Insights into the Mechanisms of Action of MDA-7/IL-24: A Ubiquitous Cancer-Suppressing Protein
Abstract
1. Introduction
2. Identification and Structure of MDA-7/IL-24
3. Transcriptional Regulation of MDA-7/IL-24
4. Tumor Suppressor Role of MDA-7/IL-24
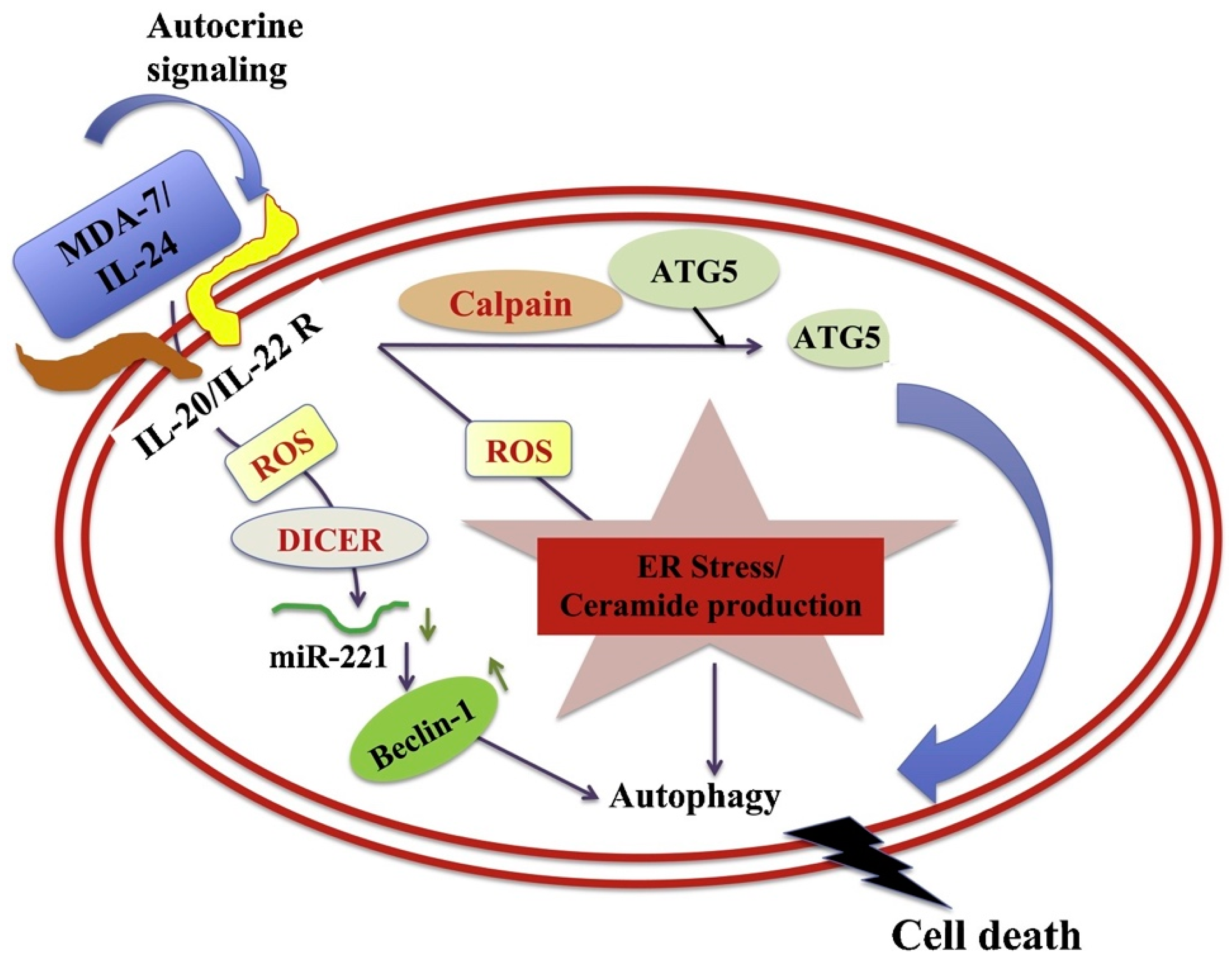
4.1. Apoptosis and Autophagy
4.2. Anti-Angiogenesis
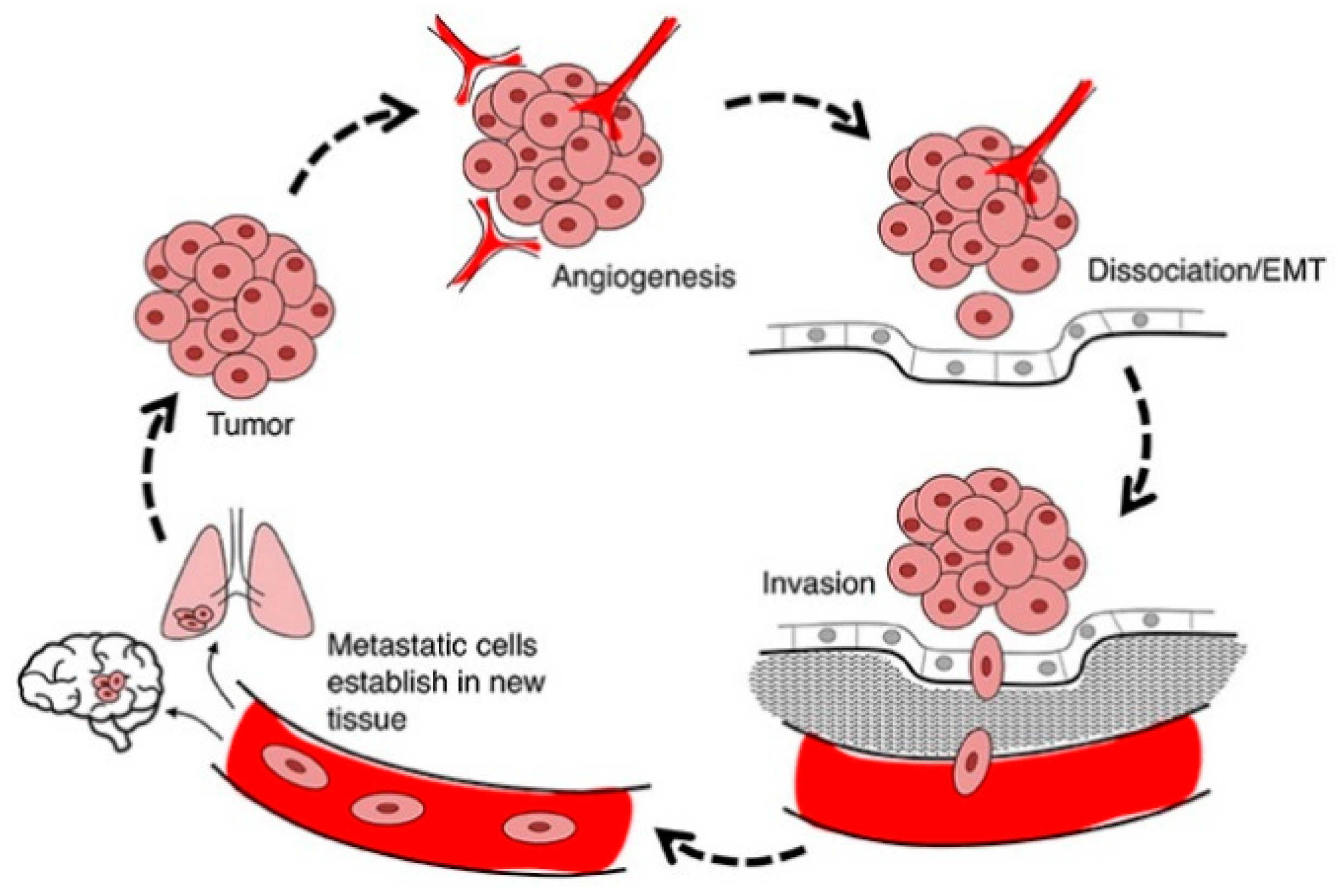
4.3. Anti-Metastasis and Anti-Invasion
4.4. Bystander Effect
4.5. Immunogenic Cell Death
5. MDA-7/IL-24 as a Single Therapeutic
5.1. Virus-Mediated Gene Delivery
5.1.1. Tropism Modification
5.1.2. Conditionally Replication Competent Ad (CRCA)
5.1.3. Ultrasound-Targeted Microbubble-Destruction (UTMD): A Strategy for Targeted Delivery of Therapeutic Agents
5.2. T Cells Expressing MDA-7/IL-24
5.3. Recombinant MDA-7/IL-24 Protein
5.4. Nanoparticle-Mediated Delivery
6. Combination Effects of MDA-7/IL-24 with Other Therapeutic Agents
7. Phase I Clinical Trial of MDA-7/IL-24 (Ad.5-mda-7:INGN-241)
8. Conclusions and Future Prospective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclosure
References
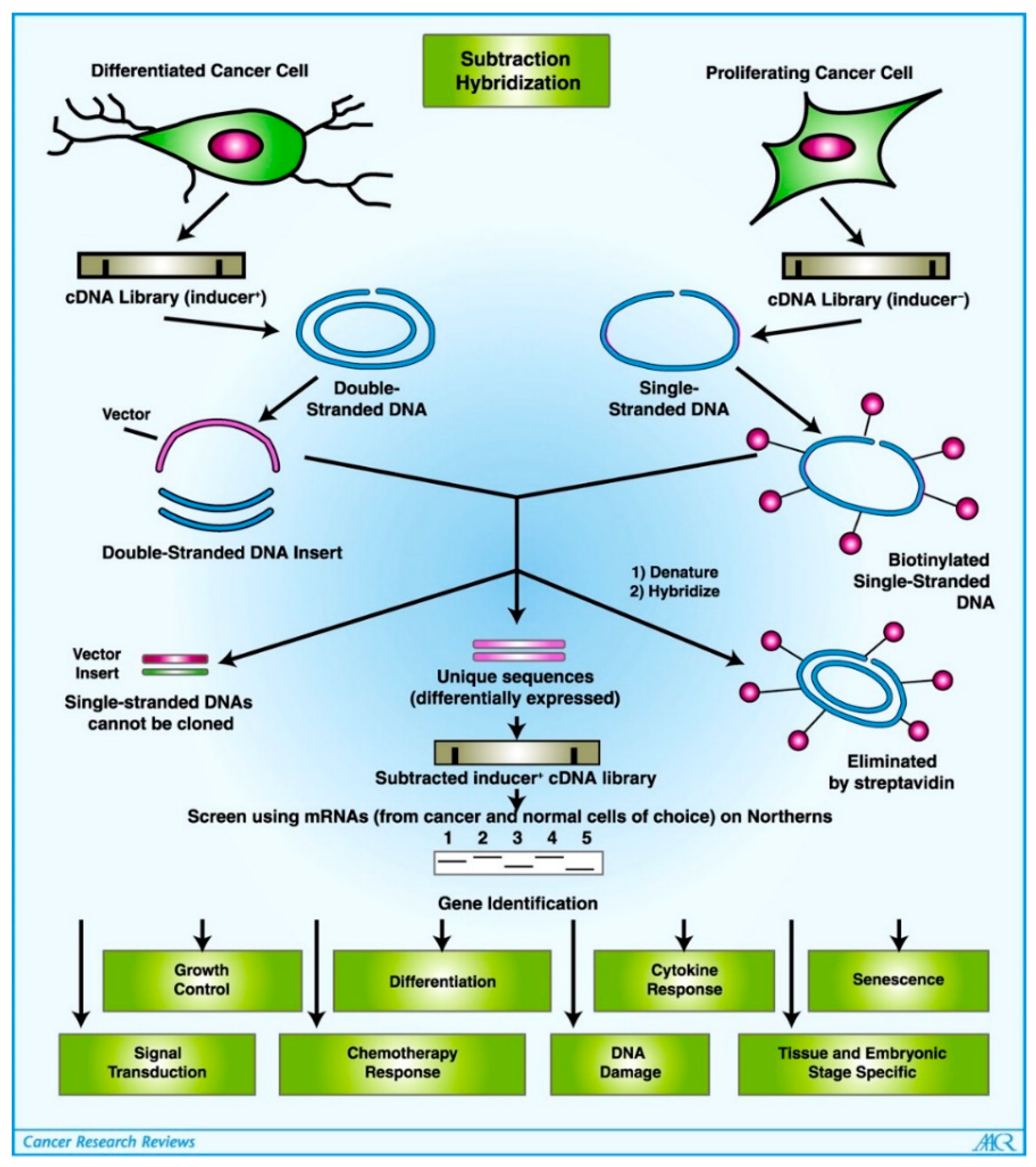
- Jiang, H.; Fisher, P.B. Use of a Sensitive and Efficient Subtraction Hybridization Protocol for the Identification of Genes Differentially Regulated during the Induction of Differentiation in Human Melanoma Cells. Mol. Cell. Differ. 1993, 1, 285–299. [Google Scholar]
- Jiang, H.; Lin, J.J.; Su, Z.Z.; Goldstein, N.I.; Fisher, P.B. Subtraction Hybridization Identifies a Novel Melanoma Differentiation Associated Gene, Mda-7, Modulated during Human Melanoma Differentiation, Growth and Progression. Oncogene 1995, 11, 2477–2486. [Google Scholar]
- Pestka, S.; Krause, C.D.; Sarkar, D.; Walter, M.R.; Shi, Y.; Fisher, P.B. Interleukin-10 and related cytokines and receptors. Annu. Rev. Immunol. 2004, 22, 929–979. [Google Scholar] [CrossRef]
- Jiang, H.; Su, Z.Z.; Lin, J.J.; Goldstein, N.I.; Young, C.S.; Fisher, P.B. The melanoma differentiation associated gene mda-7 suppresses cancer cell growth. Proc. Natl. Acad. Sci. USA 1996, 93, 9160–9165. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.Z.; Madireddi, M.T.; Lin, J.J.; Young, C.S.; Kitada, S.; Reed, J.C.; Goldstein, N.I.; Fisher, P.B. The cancer growth suppressor gene mda-7 selectively induces apoptosis in human breast cancer cells and inhibits tumor growth in nude mice. Proc. Natl. Acad. Sci. USA 1998, 95, 14400–14405. [Google Scholar] [CrossRef] [PubMed]
- Menezes, M.E.; Bhoopathi, P.; Pradhan, A.K.; Emdad, L.; Das, S.K.; Guo, C.; Wang, X.Y.; Sarkar, D.; Fisher, P.B. Role of MDA-7/IL-24 a Multifunction Protein in Human Diseases. Adv. Cancer Res. 2018, 138, 143–182. [Google Scholar] [CrossRef] [PubMed]
- Emdad, L.; Bhoopathi, P.; Talukdar, S.; Pradhan, A.K.; Sarkar, D.; Wang, X.-Y.; Das, S.K.; Fisher, P.B. Recent Insights into Apoptosis and Toxic Autophagy: The Roles of MDA-7/IL-24, a Multidimensional Anti-Cancer Therapeutic. Semin. Cancer Biol. 2020, 66, 140–154. [Google Scholar] [CrossRef]
- Das, S.K.; Sarkar, S.; Dash, R.; Dent, P.; Wang, X.Y.; Sarkar, D.; Fisher, P.B. Cancer Terminator Viruses and Approaches for Enhancing Therapeutic Outcomes. Adv. Cancer Res. 2012, 115, 1–38. [Google Scholar] [CrossRef]
- Emdad, L.; Das, S.K.; Wang, X.-Y.; Sarkar, D.; Fisher, P.B. Cancer Terminator Viruses (CTV): A Better Solution for Viral-Based Therapy of Cancer. J. Cell. Physiol. 2018, 233, 5684–5695. [Google Scholar] [CrossRef]
- Sarkar, D.; Su, Z.Z.; Fisher, P.B. Unique Conditionally Replication Competent Bipartite Adenoviruses-Cancer Terminator Viruses (CTV): Efficacious Reagents for Cancer Gene Therapy. Cell Cycle 2006, 5, 1531–1536. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, Z.; Guo, C.; Das, S.K.; Yu, X.; Pradhan, A.K.; Li, X.; Ning, Y.; Chen, S.; Liu, W.; Windle, J.J.; et al. Engineering T Cells to Express Tumoricidal MDA-7/IL24 Enhances Cancer Immunotherapy. Cancer Res. 2021, 81, 2429–2441. [Google Scholar] [CrossRef] [PubMed]
- Lebedeva, I.V.; Su, Z.Z.; Sarkar, D.; Fisher, P.B. Restoring Apoptosis as a Strategy for Cancer Gene Therapy: Focus on P53 and Mda-7. Semin. Cancer Biol. 2003, 13, 169–178. [Google Scholar] [CrossRef]
- Leszczyniecka, M.; Roberts, T.; Dent, P.; Grant, S.; Fisher, P.B. Differentiation Therapy of Human Cancer: Basic Science and Clinical Applications. Pharmacol. Ther. 2001, 90, 105–156. [Google Scholar] [CrossRef]
- Huang, F.; Adelman, J.; Jiang, H.; Goldstein, N.I.; Fisher, P.B. Identification and Temporal Expression Pattern of Genes Modulated during Irreversible Growth Arrest and Terminal Differentiation in Human Melanoma Cells. Oncogene 1999, 18, 3546–3552. [Google Scholar] [CrossRef]
- Huang, F.; Adelman, J.; Jiang, H.; Goldstein, N.I.; Fisher, P.B. Differentiation Induction Subtraction Hybridization (DISH): A Strategy for Cloning Genes Displaying Differential Expression during Growth Arrest and Terminal Differentiation. Gene 1999, 236, 125–131. [Google Scholar] [CrossRef]
- Fisher, P.B.; Gopalkrishnan, R.V.; Chada, S.; Ramesh, R.; Grimm, E.A.; Rosenfeld, M.R.; Curiel, D.T.; Dent, P. Mda-7/IL-24, a Novel Cancer Selective Apoptosis Inducing Cytokine Gene: From the Laboratory into the Clinic. Cancer Biol. Ther. 2003, 2, S23–S37. [Google Scholar] [CrossRef]
- Fisher, P.B. Is Mda-7/IL-24 a “Magic Bullet” for Cancer? Cancer Res. 2005, 65, 10128–10138. [Google Scholar] [CrossRef]
- Gupta, P.; Su, Z.Z.; Lebedeva, I.V.; Sarkar, D.; Sauane, M.; Emdad, L.; Bachelor, M.A.; Grant, S.; Curiel, D.T.; Dent, P.; et al. Mda-7/IL-24: Multifunctional Cancer-Specific Apoptosis-Inducing Cytokine. Pharmacol. Ther. 2006, 111, 596–628. [Google Scholar] [CrossRef]
- Menezes, M.E.; Bhatia, S.; Bhoopathi, P.; Das, S.K.; Emdad, L.; Dasgupta, S.; Dent, P.; Wang, X.-Y.; Sarkar, D.; Fisher, P.B. MDA-7/IL-24: Multifunctional Cancer Killing Cytokine. Adv. Exp. Med. Biol. 2014, 818, 127–153. [Google Scholar] [CrossRef]
