4′-Methylflavanone Glycosides Obtained Using Biotransformation in the Entomopathogenic Filamentous Fungi Cultures as Potential Anticarcinogenic, Antimicrobial, and Hepatoprotective Agents
Abstract
:1. Introduction
2. Results and Discussion
2.1. Biotransformation of 4′-Methylflavanone (4) in the Culture of I. fumosorosea KCH J2
2.2. Biotransformation of 4′-Methylflavanone (4) in the Culture of B. bassiana KCH J1.5
2.3. Biotransformation of 4′-Methylflavanone (4) in the Culture of I. farinosa KCH J2.1
2.4. Pharmacokinetics, Drug-Likeness, and Biological-Activity Prediction
2.4.1. SwissADME
2.4.2. Way2Drug Pass Online
3. Materials and Methods
3.1. Substrates
4′-Methylflavanone (4)
3.2. Microorganisms
3.3. Analysis
3.4. Screening Procedure
3.5. The Semipreparative Biotransformations
3.5.1. Flavanone 4′-methylene-O-β-D-(4″-O-methyl)-glucopyranoside (4a)
3.5.2. 2-Phenyl-(4′-hydroxymethyl)-4-hydroxychromane (4b)
3.5.3. Flavanone 4′-carboxylic Acid (4c)
3.5.4. 4′-Hydroxymethylflavanone 4-O-β-D-(4″-O-methyl)-glucopyranoside (4d)
3.6. Pharmacokinetics, Drug-Likeness, Biological Activity Prediction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, T.Y.; Li, Q.; Bi, K. Bioactive Flavonoids in Medicinal Plants: Structure, Activity and Biological Fate. Asian J. Pharm. Sci. 2018, 13, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Muzashvili, T.S.; Georgiev, M.I. Advances in the Biotechnological Glycosylation of Valuable Flavonoids. Biotechnol. Adv. 2014, 32, 1145–1156. [Google Scholar] [CrossRef] [PubMed]
- Thilakarathna, S.H.; Rupasinghe, V.H.P. Flavonoid Bioavailability and Attempts for Bioavailability Enhancement. Nutrients 2013, 5, 3367–3387. [Google Scholar] [CrossRef]
- Xiao, J. Dietary Flavonoid Aglycones and Their Glycosides: Which Show Better Biological Significance? Crit. Rev. Food. Sci. Nutr. 2017, 57, 1874–1905. [Google Scholar] [CrossRef]
- Vazhappilly, C.G.; Amararathna, M.; Cyril, A.C.; Linger, R.; Matar, R.; Merheb, M.; Ramadan, W.S.; Radhakrishnan, R.; Rupasinghe, H.P.V. Current Methodologies to Refine Bioavailability, Delivery, and Therapeutic Efficacy of Plant Flavonoids in Cancer Treatment. J. Nutr. Biochem. 2021, 94, 108623. [Google Scholar] [CrossRef]
