A Novel Photoswitchable Azobenzene-Containing Local Anesthetic Ethercaine with Light-Controlled Biological Activity In Vivo
Abstract
:1. Introduction
2. Results and Discussion
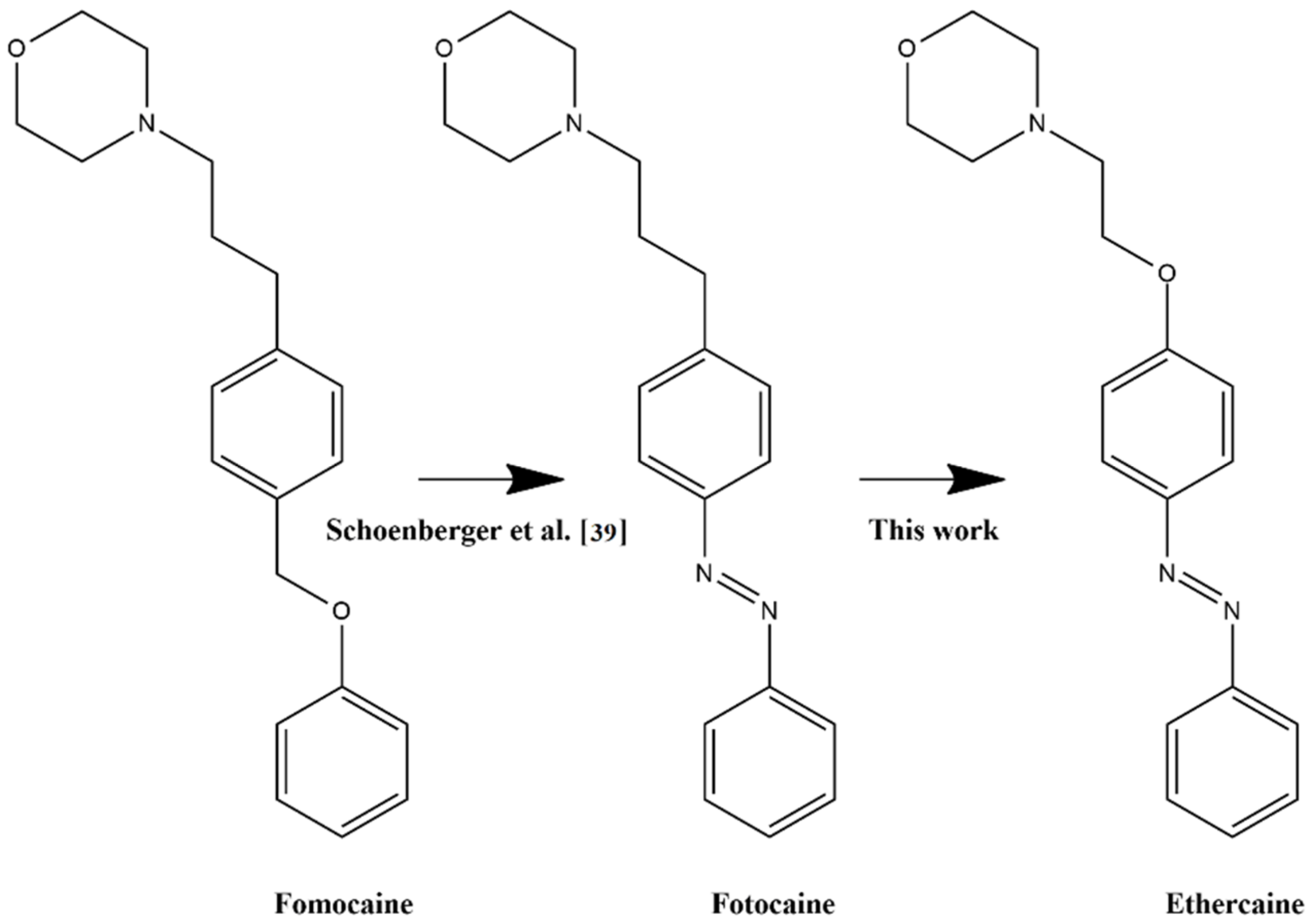
2.1. Design
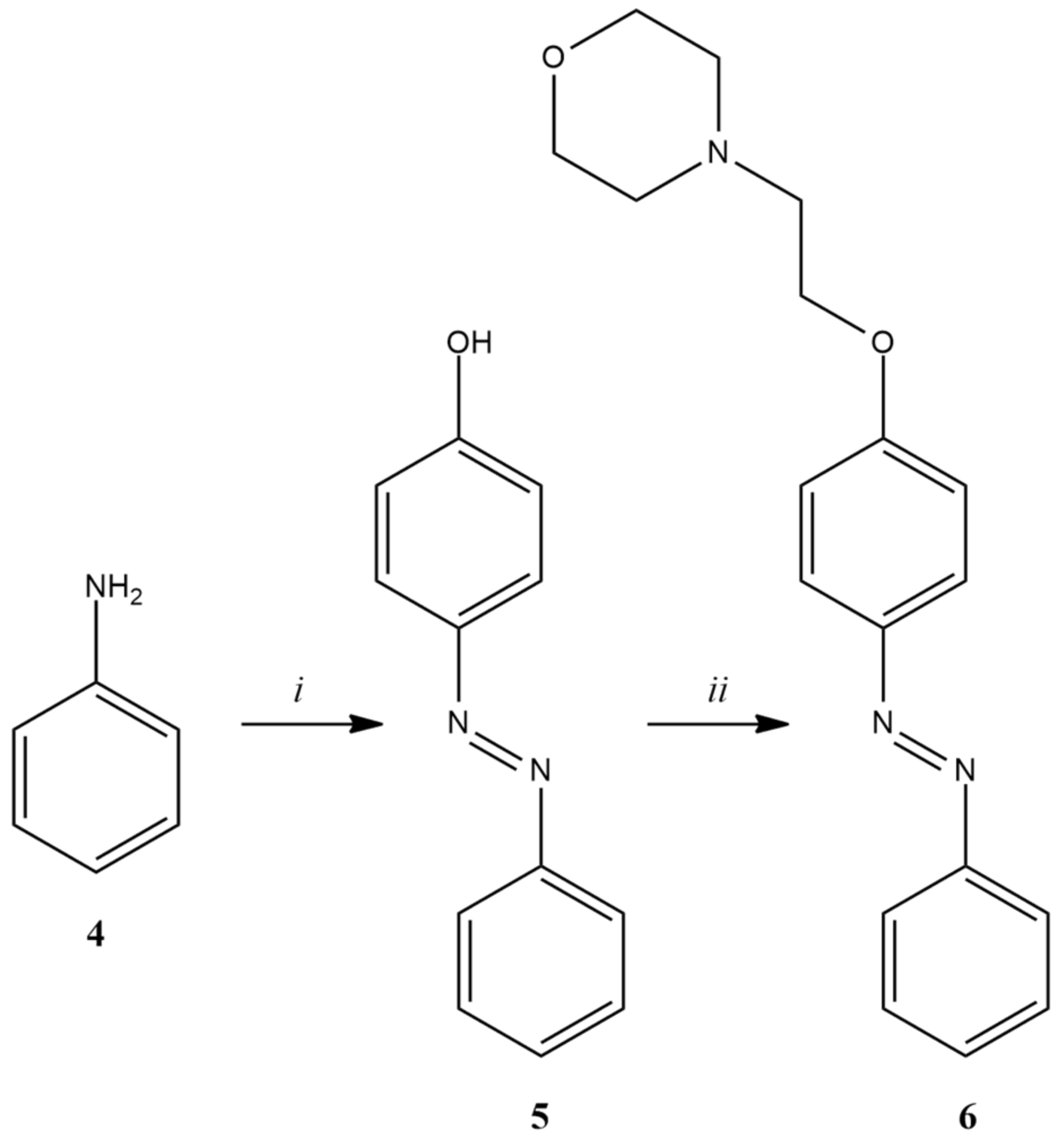
2.2. Chemistry
2.3. Photoswitching Properties
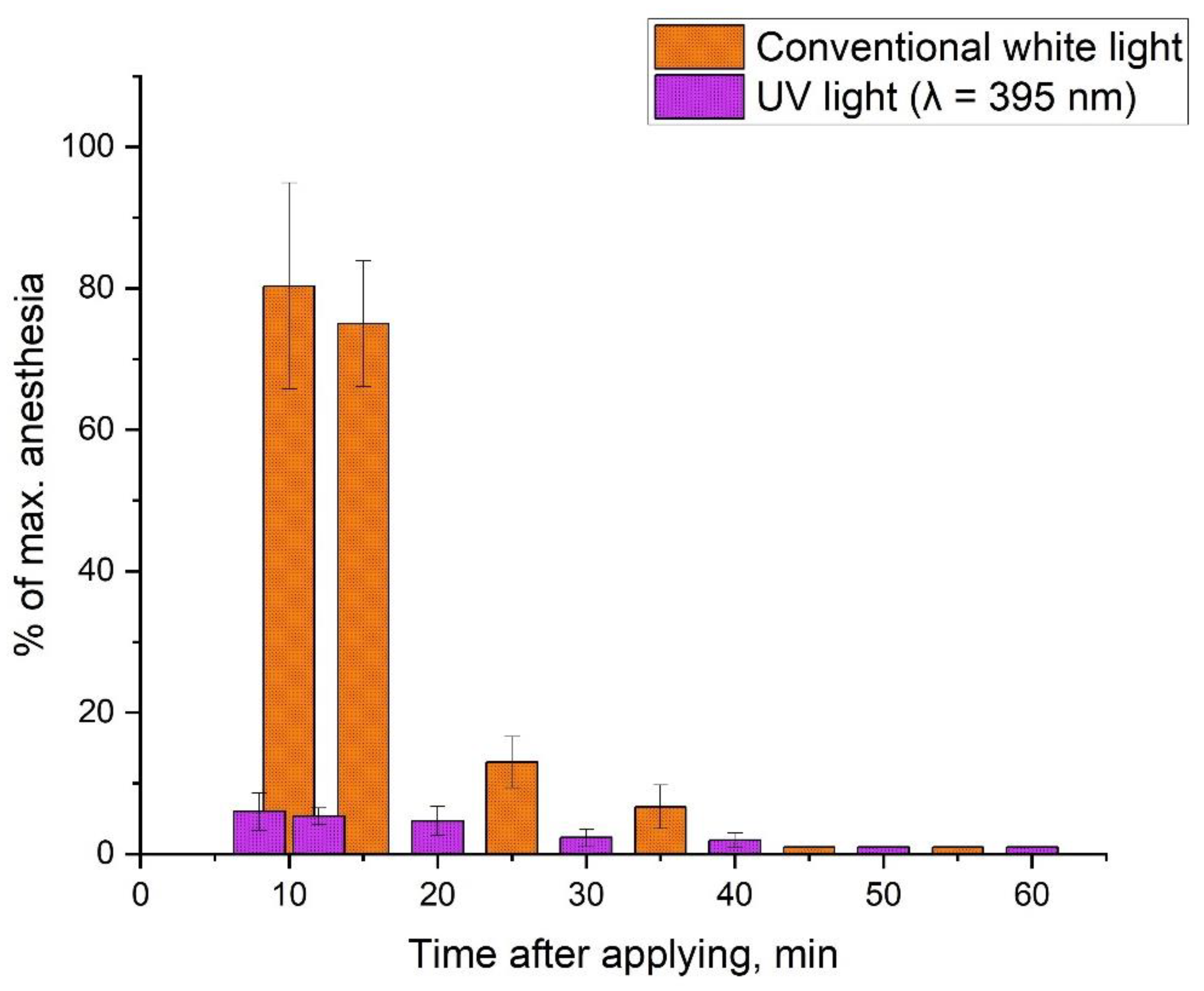
2.4. Biology
3. Materials and Methods
3.1. Materials
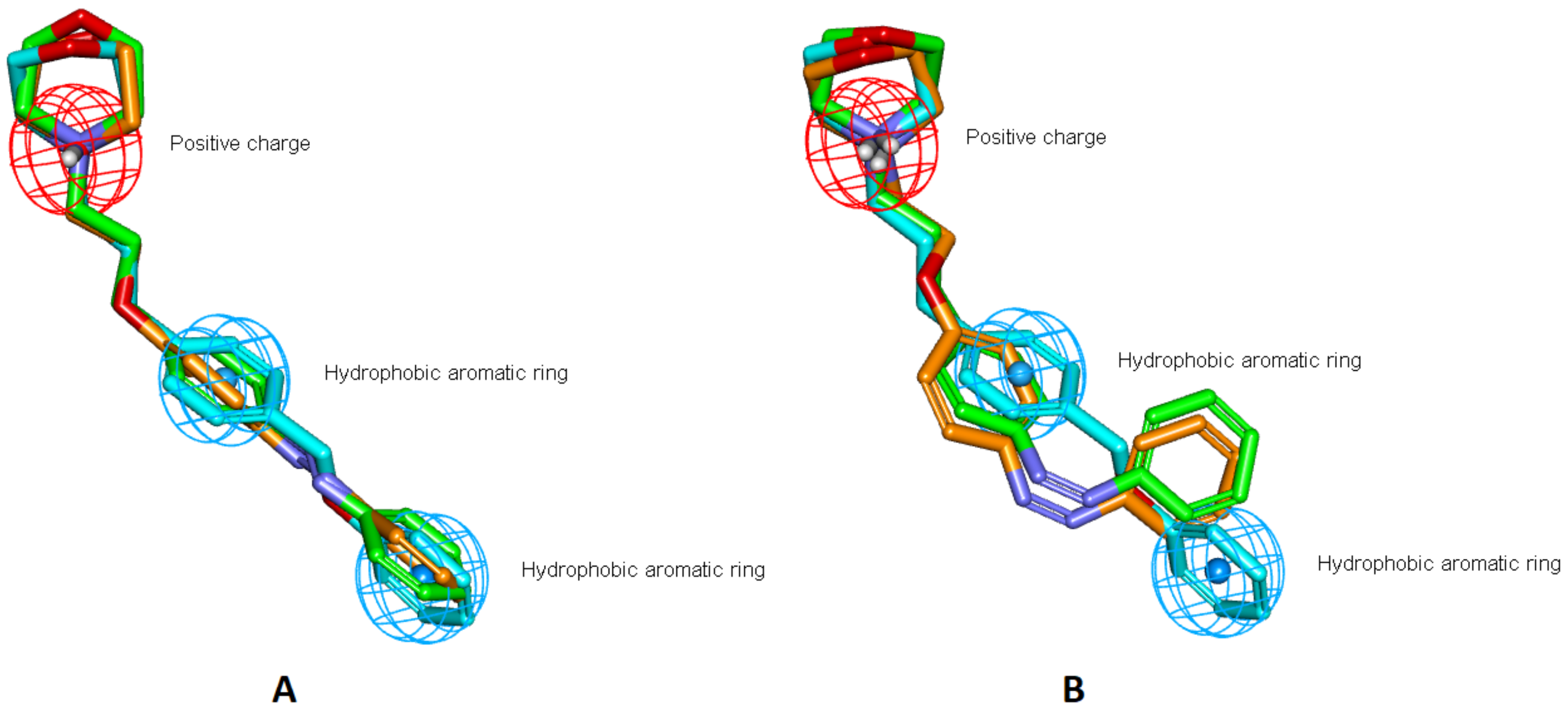
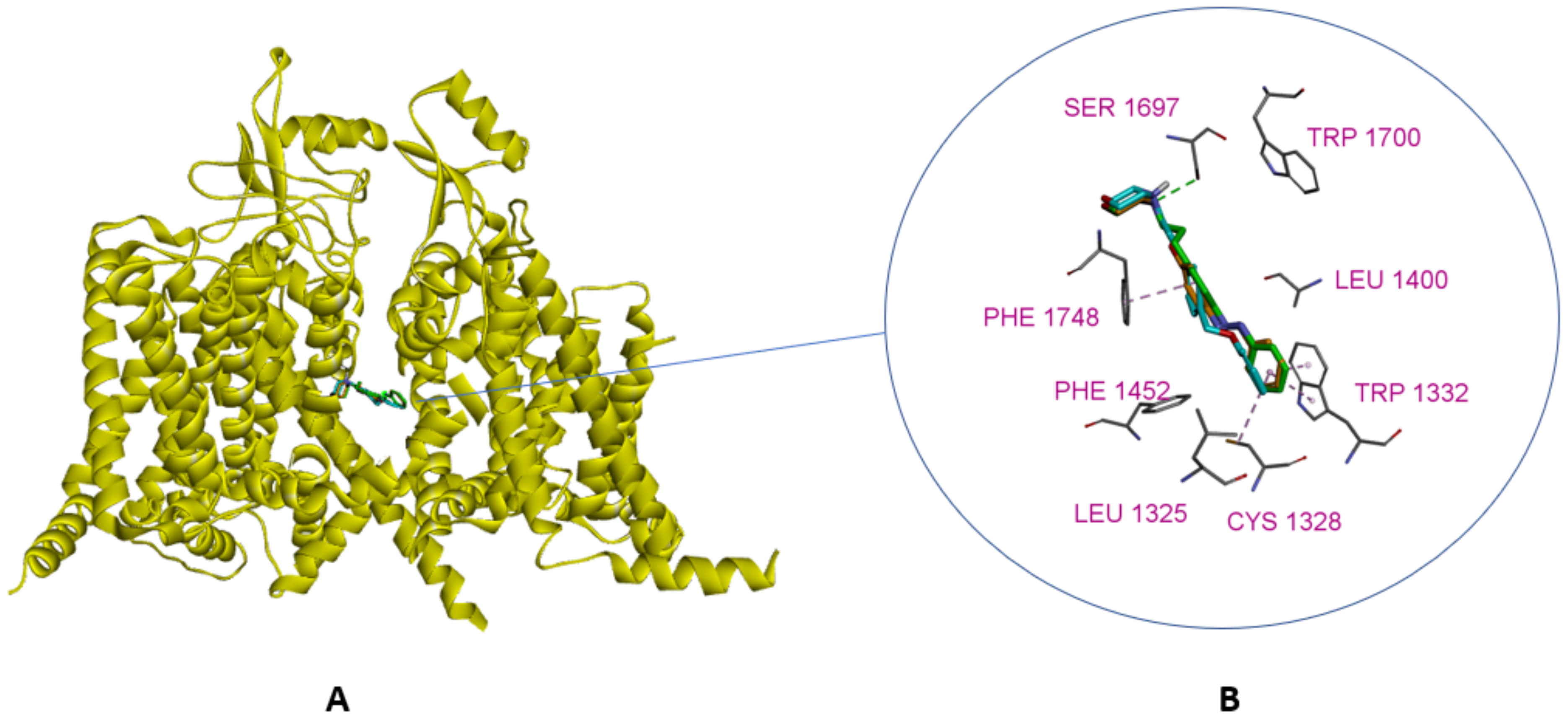
3.2. Design
3.2.1. Ligands 3D-Structures Alignment
3.2.2. Molecular Docking Calculations
3.3. Synthesis
3.3.1. Synthesis of N-(2-Ethanol)-Morpholine (2)
3.3.2. Synthesis of N-(2-Chloroethyl)-Morpholine Hydrochloride (3)
3.3.3. Synthesis of 4-Hydroxyazobenzene (5)
3.3.4. Synthesis of 4-(2-(N-Morpholino)-Ethoxy)-Azobenzene (6)
3.3.5. Preparation of a Micellar Solution of 4-(2-(N-Morpholino)-Ethoxy)-Azobenzene (6)
3.3.6. Measurement of the Hydrodynamic Size of Ethercaine Micelles Using Dynamic Light Scattering (DLS) Method
3.4. Biology
3.4.1. Preparation of Animals and Arrangement of Groups
3.4.2. Irradiation Modes
3.4.3. Determination of the Rabbit Eye’s Cornea Sensitivity Using the Surface Anesthesia Method
3.4.4. Determination of the Rabbit Eye’s Cornea Sensitivity Using the Surface Anesthesia Model during Multiple Reversible Photoswitching
3.4.5. Determination of the Local Irritating Effect
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Daubländer, M.; Müller, R.; Lipp, M.D. The Incidence of Complications Associated with Local Anesthesia in Dentistry. Anesth. Prog. 1997, 44, 132–141. [Google Scholar] [PubMed]
- Keskin Yalcin, B. Complications Associated with Local Anesthesia in Oral and Maxillofacial Surgery. In Topics in Local Anesthetics; IntechOpen: London, UK, 2020. [Google Scholar]
