Profiling of Non-Coding Regulators and Their Targets in Epicardial Fat from Patients with Coronary Artery Disease
Abstract
:1. Introduction
2. Results
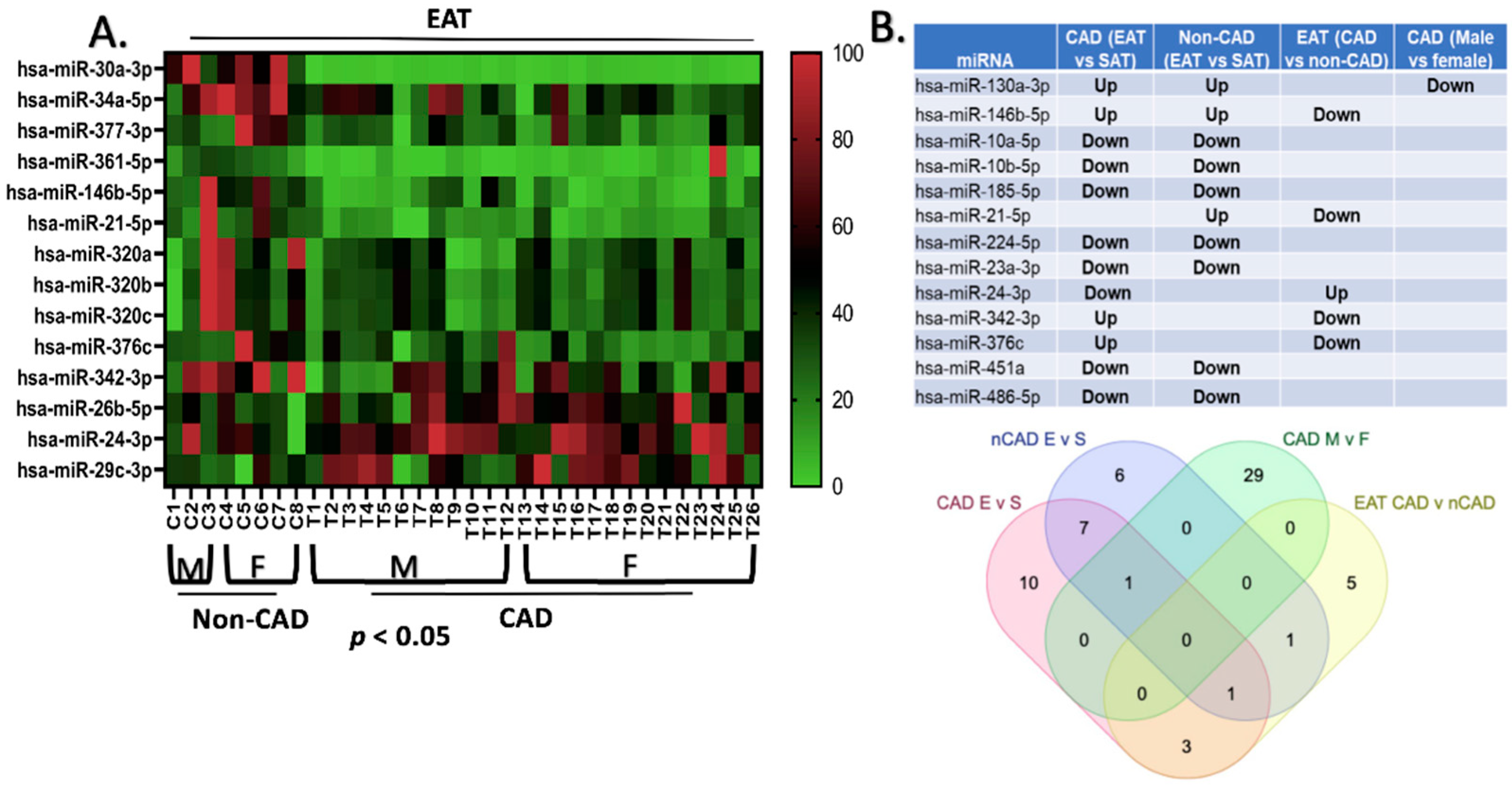
2.1. Differential Expression of miRNAs between EAT and SAT in CAD and Non-CAD Patients
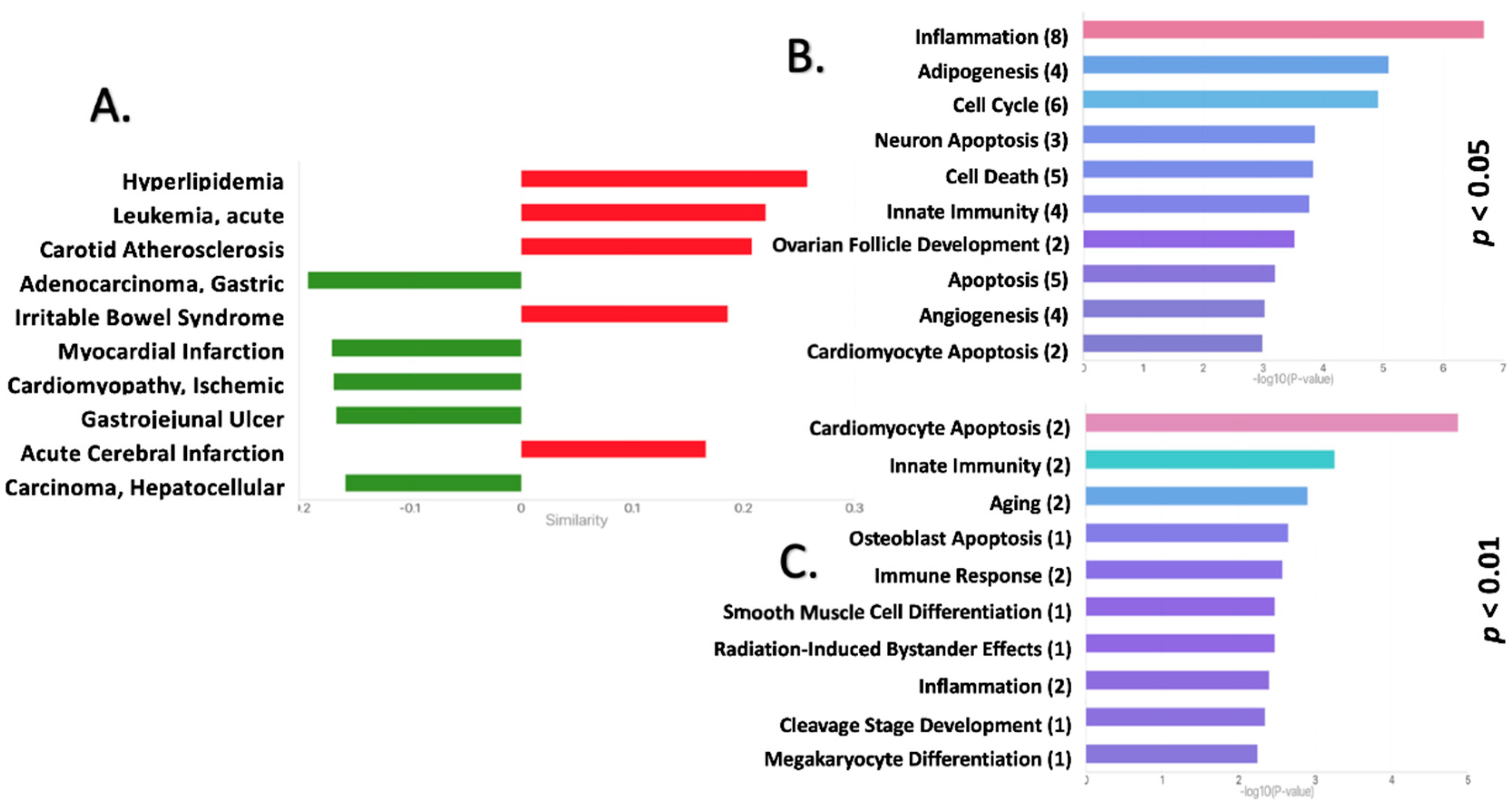
2.2. Functional Pathway Analysis Reveals Potential Roles for the DEmiRNAs in the EAT of CAD Patients
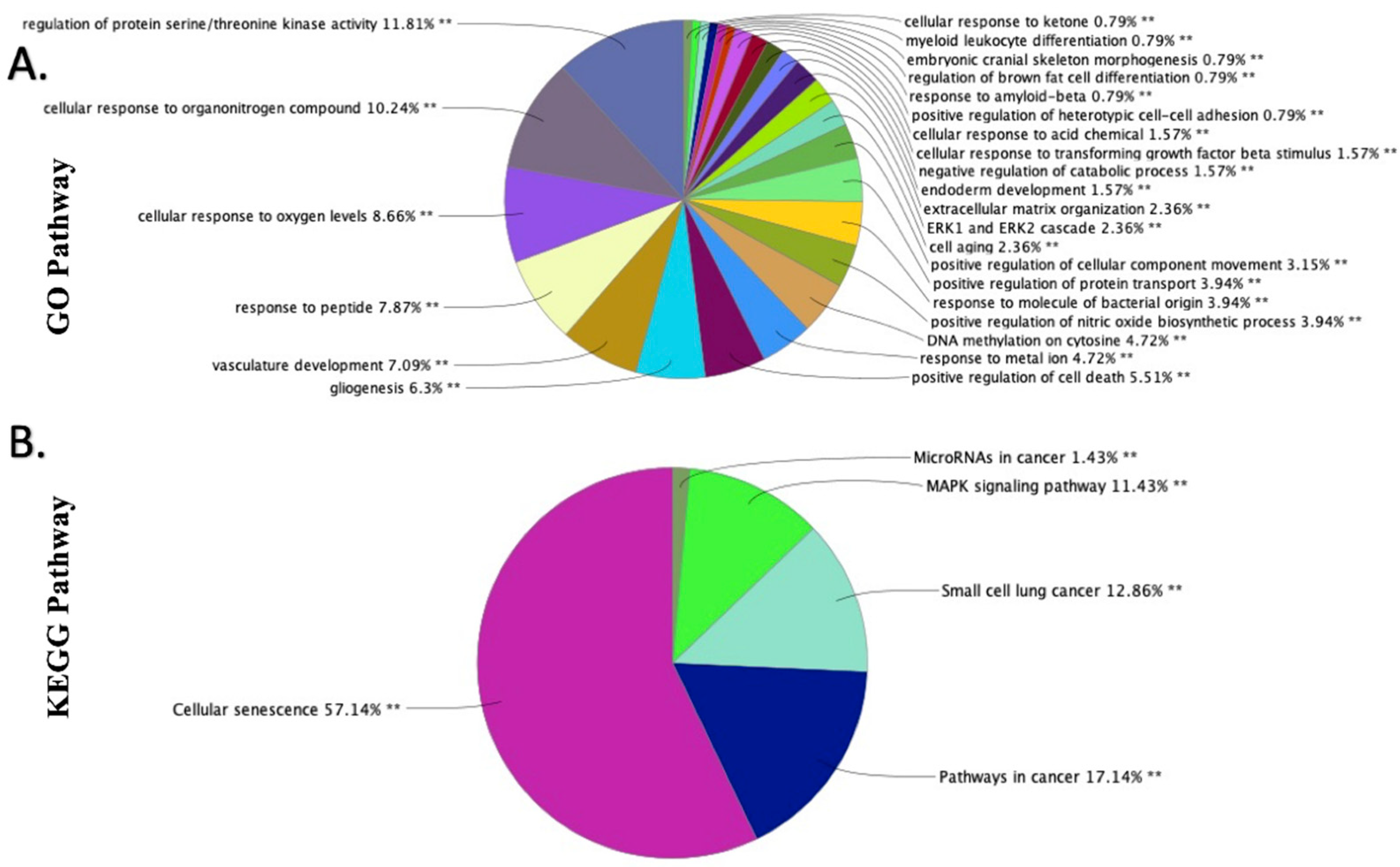
2.3. GO and KEGG Functional Enrichment Analyses of the DEmiRNAs in EAT
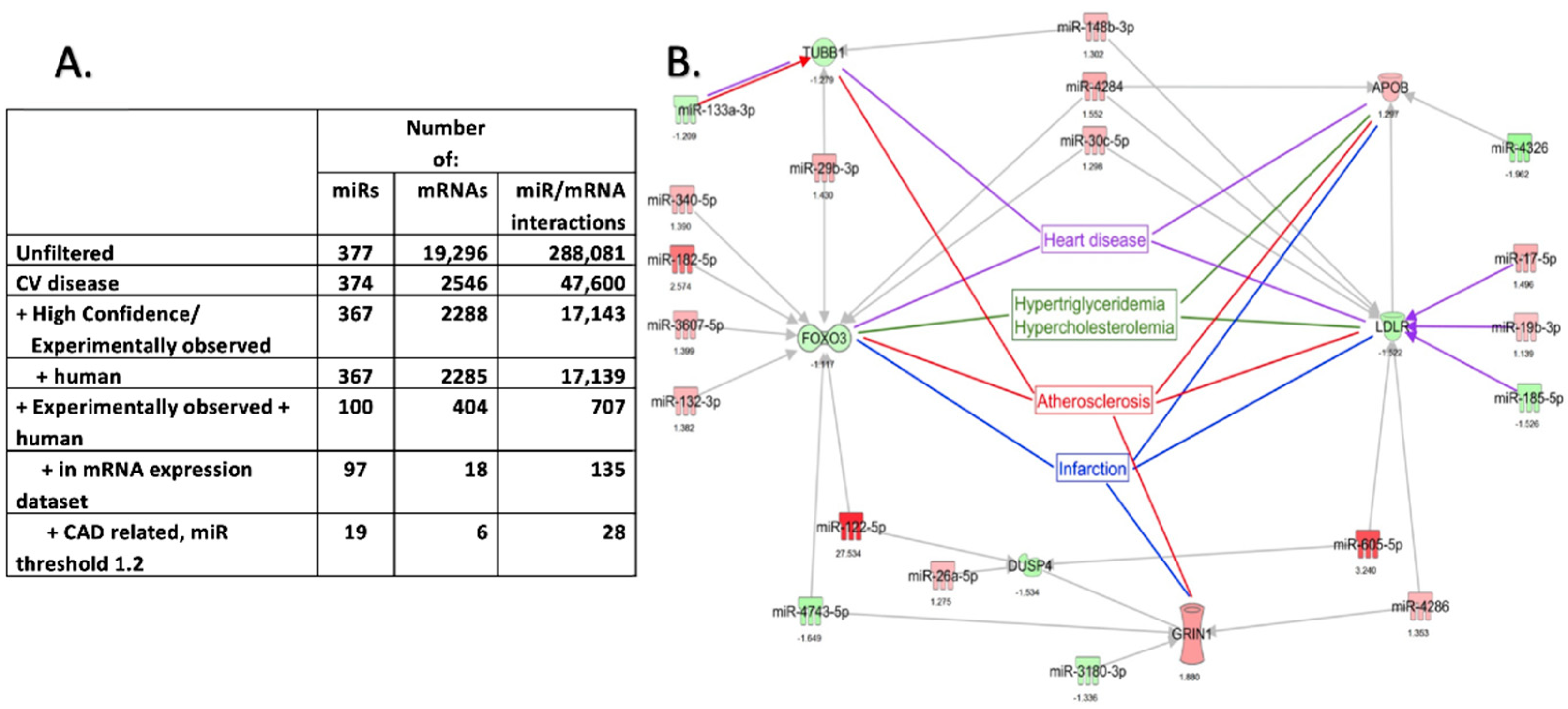
2.4. Pathway Enrichment Analysis of DEmiRNAs and DEmRNA Interactions in EAT
2.5. qPCR Validation of the Differentially Expressed mRNAs in the EAT of Patients with CAD
2.6. qPCR Validation of the Differentially Expressed miRNAs in the EAT of Patients with CAD
2.7. LncRNA Array Shows Differential Expression of Long Non-Coding RNAs
3. Discussion
4. Materials and Methods
4.1. Sample Collection and Clinical Characteristics
4.2. miRNA Microarray of EAT and SAT
4.3. Next-Generation Sequencing of EAT and SAT
4.4. LncRNA Array
4.5. qPCR Validation of DEmRNAs
4.6. qPCR Validation of DEmiRNAs
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics—2020 Update: A Report from the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, G. Local and systemic effects of the multifaceted epicardial adipose tissue depot. Nat. Rev. Endocrinol. 2015, 11, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.B.; Shah, S.; Verma, S.; Oudit, G.Y. Epicardial adipose tissue as a metabolic transducer: Role in heart failure and coronary artery disease. Heart Fail. Rev. 2017, 22, 889–902. [Google Scholar] [CrossRef] [PubMed]
- Camarena, V.; Sant, D.; Mohseni, M.; Salerno, T.; Zaleski, M.; Wang, G.; Iacobellis, G. Novel atherogenic pathways from the differential transcriptome analysis of diabetic epicardial adipose tissue. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 739–750. [Google Scholar] [CrossRef]
- Hirata, Y.; Tabata, M.; Kurobe, H.; Motoki, T.; Akaike, M.; Nishio, C.; Higashida, M.; Mikasa, H.; Nakaya, Y.; Takanashi, S.; et al. Coronary Atherosclerosis Is Associated with Macrophage Polarization in Epicardial Adipose Tissue. J. Am. Coll. Cardiol. 2011, 58, 248–255. [Google Scholar] [CrossRef] [Green Version]
- Iacobellis, G. Coronary Artery Disease and Epicardial Adipose Tissue. In Epicardial Adipose Tissue: From Cell to Clinic; Iacobellis, G., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 77–90. [Google Scholar] [CrossRef]
- Iacobellis, G.; Diaz, S.; Mendez, A.; Goldberg, R. Increased epicardial fat and plasma leptin in type 1 diabetes independently of obesity. Nutr. Metab. Cardiovasc. Dis. 2013, 24, 725–729. [Google Scholar] [CrossRef]
