Golden and Silver–Golden Chitosan Hydrogels and Fabrics Modified with Golden Chitosan Hydrogels
Abstract
:1. Introduction
2. Results and Discussion
2.1. Sol–Gel Analysis: Macro-Gels
2.2. Vis Spectrophotometry and DLS Measurements: Micro- and Nano-Gels
2.3. Flame Atomic Absorption Spectrophotometry (FAAS) of Chitosan–Au Hydrogels
2.4. Modification of Textiles—Reflectance Analysis
2.5. SEM-EDS Analysis of the Modified Textiles
2.6. ICP-MS of the Modified Textiles
2.7. Chitosan–Au–Ag Hydrogels
3. Materials and Methods
3.1. Preparation of Chitosan–Au Hydrogel
3.2. Sol-Gel Analysis
3.3. Vis Spectrophotometry Measurements
3.4. Dynamic Light Scattering Measurements
3.5. Modification of Fabric with Chitosan-Au Hydrogel
3.6. Chitosan–Au–Ag Hydrogels
3.7. AAS
3.8. ICP-QMS
3.9. Statistical and Chemometric Analysis
3.10. Reflectance Measurements
3.11. Microscopic Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wichterle, O.; Lím, D. Hydrophilic gels for biological use. Nature 1960, 185, 117–118. [Google Scholar] [CrossRef]
- Peppas, N. Hydrogels in Medicine and Pharmacy; Volume 1 Fundamentals; CRC Press Inc.: Boca Raton, FL, USA, 1986. [Google Scholar] [CrossRef]
- Ulanski, P.; Rosiak, J.M. The use of radiation technique in the synthesis of polymeric nanogels. Nucl. Instrum. Meth. B 1999, 151, 356–360. [Google Scholar] [CrossRef]
- Kozicki, M. How do monomeric components of a polymer gel dosimeter respond to ionising radiation: A steady-state radiolysis towards preparation of a 3D polymer gel dosimeter. Radiat. Phys. Chem. 2011, 80, 1419–1436. [Google Scholar] [CrossRef]
- Kouvati, K.; Jaszczak, M.; Papagiannis, P.; Kadlubowski, S.; Wach, R.; Maras, P.; Dudek, M.; Kozicki, M. Leuco crystal violet-Pluronic F-127 3D radiochromic gel dosimeter. Phys. Med. Biol. 2019, 64, 175017. [Google Scholar] [CrossRef] [PubMed]
- Muzzarelli, R.A.A. Genipin-crosslinked chitosan hydrogels as biomedical and pharmaceutical aids. Carbohydr. Polym. 2009, 77, 1–9. [Google Scholar] [CrossRef]
- Billiet, T.; Vandenhaute, M.; Schelfhout, J.; Van Vlierberghe, S.; Dubruel, P. A review of trends and limitations in hydrogel-rapid prototyping for tissue engineering. Biomaterials 2012, 33, 6020–6041. [Google Scholar] [CrossRef]
- Verhulsel, M.; Vignes, M.; Descroix, S.; Malaquin, L.; Vignjevic, D.M.; Viovy, J.-L. A review of microfabrication and hydrogel engineering for micro-organs on chips. Biomaterials 2014, 35, 1816–1832. [Google Scholar] [CrossRef]