- Bezerra, M.M.; Leão, R.A.C.; Miranda, L.S.M.; De Souza, R.O.M.A. A Brief History behind the Most Used Local Anesthetics. Tetrahedron 2020, 76, 131628. [Google Scholar] [CrossRef]
- McCaughey, W. Adverse Effects of Local Anaesthetics. Drug Saf. 1992, 7, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Zink, W.; Graf, B.M. Local Anesthetic Myotoxicity. Reg. Anesth. Pain Med. 2004, 29, 333–340. [Google Scholar] [CrossRef]
- Wolfe, J.W.; Butterworth, J.F. Local Anesthetic Systemic Toxicity: Update on Mechanisms and Treatment. Curr. Opin. Anaesthesiol. 2011, 24, 561–566. [Google Scholar] [CrossRef]
- Sekimoto, K.; Tobe, M.; Saito, S. Local Anesthetic Toxicity: Acute and Chronic Management. Acute Med. Surg. 2017, 4, 152–160. [Google Scholar] [CrossRef]
- Eggleston, S.T.; Lush, L.W. Adverse Reactions Aromatic—Intermediate Chain—Amine Salt. Ann. Pharmacother. 1996, 30, 851–857. [Google Scholar] [CrossRef]
- Bourne, E.; Wright, C.; Royse, C. A Review of Local Anesthetic Cardiotoxicity and Treatment with Lipid Emulsion. Local Reg. Anesth. 2010, 3, 11. [Google Scholar] [CrossRef] [Green Version]
- Bhansali, D.; Teng, S.L.; Lee, C.S.; Schmidt, B.L.; Bunnett, N.W.; Leong, K.W. Nanotechnology for Pain Management: Current and Future Therapeutic Interventions. Nano Today 2021, 39, 101223. [Google Scholar] [CrossRef]
- He, Y.; Qin, L.; Huang, Y.; Ma, C. Advances of Nano-Structured Extended-Release Local Anesthetics. Nanoscale Res. Lett. 2020, 15, 13. [Google Scholar] [CrossRef] [Green Version]
- Prabhakar, A.; Ward, C.T.; Watson, M.; Sanford, J.; Fiza, B.; Moll, V.; Kaye, R.J.; Morgan Hall, O.; Cornett, E.M.; Urman, R.D.; et al. Liposomal Bupivacaine and Novel Local Anesthetic Formulations. Best Pract. Res. Clin. Anaesthesiol. 2019, 33, 425–432. [Google Scholar] [CrossRef]