- Karastergiou, K.; Evans, I.; Ogston, N.; Miheisi, N.; Nair, D.; Kaski, J.-C.; Jahangiri, M.; Mohamed-Ali, V. Epicardial Adipokines in Obesity and Coronary Artery Disease Induce Atherogenic Changes in Monocytes and Endothelial Cells. Arter. Thromb. Vasc. Biol. 2010, 30, 1340–1346. [Google Scholar] [CrossRef] [Green Version]
- McAninch, E.; Fonseca, T.L.; Poggioli, R.; Panos, A.; Salerno, T.A.; Deng, Y.; Li, Y.; Bianco, A.; Iacobellis, G. Epicardial adipose tissue has a unique transcriptome modified in severe coronary artery disease. Obesity 2015, 23, 1267–1278. [Google Scholar] [CrossRef]
- Nasarre, L.; Juan-Babot, O.; Gastelurrutia, P.; Llucia-Valldeperas, A.; Badimon, L.; Bayes-Genis, A.; Llorente-Cortés, V. Low density lipoprotein receptor–related protein 1 is upregulated in epicardial fat from type 2 diabetes mellitus patients and correlates with glucose and triglyceride plasma levels. Geol. Rundsch. 2012, 51, 23–30. [Google Scholar] [CrossRef]
- Packer, M. Epicardial Adipose Tissue May Mediate Deleterious Effects of Obesity and Inflammation on the Myocardium. J. Am. Coll. Cardiol. 2018, 71, 2360–2372. [Google Scholar] [CrossRef] [PubMed]
- Chechi, K.; Richard, D. Thermogenic potential and physiological relevance of human epicardial adipose tissue. Int. J. Obes. Suppl. 2015, 5 (Suppl. S1), S28–S34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marchington, J.; Pond, C.M. Site-specific properties of pericardial and epicardial adipose tissue: The effects of insulin and high-fat feeding on lipogenesis and the incorporation of fatty acids in vitro. Int. J. Obes. 1990, 14, 1013–1022. [Google Scholar] [PubMed]
- Sacks, H.S.; Fain, J.N.; Holman, B.; Cheema, P.; Chary, A.; Parks, F.; Karas, J.; Optican, R.; Bahouth, S.W.; Garrett, E.; et al. Uncoupling protein-1 and related messenger ribonucleic acids in human epicardial and other adipose tissues: Epi-cardial fat functioning as brown fat. J. Clin. Endocrinol. Metab. 2009, 94, 3611–3615. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, L.; Foglio, E.; Pontemezzo, E.; Germani, A.; Russo, M.A.; Limana, F. Cardiac Repair: The Intricate Crosstalk between the Epicardium and the Myocardium. Curr. Stem Cell Res. Ther. 2020, 15, 661–673. [Google Scholar] [CrossRef]
- Thomou, T.; Mori, M.A.; Dreyfuss, J.M.; Konishi, M.; Sakaguchi, M.; Wolfrum, C.; Rao, T.N.; Winnay, J.N.; Garcia-Martin, R.; Grinspoon, S.K.; et al. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature 2017, 542, 450–455. [Google Scholar] [CrossRef]
- Wen, Z.; Li, J.; Fu, Y.; Zheng, Y.; Ma, M.; Wang, C. Hypertrophic Adipocyte–Derived Exosomal miR-802-5p Contributes to Insulin Resistance in Cardiac Myocytes through Targeting HSP60. Obesity 2020, 28, 1932–1940. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef] [Green Version]
- Vasudevan, S. Posttranscriptional Upregulation by MicroRNAs. Wiley Interdiscip. Rev. RNA 2011, 3, 311–330. [Google Scholar] [CrossRef]