- Emdad, L.; Lebedeva, I.V.; Su, Z.; Gupta, P.; Sauane, M.; Dash, R.; Grant, S.; Dent, P.; Curiel, D.T.; Sarkar, D.; et al. Historical Perspective and Recent Insights into Our Understanding of the Molecular and Biochemical Basis of the Antitumor Properties of Mda-7/IL-24. Cancer Biol. Ther. 2009, 8, 391–400. [Google Scholar] [CrossRef]
- Dent, P.; Yacoub, A.; Hamed, H.A.; Park, M.A.; Dash, R.; Bhutia, S.K.; Sarkar, D.; Gupta, P.; Emdad, L.; Lebedeva, I.V.; et al. MDA-7/IL-24 as a Cancer Therapeutic: From Bench to Bedside. Anticancer Drugs 2010, 21, 725–731. [Google Scholar] [CrossRef]
- Wang, M.; Tan, Z.; Zhang, R.; Kotenko, S.V.; Liang, P. Interleukin 24 (MDA-7/MOB-5) Signals through Two Heterodimeric Receptors, IL-22R1/IL-20R2 and IL-20R1/IL-20R2. J. Biol. Chem. 2002, 277, 7341–7347. [Google Scholar] [CrossRef] [PubMed]
- Dash, R.; Bhoopathi, P.; Das, S.K.; Sarkar, S.; Emdad, L.; Dasgupta, S.; Sarkar, D.; Fisher, P.B. Novel Mechanism of MDA-7/IL-24 Cancer-Specific Apoptosis through SARI Induction. Cancer Res. 2014, 74, 563–574. [Google Scholar] [CrossRef]
- Soo, C.; Shaw, W.W.; Freymiller, E.; Longaker, M.T.; Bertolami, C.N.; Chiu, R.; Tieu, A.; Ting, K. Cutaneous rat wounds express c49a, a novel gene with homology to the human melanoma differentiation associated gene, mda-7. J. Cell Biochem. 1999, 74, 1–10. [Google Scholar] [CrossRef]
- Zhang, R.; Tan, Z.; Liang, P. Identification of a novel ligand-receptor pair constitutively activated by ras oncogenes. J. Biol. Chem. 2000, 275, 24436–24443. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Tan, Z.; Thomas, E.K.; Liang, P. Conservation of the Genomic Structure and Receptor-Mediated Signaling between Human and Rat IL-24. Genes Immun. 2004, 5, 363–370. [Google Scholar] [CrossRef][Green Version]
- Schaefer, G.; Venkataraman, C.; Schindler, U. Cutting edge: FISP (IL-4-induced secreted protein), a novel cytokine-like molecule secreted by Th2 cells. J. Immunol. 2001, 166, 5859–5863. [Google Scholar] [CrossRef]
- Sandey, M.; Bird, R.C.; Das, S.K.; Sarkar, D.; Curiel, D.T.; Fisher, P.B.; Smith, B.F. Characterization of the canine mda-7 gene, transcripts and expression patterns. Gene 2014, 547, 23–33. [Google Scholar] [CrossRef]
- Huang, E.Y.; Madireddi, M.T.; Gopalkrishnan, R.V.; Leszczyniecka, M.; Su, Z.Z.; Lebedeva, I.V.; Kang, D.C.; Jiang, H.; Lin, J.J.; Alexandre, D.; et al. Genomic Structure, Chromosomal Localization and Expression Profile of a Novel Melanoma Differentiation Associated (Mda-7) Gene with Cancer Specific Growth Suppressing and Apoptosis Inducing Properties. Oncogene 2001, 20, 7051–7063. [Google Scholar] [CrossRef] [PubMed]
- Dumoutier, L.; Van Roost, E.; Ameye, G.; Michaux, L.; Renauld, J.C. IL-TIF/IL-22: Genomic Organization and Mapping of the Human and Mouse Genes. Genes Immun. 2000, 1, 488–494. [Google Scholar] [CrossRef]
- Knappe, A.; Hör, S.; Wittmann, S.; Fickenscher, H. Induction of a Novel Cellular Homolog of Interleukin-10, AK155, by Transformation of T Lymphocytes with Herpesvirus Saimiri. J. Virol. 2000, 74, 3881–3887. [Google Scholar] [CrossRef]
- Lubkowski, J.; Sonmez, C.; Smirnov, S.V.; Anishkin, A.; Kotenko, S.V.; Wlodawer, A. Crystal Structure of the Labile Complex of IL-24 with the Extracellular Domains of IL-22R1 and IL-20R2. J. Immunol. 2018, 201, 2082–2093. [Google Scholar] [CrossRef] [PubMed]
- Sauane, M.; Gupta, P.; Lebedeva, I.V.; Su, Z.-Z.; Sarkar, D.; Randolph, A.; Valerie, K.; Gopalkrishnan, R.V.; Fisher, P.B. N-Glycosylation of MDA-7/IL-24 Is Dispensable for Tumor Cell-Specific Apoptosis and “Bystander” Antitumor Activity. Cancer Res. 2006, 66, 11869–11877. [Google Scholar] [CrossRef]
- Madireddi, M.T.; Dent, P.; Fisher, P.B. AP-1 and C/EBP Transcription Factors Contribute to Mda-7 Gene Promoter Activity during Human Melanoma Differentiation. J. Cell. Physiol. 2000, 185, 36–46. [Google Scholar] [CrossRef]
- Madireddi, M.T.; Dent, P.; Fisher, P.B. Regulation of Mda-7 Gene Expression during Human Melanoma Differentiation. Oncogene 2000, 19, 1362–1368. [Google Scholar] [CrossRef]
- Otkjaer, K.; Holtmann, H.; Kragstrup, T.W.; Paludan, S.R.; Johansen, C.; Gaestel, M.; Kragballe, K.; Iversen, L. The P38 MAPK Regulates IL-24 Expression by Stabilization of the 3′ UTR of IL-24 MRNA. PLoS ONE 2010, 5, e8671. [Google Scholar] [CrossRef] [PubMed]
- Andoh, A.; Shioya, M.; Nishida, A.; Bamba, S.; Tsujikawa, T.; Kim-Mitsuyama, S.; Fujiyama, Y. Expression of IL-24, an Activator of the JAK1/STAT3/SOCS3 Cascade, Is Enhanced in Inflammatory Bowel Disease. J. Immunol. 2009, 183, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Pan, H.; Jiang, H.; Du, J.; Wang, X.; Huang, B.; Lu, J. HDAC4 Inhibits the Transcriptional Activation of Mda-7/IL-24 Induced by Sp1. Cell. Mol. Immunol. 2010, 7, 221–226. [Google Scholar] [CrossRef]
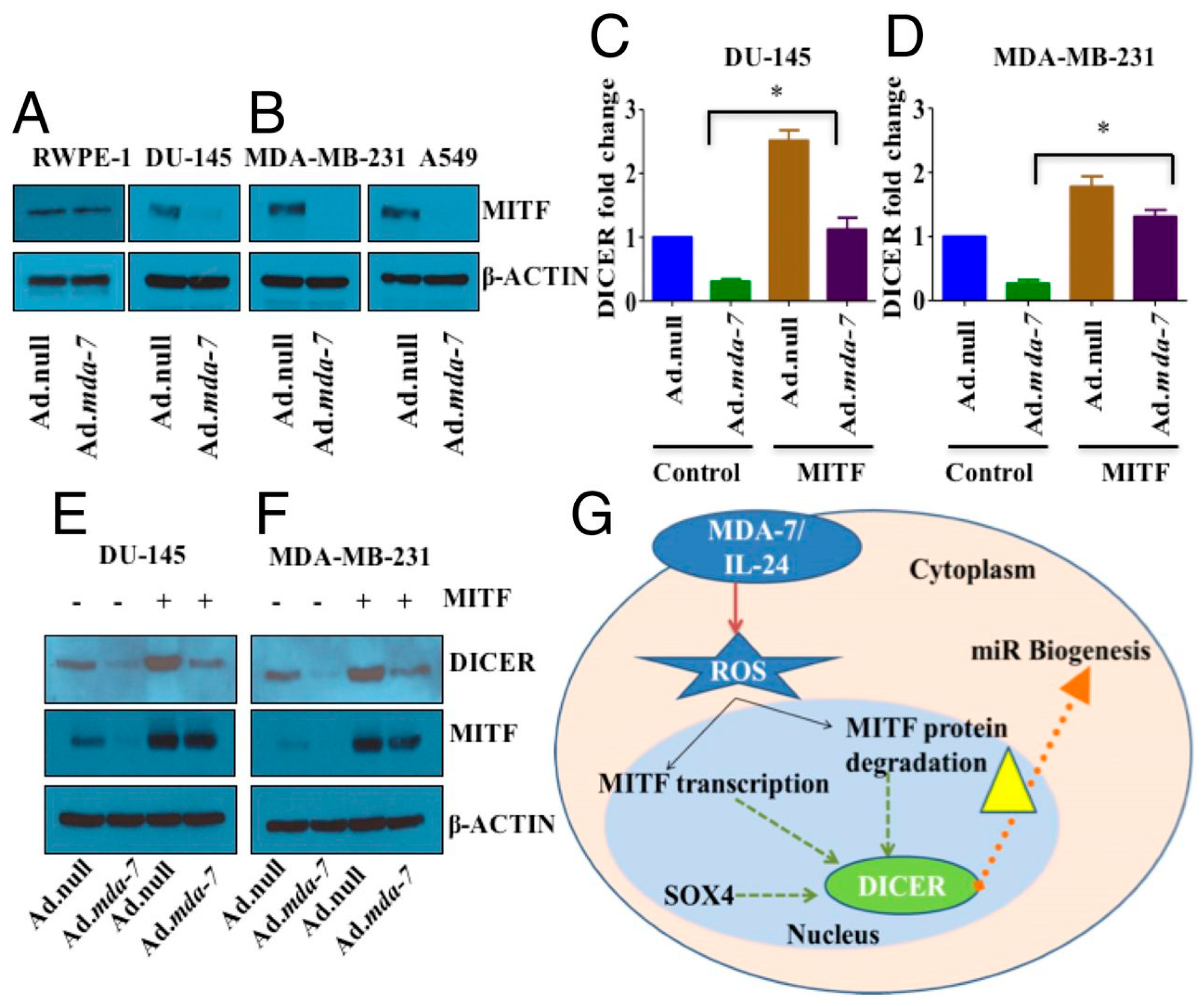
- Pradhan, A.K.; Bhoopathi, P.; Talukdar, S.; Scheunemann, D.; Sarkar, D.; Cavenee, W.K.; Das, S.K.; Emdad, L.; Fisher, P.B. MDA-7/IL-24 Regulates the MiRNA Processing Enzyme DICER through Downregulation of MITF. Proc. Natl. Acad. Sci. USA 2019, 116, 5687–5692. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef]
- Vrana, J.A.; Decker, R.H.; Johnson, C.R.; Wang, Z.; Jarvis, W.D.; Richon, V.M.; Ehinger, M.; Fisher, P.B.; Grant, S. Induction of apoptosis in U937 human leukemia cells by suberoylanilide hydroxamic acid (SAHA) proceeds through pathways that are regulated by Bcl-2/Bcl-XL, c-Jun, and p21CIP1, but independent of p53. Oncogene 1999, 18, 7016–7025. [Google Scholar] [CrossRef] [PubMed]
- Hamed, H.A.; Yacoub, A.; Park, M.A.; Archer, K.; Das, S.K.; Sarkar, D.; Grant, S.; Fisher, P.B.; Dent, P. Histone Deacetylase Inhibitors Interact with Melanoma Differentiation Associated-7/Interleukin-24 to Kill Primary Human Glioblastoma Cells. Mol. Pharmacol. 2013, 84, 171–181. [Google Scholar] [CrossRef]
- Hamed, H.A.; Das, S.K.; Sokhi, U.K.; Park, M.A.; Cruickshanks, N.; Archer, K.; Ogretmen, B.; Grant, S.; Sarkar, D.; Fisher, P.B.; et al. Combining Histone Deacetylase Inhibitors with MDA-7/IL-24 Enhances Killing of Renal Carcinoma Cells. Cancer Biol. Ther. 2013, 14, 1039–1049. [Google Scholar] [CrossRef]