- Mendoza, G.; Arruebo, M. Light-Triggered Nanoparticles for Pain Management. Expert Opin. Drug Deliv. 2020, 17, 627–633. [Google Scholar] [CrossRef]
- Filonenko, E.V. Clinical Implementation and Scientific Development of Photodynamic Therapy in Russia in 2010–2020. Biomed. Photonics 2022, 10, 4–22. [Google Scholar] [CrossRef]
- Pang, Q.; Zhao, J.; Zhang, S.; Zhang, X. Near-Infrared Triggered on-Demand Local Anesthesia Using a Jammed Microgels System. J. Biomater. Sci. Polym. Ed. 2020, 31, 2252–2267. [Google Scholar] [CrossRef]
- Rwei, A.Y.; Wang, B.Y.; Ji, T.; Zhan, C.; Kohane, D.S. Enhanced Triggering of Local Anesthetic Particles by Photosensitization and Photothermal Effect Using a Common Wavelength. Nano Lett. 2017, 17, 7138–7145. [Google Scholar] [CrossRef]
- Chen, M.C.; Chan, H.A.; Ling, M.H.; Su, L.C. Implantable Polymeric Microneedles with Phototriggerable Properties as a Patient-Controlled Transdermal Analgesia System. J. Mater. Chem. B 2017, 5, 496–503. [Google Scholar] [CrossRef]
- Weiser, B.P.; Kelz, M.B.; Eckenhoff, R.G. In Vivo Activation of Azipropofol Prolongs Anesthesia and Reveals Synaptic Targets. J. Biol. Chem. 2013, 288, 1279–1285. [Google Scholar] [CrossRef] [Green Version]
- Shalabi, A.R.; Yu, Z.; Zhou, X.; Jounaidi, Y.; Chen, H.; Dai, J.; Kent, D.E.; Feng, H.-J.; Forman, S.A.; Cohen, J.B.; et al. A Potent Photoreactive General Anesthetic with Novel Binding Site Selectivity for GABAA Receptors. Eur. J. Med. Chem. 2020, 194, 112261. [Google Scholar] [CrossRef]