- Gebert, L.F.R.; Macrae, I.J. Regulation of microRNA function in animals. Nat. Rev. Mol. Cell Biol. 2019, 20, 21–37. [Google Scholar] [CrossRef]
- Cui, M.; Wang, H.; Yao, X.; Zhang, D.; Xie, Y.; Cui, R.; Zhang, X. Circulating MicroRNAs in Cancer: Potential and Challenge. Front. Genet. 2019, 10, 626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felekkis, K.; Papaneophytou, C. Challenges in Using Circulating Micro-RNAs as Biomarkers for Cardiovascular Diseases. Int. J. Mol. Sci. 2020, 21, 561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mi, S.; Zhang, J.; Zhang, W.; Huang, R.S. Circulating MicroRNAs as Biomarkers for Inflammatory Diseases. MicroRNA 2013, 2, 64–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Fu, W.; Lu, M.; Huai, S.; Song, Y.; Wei, Y. Role of miRNAs in Epicardial Adipose Tissue in CAD Patients with T2DM. BioMed Res. Int. 2016, 2016, 222. [Google Scholar] [CrossRef] [Green Version]
- Oclon, E.A.; Latacz, A.; Zubel–Łojek, J.; Pierzchała–Koziec, K. Hyperglycemia-induced changes in miRNA expression patterns in epicardial adipose tissue of piglets. J. Endocrinol. 2016, 229, 259–266. [Google Scholar] [CrossRef] [Green Version]
- Fitzgibbons, T.P.; Lee, N.; Tran, K.-V.; Nicoloro, S.; Kelly, M.; Tam, S.K.; Czech, M.P. Coronary disease is not associated with robust alterations in inflammatory gene expression in human epicardial fat. JCI Insight 2019, 4, e124859. [Google Scholar] [CrossRef]
- de Gonzalo-Calvo, D. Plasma microRNA Profiling Reveals Novel Biomarkers of Epicardial Adipose Tissue: A Multidetector Computed Tomography Study. J. Clin. Med. 2019, 8, 780. [Google Scholar] [CrossRef] [Green Version]
- Tan, L.; Xu, Q.; Wang, Q.; Shi, R.; Zhang, G. Identification of key genes and pathways affected in epicardial adipose tissue from patients with coronary artery disease by integrated bioinformatics analysis. PeerJ 2020, 8, e8763. [Google Scholar] [CrossRef]
- Vacca, M.; DI Eusanio, M.; Cariello, M.; Graziano, G.; D’Amore, S.; Petridis, F.D.; D’Orazio, A.; Salvatore, L.; Tamburro, A.; Folesani, G.; et al. Integrative miRNA and whole-genome analyses of epicardial adipose tissue in patients with coronary atherosclerosis. Cardiovasc. Res. 2015, 109, 228–239. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, J.C.R.; Acuña, S.M.; Aoki, J.I.; Floeter-Winter, L.M.; Muxel, S.M. Long Non-Coding RNAs in the Regulation of Gene Expression: Physiology and Disease. Non-Coding RNA 2019, 5, 17. [Google Scholar] [CrossRef] [Green Version]
- Ramón y Cajal, S.; Segura, M.F.; Hümmer, S. Interplay Between ncRNAs and Cellular Communication: A Proposal for Understanding Cell-Specific Signaling Pathways. Front. Genet. 2019, 10, 281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Han, X.; Wan, Y.; Zhang, S.; Zhao, Y.; Fan, R.; Cui, Q.; Zhou, Y. TAM 2.0: Tool for MicroRNA set analysis. Nucleic Acids Res. 2018, 46, W180–W185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bindea, G.; Galon, J.; Mlecnik, B. CluePedia Cytoscape plugin: Pathway insights using integrated experimental and in silico data. Bioinformatics 2013, 29, 661–663. [Google Scholar] [CrossRef] [PubMed]