- Lou, W.; Chen, Q.; Ma, L.; Liu, J.; Yang, Z.; Shen, J.; Cui, Y.; Bian, X.W.; Qian, C. Oncolytic Adenovirus Co-Expressing MiRNA-34a and IL-24 Induces Superior Antitumor Activity in Experimental Tumor Model. J. Mol. Med. 2013, 91, 715–725. [Google Scholar] [CrossRef]
- Jan, R.; Chaudhry, G.E. Understanding Apoptosis and Apoptotic Pathways Targeted Cancer Therapeutics. Adv. Pharm. Bull. 2019, 9, 205–218. [Google Scholar] [CrossRef]
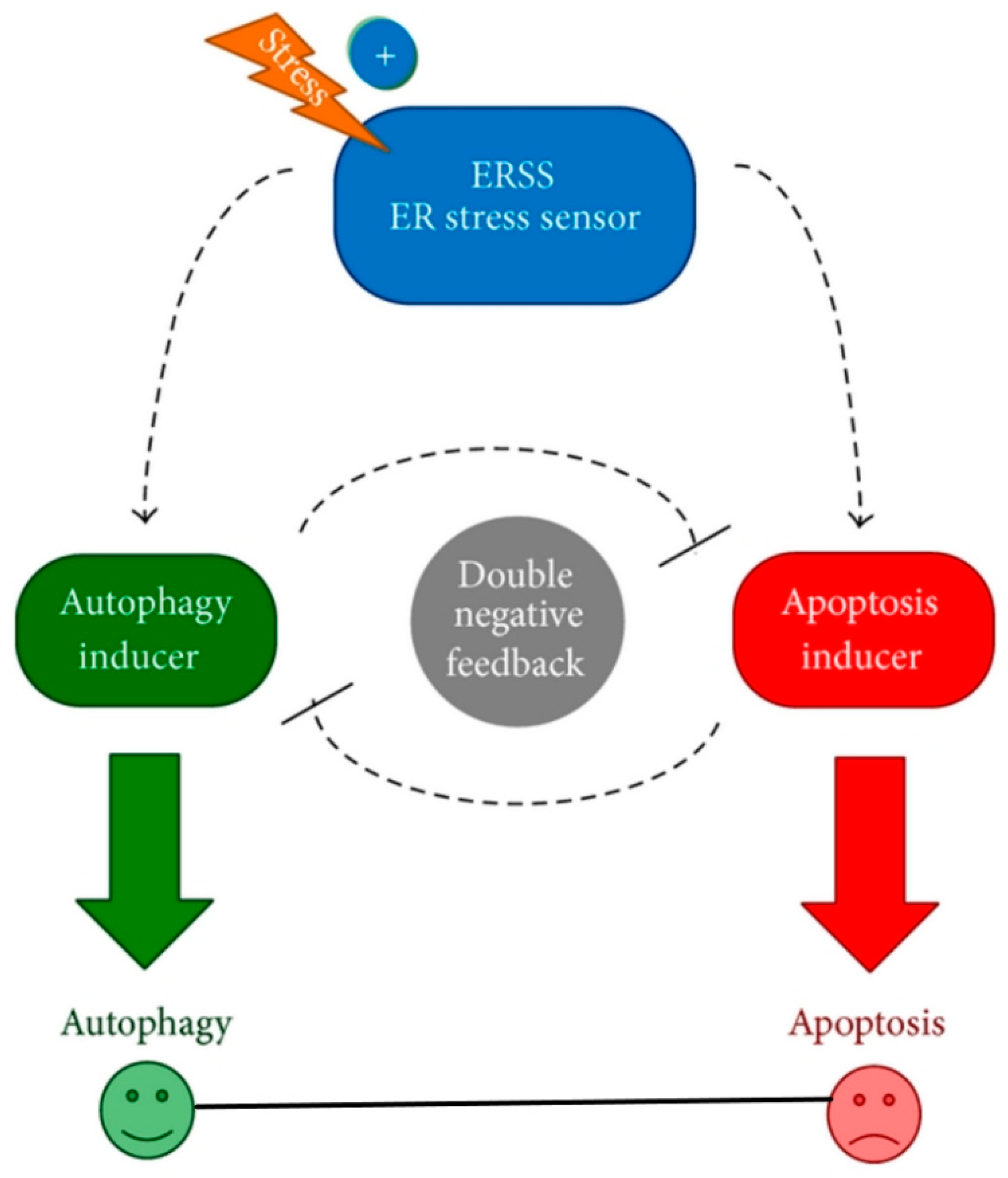
- Holczer, M.; Márton, M.; Kurucz, A.; Bánhegyi, G.; Kapuy, O. A Comprehensive Systems Biological Study of Autophagy-Apoptosis Crosstalk during Endoplasmic Reticulum Stress. BioMed Res. Int. 2015, 2015, 319589. [Google Scholar] [CrossRef] [PubMed]
- Kale, J.; Osterlund, E.J.; Andrews, D.W. BCL-2 Family Proteins: Changing Partners in the Dance towards Death. Cell Death Differ. 2017, 25, 65–80. [Google Scholar] [CrossRef]
- McIlwain, D.R.; Berger, T.; Mak, T.W. Caspase Functions in Cell Death and Disease. Cold Spring Harb. Perspect. Biol. 2015, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Tait, S.W.; Green, D.R. Mitochondria and Cell Death: Outer Membrane Permeabilization and Beyond. Nat. Rev. Mol. Cell. Biol. 2010, 11, 621–632. [Google Scholar] [CrossRef]
- Shamas-Din, A.; Kale, J.; Leber, B.; Andrews, D.W. Mechanisms of Action of Bcl-2 Family Proteins. Cold Spring Harb. Perspect. Biol. 2013, 5, 1–21. [Google Scholar] [CrossRef]
- Wang, C.; Youle, R.J. The Role of Mitochondria in Apoptosis. Annu. Rev. Genet. 2009, 43, 95. [Google Scholar] [CrossRef]
- Roufayel, R. Regulation of Stressed-Induced Cell Death by the Bcl-2 Family of Apoptotic Proteins. Mol. Membr. Biol. 2017, 33, 89–99. [Google Scholar] [CrossRef]
- Sieger, K.A.; Mhashilkar, A.M.; Stewart, A.; Sutton, R.B.; Strube, R.W.; Chen, S.Y.; Pataer, A.; Swisher, S.G.; Grimm, E.A.; Ramesh, R.; et al. The Tumor Suppressor Activity of MDA-7/IL-24 Is Mediated by Intracellular Protein Expression in NSCLC Cells. Mol. Ther. 2004, 9, 355–367. [Google Scholar] [CrossRef]
- Lebedeva, I.V.; Su, Z.Z.; Chang, Y.; Kitada, S.; Reed, J.C.; Fisher, P.B. The Cancer Growth Suppressing Gene Mda-7 Induces Apoptosis Selectively in Human Melanoma Cells. Oncogene 2002, 21, 708–718. [Google Scholar] [CrossRef]
- Dash, R.; Bhutia, S.K.; Azab, B.; Su, Z.Z.; Quinn, B.A.; Kegelmen, T.P.; Das, S.K.; Kim, K.; Lee, S.G.; Park, M.A.; et al. mda-7/IL-24: A unique member of the IL-10 gene family promoting cancer-targeted toxicity. Cytokine Growth Factor Rev. 2010, 21, 381–391. [Google Scholar] [CrossRef] [PubMed]
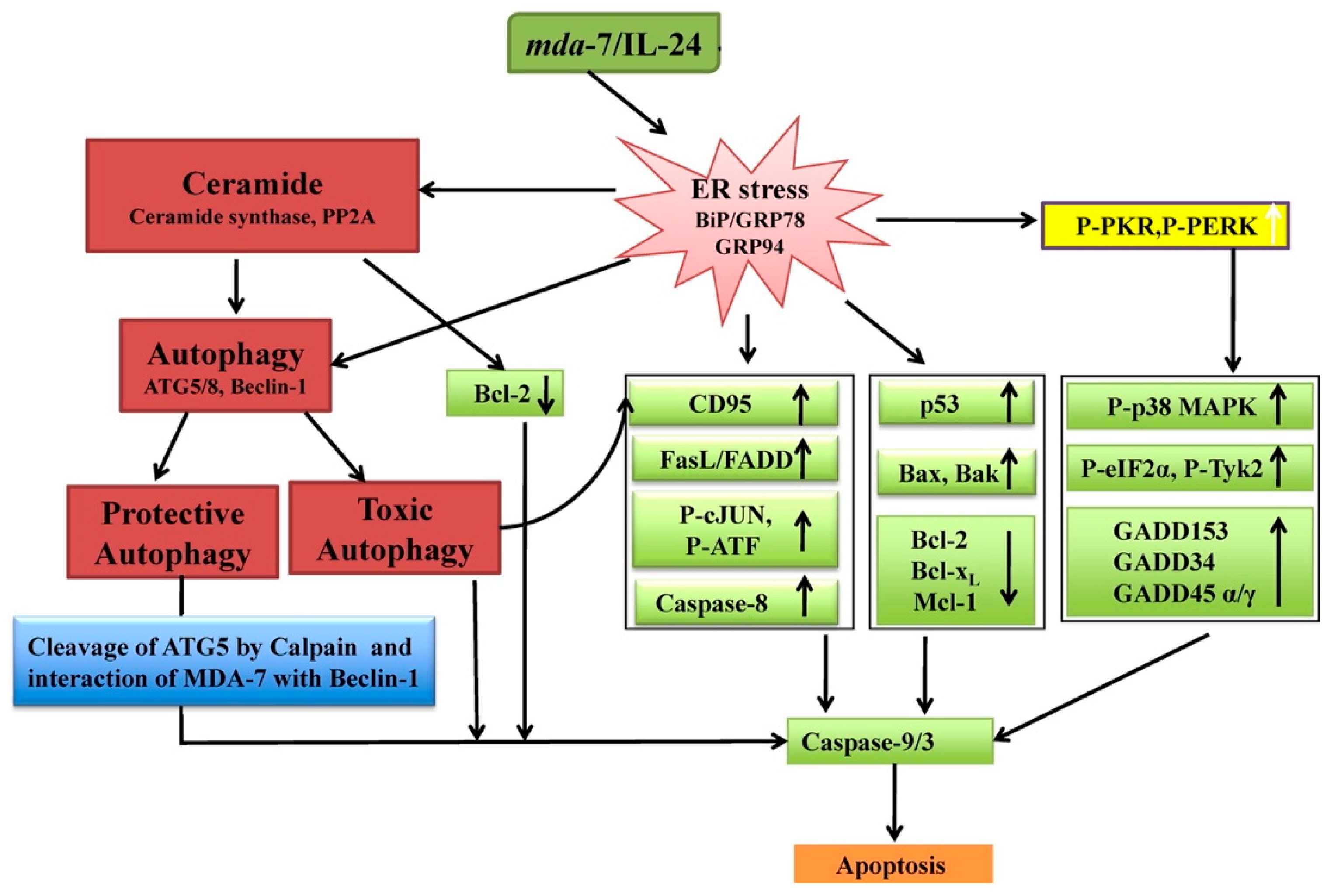
- Sauane, M.; Su, Z.Z.; Dash, R.; Liu, X.; Norris, J.S.; Sarkar, D.; Lee, S.G.; Allegood, J.C.; Dent, P.; Spiegel, S.; et al. Ceramide Plays a Prominent Role in MDA-7/IL-24-Induced Cancer-Specific Apoptosis. J. Cell. Physiol. 2010, 222, 546–555. [Google Scholar] [CrossRef]
- Park, M.A.; Walker, T.; Martin, A.P.; Allegood, J.; Vozhilla, N.; Emdad, L.; Sarkar, D.; Rahmani, M.; Graf, M.; Yacoub, A.; et al. MDA-7/IL-24-induced cell killing in malignant renal carcinoma cells occurs by a ceramide/CD95/PERK-dependent mechanism. Mol. Cancer Ther. 2009, 8, 1280–1291. [Google Scholar] [CrossRef] [PubMed]
- Park, M.A.; Hamed, H.A.; Mitchell, C.; Cruickshanks, N.; Dash, R.; Allegood, J.; Dmitriev, I.P.; Tye, G.; Ogretmen, B.; Spiegel, S.; et al. A serotype 5/3 adenovirus expressing MDA-7/IL-24 infects renal carcinoma cells and promotes toxicity of agents that increase ROS and ceramide levels. Mol. Pharmacol. 2011, 79, 368–380. [Google Scholar] [CrossRef]
- Pradhan, A.K.; Talukdar, S.; Bhoopathi, P.; Shen, X.N.; Emdad, L.; Das, S.K.; Sarkar, D.; Fisher, P.B. Mda-7/IL-24 Mediates Cancer Cell-Specific Death via Regulation of MiR-221 and the Beclin-1 Axis. Cancer Res. 2017, 77, 949–959. [Google Scholar] [CrossRef]
- Wechman, S.L.; Pradhan, A.K.; DeSalle, R.; Das, S.K.; Emdad, L.; Sarkar, D.; Fisher, P.B. New Insights Into Beclin-1: Evolution and Pan-Malignancy Inhibitor Activity. Adv. Cancer Res. 2018, 137, 77–114. [Google Scholar] [CrossRef] [PubMed]
- Nishida, N.; Yano, H.; Nishida, T.; Kamura, T.; Kojiro, M. Angiogenesis in Cancer. Vasc. Health Risk Manag. 2006, 2, 213–219. [Google Scholar] [CrossRef]
- Tonini, T.; Rossi, F.; Claudio, P.P. Molecular Basis of Angiogenesis and Cancer. Oncogene 2003, 22, 6549–6556. [Google Scholar] [CrossRef]
- Ramesh, R.; Mhashilkar, A.M.; Tanaka, F.; Saito, Y.; Branch, C.D.; Sieger, K.; Mumm, J.B.; Stewart, A.L.; Boquoi, A.; Dumoutier, L.; et al. Melanoma Differentiation-Associated Gene 7/Interleukin (IL)-24 Is a Novel Ligand That Regulates Angiogenesis via the IL-22 Receptor. Cancer Res. 2003, 63, 5105–5113. [Google Scholar]
- Chada, S.; Sutton, R.B.; Ekmekcioglu, S.; Ellerhorst, J.; Mumm, J.B.; Leitner, W.W.; Yang, H.-Y.; Sahin, A.A.; Hunt, K.K.; Fuson, K.L.; et al. MDA-7/IL-24 Is a Unique Cytokine--Tumor Suppressor in the IL-10 Family. Int. Immunopharmacol. 2004, 4, 649–667. [Google Scholar] [CrossRef] [PubMed]
- Lebedeva, I.V.; Emdad, L.; Su, Z.-Z.; Gupta, P.; Sauane, M.; Sarkar, D.; Staudt, M.R.; Liu, S.-J.; Taher, M.M.; Xiao, R.; et al. Mda-7/IL-24, Novel Anticancer Cytokine: Focus on Bystander Antitumor, Radiosensitization and Antiangiogenic Properties and Overview of the Phase I Clinical Experience (Review). Int. J. Oncol. 2007, 31, 985–1007. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bhutia, S.K.; Das, S.K.; Kegelman, T.P.; Azab, B.; Dash, R.; Su, Z.Z.; Wang, X.Y.; Rizzi, F.; Bettuzzi, S.; Lee, S.G.; et al. mda-7/IL-24 differentially regulates soluble and nuclear clusterin in prostate cancer. J. Cell. Physiol. 2012, 227, 1805–1813. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, T.; Ramesh, R.; Munshi, A.; Chada, S.; Meyn, R.E. Adenovirus-Mediated Mda-7 (IL24) Gene Therapy Suppresses Angiogenesis and Sensitizes NSCLC Xenograft Tumors to Radiation. Mol. Ther. 2004, 9, 818–828. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.; Branch, C.D.; Gallick, G.E.; Chada, S.; Ramesh, R. Inhibition of Src kinase activity by Ad-mda7 suppresses vascular endothelial growth factor expression in prostate carcinoma cells. Mol. Ther. 2005, 12, 707–715. [Google Scholar] [CrossRef]