- Zhang, W.; Ji, T.; Li, Y.; Zheng, Y.; Mehta, M.; Zhao, C.; Liu, A.; Kohane, D.S. Light-Triggered Release of Conventional Local Anesthetics from a Macromolecular Prodrug for on-Demand Local Anesthesia. Nat. Commun. 2020, 11, 2323. [Google Scholar] [CrossRef]
- Font, J.; López-Cano, M.; Notartomaso, S.; Scarselli, P.; Di Pietro, P.; Bresolí-Obach, R.; Battaglia, G.; Malhaire, F.; Rovira, X.; Catena, J.; et al. Optical Control of Pain in Vivo with a Photoactive MGlu5 Receptor Negative Allosteric Modulator. Elife 2017, 6, e23545. [Google Scholar] [CrossRef] [Green Version]
- López-Cano, M.; Font, J.; Aso, E.; Sahlholm, K.; Cabré, G.; Giraldo, J.; De Koninck, Y.; Hernando, J.; Llebaria, A.; Fernández-Dueñas, V.; et al. Remote Local Photoactivation of Morphine Produces Analgesia without Opioid-related Adverse Effects. Br. J. Pharmacol. 2021. Online Ahead of Print. [Google Scholar] [CrossRef]
- Wang, F.; Bélanger, E.; Paquet, M.E.; Côté, D.C.; De Koninck, Y. Probing Pain Pathways with Light. Neuroscience 2016, 338, 248–271. [Google Scholar] [CrossRef] [Green Version]
- Gazerani, P. Shedding Light on Photo-Switchable Analgesics for Pain. Pain Manag. 2017, 7, 71–74. [Google Scholar] [CrossRef] [Green Version]
- Kienzler, M.A.; Isacoff, E.Y. Precise Modulation of Neuronal Activity with Synthetic Photoswitchable Ligands. Curr. Opin. Neurobiol. 2017, 45, 202–209. [Google Scholar] [CrossRef]
- Jarrin, S.; Finn, D.P. Optogenetics and Its Application in Pain and Anxiety Research. Neurosci. Biobehav. Rev. 2019, 105, 200–211. [Google Scholar] [CrossRef]
- Beaudry, H.; Daou, I.; Ribeiro-da-Silva, A.; Séguéla, P. Can We Use Optogenetics to Treat Chronic Pain? Douleur Analg. 2016, 29, 232–240. [Google Scholar] [CrossRef]
- Deisseroth, K. Optogenetics. Nat. Methods 2011, 8, 26–29. [Google Scholar] [CrossRef]
