussion
4. Materials and Methods
4.1. Mice and Diets
4.2. Antisense Oligonucleotides (ASOs)
4.3. Experimental Design
4.4. Hematoxylin and Eosin Staining in SAT and VAT
4.5. Functional Studies in Isolated Adipocytes from SAT and VAT
4.6. Blood Glucose and Plasma Biochemistry
4.7. RNA Isolation and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Obesity and Overweight Fact Sheet N°311. January 2015. Retrieved 2 February 2016. Available online: https://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 17 November 2021).
- Bray, G.A.; Kim, K.K.; Wilding, J.P.H.; World Obesity Federation. Obesity: A chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes. Rev. 2017, 18, 715–723. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.; Li, H. Obesity: Epidemiology, Pathophysiology, and Therapeutics. Front. Endocrinol. 2021, 6, 1070. [Google Scholar] [CrossRef] [PubMed]
- Watson, K.E. Plasma lipoprotein concentrations in ethnic populations. Curr. Cardiol. Rep. 2003, 5, 483–487. [Google Scholar] [CrossRef]
- Raposo, H.F.; Paiva, A.A.; Kato, L.S.; De Oliveira, H.C. Apolipoprotein CIII overexpression exacerbates diet-induced obesity due to adipose tissue higher exogenous lipid uptake and retention and lower lipolysis rates. Nutr. Metab. 2015, 12, 61. [Google Scholar] [CrossRef] [Green Version]
- Paiva, A.A.; Raposo, H.F.; Wanschel, A.C.; Nardelli, T.R.; Oliveira, H.C. Apolipoprotein CIII Overexpression-Induced Hy-pertriglyceridemia Increases Nonalcoholic Fatty Liver Disease in Association with Inflammation and Cell Death. Oxid. Med. Cell. Longev. 2017, 2017, 1838679. [Google Scholar] [CrossRef]
- Altomonte, J.; Cong, L.; Harbaran, S.; Richter, A.; Xu, J.; Meseck, M.; Dong, H.H. Foxo1 mediates insulin action on apoC-III and triglyceride metabolism. J. Clin. Investig. 2004, 114, 1493–1503. [Google Scholar] [CrossRef] [Green Version]
- Caron, S.; Verrijken, A.; Mertens, I.; Samanez, C.H.; Mautino, G.; Haas, J.T.; Duran-Sandoval, D.; Prawitt, J.; Francque, S.; Vallez, E.; et al. Transcriptional Activation of Apolipoprotein CIII Expression by Glucose May Contribute to Diabetic Dyslipidemia. Arter. Thromb. Vasc. Biol. 2011, 31, 513–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valladolid-Acebes, I.; Åvall, K.; Recio-López, P.; Moruzzi, N.; Bryzgalova, G.; Björnholm, M.; Krook, A.; Alonso, E.F.; Ericsson, M.; Landfors, F.; et al. Lowering apolipoprotein CIII protects against high-fat di-et-induced metabolic derangements. Sci. Adv. 2021, 7, eabc2931. [Google Scholar] [CrossRef]
- Åvall, K.; Ali, Y.; Leibiger, I.B.; Leibiger, B.; Moede, T.; Paschen, M.; Dicker, A.; Daré, E.; Köhler, M.; Ilegems, E.; et al. Apolipoprotein CIII links islet insulin resistance to β-cell failure in diabetes. Proc. Natl. Acad. Sci. USA 2015, 112, E2611–E2619. [Google Scholar] [CrossRef] [Green Version]
- Duivenvoorden, I.; Teusink, B.; Rensen, P.C.; Romijn, J.A.; Havekes, L.M.; Voshol, P.J. Apolipoprotein C3 Deficiency Results in Diet-Induced Obesity and Aggravated Insulin Resistance in Mice. Diabetes 2005, 54, 664–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borén, J.; Packard, C.J.; Taskinen, M.R. The Roles of ApoC-III on the Metabolism of Triglyceride-Rich Lipoproteins in Humans. Front. Endocrinol. 2020, 11, 474. [Google Scholar] [CrossRef] [PubMed]
- Graham, M.J.; Lee, R.G.; Bell, T.A., III; Fu, W.; Mullick, A.E.; Alexander, V.J.; Singleton, W.; Viney, N.; Geary, R.; Su, J.; et al. Antisense oligonucleotide inhibition of apolipoprotein C-III reduces plasma tri-glycerides in rodents, nonhuman primates, and humans. Circ. Res. 2013, 112, 1479–1490. [Google Scholar] [CrossRef] [PubMed]