- Ramesh, R.; Ito, I.; Gopalan, B.; Saito, Y.; Mhashilkar, A.M.; Chada, S. Ectopic Production of MDA-7/IL-24 Inhibits Invasion and Migration of Human Lung Cancer Cells. Mol. Ther. 2004, 9, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Wei, L.-L.; Yuan, C.-F.; Yang, J.-X.; Yi, F.-P.; Ma, Y.-P.; Song, F.-Z. Melanoma Differentiation-Associated Gene-7/Interleukin 24 Inhibits Invasion and Migration of Human Cervical Cancer Cells in Vitro. Saudi Med. J. 2007, 28, 1671–1675. [Google Scholar]
- Huo, W.; Li, Z.M.; Zhu, X.M.; Bao, Y.M.; An, L.J. MDA-7/IL-24 suppresses tumor adhesion and invasive potential in hepatocellular carcinoma cell lines. Oncol. Rep. 2013, 30, 986–992. [Google Scholar] [CrossRef][Green Version]
- Wang, C.J.; Zhang, H.; Chen, K.; Zheng, J.W.; Xiao, C.W.; Ji, W.W.; Yu, Y.; Hu, H.Y.; Li, Y.; Xue, X.B. Ad.mda-7 (IL-24) selectively induces apoptosis in hepatocellular carcinoma cell lines, suppresses metastasis, and enhances the effect of doxorubicin on xenograft tumors. Oncol. Res. 2010, 18, 561–574. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, A.K.; Bhoopathi, P.; Talukdar, S.; Shen, X.N.; Emdad, L.; Das, S.K.; Sarkar, D.; Fisher, P.B. Recombinant MDA-7/IL24 Suppresses Prostate Cancer Bone Metastasis through Downregulation of the Akt/Mcl-1 Pathway. Mol. Cancer Ther. 2018, 17, 1951–1960. [Google Scholar] [CrossRef] [PubMed]
- De Ieso, M.L.; Yool, A.J. Mechanisms of Aquaporin-Facilitated Cancer Invasion and Metastasis. Front. Chem. 2018, 6, 135. [Google Scholar] [CrossRef]
- Su, Z.; Emdad, L.; Sauane, M.; Lebedeva, I.V.; Sarkar, D.; Gupta, P.; James, C.D.; Randolph, A.; Valerie, K.; Walter, M.R.; et al. Unique Aspects of Mda-7/IL-24 Antitumor Bystander Activity: Establishing a Role for Secretion of MDA-7/IL-24 Protein by Normal Cells. Oncogene 2005, 24, 7552–7566. [Google Scholar] [CrossRef]
- Sauane, M.; Su, Z.Z.; Gupta, P.; Lebedeva, I.V.; Dent, P.; Sarkar, D.; Fisher, P.B. Autocrine regulation of mda-7/IL-24 mediates cancer-specific apoptosis. Proc. Natl. Acad. Sci. USA 2008, 105, 9763–9768. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, A.K.; Bhoopathi, P.; Talukdar, S.; Das, S.K.; Emdad, L.; Sarkar, D.; Ivanov, A.I.; Fisher, P.B. Mechanism of Internalization of MDA-7/IL-24 Protein and Its Cognate Receptors Following Ligand-Receptor Docking. Oncotarget 2019, 10, 5103. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Su, Z.; Lebedeva, I.V.; Gopalkrishnan, R.V.; Goldstein, N.I.; Stein, C.A.; Reed, J.C.; Dent, P.; Fisher, P.B. A combinatorial approach for selectively inducing programmed cell death in human pancreatic cancer cells. Proc. Natl. Acad. Sci. USA 2001, 98, 10332–10337. [Google Scholar] [CrossRef]
- Lebedeva, I.V.; Sarkar, D.; Su, Z.Z.; Gopalkrishnan, R.V.; Athar, M.; Randolph, A.; Valerie, K.; Dent, P.; Fisher, P.B. Molecular target-based therapy of pancreatic cancer. Cancer Res. 2006, 66, 2403–2413. [Google Scholar] [CrossRef][Green Version]
- Quinn, B.A.; Lee, N.A.; Kegelman, T.P.; Bhoopathi, P.; Emdad, L.; Das, S.K.; Pellecchia, M.; Sarkar, D.; Fisher, P.B. The Quest for an Effective Treatment for an Intractable Cancer: Established and Novel Therapies for Pancreatic Adenocarcinoma. Adv. Cancer Res. 2015, 127, 283–306. [Google Scholar] [CrossRef]
- Buscail, L.; Bournet, B.; Cordelier, P. Role of Oncogenic KRAS in the Diagnosis, Prognosis and Treatment of Pancreatic Cancer. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 153–168. [Google Scholar] [CrossRef]
- Chada, S.; Mhashilkar, A.M.; Ramesh, R.; Mumm, J.B.; Sutton, R.B.; Bocangel, D.; Zheng, M.; Grimm, E.A.; Ekmekcioglu, S. Bystander Activity of Ad-Mda7: Human MDA-7 Protein Kills Melanoma Cells via an IL-20 Receptor-Dependent but STAT3-Independent Mechanism. Mol. Ther. 2004, 10, 1085–1095. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D.; Su, Z.Z.; Vozhilla, N.; Park, E.S.; Gupta, P.; Fisher, P.B. Dual cancer-specific targeting strategy cures primary and distant breast carcinomas in nude mice. Proc. Natl. Acad. Sci. USA 2005, 102, 14034–14039. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D.; Su, Z.Z.; Park, E.S.; Vozhilla, N.; Dent, P.; Curiel, D.T.; Fisher, P.B. A cancer terminator virus eradicates both primary and distant human melanomas. Cancer Gene Ther. 2008, 15, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Persaud, L.; De Jesus, D.; Brannigan, O.; Richiez-Paredes, M.; Huaman, J.; Alvarado, G.; Riker, L.; Mendez, G.; Dejoie, J.; Sauane, M. Mechanism of Action and Applications of Interleukin 24 in Immunotherapy. Int. J. Mol. Sci. 2016, 17, 869. [Google Scholar] [CrossRef]
- Caudell, E.G.; Mumm, J.B.; Poindexter, N.; Ekmekcioglu, S.; Mhashilkar, A.M.; Yang, X.H.; Retter, M.W.; Hill, P.; Chada, S.; Grimm, E.A. The protein product of the tumor suppressor gene, melanoma differentiation-associated gene 7, exhibits immunostimulatory activity and is designated IL-24. J. Immunol. 2002, 168, 6041–6046. [Google Scholar] [CrossRef]
- Miyahara, R.; Banerjee, S.; Kawano, K.; Efferson, C.; Tsuda, N.; Miyahara, Y.; Ioannides, C.G.; Chada, S.; Ramesh, R. Melanoma Differentiation-Associated Gene-7 (Mda-7)/Interleukin (IL)-24 Induces Anticancer Immunity in a Syngeneic Murine Model. Cancer Gene Ther. 2006, 13, 753–761. [Google Scholar] [CrossRef]
- Ma, Y.F.; Ren, Y.; Wu, C.J.; Zhao, X.H.; Xu, H.; Wu, D.Z.; Xu, J.; Zhang, X.L.; Ji, Y. Interleukin (IL)-24 transforms the tumor microenvironment and induces anticancer immunity in a murine model of colon cancer. Mol. Immunol. 2016, 75, 11–20. [Google Scholar] [CrossRef]
- Gao, P.; Sun, X.; Chen, X.; Wang, Y.; Foster, B.A.; Subjeck, J.; Fisher, P.B.; Wang, X.-Y. Secretable Chaperone Grp170 Enhances Therapeutic Activity of a Novel Tumor Suppressor, Mda-7/IL-24. Cancer Res. 2008, 68, 3890–3898. [Google Scholar] [CrossRef]