- Bonin, R.P.; Wang, F.; Desrochers-Couture, M.; Ga¸secka, A.; Boulanger, M.-E.; Côté, D.C.; De Koninck, Y. Epidural Optogenetics for Controlled Analgesia. Mol. Pain 2016, 12, 1744806916629051. [Google Scholar] [CrossRef] [Green Version]
- Broichhagen, J.; Frank, J.A.; Trauner, D. A Roadmap to Success in Photopharmacology. Acc. Chem. Res. 2015, 48, 1947–1960. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Santacana, X.; Pittolo, S.; Rovira, X.; Lopez, M.; Zussy, C.; Dalton, J.A.R.; Faucherre, A.; Jopling, C.; Pin, J.P.; Ciruela, F.; et al. Illuminating Phenylazopyridines to Photoswitch Metabotropic Glutamate Receptors: From the Flask to the Animals. ACS Cent. Sci. 2017, 3, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Pittolo, S.; Gómez-Santacana, X.; Eckelt, K.; Rovira, X.; Dalton, J.; Goudet, C.; Pin, J.-P.; Llobet, A.; Giraldo, J.; Llebaria, A.; et al. An Allosteric Modulator to Control Endogenous G Protein-Coupled Receptors with Light. Nat. Chem. Biol. 2014, 10, 813–815. [Google Scholar] [CrossRef] [PubMed]
- Rovira, X.; Trapero, A.; Pittolo, S.; Zussy, C.; Faucherre, A.; Jopling, C.; Giraldo, J.; Pin, J.P.; Gorostiza, P.; Goudet, C.; et al. OptoGluNAM4.1, a Photoswitchable Allosteric Antagonist for Real-Time Control of MGlu4 Receptor Activity. Cell Chem. Biol. 2016, 23, 929–934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stein, M.; Middendorp, S.J.; Carta, V.; Pejo, E.; Raines, D.E.; Forman, S.A.; Sigel, E.; Trauner, D. Azo-Propofols: Photochromic Potentiators of GABAA Receptors. Angew. Chem. Int. Ed. 2012, 51, 10500–10504. [Google Scholar] [CrossRef] [Green Version]
- Schönberger, M.; Trauner, D. A Photochromic Agonist for μ-Opioid Receptors. Angew. Chem. Int. Ed. 2014, 53, 3264–3267. [Google Scholar] [CrossRef]