- De Deene, Y.; Vergote, K.; Claeys, C.; De Wagter, C. The fundamental radiation properties of normoxic polymer gel dosimeters: A comparison between a methacrylic acid based gel and acrylamide based gels. Phys. Med. Biol. 2006, 51, 653–673. [Google Scholar] [CrossRef]
- Kozicki, M.; Jaszczak, M.; Maras, P.; Dudek, M.; Cłapa, M. On the development of a VIPARnd radiotherapy 3D polymer gel dosimeter. Phys. Med. Biol. 2017, 62, 986–1008. [Google Scholar] [CrossRef]
- Joshi, C.; Schreiner, L.J. High dose rate brachytherapy three-dimensional gel dosimetry using optical computed tomography readout. J. Phys. Conf. Ser. 2019, 1305, 012051. [Google Scholar] [CrossRef] [Green Version]
- Kozicki, M.; Kwiatos, K.; Dudek, M.; Stempien, Z. Radiochromic gels for UV radiation measurements in 3D. J. Photochem. Photobiol. A 2018, 351, 197–207. [Google Scholar] [CrossRef]
- Kozicki, M.; Kołodziejczyk, M.; Szynkowska, M.; Pawlaczyk, A.; Lesniewska, E.; Matusiak, A.; Adamus, A.; Karolczak, A. Hydrogels made from chitosan and silver nitrate. Carbohydr. Polym. 2016, 140, 74–87. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A.; Boudrant, J.; Meyer, D.; Manno, N.; DeMarchis, M.; Paoletti, M.G. Current views on fungal chitin/chitosan, human chitinases, food preservation, glucans, pectins and inulin: A tribute to Henri Braconnot, precursor of the carbohydrate polymers science, on the chitin bicentennial. Carbohydr. Polym. 2012, 87, 995–1012. [Google Scholar] [CrossRef]
- Modrzejewska, Z.; Skwarczyńska, A.; Douglas, T.E.L.; Biniaś, D.; Maniukiewicz, W.; Sielski, J. Structure of chitosan gels mineralized by sorption. J. Mol. Struct. 2015, 1098, 101–109. [Google Scholar] [CrossRef]
- Toppazzini, M.; Coslovi, A.; Boschelle, M.; Marsich, E.; Benincasa, M.; Gennaro, R.; Paoletti, S. Can the interaction between the antimicrobial peptide LL-37 and alginate be exploited for the formulation of new biomaterials with antimicrobial properties? Carbohydr. Polym. 2011, 83, 578–585. [Google Scholar] [CrossRef]
- Paulino, A.T.; Guilherme, M.R.; de Almeida, E.A.S.; Pereira, A.G.B.; Muniz, E.C.; Tambourgi, E.B. One-pot synthesis of a chitosan-based hydrogel as a potential device for magnetic biomaterial. J. Magn. Magn. Mater. 2009, 321, 2636–2642. [Google Scholar] [CrossRef]
- Yan, H.; Dai, J.; Yang, Z.; Yang, H.; Cheng, R. Enhanced and selective adsorption of copper(II) ions on surface carboxymethylated chitosan hydrogel beads. Chem. Eng. J. 2011, 174, 586–594. [Google Scholar] [CrossRef]