- Gaudet, D.; Brisson, D.; Tremblay, K.; Alexander, V.J.; Singleton, W.; Hughes, S.G.; Geary, R.S.; Baker, B.F.; Graham, M.J.; Crooke, R.M.; et al. Targeting APOC3 in the Familial Chylomicronemia Syndrome. N. Engl. J. Med. 2014, 371, 2200–2206. [Google Scholar] [CrossRef] [PubMed]
- Li, F.H.; Wang, Y.; Liu, X.L.; Xu, Q. The silencing of ApoC3 suppresses oxidative stress and inflammatory responses in placenta cells from mice with preeclampsia via inhibition of the NF-κB signaling pathway. Biomed. Pharm. 2018, 107, 1377–1384. [Google Scholar] [CrossRef]
- Brown, W.V.; Levy, R.I.; Fredrickson, D.S. Studies of the Proteins in Human Plasma Very Low Density Lipoproteins. J. Biol. Chem. 1969, 244, 5687–5694. [Google Scholar] [CrossRef]
- Taskinen, M.R.; Borén, J. Why Is Apolipoprotein CIII Emerging as a Novel Therapeutic Target to Reduce the Burden of Car-diovascular Disease? Curr. Atheroscler. Rep. 2016, 18, 59. [Google Scholar] [CrossRef] [Green Version]
- Larsson, M.; Allan, C.M.; Jung, R.S.; Heizer, P.J.; Beigneux, A.P.; Young, S.G.; Fong, L.G. Apolipoprotein C-III inhibits tri-glyceride hydrolysis by GPIHBP1-bound LPL. J. Lipid Res. 2017, 58, 1893–1902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gouni-Berthold, I.; Alexander, V.J.; Yang, Q.; Hurh, E.; Steinhagen-Thiessen, E.; Moriarty, P.M.; Hughes, S.G.; Gaudet, D.; Hegele, R.A.; O’Dea, L.S.L.; et al. Efficacy and safety of vo-lanesorsen in patients with multifactorial chylomicronaemia (COMPASS): A multicentre, double-blind, randomised, place-bo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2021, 9, 264–275. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Misra, A.; Vikram, N.K. Clinical and pathophysiological consequences of abdominal adiposity and abdominal adipose tissue depots. Nutrition 2003, 19, 457–466. [Google Scholar] [CrossRef]
- Suárez-Cuenca, J.A.; De La Peña-Sosa, G.; De La Vega-Moreno, K.; Banderas-Lares, D.Z.; Salamanca-García, M.; Mar-tínez-Hernández, J.E.; Vera-Gómez, E.; Hernández-Patricio, A.; Zamora-Alemán, C.R.; Domínguez-Pérez, G.A.; et al. Enlarged adipocytes from subcutaneous vc. visceral adipose tissue differentially contribute to metabolic dysfunction and atherogenic risk of patients with obesity. Sci. Rep. 2021, 11, 1831. [Google Scholar] [CrossRef]
- Reyes-Farias, M.; Fos-Domenech, J.; Serra, D.; Herrero, L.; Sánchez-Infantes, D. White adipose tissue dysfunction in obesity and aging. Biochem. Pharmacol. 2021, 192, 114723. [Google Scholar] [CrossRef] [PubMed]
- Philipsen, A.; Jørgensen, M.E.; Vistisen, D.; Sandbaek, A.; Almdal, T.P.; Christiansen, J.S.; Lauritzen, T.; Witte, D.R. Associa-tions between ultrasound measures of abdominal fat distribution and indices of glucose metabolism in a population at high risk of type 2 diabetes: The ADDITION-PRO study. PLoS ONE 2015, 10, e0123062. [Google Scholar]
- Majka, S.M.; Miller, H.L.; Helm, K.M.; Acosta, A.S.; Childs, C.R.; Kong, R.; Klemm, D.J. Analysis and Isolation of Adipocytes by Flow Cytometry. Methods Enzymol. 2014, 537, 281–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Z.; Bodkin, N.L.; Ortmeyer, H.K.; Hansen, B.C.; Shuldiner, A.R. Hyperinsulinemia is associated with altered insulin receptor mRNA splicing in muscle of the spontaneously obese diabetic rhesus monkey. J. Clin. Investig. 1994, 94, 1289–1296. [Google Scholar] [CrossRef] [Green Version]
- Rodbell, M. Metabolism of Isolated Fat Cells. I. Effects of Hormones on Glucose Metabolism and Lipolysis. J. Biol. Chem. 1964, 239, 375–380. [Google Scholar] [CrossRef]