- Mostyn, S.N.; Sarker, S.; Muthuraman, P.; Raja, A.; Shimmon, S.; Rawling, T.; Cioffi, C.L.; Vandenberg, R.J. Photoswitchable ORG25543 Congener Enables Optical Control of Glycine Transporter 2. ACS Chem. Neurosci. 2020, 11, 1250–1258. [Google Scholar] [CrossRef] [Green Version]
- Frank, J.A.; Moroni, M.; Moshourab, R.; Sumser, M.; Lewin, G.R.; Trauner, D. Photoswitchable Fatty Acids Enable Optical Control of TRPV1. Nat. Commun. 2015, 6, 7118. [Google Scholar] [CrossRef] [Green Version]
- Mourot, A.; Fehrentz, T.; Le Feuvre, Y.; Smith, C.M.; Herold, C.; Dalkara, D.; Nagy, F.; Trauner, D.; Kramer, R.H. Rapid Optical Control of Nociception with an Ion-Channel Photoswitch. Nat. Methods 2012, 9, 396–402. [Google Scholar] [CrossRef]
- Schoenberger, M.; Damijonaitis, A.; Zhang, Z.; Nagel, D.; Trauner, D. Development of a New Photochromic Ion Channel Blocker via Azologization of Fomocaine. ACS Chem. Neurosci. 2014, 5, 514–518. [Google Scholar] [CrossRef]
- Vetter, I.; Deuis, J.R.; Mueller, A.; Israel, M.R.; Starobova, H.; Zhang, A.; Rash, L.D.; Mobli, M. NaV1.7 as a Pain Target—From Gene to Pharmacology. Pharmacol. Ther. 2017, 172, 73–100. [Google Scholar] [CrossRef] [Green Version]
- Shen, H.; Liu, D.; Wu, K.; Lei, J.; Yan, N. Structures of Human Na v 1.7 Channel in Complex with Auxiliary Subunits and Animal Toxins. Science 2019, 363, 1303–1308. [Google Scholar] [CrossRef]
- Panigel, J.; Cook, S.P. A Point Mutation at F1737 of the Human Na v 1.7 Sodium Channel Decreases Inhibition by Local Anesthetics. J. Neurogenet. 2011, 25, 134–139. [Google Scholar] [CrossRef]
- Fozzard, H.A.; Sheets, M.F.; Hanck, D.A. The Sodium Channel as a Target for Local Anesthetic Drugs. Front. Pharmacol. 2011, 2, 68. [Google Scholar] [CrossRef] [Green Version]