- Bindea, G.; Mlecnik, B.; Hackl, H.; Charoentong, P.; Tosolini, M.; Kirilovsky, A.; Fridman, W.-H.; Pagès, F.; Trajanoski, Z.; Galon, J. ClueGO: A Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 2009, 25, 1091–1093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.-Y.; Lin, Y.-C.-D.; Li, J.; Huang, K.-Y.; Shrestha, S.; Hong, H.-C.; Tang, Y.; Chen, Y.-G.; Jin, C.-N.; Yu, Y.; et al. miRTarBase 2020: Updates to the experimentally validated microRNA–target interaction database. Nucleic Acids Res. 2020, 48, D148–D154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, V.; Bell, G.W.; Nam, J.-W.; Bartel, D.P. Predicting effective microRNA target sites in mammalian mRNAs. eLife 2015, 4, e05005. [Google Scholar] [CrossRef]
- Milanese, G.; Silva, M.; Ledda, R.E.; Goldoni, M.; Nayak, S.; Bruno, L.; Rossi, E.; Maffei, E.; Cademartiri, F.; Sverzellati, N. Validity of epicardial fat volume as biomarker of coronary artery disease in symptomatic individuals: Results from the ALTER-BIO registry. Int. J. Cardiol. 2020, 314, 20–24. [Google Scholar] [CrossRef]
- Yu, W.; Liu, B.; Zhang, F.; Wang, J.; Shao, X.; Yang, X.; Shi, Y.; Wang, B.; Xu, Y.; Wang, Y. Association of Epicardial Fat Volume with Increased Risk of Obstructive Coronary Artery Disease in Chinese Patients with Suspected Coronary Artery Disease. J. Am. Heart Assoc. 2021, 10, e018080. [Google Scholar] [CrossRef]
- Sacks, H.S.; Fain, J.N.; Bahouth, S.W.; Ojha, S.; Frontini, A.; Budge, H.; Cinti, S.; Symonds, M. Adult Epicardial Fat Exhibits Beige Features. J. Clin. Endocrinol. Metab. 2013, 98, E1448–E1455. [Google Scholar] [CrossRef]
- Talman, A.H.; Psaltis, P.J.; Cameron, J.D.; Meredith, I.T.; Seneviratne, S.K.; Wong, D.T.L. Epicardial adipose tissue: Far more than a fat depot. Cardiovasc. Diagn. Ther. 2014, 4, 416–429. [Google Scholar] [CrossRef]
- Colom, C.; Viladés, D.; Pérez-Cuellar, M.; Leta, R.; Rivas-Urbina, A.; Carreras, G.; Ordóñez-Llanos, J.; Pérez, A.; Sánchez-Quesada, J.L. Associations between epicardial adipose tissue, subclinical atherosclerosis and high-density lipoprotein composition in type 1 diabetes. Cardiovasc. Diabetol. 2018, 17, 156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dönmez, Y.; Bulut, A. Epicardial fat thickness is significantly increased and related to LDL cholesterol level in patients with familial hypercholesterolemia. J. Ultrasound 2019, 22, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Paik, J.-H. FOXOs in the maintenance of vascular homoeostasis. Biochem. Soc. Trans. 