- Gao, J.; Liu, R.; Wu, J.; Liu, Z.; Li, J.; Zhou, J.; Hao, T.; Wang, Y.; Du, Z.; Duan, C.; et al. The use of chitosan based hydrogel for enhancing the therapeutic benefits of adipose-derived MSCs for acute kidney injury. Biomaterials 2012, 33, 3673–3681. [Google Scholar] [CrossRef]
- Yadollahi, M.; Farhoudian, S.; Namazi, H. One-pot synthesis of antibacterial chitosan/silver bio-nanocomposite hydrogel beads as drug delivery systems. Int. J. Biol. Macromol. 2015, 79, 37–43. [Google Scholar] [CrossRef]
- Caswell, K.K.; Bender, C.M.; Murphy, C.J. Seedless, surfactantless wet chemical synthesis of silver nanowires. Nano Lett. 2003, 3, 667–669. [Google Scholar] [CrossRef]
- Choi, S.-H.; Lee, S.-H.; Hwang, Y.-M.; Lee, K.-P.; Kang, H.-D. Interaction between the surface of the silver nanoparticles prepared by γ-irradiation and organic molecules containing thiol group. Radiat. Phys. Chem. 2003, 67, 517–521. [Google Scholar] [CrossRef]
- Hussain, I.; Brust, M.; Papworth, A.J.; Cooper, A.I. Preparation of acrylate-stabilized gold and silver hydrosols and gold–polymer composite films. Langmuir 2003, 19, 4831–4835. [Google Scholar] [CrossRef]
- Nersisyan, H.H.; Lee, J.H.; Son, H.T.; Won, C.W.; Maeng, D.Y. A new and effective chemical reduction method for preparation of nanosized silver powder and colloid dispersion. Mater. Res. Bull. 2003, 38, 949–956. [Google Scholar] [CrossRef]
- Pan, A.L.; Zheng, H.G.; Yang, Z.P.; Liu, F.X.; Ding, Z.J.; Qian, Y.T. Gamma-irradiation-induced Ag/SiO2composite films and their optical absorption properties. Mater. Res. Bull. 2003, 38, 789–796. [Google Scholar] [CrossRef]
- Suber, L.; Sondi, I.; Matijevic, E.; Goia, D.V. Preparation and the mechanisms of formation of silver particles of different morphologies inhomogeneous solutions. J. Coll. Interface Sci. 2005, 288, 489–495. [Google Scholar] [CrossRef]
- Trapalis, C.C.; Vaimakis, T.; Kharlamov, A.; Kokkoris, M.; Kordas, G. Nanostructured MeSiO2 (Me = Ag, Cu) coatings with antibacterial activity. In Nanostructured Materials and Coatings for Biomedical and Sensor Applications; Gogotsi, Y.G., Uvarova, I.V., Eds.; Kluwer Academic Publishers: Alphen aan den Rijn, The Netherlands, 2003; pp. 41–49. [Google Scholar]
- Wei, D.; Sun, W.; Qian, W.; Ye, Y.; Ma, X. The synthesis of chitosan-based silver nanoparticles and their antibacterial activity. Carbohydr. Res. 2009, 344, 2375–2382. [Google Scholar] [CrossRef]
- Atiyeh, B.S.; Costagliola, M.; Hayek, S.N.; Dibo, S.A. Effect of silver onburn wound infection control and healing: Review of the literature. Burns 2007, 33, 139–148. [Google Scholar] [CrossRef]