- Ramos, M.P.; Crespo-Solans, M.D.; Del Campo, S.; Cacho, J.; Herrera, E. Fat accumulation in the rat during early pregnancy is modulated by enhanced insulin responsiveness. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E318–E328. [Google Scholar] [CrossRef] [Green Version]
- Sevillano, J.; Sánchez-Alonso, M.G.; Zapatería, B.; Calderón, M.; Alcalá, M.; Limones, M.; Pita, J.; Gramage, E.; Vicen-te-Rodríguez, M.; Horrillo, D.; et al. Pleiotrophin deletion alters glucose homeostasis, energy metabolism and brown fat thermogenic function in mice. Diabetologia 2019, 62, 123–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Gene ID | Primer Strand | Primer Sequence (5′→3′) | PCR Size (bp) |
|---|---|---|---|
| apoCIII | Forward | CGCTAAGTAGCGTGCAGGA | 68 |
| Reverse | TCTGAAGTGATTGTCCATCCAG | ||
| ATGL | Forward | GGTCCTCCGAGAGATGTGC | 75 |
| Reverse | TGGTTCAGTAGGCCATTCCTC | ||
| CD137 | Forward | GTCGACCCTGGACGAACTGCTCT | 134 |
| Reverse | CCTCTGGAGTCACACAGAAATGGTGGTA | ||
| CIDEA | Forward | ATCACAACTGGCCTGGTTACG | 136 |
| Reverse | TACTACCCGGTGTCCATTTCT | ||
| DIO2 | Forward | TTGGGGTAGGGAATGTTGGC | 99 |
| Reverse | TCCGTTTCCTCTTTCCGGTG | ||
| HPRT | Forward | CAGTCCCAGCGTCGTGATTA | 167 |
| Reverse | GGCCTCCCATCTCCTTCATG | ||
| HSL | Forward | TCCTCAGAGACCTCCGACTG | 135 |
| Reverse | ACACACTCCTGCGCATAGAC | ||
| IL-1β | Forward | TGGACCTTCCAGGATGAGGACA | 148 |
| Reverse | GTTCATCTCGGAGCCTGTAGTG | ||
| IL-6 | Forward | TACCACTTCACAAGTCGGAGGC | 116 |
| Reverse | CTGCAAGTGCATCATCGTTGTTC | ||
| PGC1α | Forward | CCCTGCCATTGTTAAGACC | 161 |
| Reverse | TGCTGCTGTTCCTGTTTTC | ||
| PPIA | Forward | GGGTTCCTCCTTTCACAGAA | 145 |
| Reverse | GATGCCAGGACCTGTATGCT | ||
| PRDM16 | Forward | CAGCACGGTGAAGCCATTC | 87 |
| Reverse | GCGTGCATCCGCTTGTG | ||
| TBP | Forward | TGCTGTTGGTGATTGTTGGT | 97 |
| Reverse | CTGGCTTGTGTGGGAAAGAT | ||
| Tbx1 | Forward | GGCAGGCAGACGAATGTTC | 102 |
| Reverse | TTGTCATCTACGGGCACAAAG | ||
| TMEM26 | Forward | GAAACCAGTATTGCAGCACCCAAT | 205 |
| Reverse | AATATTAGCAGGAGTGTTTGGTGGA | ||
| TNF | Forward | GGTGCCTATGTCTCAGCCTCTT | 139 |
| Reverse | GCCATAGAACTGATGAGAGGGAG | ||
| UCP-1 | Forward | ACTGCCACACCTCCAGTCATT | 123 |
| Reverse | CTTTGCCTCACTCAGGATTGG | ||
| β-Actin | Forward | CTAAGGCCAACCGTGAAAAG | 104 |
| Reverse | ACCAGAGGCATACAGGGACA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Recio-López, P.; Valladolid-Acebes, I.; Berggren, P.-O.; Juntti-Berggren, L. Apolipoprotein CIII Reduction Protects White Adipose Tissues against Obesity-Induced Inflammation and Insulin Resistance in Mice. Int. J. Mol. Sci. 2022, 23, 62. https://doi.org/10.3390/ijms23010062
Recio-López P, Valladolid-Acebes I, Berggren P-O, Juntti-Berggren L. Apolipoprotein CIII Reduction Protects White Adipose Tissues against Obesity-Induced Inflammation and Insulin Resistance in Mice. International Journal of Molecular Sciences. 2022; 23(1):62. https://doi.org/10.3390/ijms23010062
Chicago/Turabian StyleRecio-López, Patricia, Ismael Valladolid-Acebes, Per-Olof Berggren, and Lisa Juntti-Berggren. 2022. "Apolipoprotein CIII Reduction Protects White Adipose Tissues against Obesity-Induced Inflammation and Insulin Resistance in Mice" International Journal of Molecular Sciences 23, no. 1: 62. https://doi.org/10.3390/ijms23010062
APA StyleRecio-López, P., Valladolid-Acebes, I., Berggren, P.-O., & Juntti-Berggren, L. (2022). Apolipoprotein CIII Reduction Protects White Adipose Tissues against Obesity-Induced Inflammation and Insulin Resistance in Mice. International Journal of Molecular Sciences, 23(1), 62. https://doi.org/10.3390/ijms23010062