2006, 34, 731–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sengupta, A.; Molkentin, J.D.; Yutzey, K.E. FoxO Transcription Factors Promote Autophagy in Cardiomyocytes. J. Biol. Chem. 2009, 284, 28319–28331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Wang, Z.; Wang, C.; Ma, Q.; Zhao, Y. Perivascular Adipose Tissue-Derived Adiponectin Inhibits Collar-Induced Carotid Atherosclerosis by Promoting Macrophage Autophagy. PLoS ONE 2015, 10, e0124031. [Google Scholar] [CrossRef] [Green Version]
- Little, A.; Hu, Y.; Sun, Q.; Jain, D.; Broome, J.; Chen, M.-H.; Thibord, F.; McHugh, C.; Surendran, P.; Blackwell, T.W.; et al. Whole genome sequence analysis of platelet traits in the NHLBI trans-omics for precision medicine initiative. Hum. Mol. Genet. 2022, 31, 347–361. [Google Scholar] [CrossRef] [PubMed]
- Freson, K.; De Vos, R.; Wittevrongel, C.; Thys, C.; Defoor, J.; Vanhees, L.; Vermylen, J.; Peerlinck, K.; Van Geet, C. The TUBB1 Q43P functional polymorphism reduces the risk of cardiovascular disease in men by modulating platelet function and structure. Blood 2005, 106, 2356–2362. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Tang, R.; Wang, S.; Wang, W.; Zhang, K.; Li, J.; Li, P.; Tang, Y.-D. SAHA could inhibit TGF-β1/p38 pathway in MI-induced cardiac fibrosis through DUSP4 overexpression. Heart Vessel. 2021, 37, 152–160. [Google Scholar] [CrossRef]
- Choi, J.C.; Wu, W.; Muchir, A.; Iwata, S.; Homma, S.; Worman, H.J. Dual Specificity Phosphatase 4 Mediates Cardiomyopathy Caused by Lamin A/C (LMNA) Gene Mutation. J. Biol. Chem. 2012, 287, 40513–40524. [Google Scholar] [CrossRef] [Green Version]
- Barajas-Espinosa, A.; Basye, A.; Angelos, M.G.; Chen, C.-A. Modulation of p38 kinase by DUSP4 is important in regulating cardiovascular function under oxidative stress. Free Radic. Biol. Med. 2015, 89, 170–181. [Google Scholar] [CrossRef] [Green Version]
- Xie, D.; Xiong, K.; Su, X.; Wang, G.; Ji, Q.; Zou, Q.; Wang, L.; Liu, Y.; Liang, D.; Xue, J.; et al. Identification of an endogenous glutamatergic transmitter system controlling excitability and conductivity of atrial cardiomyocytes. Cell Res. 2021, 31, 951–964. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-L.; Li, Y.-H.; Shi, G.-Y.; Tang, S.-H.; Jiang, S.-J.; Huang, C.-W.; Liu, P.-Y.; Hong, J.-S.; Wu, H.-L. Dextromethorphan reduces oxidative stress and inhibits atherosclerosis and neointima formation in mice. Cardiovasc. Res. 2009, 82, 161–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siu, D. A new way of targeting to treat coronary artery disease. J. Cardiovasc. Med. 2010, 11, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Marquard, J.; Stirban, A.; Schliess, F.; Sievers, F.; Welters, A.; Otter, S.; Fischer, A.J.; Wnendt, S.; Meissner, T.; Heise, T.; et al. Effects of dextromethorphan as add-on to sitagliptin on blood glucose and serum insulin concentrations in individuals with type 2 diabetes mellitus: A randomized, placebo-controlled, double-blinded, multiple crossover, single-dose clinical trial. Diabetes Obes. Metab. 