- Choi, J.-H.; Lee, S.-W.; Jeong, J.-H.; Choi, D.-G.; Lee, E.-S. Direct imprint of conductive silver patterns using nanosilver particles and UV curable resin. Microelectron. Eng. 2009, 86, 622–627. [Google Scholar] [CrossRef]
- Klasen, H.J. A historical review of the use of silver in the treatment of burns. II. Renewed interest for silver. Burns 2000, 26, 131–138. [Google Scholar] [CrossRef]
- Wei, D.; Qian, W. Facile synthesis of Ag and Au nanoparticles utilizing chitosan as a mediator agent. Colloids Surf. B 2008, 62, 136–142. [Google Scholar] [CrossRef]
- Yang, X.; Wang, L. Silver nanocrystals modified microstructured polymer optical fibres for chemical and optical sensing. Opt. Commun. 2007, 280, 368–373. [Google Scholar] [CrossRef]
- Chen, R.; Chen, Q.; Huo, D.; Ding, Y.; Hu, Y.; Jiang, X. In situ formation of chitosan–gold hybrid hydrogel and its application for drug delivery. Colloids Surf. B 2012, 97, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Marsich, E.; Travan, A.; Donati, I.; Di Luca, A.; Benincasa, M.; Crosera, M.; Paoletti, S. Biological response of hydrogels embedding gold nanoparticles. Colloids Surf. B 2011, 83, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Sacco, P.; Travan, A.; Borgogna, M.; Paoletti, S.; Marsich, E. Silver-containing antimicrobial membrane based on chitosan–TPP hydrogel for the treatment of wounds. J. Mater. Sci.—Mater. Electron. 2015, 26, 128. [Google Scholar] [CrossRef] [PubMed]
- Tyliszczak, B.; Drabczyk, A.; Kudłacik-Kramarczyk, S.; Bialak-Wąs, K.; Sobczak-Kupiec, A. In vitro cytotoxicity of hydrogels based on chitosan and modified with gold nanoparticles. J. Polym. Res. 2017, 24, 153. [Google Scholar] [CrossRef] [Green Version]
- Giri, T.K.; Thakur, A.; Alexander, A.; Badwaik, A.H.; Tripathi, D.K. Modified chitosan hydrogels as drug delivery and tissue engineering systems: Present status and applications. Acta Pharm. Sinic. B 2012, 2, 439–449. [Google Scholar] [CrossRef] [Green Version]
- Futyra, A.R.; Liskiewicz, M.K.; Sebastian, V.; Irusta, S.; Arruebo, M.; Stochel, G.; Kyziol, A. Development of non cytotoxic chitosan-gold nanocomposites as efficient antibacterial materials. ACS Appl. Mater. Interfaces 2015, 7, 1087–1099. [Google Scholar] [CrossRef]
- Mohandas, A.; Deepthi, S.; Biswas, R.; Jayakumar, R. Chitosan based metallic nanocomposite scaffolds as antimicrobial wound dressings. Bioact. Mater. 2018, 3, 267–277. [Google Scholar] [CrossRef]