- Menezes, M.E.; Shen, X.N.; Das, S.K.; Emdad, L.; Guo, C.; Yuan, F.; Li, Y.J.; Archer, M.C.; Zacksenhaus, E.; Windle, J.J.; et al. MDA-7/IL-24 functions as a tumor suppressor gene in vivo in transgenic mouse models of breast cancer. Oncotarget 2015, 6, 36928–36942. [Google Scholar] [CrossRef]
- Glasgow, J.N.; Bauerschmitz, G.J.; Curiel, D.T.; Hemminki, A. Transductional and transcriptional targeting of adenovirus for clinical applications. Curr. Gene Ther. 2004, 4, 1–14. [Google Scholar] [CrossRef]
- Dash, R.; Dmitriev, I.; Su, Z.Z.; Bhutia, S.K.; Azab, B.; Vozhilla, N.; Yacoub, A.; Dent, P.; Curiel, D.T.; Sarkar, D.; et al. Enhanced delivery of mda-7/IL-24 using a serotype chimeric adenovirus (Ad.5/3) improves therapeutic efficacy in low CAR prostate cancer cells. Cancer Gene Ther. 2010, 17, 447–456. [Google Scholar] [CrossRef][Green Version]
- Hamed, H.A.; Yacoub, A.; Park, M.A.; Eulitt, P.J.; Dash, R.; Sarkar, D.; Dmitriev, I.P.; Lesniak, M.S.; Shah, K.; Grant, S.; et al. Inhibition of multiple protective signaling pathways and Ad.5/3 delivery enhances mda-7/IL-24 therapy of malignant glioma. Mol. Ther. 2010, 18, 1130–1142. [Google Scholar] [CrossRef]
- Azab, B.; Dash, R.; Das, S.K.; Bhutia, S.K.; Shen, X.N.; Quinn, B.A.; Sarkar, S.; Wang, X.Y.; Hedvat, M.; Dmitriev, I.P.; et al. Enhanced delivery of mda-7/IL-24 using a serotype chimeric adenovirus (Ad.5/3) in combination with the Apogossypol derivative BI-97C1 (Sabutoclax) improves therapeutic efficacy in low CAR colorectal cancer cells. J. Cell. Physiol. 2012, 227, 2145–2153. [Google Scholar] [CrossRef]
- Heise, C.; Kirn, D.H. Replication-selective adenoviruses as oncolytic agents. J. Clin. Investig. 2000, 105, 847–851. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D.; Lebedeva, I.V.; Su, Z.Z.; Park, E.S.; Chatman, L.; Vozhilla, N.; Dent, P.; Curiel, D.T.; Fisher, P.B. Eradication of therapy-resistant human prostate tumors using a cancer terminator virus. Cancer Res. 2007, 67, 5434–5442. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.Z.; Sarkar, D.; Emdad, L.; Duigou, G.J.; Young, C.S.; Ware, J.; Randolph, A.; Valerie, K.; Fisher, P.B. Targeting gene expression selectively in cancer cells by using the progression-elevated gene-3 promoter. Proc. Natl. Acad. Sci. USA 2005, 102, 1059–1064. [Google Scholar] [CrossRef]
- Bhang, H.E.; Gabrielson, K.L.; Laterra, J.; Fisher, P.B.; Pomper, M.G. Tumor-specific imaging through progression elevated gene-3 promoter-driven gene expression. Nat. Med. 2011, 17, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Azab, B.M.; Dash, R.; Das, S.K.; Bhutia, S.K.; Sarkar, S.; Shen, X.N.; Quinn, B.A.; Dent, P.; Dmitriev, I.P.; Wang, X.Y.; et al. Enhanced prostate cancer gene transfer and therapy using a novel serotype chimera cancer terminator virus (Ad.5/3-CTV). J. Cell. Physiol. 2014, 229, 34–43. [Google Scholar] [CrossRef]
- Bhoopathi, P.; Pradhan, A.K.; Maji, S.; Das, S.K.; Emdad, L.; Fisher, P.B. Theranostic Tripartite Cancer Terminator Virus for Cancer Therapy and Imaging. Cancers 2021, 13, 857. [Google Scholar] [CrossRef] [PubMed]
- Bhoopathi, P.; Lee, N.; Pradhan, A.K.; Shen, X.N.; Das, S.K.; Sarkar, D.; Emdad, L.; Fisher, P.B. mda-7/IL-24 Induces Cell Death in Neuroblastoma through a Novel Mechanism Involving AIF and ATM. Cancer Res. 2016, 76, 3572–3582. [Google Scholar] [CrossRef]
- Zhao, L.; Gu, J.; Dong, A.; Zhang, Y.; Zhong, L.; He, L.; Wang, Y.; Zhang, J.; Zhang, Z.; Huiwang, J.; et al. Potent antitumor activity of oncolytic adenovirus expressing mda-7/IL-24 for colorectal cancer. Hum. Gene Ther. 2005, 16, 845–858. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Liu, J.; Tong, Y.; Yan, S.; Yang, C.; Yang, M.; Liu, X. Enhanced antitumor activity by a selective conditionally replicating adenovirus combining with MDA-7/interleukin-24 for B-lymphoblastic leukemia via induction of apoptosis. Leukemia 2008, 22, 361–369. [Google Scholar] [CrossRef]
- Yang, C.; Tong, Y.; Ni, W.; Liu, J.; Xu, W.; Li, L.; Liu, X.; Meng, H.; Qian, W. Inhibition of autophagy induced by overexpression of mda-7/interleukin-24 strongly augments the antileukemia activity in vitro and in vivo. Cancer Gene Ther. 2010, 17, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Cheng, Q.; Bai, J.; Qi, Y.D.; Liu, J.J.; Li, L.T.; Zheng, J.N. An oncolytic adenovirus expressing interleukin-24 enhances antitumor activities in combination with paclitaxel in breast cancer cells. Mol. Med. Rep. 2013, 8, 1416–1424. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Yang, C.S.; Xu, D.; Sun, C.; Zheng, J.N.; Lei, T.C.; Liu, Y.Q. Potent anti-tumour activity of a novel conditionally replicating adenovirus for melanoma via inhibition of migration and invasion. Br. J. Cancer 2014, 110, 2496–2505. [Google Scholar] [CrossRef] [PubMed]
- Howard, C.M.; Forsberg, F.; Minimo, C.; Liu, J.B.; Merton, D.A.; Claudio, P.P. Ultrasound guided site specific gene delivery system using adenoviral vectors and commercial ultrasound contrast agents. J. Cell. Physiol. 2006, 209, 413–421. [Google Scholar] [CrossRef]
- Wu, J.; Li, R.K. Ultrasound-targeted microbubble destruction in gene therapy: A new tool to cure human diseases. Genes Dis. 2016, 4, 64–74. [Google Scholar] [CrossRef]
- Mayer, C.R.; Geis, N.A.; Katus, H.A.; Bekeredjian, R. Ultrasound targeted microbubble destruction for drug and gene delivery. Expert Opin. Drug Deliv. 2008, 5, 1121–1138. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Yang, F.; Lin, Y.; Zhang, J.S.; Qiu, R.X.; Jiang, L.; Zhou, X.X.; Yu, J.X. New development and application of ultrasound targeted microbubble destruction in gene therapy and drug delivery. Curr. Gene Ther. 2013, 13, 250–274. [Google Scholar] [CrossRef]