- Noreng, S.; Li, T.; Payandeh, J. Structural Pharmacology of Voltage-Gated Sodium Channels. J. Mol. Biol. 2021, 433, 166967. [Google Scholar] [CrossRef]
- Körner, J.; Albani, S.; Sudha Bhagavath Eswaran, V.; Roehl, A.B.; Rossetti, G.; Lampert, A. Sodium Channels and Local Anesthetics—Old Friends With New Perspectives. Front. Pharmacol. 2022, 13, 837088. [Google Scholar] [CrossRef]
- Skarga, V.V.; Nevezhin, E.V.; Matrosov, A.A.; Negrebetsky, V.V.; Malakhov, M. V Detection of hydroperoxides in solutions of photooxidized psoralen. Fine Chem. Technol. 2019, 14, 32–38. [Google Scholar] [CrossRef]
- Beveridge, D.L.; Jaffé, H.H. The Electronic Structure and Spectra of Cis- and Trans-Azobenzene. J. Am. Chem. Soc. 1966, 88, 1948–1953. [Google Scholar] [CrossRef]
- Vetráková, Ľ.; Ladányi, V.; Al Anshori, J.; Dvořák, P.; Wirz, J.; Heger, D. The Absorption Spectrum of Cis -Azobenzene. Photochem. Photobiol. Sci. 2017, 16, 1749–1756. [Google Scholar] [CrossRef]
- Vogel, H.G. Drug Discovery and Evaluation, 3rd ed.; Vogel, H.G., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; ISBN 978-3-540-71420-0. [Google Scholar]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Huang, Y.; Xiao, Z.; Ren, X.; Yang, C. Guide for the Care and Use of Laboratory Animals; National Academies Press: Washington, DC, USA, 2011; ISBN 978-0-309-15400-0. [Google Scholar]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE Guidelines 2.0: Updated Guidelines for Reporting Animal Research. Exp. Physiol. 2020, 105, 1459–1466. [Google Scholar] [CrossRef]



| Compound Under Study | Regnier Index (min—13, max—1300) | |
|---|---|---|
| In the Dark | Under Irradiation (λ = 395 nm) | |