- Kozicki, M.; Sąsiadek, E.; Kadlubowski, S.; Dudek, M.; Karbownik, I. Radiation sensitive polyacrylonitrile microfibres doped with PDA nanoparticles. Radiat. Phys. Chem. 2020, 169, 107751. [Google Scholar] [CrossRef]
- Kozicki, M.; Sasiadek, E. Textile UV detector with 2,3,5-triphenyltetrazolium chloride as an active compound. Radiat. Meas. 2011, 46, 510–526. [Google Scholar] [CrossRef]
- Sąsiadek, E.; Jaszczak, M.; Skwarek, J.; Kozicki, M. NBT-Pluronic F-127 hydrogels printed on flat textiles as UV radiation sensors. Materials 2021, 14, 3435. [Google Scholar] [CrossRef]
- Stempien, Z.; Khalid, M.; Kozicki, M.; Kozanecki, M.; Varela, H.; Filipczak, P.; Pawlak, R.; Korzeniewska, E.; Sąsiadek, E. In-situ deposition of reduced graphene oxide layers on textile surfaces by the reactive inkjet printing technique and their use in supercapacitor applications. Synth. Met. 2019, 256, 116144. [Google Scholar] [CrossRef]
- Haiss, W.; Thanh, N.T.K.; Aveyard, J.; Fernig, D.G. Determination of size and concentration of gold nanoparticles from UV-Vis spectra. Anal. Chem. 2007, 79, 4215–4221. [Google Scholar] [CrossRef]
- Grancaric, A.M.; Tarbuk, A.; Pusic, T. Electrokinetic properties of textile fabrics. Color. Technol. 2006, 121, 221–227. [Google Scholar] [CrossRef]
- Luxbacher, T.; Pušić, T.; Bukšek, H.; Petrinić, I. The zeta potential of textile fabrics: A review. Tekstil 2016, 65, 346–351. [Google Scholar]
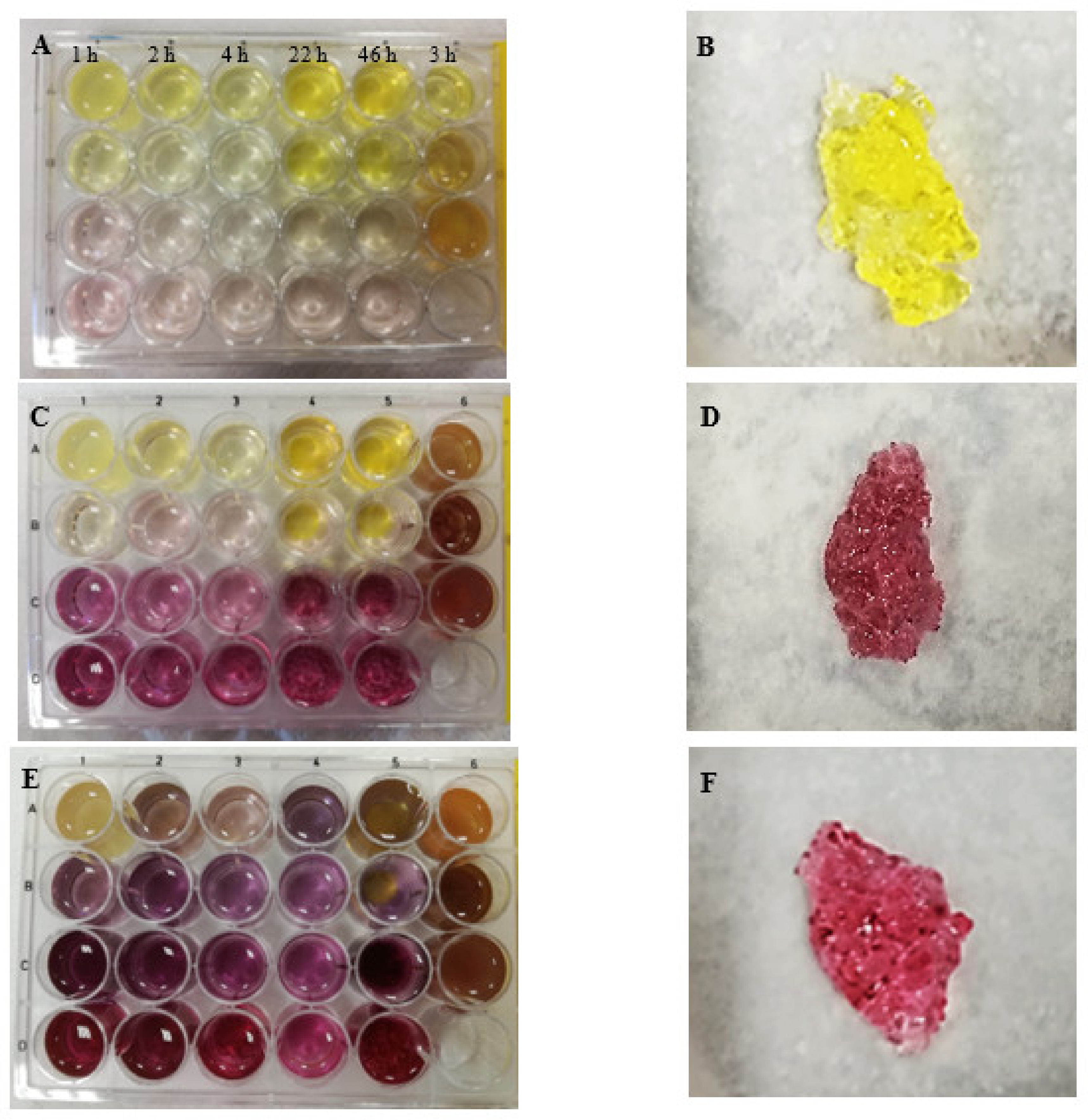




| Concentration of HAuCl4 | Sample Number | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| Reaction Time between 2% Chitosan and HAuCl4 | |||||||
| 1 h | 2 h | 4 h | 22 h | 46 h | 3 h | ||
| 1% HAuCl4 + Chitosan | |||||||
| A 1% | Intense yellow gel Viscous, liquid jelly-like structure that was hard to transfer to a beaker | Yellow gel Firm gel resembling gelatine jelly | Yellow gel Firm gel resembling gelatine jelly | Yellow gel Very firm gel from wall-to-wall | Yellow gel Very firm gel from wall-to-wall | Yellow gel Very firm gel from wall-to-wall that was easy to transfer to a beaker | 1.5% chitosan |
| B 0.5% | Bright yellow gel Gel resembling light gelatine jelly; it was possible to transfer the whole gel to a beaker | Bright yellow gel Firm gel resembling gelatine jelly (from wall-to-wall) | Bright yellow gel Firm gel resembling gelatine jelly | Yellow gel Firm gel resembling gelatine jelly (not from wall-to-wall) | Yellow very firm gel Gel from wall-to-wall. This gel was the stiffest out of the other gels in series 5 | Yellow-brownish bright gel Viscous gel that was hard to transfer to a beaker | 1% chitosan |