- Dias, C.; Nylandsted, J. Plasma Membrane Integrity in Health and Disease: Significance and Therapeutic Potential. Cell Discov. 2021, 7, 1–18. [Google Scholar] [CrossRef]
- Forni, C.; Rossi, M.; Borromeo, I.; Feriotto, G.; Platamone, G.; Tabolacci, C.; Mischiati, C.; Beninati, S. Flavonoids: A Myth or a Reality for Cancer Therapy? Molecules 2021, 26, 3583. [Google Scholar] [CrossRef]
- Ponte, L.G.S.; Pavan, I.C.B.; Mancini, M.C.S.; da Silva, L.G.S.; Morelli, A.P.; Severino, M.B.; Bezerra, R.M.N.; Simabuco, F.M. The Hallmarks of Flavonoids in Cancer. Molecules 2021, 26, 2029. [Google Scholar] [CrossRef]
- Ferdous, U.T.; Balia Yusof, Z.N. Insight into Potential Anticancer Activity of Algal Flavonoids: Current Status and Challenges. Molecules 2021, 26, 6844. [Google Scholar] [CrossRef]
- Lata, S.; Mittal, S.K. In Vitro and in Vivo Hepatoprotective Activity of Flavonoids Rich Extracts on Cucumis Dipsaceus Ehrenb. (Fruit). Int. J. Pharmacol. 2017, 13, 563–572. [Google Scholar] [CrossRef]
- Senosy, W.R.; Kamal, A.M.; El-Toumy, S.A.; ElGendy, E.A. Phenolic Compounds and Hepatoprotective Activity of Centaurea Aegyptiaca, L. on Carbon Tetrachloride-Induced Hepatotoxicity in Rats. J. Adv. Pharm. Res. 2018, 2, 123–132. [Google Scholar] [CrossRef] [Green Version]
- Tabeshpour, J.; Hosseinzadeh, H.; Hashemzaei, M.; Karimi, G. A Review of the Hepatoprotective Effects of Hesperidin, a Flavanone Glycoside in Citrus Fruits, against Natural and Chemical Toxicities. DARU J. Pharm. Sci. 2020, 28, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Adinew, G.M.; Taka, E.; Mendonca, P.; Messeha, S.S.; Soliman, K.F.A. The Anticancer Effects of Flavonoids through MiRNAs Modulations in Triple-Negative Breast Cancer. Nutrients 2021, 13, 1212. [Google Scholar] [CrossRef]
- Zhai, K.; Mazurakova, A.; Koklesova, L.; Kubatka, P.; Büsselberg, D. Flavonoids Synergistically Enhance the Anti-Glioblastoma Effects of Chemotherapeutic Drugs. Biomolecules 2021, 11, 1841. [Google Scholar] [CrossRef]