- Greco, A.; Di Benedetto, A.; Howard, C.M.; Kelly, S.; Nande, R.; Dementieva, Y.; Miranda, M.; Brunetti, A.; Salvatore, M.; Claudio, L.; et al. Eradication of therapy-resistant human prostate tumors using an ultrasound-guided site-specific cancer terminator virus delivery approach. Mol. Ther. 2010, 18, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Dash, R.; Azab, B.; Quinn, B.A.; Shen, X.; Wang, X.-Y.; Das, S.K.; Rahmani, M.; Wei, J.; Hedvat, M.; Dent, P.; et al. Apogossypol Derivative BI-97C1 (Sabutoclax) Targeting Mcl-1 Sensitizes Prostate Cancer Cells to Mda-7/IL-24-Mediated Toxicity. Proc. Natl. Acad. Sci. USA 2011, 108, 8785–8790. [Google Scholar] [CrossRef]
- Dash, R.; Azab, B.; Shen, X.N.; Sokhi, U.K.; Sarkar, S.; Su, Z.Z.; Wang, X.Y.; Claudio, P.P.; Dent, P.; Dmitriev, I.P.; et al. Developing an effective gene therapy for prostate cancer: New technologies with potential to translate from the laboratory into the clinic. Discov. Med. 2011, 11, 46–56. [Google Scholar] [PubMed]
- Sarkar, S.; Quinn, B.A.; Shen, X.N.; Dash, R.; Das, S.K.; Emdad, L.; Klibanov, A.L.; Wang, X.Y.; Pellecchia, M.; Sarkar, D.; et al. Therapy of prostate cancer using a novel cancer terminator virus and a small molecule BH-3 mimetic. Oncotarget 2015, 6, 10712–10727. [Google Scholar] [CrossRef] [PubMed]
- June, C.H.; O’Connor, R.S.; Kawalekar, O.U.; Ghassemi, S.; Milone, M.C. CAR T cell immunotherapy for human cancer. Science 2018, 359, 1361–1365. [Google Scholar] [CrossRef]
- Mirzaei, H.R.; Rodriguez, A.; Shepphird, J.; Brown, C.E.; Badie, B. Chimeric Antigen Receptors T Cell Therapy in Solid Tumor: Challenges and Clinical Applications. Front. Immunol. 2017, 8, 1850. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Menezes, M.E.; Bhatia, S.; Wang, X.Y.; Emdad, L.; Sarkar, D.; Fisher, P.B. Gene Therapies for Cancer: Strategies, Challenges and Successes. J. Cell. Physiol. 2015, 230, 259. [Google Scholar] [CrossRef]
- Zhu, Q.; Pan, X.; Sun, Y.; Wang, Z.; Liu, F.; Li, A.; Zhao, Z.; Wang, Y.; Li, K.; Mi, L. Biological Nanoparticles Carrying the Hmda-7 Gene Are Effective in Inhibiting Pancreatic Cancer in Vitro and in Vivo. PLoS ONE 2017, 12, e0185507. [Google Scholar] [CrossRef]
- Ramesh, R.; Ito, I.; Saito, Y.; Wu, Z.; Mhashikar, A.M.; Wilson, D.R.; Branch, C.D.; Roth, J.A.; Chada, S. Local and Systemic Inhibition of Lung Tumor Growth after Nanoparticle-Mediated Mda-7/IL-24 Gene Delivery. DNA Cell Biol. 2004, 23, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Inoue, S.; Hartman, A.; Branch, C.D.; Bucana, C.D.; Bekele, B.N.; Stephens, L.C.; Chada, S.; Ramesh, R. mda-7 In combination with bevacizumab treatment produces a synergistic and complete inhibitory effect on lung tumor xenograft. Mol. Ther. 2007, 15, 287–294. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, T.; Liu, Y.; Fanale, M.; Swisher, S.G.; Chada, S.; Hunt, K.K. Combination Therapy of Ad-Mda7 and Trastuzumab Increases Cell Death in Her-2/Neu-Overexpressing Breast Cancer Cells. Surgery 2004, 136, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Chada, S.; Mhashilkar, A.M.; Liu, Y.; Nishikawa, T.; Bocangel, D.; Zheng, M.; Vorburger, S.A.; Pataer, A.; Swisher, S.G.; Ramesh, R.; et al. Mda-7 Gene Transfer Sensitizes Breast Carcinoma Cells to Chemotherapy, Biologic Therapies and Radiotherapy: Correlation with Expression of Bcl-2 Family Members. Cancer Gene Ther. 2006, 13, 490–502. [Google Scholar] [CrossRef] [PubMed]
- Suh, Y.-J.; Chada, S.; McKenzie, T.; Liu, Y.; Swisher, S.G.; Lucci, A.; Hunt, K.K. Synergistic Tumoricidal Effect between Celecoxib and Adenoviral-Mediated Delivery of Mda-7 in Human Breast Cancer Cells. Surgery 2005, 138, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Yacoub, A.; Liu, R.; Park, M.A.; Hamed, H.A.; Dash, R.; Schramm, D.N.; Sarkar, D.; Dimitriev, I.P.; Bell, J.K.; Grant, S.; et al. Cisplatin Enhances Protein Kinase R-like Endoplasmic Reticulum Kinase- and CD95-Dependent Melanoma Differentiation-Associated Gene-7/Interleukin-24-Induced Killing in Ovarian Carcinoma Cells. Mol. Pharmacol. 2010, 77, 298–310. [Google Scholar] [CrossRef]
- Yang, M.; Yang, C.; Tao, Y.; Tang, J.; Huang, Q.; Guo, W.; Feng, S.; Jiang, A.; Xu, X.; Jiang, G.; et al. Combination Therapy with F5/35 Fiber Chimeric Conditionally Replicative Adenoviruses Expressing IL-24 Enhances the Antitumor Effect of Temozolomide against Melanoma. Cancer Med. 2018, 7, 5928–5942. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lv, J.; Zhang, T. Combination of IL-24 and Cisplatin Inhibits Angiogenesis and Lymphangiogenesis of Cervical Cancer Xenografts in a Nude Mouse Model by Inhibiting VEGF, VEGF-C and PDGF-B. Oncol. Rep. 2015, 33, 2468–2476. [Google Scholar] [CrossRef]
- Xiao, L.; Li, X.; Niu, N.; Qian, J.; Xie, G.; Wang, Y. Dichloroacetate (DCA) Enhances Tumor Cell Death in Combination with Oncolytic Adenovirus Armed with MDA-7/IL-24. Mol. Cell. Biochem. 2010, 340, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.-G.; Kwon, J.; Ekmekcioglu, S.; Poindexter, N.J.; Grimm, E.A. IL-24 Gene Transfer Sensitizes Melanoma Cells to Erlotinib through Modulation of the Apaf-1 and Akt Signaling Pathways. Melanoma Res. 2011, 21, 44–56. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, R.; Tao, X.; Dong, Y.; Lv, X.; Sun, A.; Wei, D. TAT-IL-24-KDEL-Induced Apoptosis Is Inhibited by Survivin but Restored by the Small Molecular Survivin Inhibitor, YM155, in Cancer Cells. Oncotarget 2016, 7, 37030–37042. [Google Scholar] [CrossRef]
- Wang, C.; Li, Q.; Xiao, B.; Fang, H.; Huang, B.; Huang, F.; Wang, Y. Luteolin Enhances the Antitumor Efficacy of Oncolytic Vaccinia Virus That Harbors IL-24 Gene in Liver Cancer Cells. J. Clin. Lab. Anal. 2021, 35, e23677. [Google Scholar] [CrossRef]
- Emdad, L.; Lebedeva, I.V.; Su, Z.-Z.; Gupta, P.; Sarkar, D.; Settleman, J.; Fisher, P.B. Combinatorial Treatment of Non-Small-Cell Lung Cancers with Gefitinib and Ad.Mda-7 Enhances Apoptosis-Induction and Reverses Resistance to a Single Therapy. J. Cell. Physiol. 2007, 210, 549–559. [Google Scholar] [CrossRef]