2015, 18, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Husi, H.; Ward, M.A.; Choudhary, J.S.; Blackstock, W.P.; Grant, S.G.N. Proteomic analysis of NMDA receptor–adhesion protein signaling complexes. Nat. Neurosci. 2000, 3, 661–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villasante Fricke, A.C.; Iacobellis, G. Epicardial Adipose Tissue: Clinical Biomarker of Cardio-Metabolic Risk. Int. J. Mol. Sci. 2019, 20, 5989. [Google Scholar] [CrossRef] [Green Version]
- Flinn, B.; Royce, N.; Gress, T.; Chowdhury, N.; Santanam, N. Dual role for angiotensin-converting enzyme 2 in Severe Acute Respiratory Syndrome Coronavirus 2 infection and cardiac fat. Obes. Rev. 2021, 22, e13225. [Google Scholar] [CrossRef]
- Pan, X.; Shao, Y.; Wu, F.; Wang, Y.; Xiong, R.; Zheng, J.; Tian, H.; Wang, B.; Wang, Y.; Zhang, Y.; et al. FGF21 Prevents Angiotensin II-Induced Hypertension and Vascular Dysfunction by Activation of ACE2/Angiotensin-(1–7) Axis in Mice. Cell Metab. 2018, 27, 1323–1337.e5. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Chen, X.; Su, T.; Li, H.; Huang, Q.; Wu, D.; Yang, C.; Han, Z. Circulating miR-499 are novel and sensitive biomarker of acute myocardial infarction. J. Thorac. Dis. 2015, 7, 303–308. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Yuan, S.; Li, H.; Zhan, J.; Wang, F.; Fan, J.; Nie, X.; Wang, Y.; Wen, Z.; Chen, Y.; et al. The double face of miR-320: Cardiomyocytes-derived miR-320 deteriorated while fibroblasts-derived miR-320 protected against heart failure induced by transverse aortic constriction. Signal Transduct. Target. Ther. 2021, 6, 69. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, J.; Wu, M.; Xu, H. Identification of key miRNAs and mRNAs related to coronary artery disease by meta-analysis. BMC Cardiovasc. Disord. 2021, 21, 443. [Google Scholar] [CrossRef] [PubMed]
- Fortunato, O.; Borzi, C.; Milione, M.; Centonze, G.; Conte, D.; Boeri, M.; Verri, C.; Moro, M.; Facchinetti, F.; Andriani, F.; et al. Circulating mir-320a promotes immunosuppressive macrophages M2 phenotype associated with lung cancer risk. Int. J. Cancer 2018, 144, 2746–2761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, P.T.; Wheelwright, M.; Teles, R.; Komisopoulou, E.; Edfeldt, K.; Ferguson, B.; Mehta, M.D.; Vazirnia, A.; Rea, T.H.; Sarno, E.N.; et al. MicroRNA-21 targets the vitamin D–dependent antimicrobial pathway in leprosy. Nat. Med. 2012, 18, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Stöllberger, C.; Finsterer, J. Role of Infectious and Immune Factors in Coronary and Cerebrovascular Arteriosclerosis. Clin. Vaccine Immunol. 