- Walle, T. Methylation of Dietary Flavones Increases Their Metabolic Stability and Chemopreventive Effects. Int. J. Mol. Sci. 2009, 10, 5002–5019. [Google Scholar] [CrossRef] [PubMed]
- Koirala, N.; Thuan, N.H.; Ghimire, G.P.; Thang, D.V.; Sohng, J.K. Methylation of Flavonoids: Chemical Structures, Bioactivities, Progress and Perspectives for Biotechnological Production. Enzyme Microb. Technol. 2016, 86, 103–116. [Google Scholar] [CrossRef]
- Wen, L.; Jiang, Y.; Yang, J.; Zhao, Y.; Tian, M.; Yang, B. Structure, Bioactivity, and Synthesis of Methylated Flavonoids. Ann. N.Y. Acad. Sci. 2017, 1398, 120–129. [Google Scholar] [CrossRef]
- Kondhare, D.D.; Gyananath, G.; Tamboli, Y.; Kumbhar, S.S.; Choudhari, P.B.; Bhatia, M.S.; Zubaidha, P.K. An Efficient Synthesis of Flavanones and Their Docking Studies with Aldose Reductase. Med. Chem. Res. 2017, 26, 987–998. [Google Scholar] [CrossRef]
- Chimenti, F.; Fioravanti, R.; Bolasco, A.; Chimenti, P.; Secci, D.; Rossi, F.; Yáñez, M.; Orallo, F.; Ortuso, F.; Alcaro, S.; et al. A New Series of Flavones, Thioflavones, and Flavanones as Selective Monoamine Oxidase-B Inhibitors. Bioorg. Med. Chem. 2010, 18, 1273–1279. [Google Scholar] [CrossRef] [PubMed]
- Dymarska, M.; Janeczko, T.; Kostrzewa-Susłow, E. Biotransformations of Flavones and an Isoflavone (Daidzein) in Cultures of Entomopathogenic Filamentous Fungi. Molecules 2018, 23, 1356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dou, F.; Wang, Z.; Li, G.; Dun, B. Microbial Transformation of Flavonoids by Isaria Fumosorosea ACCC 37814. Molecules 2019, 24, 1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krawczyk-Łebek, A.; Dymarska, M.; Janeczko, T.; Kostrzewa-Susłow, E. Entomopathogenic Filamentous Fungi as Biocatalysts in Glycosylation of Methylflavonoids. Catalysts 2020, 10, 1148. [Google Scholar] [CrossRef]
- Krawczyk-Łebek, A.; Dymarska, M.; Janeczko, T.; Kostrzewa-Susłow, E. New Glycosylated Dihydrochalcones Obtained by Biotransformation of 2′-Hydroxy-2-Methylchalcone in Cultures of Entomopathogenic Filamentous Fungi. Int. J. Mol. Sci. 2021, 22, 9619. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk-Łebek, A.; Dymarska, M.; Janeczko, T.; Kostrzewa-Susłow, E. Fungal Biotransformation of 2′-Methylflavanone and 2′-Methylflavone as a Method to Obtain Glycosylated Derivatives. Int. J. Mol. Sci. 2021, 22, 9617. [Google Scholar] [CrossRef]