- Lebedeva, I.V.; Washington, I.; Sarkar, D.; Clark, J.A.; Fine, R.L.; Dent, P.; Curiel, D.T.; Turro, N.J.; Fisher, P.B. Strategy for Reversing Resistance to a Single Anticancer Agent in Human Prostate and Pancreatic Carcinomas. Proc. Natl. Acad. Sci. USA 2007, 104, 3484–3489. [Google Scholar] [CrossRef] [PubMed]
- Lebedeva, I.V.; Su, Z.Z.; Vozhilla, N.; Chatman, L.; Sarkar, D.; Dent, P.; Athar, M.; Fisher, P.B. Mechanism of in vitro pancreatic cancer cell growth inhibition by melanoma differentiation-associated gene-7/interleukin-24 and perillyl alcohol. Cancer Res. 2008, 68, 7439–7447. [Google Scholar] [CrossRef]
- Lebedeva, I.V.; Su, Z.Z.; Vozhilla, N.; Chatman, L.; Sarkar, D.; Dent, P.; Athar, M.; Fisher, P.B. Chemoprevention by perillyl alcohol coupled with viral gene therapy reduces pancreatic cancer pathogenesis. Mol. Cancer Ther. 2008, 7, 2042–2050. [Google Scholar] [CrossRef] [PubMed]
- Lebedeva, I.V.; Su, Z.Z.; Sarkar, D.; Gopalkrishnan, R.V.; Waxman, S.; Yacoub, A.; Dent, P.; Fisher, P.B. Induction of reactive oxygen species renders mutant and wild-type K-ras pancreatic carcinoma cells susceptible to Ad.mda-7-induced apoptosis. Oncogene 2005, 24, 585–596. [Google Scholar] [CrossRef]
- Yacoub, A.; Mitchell, C.; Brannon, J.; Rosenberg, E.; Qiao, L.; McKinstry, R.; Linehan, W.M.; Su, Z.Z.; Sarkar, D.; Lebedeva, I.V.; et al. MDA-7 (interleukin-24) inhibits the proliferation of renal carcinoma cells and interacts with free radicals to promote cell death and loss of reproductive capacity. Mol. Cancer Ther. 2003, 2, 623–632. [Google Scholar]
- Su, Z.Z.; Lebedeva, I.V.; Sarkar, D.; Gopalkrishnan, R.V.; Sauane, M.; Sigmon, C.; Yacoub, A.; Valerie, K.; Dent, P.; Fisher, P.B. Melanoma differentiation associated gene-7, mda-7/IL-24, selectively induces growth suppression, apoptosis and radiosensitization in malignant gliomas in a p53-independent manner. Oncogene 2003, 22, 1164–1180. [Google Scholar] [CrossRef]
- Yacoub, A.; Mitchell, C.; Hong, Y.; Gopalkrishnan, R.V.; Su, Z.Z.; Gupta, P.; Sauane, M.; Lebedeva, I.V.; Curiel, D.T.; Mahasreshti, P.J.; et al. MDA-7 regulates cell growth and radiosensitivity in vitro of primary (non-established) human glioma cells. Cancer Biol. Ther. 2004, 3, 739–751. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nishikawa, T.; Munshi, A.; Story, M.D.; Ismail, S.; Stevens, C.; Chada, S.; Meyn, R.E. Adenoviral-mediated mda-7 expression suppresses DNA repair capacity and radiosensitizes non-small-cell lung cancer cells. Oncogene 2004, 23, 7125–7131. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Su, Z.Z.; Lebedeva, I.V.; Sarkar, D.; Emdad, L.; Gupta, P.; Kitada, S.; Dent, P.; Reed, J.C.; Fisher, P.B. Ionizing radiation enhances therapeutic activity of mda-7/IL-24: Overcoming radiation- and mda-7/IL-24-resistance in prostate cancer cells overexpressing the antiapoptotic proteins bcl-xL or bcl-2. Oncogene 2006, 25, 2339–2348. [Google Scholar] [CrossRef] [PubMed]
- Emdad, L.; Sarkar, D.; Lebedeva, I.V.; Su, Z.Z.; Gupta, P.; Mahasreshti, P.J.; Dent, P.; Curiel, D.T.; Fisher, P.B. Ionizing radiation enhances adenoviral vector expressing mda-7/IL-24-mediated apoptosis in human ovarian cancer. J. Cell. Physiol. 2006, 208, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Yacoub, A.; Hamed, H.; Emdad, L.; Dos Santos, W.; Gupta, P.; Broaddus, W.C.; Ramakrishnan, V.; Sarkar, D.; Shah, K.; Curiel, D.T.; et al. MDA-7/IL-24 plus radiation enhance survival in animals with intracranial primary human GBM tumors. Cancer Biol. Ther. 2008, 7, 917–933. [Google Scholar] [CrossRef]
- Yacoub, A.; Mitchell, C.; Lebedeva, I.V.; Sarkar, D.; Su, Z.Z.; McKinstry, R.; Gopalkrishnan, R.V.; Grant, S.; Fisher, P.B.; Dent, P. mda-7 (IL-24) Inhibits growth and enhances radiosensitivity of glioma cells in vitro via JNK signaling. Cancer Biol. Ther. 2003, 2, 347–353. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y.; Sun, P.; Xie, Y.; Xiang, J.; Yang, J. Enhanced therapeutic efficacy of adenovirus-mediated interleukin-24 gene therapy combined with ionizing radiotherapy for nasopharyngeal carcinoma. Oncol. Rep. 2013, 30, 1165–1174. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Bocangel, D.; Ramesh, R.; Ekmekcioglu, S.; Poindexter, N.; Grimm, E.A.; Chada, S. Interleukin-24 overcomes temozolomide resistance and enhances cell death by down-regulation of O6-methylguanine-DNA methyltransferase in human melanoma cells. Mol. Cancer Ther. 2008, 7, 3842–3851. [Google Scholar] [CrossRef]
- Germano, I.M.; Emdad, L.; Qadeer, Z.A.; Binello, E.; Uzzaman, M. Embryonic stem cell (ESC)-mediated transgene delivery induces growth suppression, apoptosis and radiosensitization, and overcomes temozolomide resistance in malignant gliomas. Cancer Gene Ther. 2010, 17, 664–674. [Google Scholar] [CrossRef]
- Gupta, P.; Emdad, L.; Lebedeva, I.V.; Sarkar, D.; Dent, P.; Curiel, D.T.; Settleman, J.; Fisher, P.B. Targeted combinatorial therapy of non-small cell lung carcinoma using a GST-fusion protein of full-length or truncated MDA-7/IL-24 with Tarceva. J. Cell. Physiol. 2008, 215, 827–836. [Google Scholar] [CrossRef]
- Wu, Y.M.; Zhang, K.J.; Yue, X.T.; Wang, Y.Q.; Yang, Y.; Li, G.C.; Li, N.; Wang, Y.G. Enhancement of tumor cell death by combining cisplatin with an oncolytic adenovirus carrying MDA-7/IL-24. Acta Pharmacol. Sin. 2009, 30, 467–477. [Google Scholar] [CrossRef]
- Eulitt, P.J.; Park, M.A.; Hamed, H.A.; Cruikshanks, N.; Yang, C.; Dmitriev, I.P.; Yacoub, A.; Curiel, D.T.; Fisher, P.B.; Dent, P. Enhancing Mda-7/IL-24 Therapy in Renal Carcinoma Cells by Inhibiting Multiple Protective Signaling Pathways Using Sorafenib and by Ad.5/3 Gene Delivery. Cancer Biol. Ther. 2010, 10, 1290–1305. [Google Scholar] [CrossRef]
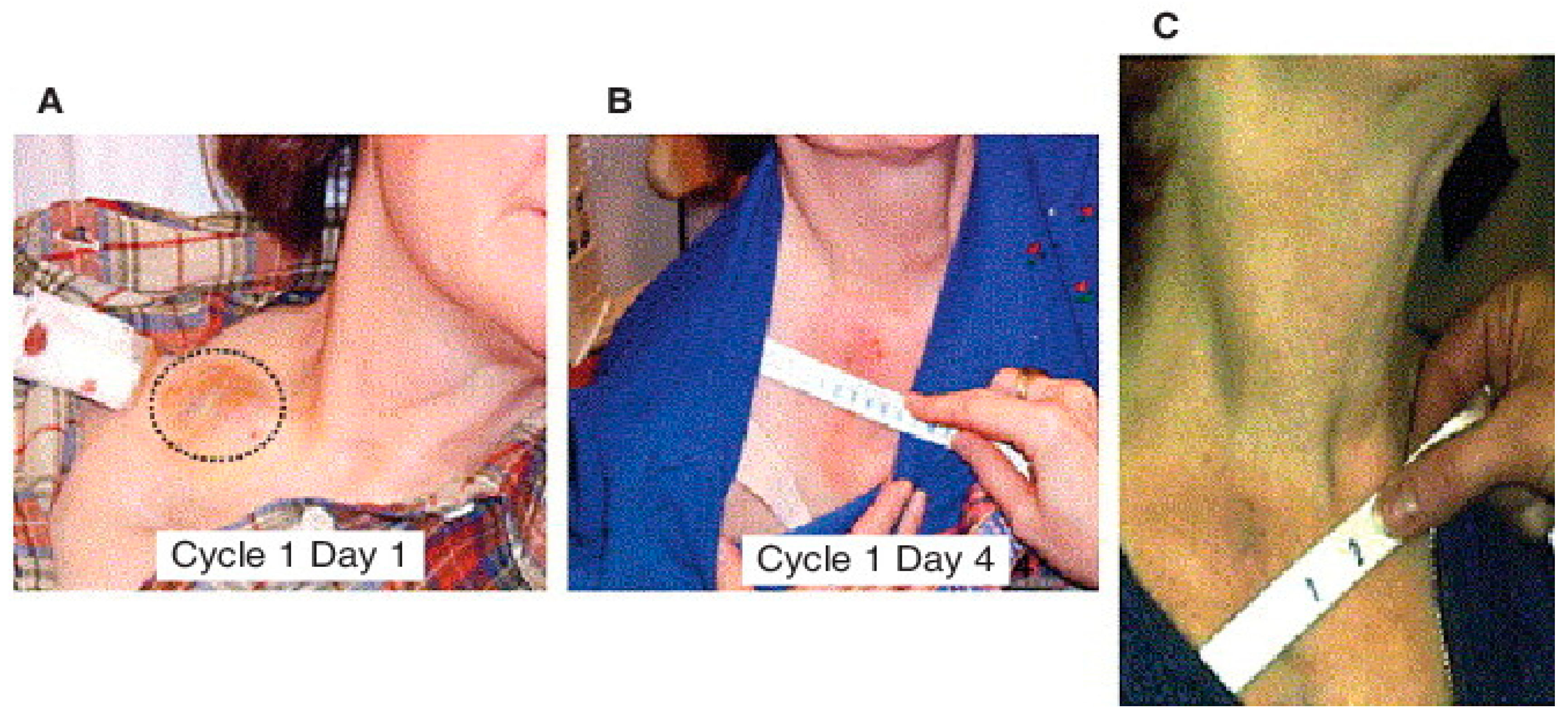