2002, 9, 207–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afonso, M.S.; Verma, N.; van Solingen, C.; Cyr, Y.; Sharma, M.; Perie, L.; Corr, E.M.; Schlegel, M.; Shanley, L.C.; Peled, D.; et al. MicroRNA-33 Inhibits Adaptive Thermogenesis and Adipose Tissue Beiging. Arter. Thromb. Vasc. Biol. 2021, 41, 1360–1373. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, C.; Li, H.; Song, Y.; Zhao, Y.; Zhai, L.; Wang, H.; Zhong, R.; Tang, H.; Zhu, D. miR-378 Activates the Pyruvate-PEP Futile Cycle and Enhances Lipolysis to Ameliorate Obesity in Mice. EBioMedicine 2016, 5, 93–104. [Google Scholar] [CrossRef] [Green Version]
- Karere, G.M.; Cox, L.A.; Bishop, A.C.; South, A.M.; Shaltout, H.A.; Mercado-Deane, M.-G.; Cuda, S. Sex Differences in MicroRNA Expression and Cardiometabolic Risk Factors in Hispanic Adolescents with Obesity. J. Pediatr. 2021, 235, 138–143.e5. [Google Scholar] [CrossRef]
- Han, H.; Qu, G.; Han, C.; Wang, Y.; Sun, T.; Li, F.; Wang, J.; Luo, S. MiR-34a, miR-21 and miR-23a as potential biomarkers for coronary artery disease: A pilot microarray study and confirmation in a 32 patient cohort. Exp. Mol. Med. 2015, 47, e138. [Google Scholar] [CrossRef] [Green Version]
- Marí-Alexandre, J.; Barceló-Molina, M.; Sanz-Sánchez, J.; Molina, P.; Sancho, J.; Abellán, Y.; Santaolaria-Ayora, M.L.; Giner, J.; Martínez-Dolz, L.; Estelles, A.; et al. Thickness and an Altered miRNA Expression in the Epicardial Adipose Tissue Is Associated with Coronary Heart Disease in Sudden Death Victims. Rev. Española De Cardiol. (Engl. Ed.) 2019, 72, 30–39. [Google Scholar] [CrossRef]
- Du, H.; Zhao, Y.; Yin, Z.; Wang, D.W.; Chen, C. The role of miR-320 in glucose and lipid metabolism disorder-associated diseases. Int. J. Biol. Sci. 2021, 17, 402–416. [Google Scholar] [CrossRef]
- Ling, H.-Y.; Ou, H.-S.; Feng, S.-D.; Zhang, X.-Y.; Tuo, Q.-H.; Chen, L.-X.; Zhu, B.-Y.; Gao, Z.-P.; Tang, C.-K.; Yin, W.-D.; et al. Changes in microRNA (miR) Profile and Effects of miR-320 in Insulin-Resistant 3T3-L1 Adipocytes. Clin. Exp. Pharmacol. Physiol. 2009, 36, e32–e39. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, X. Downregulation of miR-320 Alleviates Endoplasmic Reticulum Stress and Inflammatory Response in 3T3-L1 Adipocytes. Exp. Clin. Endocrinol. Diabetes 2019, 129, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Fan, J.; Zhao, Y.; Zhang, X.; Dai, B.; Zhan, J.; Yin, Z.; Nie, X.; Fu, X.-D.; Chen, C.; et al. Nuclear miR-320 Mediates Diabetes-Induced Cardiac Dysfunction by Activating Transcription of Fatty Acid Metabolic Genes to Cause Lipotoxicity in the Heart. Circ. Res. 2019, 125, 1106–1120. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.-P.; Wu, J.; Wang, X.; Sartor, M.A.; Qian, J.; Jones, K.; Nicolaou, P.; Pritchard, T.J.; Fan, G.