- Włoch, A.; Strugała-Danak, P.; Pruchnik, H.; Krawczyk-Łebek, A.; Szczecka, K.; Janeczko, T.; Kostrzewa-Susłow, E. Interaction of 4′-Methylflavonoids with Biological Membranes, Liposomes, and Human Albumin. Sci. Rep. 2021, 11, 1–14. [Google Scholar] [CrossRef]
- Xie, L.; Zhang, L.; Bai, J.; Yue, Q.; Zhang, M.; Li, J.; Wang, C.; Xu, Y. Methylglucosylation of Phenolic Compounds by Fungal Glycosyltransferase-Methyltransferase Functional Modules. J. Agric. Food Chem. 2019, 67, 8573–8580. [Google Scholar] [CrossRef]
- Sordon, S.; Popłoński, J.; Tronina, T.; Huszcza, E. Regioselective O-Glycosylation of Flavonoids by Fungi Beauveria Bassiana, Absidia Coerulea and Absidia Glauca. Bioorg. Chem. 2019, 93, 102750. [Google Scholar] [CrossRef]
- Łużny, M.; Tronina, T.; Kozłowska, E.; Dymarska, M.; Popłoński, J.; Łyczko, J.; Kostrzewa-Susłow, E.; Janeczko, T. Biotransformation of Methoxyflavones by Selected Entomopathogenic Filamentous Fungi. Int. J. Mol. Sci. 2020, 21, 6121. [Google Scholar] [CrossRef]
- Dymarska, M.; Janeczko, T.; Kostrzewa-Susłow, E. Glycosylation of Methoxylated Flavonoids in the Cultures of Isaria Fumosorosea KCH J2. Molecules 2018, 23, 2578. [Google Scholar] [CrossRef] [Green Version]
- Dymarska, M.; Janeczko, T.; Kostrzewa-Susłow, E. Glycosylation of 3-Hydroxyflavone, 3-Methoxyflavone, Quercetin and Baicalein in Fungal Cultures of the Genus Isaria. Molecules 2018, 23, 2477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krawczyk-Łebek, A.; Dymarska, M.; Janeczko, T.; Kostrzewa-Susłow, E. Microbial Transformations of 6-Chloroflavone by the Strain Isaria Fumosorosea KCH J2. In Nauka i Praktyka w Świecie Flawonoidów; House of the Rzeszów University of Technology: Rzeszów, Poland, 2021; pp. 5–16. [Google Scholar]