| Lidocaine 2% | 451 ± 40 (n = 8) * | 469 ± 37 (n = 8) |
| Ethercaine (6) (0.6% solution in 4% Kolliphor ® ELP) | 232 ± 50 (n = 8) | 22 ± 3 (n = 8) |
| 4% Kolliphor ® ELP | 13 (n = 4) | 13 (n = 4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noev, A.; Kuznetsov, N.; Korenev, G.; Morozova, N.; Vasil’ev, Y.; Suvorov, N.; Diachkova, E.; Usachev, M.; Pankratov, A.; Grin, M. A Novel Photoswitchable Azobenzene-Containing Local Anesthetic Ethercaine with Light-Controlled Biological Activity In Vivo. Int. J. Mol. Sci. 2022, 23, 5352. https://doi.org/10.3390/ijms23105352
Noev A, Kuznetsov N, Korenev G, Morozova N, Vasil’ev Y, Suvorov N, Diachkova E, Usachev M, Pankratov A, Grin M. A Novel Photoswitchable Azobenzene-Containing Local Anesthetic Ethercaine with Light-Controlled Biological Activity In Vivo. International Journal of Molecular Sciences. 2022; 23(10):5352. https://doi.org/10.3390/ijms23105352
Chicago/Turabian StyleNoev, Alexey, Nikita Kuznetsov, Georgiy Korenev, Natalia Morozova, Yuriy Vasil’ev, Nikita Suvorov, Ekaterina Diachkova, Maksim Usachev, Andrei Pankratov, and Mikhail Grin. 2022. "A Novel Photoswitchable Azobenzene-Containing Local Anesthetic Ethercaine with Light-Controlled Biological Activity In Vivo" International Journal of Molecular Sciences 23, no. 10: 5352. https://doi.org/10.3390/ijms23105352
APA StyleNoev, A., Kuznetsov, N., Korenev, G., Morozova, N., Vasil’ev, Y., Suvorov, N., Diachkova, E., Usachev, M., Pankratov, A., & Grin, M. (2022). A Novel Photoswitchable Azobenzene-Containing Local Anesthetic Ethercaine with Light-Controlled Biological Activity In Vivo. International Journal of Molecular Sciences, 23(10), 5352. https://doi.org/10.3390/ijms23105352