- Tong, A.W.; Nemunaitis, J.; Su, D.; Zhang, Y.; Cunningham, C.; Senzer, N.; Netto, G.; Rich, D.; Mhashilkar, A.; Parker, K.; et al. Intratumoral Injection of INGN 241, a Nonreplicating Adenovector Expressing the Melanoma-Differentiation Associated Gene-7 (Mda-7/IL24): Biologic Outcome in Advanced Cancer Patients. Mol. Ther. 2005, 11, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, C.C.; Chada, S.; Merritt, J.A.; Tong, A.; Senzer, N.; Zhang, Y.; Mhashilkar, A.; Parker, K.; Vukelja, S.; Richards, D.; et al. Clinical and Local Biological Effects of an Intratumoral Injection of Mda-7 (IL24; INGN 241) in Patients with Advanced Carcinoma: A Phase I Study. Mol. Ther. 2005, 11, 149–159. [Google Scholar] [CrossRef]
- Dent, P.; Yacoub, A.; Hamed, H.A.; Park, M.A.; Dash, R.; Bhutia, S.K.; Sarkar, D.; Wang, X.Y.; Gupta, P.; Emdad, L.; et al. The development of MDA-7/IL-24 as a cancer therapeutic. Pharmacol. Ther. 2010, 128, 375–384. [Google Scholar] [CrossRef]
- Fisher, P.B.; Sarkar, D.; Lebedeva, I.V.; Emdad, L.; Gupta, P.; Sauane, M.; Su, Z.Z.; Grant, S.; Dent, P.; Curiel, D.T.; et al. Melanoma differentiation associated gene-7/interleukin-24 (mda-7/IL-24): Novel gene therapeutic for metastatic melanoma. Toxicol. Appl. Pharmacol. 2007, 224, 300–307. [Google Scholar] [CrossRef][Green Version]
- Lebedeva, I.V.; Sauane, M.; Gopalkrishnan, R.V.; Sarkar, D.; Su, Z.Z.; Gupta, P.; Nemunaitis, J.; Cunningham, C.; Yacoub, A.; Dent, P.; et al. mda-7/IL-24: Exploiting cancer’s Achilles’ heel. Mol. Ther. 2005, 11, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Eager, R.; Harle, L.; Nemunaitis, J. Ad-MDA-7; INGN 241: A review of preclinical and clinical experience. Expert Opin. Biol. Ther. 2008, 8, 1633–1643. [Google Scholar] [CrossRef]
- Rasoolian, M.; Kheirollahi, M.; Hosseini, S.Y. MDA-7/interleukin 24 (IL-24) in tumor gene therapy: Application of tumor penetrating/homing peptides for improvement of the effects. Expert Opin. Biol. Ther. 2019, 19, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Rastegari, M.; Shiri, A.; Behzad-Behbahani, A.; Rasoolian, M.; Zare, F.; Rafiei, G.; Mortazavi, M.; Sharifzadeh, S.; Hosseini, S.Y. The Evaluation of tLyP-1-Bound Mda-7/IL-24 Killing Activity on a Liver Tumor Cell Line. Cancer Biother. Radiopharm. 2020. [Google Scholar] [CrossRef]

| COMBINATION TREATMENT | CANCER TYPE | MECHANISM | REF |
|---|---|---|---|
| Ad.5mda-7 + bevacizumab | Lung tumor xenograft | Treated lung tumor cells showed lower VEGF ligand-receptor binding, lower cell survival, significant growth arrest and apoptosis. | [121] |
| Ad.5/3mda-7 + HDAC inhibitor | Renal cell carcinoma | This combination led to activation of CD95, dihydro-ceramide/ROS/Ca2 + generation and ER stress. | [43] |
| Ad5.mda-7 + trastuzumab (Herceptin) | Breast cancer | Inhibited the β-catenin and AKT pathway in HER-2/neu overexpressing breast cancer cells. | [122] |
| Ad5.mda-7 + radiotherapy | Breast cancer | Mda-7 expressing cells showed synergistic cytotoxicity and apoptosis due to decreased Bcl-2 expression and Bax upregulation. | [123] |
| Ad5.mda-7 + cox-2 inhibitor (celecoxib) | Breast cancer | Mda-7 treatment downregulated AKT and simultaneously inhibited Cox-2 expression, promoting apoptosis. | [124] |
| Ad.5/3.mda-7 + cisplatin/paclitaxel | Ovarian cancer | Combination of paclitaxel significantly enhanced (additive effect) the tumor cell killing by Ad5/3.mda-7 + cisplatin treatment. | [125] |
| Ad5.mda-7 + sabutoclax (BI-97C1) | Prostate cancer | Sabutoclax inhibited mcl-1 and synergized with mda-7, preventing tumor growth, angiogenesis and regulating immune responses. | [112] |
| F5/35-Zd55-IL-24 + temozolomide | Melanoma | F5/35-zd55-IL-24 and TMZ increased the level of pro-apoptotic proteins and decreased anti-apoptotic proteins. | [126] |
| IL-24 + cisplatin | Cervical cancer | IL-24 (mda-7) enhanced the tumor chemosensitivity to cisplatin by downregulating the VEGF, VEGF-c and PDGF-b expression. | [127] |
| Zd55-IL-24 + dichloroacetate | Liver cancer | This combination treatment promoted translocation of Bax from the cytoplasm to mitochondria and promoted apoptosis, without altering bcl-2 expression. | [128] |
| IL-24 + Erlotinib | Melanoma | IL-24 (mda-7) sensitized melanoma cells to Erlotinib by modulating apaf-1 and AKT pathways. | [129] |
| Tat-IL-24-kdel + survivin inhibitor (ym155) | Melanoma | Inhibition of survivin promoted the apoptosis promoting efficiency of tat-IL-24-kdel in melanoma cells. | [130] |
| vv-IL-24 + luteolin | Hepatic cancer | Luteolin promoted vv-IL-24 gene expression in liver cancer cells using in vitro and in vivo experiments. | [131] |
| Ad.mda-7 + gefitinib | Non-small cell lung cancer | This combination inhibited p-EGFR, p-ERK and p-AKT levels in NSCLC cells. | [132] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Modi, J.; Roy, A.; Pradhan, A.K.; Kumar, A.; Talukdar, S.; Bhoopathi, P.; Maji, S.; Mannangatti, P.; Sanchez De La Rosa, D.; Li, J.; et al. Insights into the Mechanisms of Action of MDA-7/IL-24: A Ubiquitous Cancer-Suppressing Protein. Int. J. Mol. Sci. 2022, 23, 72. https://doi.org/10.3390/ijms23010072
Modi J, Roy A, Pradhan AK, Kumar A, Talukdar S, Bhoopathi P, Maji S, Mannangatti P, Sanchez De La Rosa D, Li J, et al. Insights into the Mechanisms of Action of MDA-7/IL-24: A Ubiquitous Cancer-Suppressing Protein. International Journal of Molecular Sciences. 2022; 23(1):72. https://doi.org/10.3390/ijms23010072
Chicago/Turabian StyleModi, Jinkal, Abhishek Roy, Anjan K. Pradhan, Amit Kumar, Sarmistha Talukdar, Praveen Bhoopathi, Santanu Maji, Padmanabhan Mannangatti, Daniel Sanchez De La Rosa, Jiong Li, and et al. 2022. "Insights into the Mechanisms of Action of MDA-7/IL-24: A Ubiquitous Cancer-Suppressing Protein" International Journal of Molecular Sciences 23, no. 1: 72. https://doi.org/10.3390/ijms23010072
APA StyleModi, J., Roy, A., Pradhan, A. K., Kumar, A., Talukdar, S., Bhoopathi, P., Maji, S., Mannangatti, P., Sanchez De La Rosa, D., Li, J., Guo, C., Subler, M. A., Windle, J. J., Cavenee, W. K., Sarkar, D., Wang, X.-Y., Das, S. K., Emdad, L., & Fisher, P. B. (2022). Insights into the Mechanisms of Action of MDA-7/IL-24: A Ubiquitous Cancer-Suppressing Protein. International Journal of Molecular Sciences, 23(1), 72. https://doi.org/10.3390/ijms23010072