- Dymarska, M.; Grzeszczuk, J.; Urbaniak, M.; Janeczko, T.; Pląskowska, E.; Stępień, Ł.; Kostrzewa-Susłow, E. Glycosylation of 6-Methylflavone by the Strain Isaria Fumosorosea KCH J2. PLoS ONE 2017, 12, e0184885. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Zhang, L.; Wang, C.; Wang, X.; Xu, Y.; Yu, H.; Wu, P.; Li, S.; Han, L.; Gunatilaka, A.A.L.; et al. Methylglucosylation of Aromatic Amino and Phenolic Moieties of Drug-like Biosynthons by Combinatorial Biosynthesis. Proc. Natl. Acad. Sci. USA 2018, 115, E4980–E4989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kostrzewa-Susłow, E.; Janeczko, T. Microbial Transformations of 5′-Hydroxy- and 5-Methoxyflavone in Aspergillus Niger and Penicillium Chermesinum Cultures. J. Microbiol. Biotechnol. Food Sci. 2014, 3, 448–452. [Google Scholar]
- Lynch, T.; Price, A. The Effect of Cytochrome P450 Metabolism on Drug Response, Interactions, and Adverse Effects. Am. Fam. Phys. 2007, 76, 391–396. [Google Scholar]
- Verstraeten, S.V.; Keen, C.L.; Schmitz, H.H.; Fraga, C.G.; Oteiza, P.I. Flavan-3-Ols and Procyanidins Protect Liposomes against Lipid Oxidation and Disruption of the Bilayer Structure. Free Radic. Biol. Med. 2002, 34, 84–92. [Google Scholar] [CrossRef]
- Erlejman, A.G.; Verstraeten, S.V.; Fraga, C.G.; Oteiza, P.I. The Interaction of Flavonoids with Membranes: Potential Determinant of Flavonoid Antioxidant Effects. Free Radic. Res. 2004, 38, 1311–1320. [Google Scholar] [CrossRef]
- Lewis, D.A.; Shaw, G.P. A Natural Flavonoid and Synthetic Analogues Protect the Gastric Mucosa from Aspirin-Induced Erosions. J. Nutr. Biochem. 2001, 12, 95–100. [Google Scholar] [CrossRef]
- Lin, K.; Wang, Y.; Gong, J.; Tan, Y.; Deng, T.; Wei, N. Protective Effects of Total Flavonoids from Alpinia Officinarum Rhizoma against Ethanol-Induced Gastric Ulcer in Vivo and in Vitro. Pharm. Biol. 2020, 58, 854–862. [Google Scholar] [CrossRef]
- De Oliveira Santos, G.C.; Vasconcelos, C.C.; Lopes, A.J.O.; de Sousa Cartágenes, M.d.S.; Filho, A.K.D.B.; do Nascimento, F.R.F.; Ramos, R.M.; Pires, E.R.R.B.; de Andrade, M.S.; Rocha, F.M.G.; et al. Candida Infections and Therapeutic Strategies: Mechanisms of Action for Traditional and Alternative Agents. Front. Microbiol. 2018, 9, 1351. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Tran, C.L.; Vuong, C.H.; Do, T.H.T.; Le, T.D.; Mai, D.T.; Phan, N.M. Flavonoids with Hepatoprotective Activity from the Leaves of Cleome Viscosa, L. Nat. Prod. Res. 2017, 31, 2587–2592. [Google Scholar] [CrossRef]