-C. MicroRNA-320 Is Involved in the Regulation of Cardiac Ischemia/Reperfusion Injury by Targeting Heat-Shock Protein 20. Circulation 2009, 119, 2357–2366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grahn, T.H.M.; Kaur, R.; Yin, J.; Schweiger, M.; Sharma, V.M.; Lee, M.-J.; Ido, Y.; Smas, C.M.; Zechner, R.; Lass, A.; et al. Fat-specific Protein 27 (FSP27) Interacts with Adipose Triglyceride Lipase (ATGL) to Regulate Lipolysis and Insulin Sensitivity in Human Adipocytes. J. Biol. Chem. 2014, 289, 12029–12039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuji, M.; Kawasaki, T.; Matsuda, T.; Arai, T.; Gojo, S.; Takeuchi, J.K. Sexual dimorphisms of mRNA and miRNA in human/murine heart disease. PLoS ONE 2017, 12, e0177988. [Google Scholar] [CrossRef] [Green Version]
- Kocher, C.; Christiansen, M.; Martin, S.; Adams, C.; Wehner, P.; Gress, T.; Santanam, N. Sexual dimorphism in obesity-related genes in the epicardial fat during aging. J. Physiol. Biochem. 2016, 73, 215–224. [Google Scholar] [CrossRef] [Green Version]
- Meadows, S.; Seidler, A.; Wall, M.; Page, J.; Taylor, C.; Flinn, B.; Turner, R.; Santanam, N. Altered Regulation of adipomiR Editing with Aging. Int. J. Mol. Sci. 2020, 21, 6899. [Google Scholar] [CrossRef]
- Iacobollis, G.; Mahabadi, A.A. Is epicardial fat attenuation a novel marker of coronary inflammation? Atherosclerosis 2019, 284, 212–213. [Google Scholar] [CrossRef]
- Shannon, P. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Karagkouni, D.; Paraskevopoulou, M.D.; Tastsoglou, S.; Skoufos, G.; Karavangeli, A.; Pierros, V.; Zacharopoulou, E.; Hatzigeorgiou, A.G. DIANA-LncBase v3: Indexing experimentally supported miRNA targets on non-coding transcripts. Nucleic Acids Res. 2019, 48, D101–D110. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flinn, B.; Adams, C.; Chowdhury, N.; Gress, T.; Santanam, N. Profiling of Non-Coding Regulators and Their Targets in Epicardial Fat from Patients with Coronary Artery Disease. Int. J. Mol. Sci. 2022, 23, 5297. https://doi.org/10.3390/ijms23105297
Flinn B, Adams C, Chowdhury N, Gress T, Santanam N. Profiling of Non-Coding Regulators and Their Targets in Epicardial Fat from Patients with Coronary Artery Disease. International Journal of Molecular Sciences. 2022; 23(10):5297. https://doi.org/10.3390/ijms23105297
Chicago/Turabian StyleFlinn, Brendin, Christopher Adams, Nepal Chowdhury, Todd Gress, and Nalini Santanam. 2022. "Profiling of Non-Coding Regulators and Their Targets in Epicardial Fat from Patients with Coronary Artery Disease" International Journal of Molecular Sciences 23, no. 10: 5297. https://doi.org/10.3390/ijms23105297
APA StyleFlinn, B., Adams, C., Chowdhury, N., Gress, T., & Santanam, N. (2022). Profiling of Non-Coding Regulators and Their Targets in Epicardial Fat from Patients with Coronary Artery Disease. International Journal of Molecular Sciences, 23(10), 5297. https://doi.org/10.3390/ijms23105297