| C 0.1% | Bright transparent light pink viscous gel | Bright yellow gel Gelatine jelly-like structure | Bright yellow gel Gel resembling light gelatine jelly | Pink-raspberry bright gel In the vial it looked like raspberry strings but kept its shape during swelling | Raspberry-colour gel Viscous gel | No typical gel from wall-to-wall; yellow-brownish solution | 0.5% chitosan |
| D 0.05% | Bright transparent light pink viscous gel No typical gel from wall-to-wall was formed. The mixture remained as a transparent solution | Bright pink gel Viscous gel | Bright pink gel Gel resembling viscous physical gel | Bright pink gel Firm gel that looked in a vial like raspberry-colour strings | Bright raspberry-colour viscous gel | - | |
| Reaction time (h) | 1 | 2 | 4 | 22 | 46 | 3 | |
|---|---|---|---|---|---|---|---|
| HAuCl4 (%) | Options | 1 | 2 | 3 | 4 | 5 | 6 |
| 1 | A | + | + | + | + | + | 1.5% chit |
| 0.5 | B | + | + | + | + | + | 1% chit |
| 0.1 | C | + | + | + | + | + | 0.5% chit |
| 0.05 | D | + | + | + | + | + | - |
| Fabric | Crude Cotton | Bleached Cotton | Cotton/PET | PET | PA | PAN |
|---|---|---|---|---|---|---|
| Weave | plain | plain | twill | plain | plain | twill |
| Warp [1/dm] | 250 | 280 | 500 | 600 | 400 | 240 |
| Thread [1/dm] | 220 | 260 | 240 | 400 | 300 | 180 |
| Thickness [mm] | 0.40 | 0.36 | 0.36 | 0.14 | 0.12 | 0.57 |
| Surface mass [g/m2] | 150 | 140 | 185 | 80 | 65 | 220 |
| Color | beige with visible seed flakes | white | white | white | white | beige |
| Optical brightener | no | no | yes | yes | yes | no |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozicki, M.; Pawlaczyk, A.; Adamska, A.; Szynkowska-Jóźwik, M.I.; Sąsiadek-Andrzejczak, E. Golden and Silver–Golden Chitosan Hydrogels and Fabrics Modified with Golden Chitosan Hydrogels. Int. J. Mol. Sci. 2022, 23, 5406. https://doi.org/10.3390/ijms23105406
Kozicki M, Pawlaczyk A, Adamska A, Szynkowska-Jóźwik MI, Sąsiadek-Andrzejczak E. Golden and Silver–Golden Chitosan Hydrogels and Fabrics Modified with Golden Chitosan Hydrogels. International Journal of Molecular Sciences. 2022; 23(10):5406. https://doi.org/10.3390/ijms23105406
Chicago/Turabian StyleKozicki, Marek, Aleksandra Pawlaczyk, Aleksandra Adamska, Małgorzata Iwona Szynkowska-Jóźwik, and Elżbieta Sąsiadek-Andrzejczak. 2022. "Golden and Silver–Golden Chitosan Hydrogels and Fabrics Modified with Golden Chitosan Hydrogels" International Journal of Molecular Sciences 23, no. 10: 5406. https://doi.org/10.3390/ijms23105406