- Shi, Z.; Li, T.; Liu, Y.; Cai, T.; Yao, W.; Jiang, J.; He, Y.; Shan, L. Hepatoprotective and Anti-Oxidative Effects of Total Flavonoids from Qu Zhi Qiao (Fruit of Citrus Paradisi Cv.Changshanhuyou) on Nonalcoholic Steatohepatitis in Vivo and in Vitro through Nrf2-ARE Signaling Pathway. Front. Pharmacol. 2020, 11, 483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahlan, M.; Rizka Alia Hapsari, N.; Diah Pratami, K.; Cahya Khayrani, A.; Lischer, K.; Alhazmi, A.; Mohammedsaleh, Z.M.; Shater, A.F.; Saleh, F.M.; Alsanie, W.F.; et al. Potential Hepatoprotective Effects of Flavonoids Contained in Propolis from South Sulawesi against Chemotherapy Agents. Saudi J. Biol. Sci. 2021, 28, 5461–5468. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.; Meredith, T.; Swoboda, J.; Walker, S. Staphylococcus Aureus and Bacillus Subtilis W23 Make Polyribitol Wall Teichoic Acids Using Different Enzymatic Pathways. Chem. Biol. 2010, 17, 1101–1110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amparo, T.R.; Rodrigues, I.V.; Seibert, J.B.; Almeida, T.C.; Cabral, V.A.R.; de Abreu Vieira, P.M.; Brandão, G.C.; de Oliveira, M.L.G.; da Silva, G.N.; dos Santos, O.D.H.; et al. Antibacterial Substances from Leaves of Protium Spruceanum (Burseraceae): In Vitro and in Silico Evaluation. Braz. J. Pharm. Sci. 2020, 56, e18474. [Google Scholar] [CrossRef]


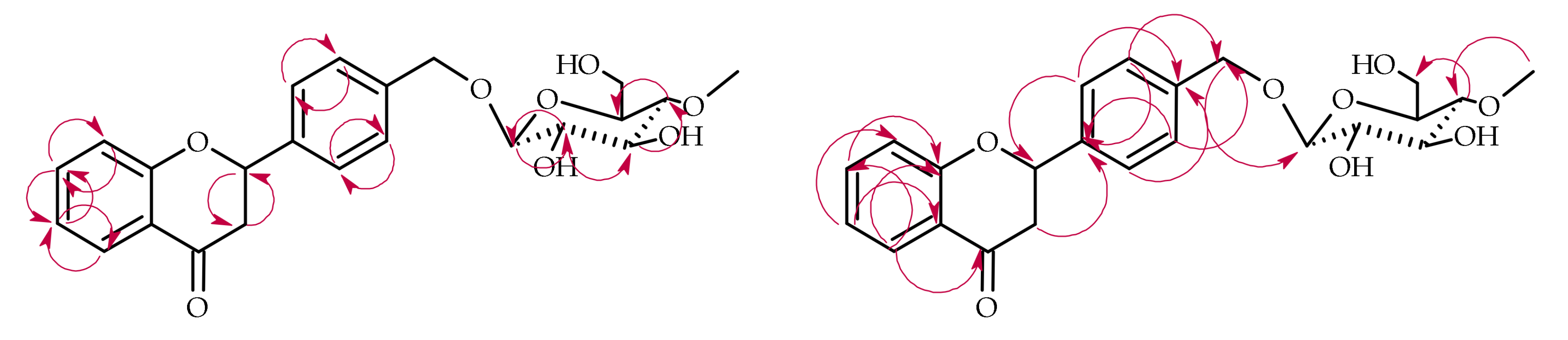
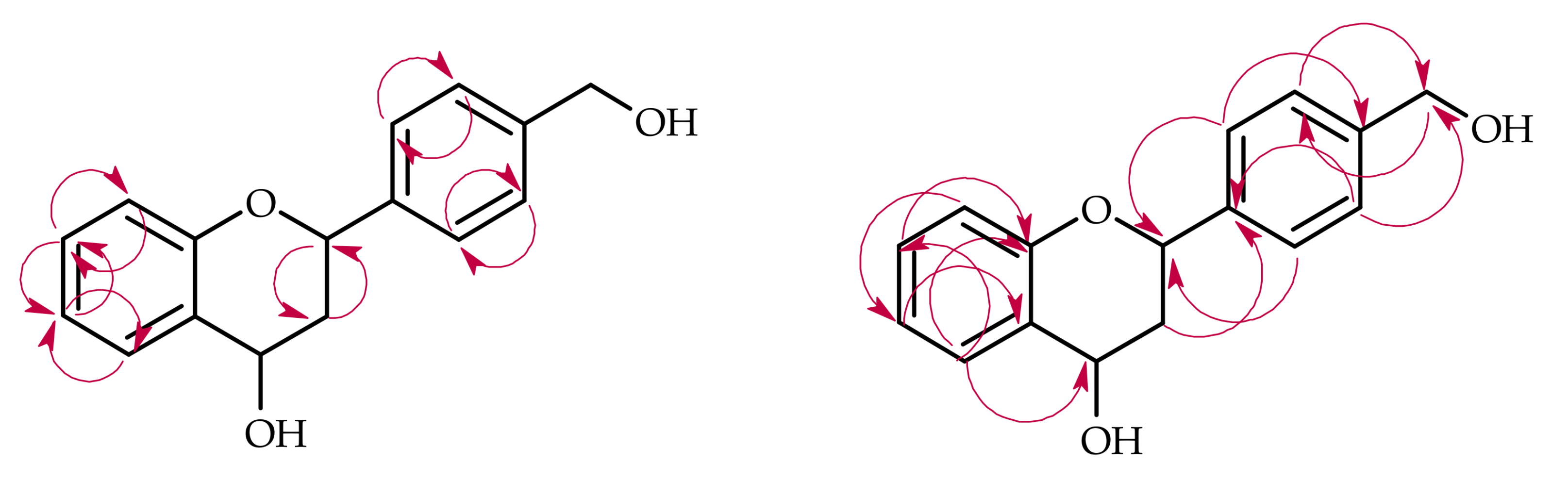
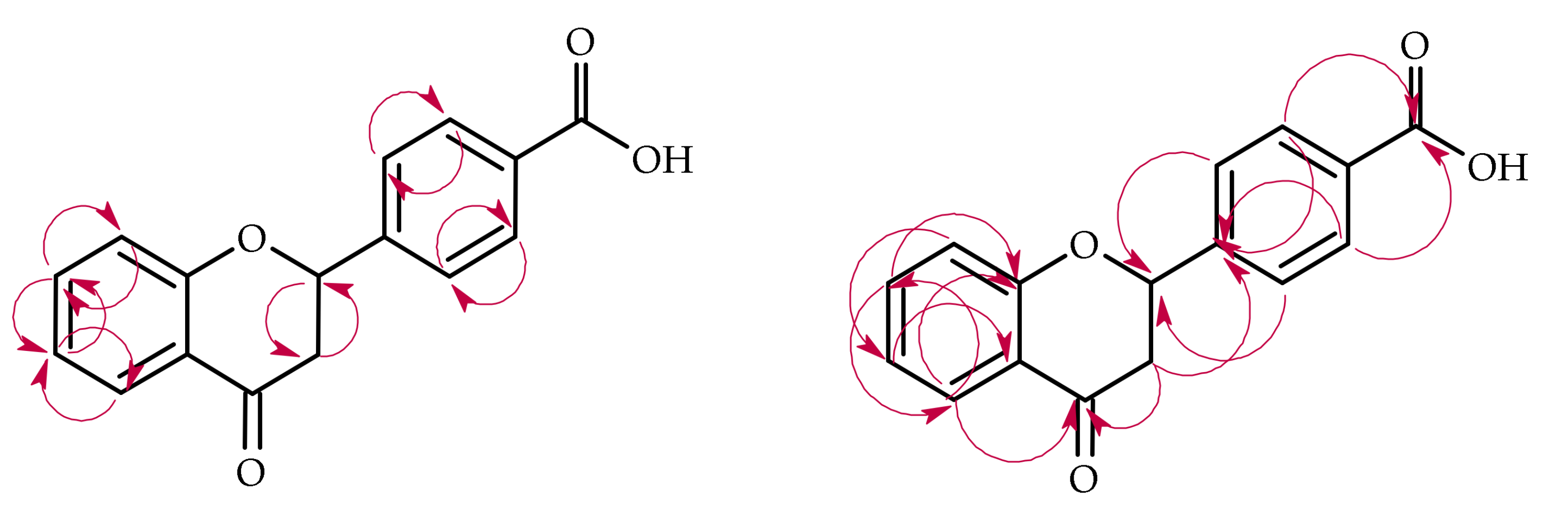





| Proton | Compound | ||||
|---|---|---|---|---|---|
| 4 | 4a | 4b | 4c | 4d | |
| H-2 | 5.59 (dd) J = 13.0, J = 2.8 | 5.63 (dd) J = 12.9, J = 2.9 | 5.30 (dd) J = 11.7, J = 2.2 | 5.77 (dd) J = 13.1, J = 2.9 | 5.30 (dd) J = 12.0, J = 1.5 |
| H-3ax | 3.15 (dd) J = 16.7, J = 13.0 | 3.14 (m) | 2.21 (dt) J = 14.0, J = 2.4 | 3.16 (dd) J = 16.8, J = 13.1 | 2.45 (dt) J = 14.5, J = 2.0 |
| H-3eq | 2.83 (dd) J = 16.7, J = 2.6 | 2.86 (dd) J = 16.8, J = 3.0 | 2.12 (ddd) J = 14.1, J = 11.7, J = 3.5 | 2.94 (dd) J = 16.7, J = 3.0 | 2.01 (dd) J = 3.1, J = 2.5 |
| H-4 | - | - | 4.78 (t) J = 2.9 | - | 4.91 (t) J = 2.6 |
| H-5 | 7.84 (dd) J = 7.8, J = 1.2 | 7.84 (dd) J = 7.8, J = 1.7 | 7.35 (dd) J = 7.6, J = 1.6 | 7.86 (dd) J = 7.7, J = 1.8 | 7.44 (dd) J = 7.7, J = 1.6 |
| H-6 | 7.08 (dd) J = 14.0, J = 7.8 | 7.09 (m) | 6.91 (td) J = 7.5, J = 1.1 | 7.12 (m) | 6.92 (td) J = 7.5, J = 1.1 |
| H-7 | 7.57 (m) | 7.58 (m) | 7.20 (ddd) J = 8.4, J = 7.4, J = 1.7 | 7.61 (ddd) J = 8.8, J = 7.2, J = 1.7 | 7.23 (m) |
| H-8 | 7.08 (dd) J = 14.0, J = 7.8 | 7.09 (m) | 6.86 (dd) J = 8.4, J = 0.8 | 7.12 (m) | 6.87 (dd) J = 8.2, J = 0.7 |
| H-2′ | 7.47 (d) J = 8.0 | 7.56 (d) J = 7.9 | 7.45 (d) J = 8.1 | 7.75 (d) J = 8.2 | 7.46 (d) J = 8.0 |
| H-3′ | 7.26 (d) J = 7.9 | 7.49 (d) J = 8.1 | 7.41 (d) J = 8.2 | 8.12 (d) J = 8.4 | 7.40 (d) J = 8.1 |
| H-5′ | 7.26 (d) J = 7.9 | 7.49 (d) J = 8.1 | 7.41 (d) J = 8.2 | 8.12 (d) J = 8.4 | 7.40 (d) J = 8.1 |
| H-6′ | 7.47 (d) J = 8.0 | 7.56 (d) J = 7.9 | 7.45 (d) J = 8.1 | 7.75 (d) J = 8.2 | 7.46 (d) J = 8.0 |
| H-1″ | - | 4.39 (d) J = 7.7 | - | - | 4.62 (d) J = 7.9 |
| H-2″ | - | 3.27 (m) | - | - | 3.21 (td) J = 8.8, J = 4.0 |
| H-3″ | - | 3.52 (m) | - | - | 3.56 (dd) J = 5.9, J = 2.6 |
| H-4″ | - | 3.14 (m) | - | - | 3.13 (dd) J = 9.5, J = 9.0 |
| H-5″ | - | 3.27 (m) | - | - | 3.35 (m) |
| H-6″ | - | 3.82 (dd) J = 11.6, J = 1.1 3.67 (dd) J = 11.6, J = 5.1 | - | - | 3.86 (dt) J = 8.3, J = 6.0, 3.70 (m) |
| C4″-OCH3 | - | 3.53 (s) | - | - | 3.54 (s) |
| C4′-CH3 | 2.36 (s) | - | - | - | - |
| 2″-OH | - | - | - | - | 4.54 (d) J = 4.0 |
| 3″-OH | - | - | - | - | 4.27 (d) J = 3.8 |
| 4′-CH2- | - | 4.92 (d) J = 12.3 4.66 (d) J = 12.3 | 4.66 (s) | - | 4.65 (d) J = 5.5 |
| 4′-CH2-OH | - | - | - | 4.25 (t) J = 5.7 | |
| Carbon | Compound | ||||
|---|---|---|---|---|---|
| 4 | 4a | 4b | 4c | 4d | |
| C-2 | 80.3 | 80.2 | 73.8 | 79.9 | 73.8 |
| C-3 | 44.9 | 44.9 | 39.7 | 44.9 | 36.2 |
| C-4 | 191.9 | 191.9 | 63.7 | 191.4 | 69.9 |
| C-4a | 122.0 | 121.9 | 125.7 | 122.0 | 122.7 |
| C-5 | 127.4 | 127.4 | 131.5 | 127.4 | 132.8 |
| C-6 | 122.2 | 122.2 | 121.1 | 122.5 | 121.0 |
| C-7 | 136.8 | 136.9 | 129.9 | 137.0 | 130.3 |
| C-8 | 118.9 | 118.9 | 117.6 | 119.0 | 117.5 |
| C-8a | 162.5 | 162.4 | 155.8 | 162.2 | 156.3 |
| C-1′ | 139.1 | 139.4 | 143.1 | 145.3 | 141.1 |
| C-2′ | 127.3 | 127.2 | 127.0 | 127.3 | 127.4 |
| C-3′ | 130.1 | 128.8 | 127.5 | 130.8 | 126.9 |
| C-4′ | 137.4 | 139.7 | 141.2 | 131.6 | 143.1 |
| C-5′ | 130.1 | 128.8 | 127.5 | 130.8 | 126.9 |
| C-6′ | 127.3 | 127.2 | 127.0 | 127.3 | 127.4 |
| C-1″ | - | 103.1 | - | - | 101.2 |
| C-2″ | - | 75.2 | - | - | 75.0 |
| C-3″ | - | 78.0 | - | - | 78.1 |
| C-4″ | - | 80.5 | - | - | 80.6 |
| C-5″ | - | 76.9 | - | - | 77.0 |
| C-6″ | - | 62.4 | - | - | 62.6 |
| 4″-OCH3 | - | 60.5 | - | - | 60.5 |
| 4′-CH3 | 21.2 | - | - | - | - |
| 4′-CH2- | - | 70.6 | 64.4 | - | 64.5 |
| 4′-COOH | - | - | - | 167.3 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krawczyk-Łebek, A.; Dymarska, M.; Janeczko, T.; Kostrzewa-Susłow, E. 4′-Methylflavanone Glycosides Obtained Using Biotransformation in the Entomopathogenic Filamentous Fungi Cultures as Potential Anticarcinogenic, Antimicrobial, and Hepatoprotective Agents. Int. J. Mol. Sci. 2022, 23, 5373. https://doi.org/10.3390/ijms23105373
Krawczyk-Łebek A, Dymarska M, Janeczko T, Kostrzewa-Susłow E. 4′-Methylflavanone Glycosides Obtained Using Biotransformation in the Entomopathogenic Filamentous Fungi Cultures as Potential Anticarcinogenic, Antimicrobial, and Hepatoprotective Agents. International Journal of Molecular Sciences. 2022; 23(10):5373. https://doi.org/10.3390/ijms23105373
Chicago/Turabian StyleKrawczyk-Łebek, Agnieszka, Monika Dymarska, Tomasz Janeczko, and Edyta Kostrzewa-Susłow. 2022. "4′-Methylflavanone Glycosides Obtained Using Biotransformation in the Entomopathogenic Filamentous Fungi Cultures as Potential Anticarcinogenic, Antimicrobial, and Hepatoprotective Agents" International Journal of Molecular Sciences 23, no. 10: 5373. https://doi.org/10.3390/ijms23105373






