Increasing Heavy Metal Tolerance by the Exogenous Application of Organic Acids
Abstract
:1. Introduction
2. Application of Organic Acids to Alleviate HM Stress
2.1. Carboxylic Acids
2.1.1. Citric Acid
2.1.2. Malic Acid
2.1.3. Oxalic Acid
2.1.4. Lipoic Acid
2.1.5. Jasmonic Acid
2.2. Phenolic Acids
2.2.1. Salicylic Acid
2.2.2. Gallic Acid
2.2.3. Caffeic Acid
3. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kim, J.J.; Kim, Y.S.; Kumar, V. Heavy Metal Toxicity: An Update of Chelating Therapeutic Strategies. J. Trace Elem. Med. Biol. 2019, 54, 226–231. [Google Scholar] [CrossRef]
- Rahman, Z.; Singh, V.P. The Relative Impact of Toxic Heavy Metals (THMs) (Arsenic (As), Cadmium (Cd), Chromium (Cr)(VI), Mercury (Hg), and Lead (Pb)) on the Total Environment: An Overview. Environ. Monit. Assess. 2019, 191, 419. [Google Scholar] [CrossRef] [PubMed]
- Muszyńska, E.; Labudda, M. Dual Role of Metallic Trace Elements in Stress Biology—from Negative to Beneficial Impact on Plants. Int. J. Mol. Sci. 2019, 20, 3117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fryzova, R.; Pohanka, M.; Martinkova, P.; Cihlarova, H.; Brtnicky, M.; Hladky, J.; Kynicky, J. Oxidative Stress and Heavy Metals in Plants. In Reviews of Environmental Contamination and Toxicology; Springer LLC: New York, NY, USA, 2018; Volume 245, pp. 129–156. [Google Scholar]
- Suvarapu, L.N.; Baek, S.O. Determination of Heavy Metals in the Ambient Atmosphere: A Review. Toxicol. Ind. Health 2017, 33, 79–96. [Google Scholar] [CrossRef]
- Liu, P.; Huang, R.; Hu, X.; Jia, Y.; Li, J.; Luo, J.; Liu, Q.; Luo, L.; Liu, G.; Chen, Z. Physiological Responses and Proteomic Changes Reveal Insights into Stylosanthes Response to Manganese Toxicity. BMC Plant Biol. 2019, 19, 212. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.; Aslam, A.; Sheraz, M.; Ali, B.; Ulhassan, Z.; Najeeb, U.; Zhou, W.; Gill, R.A. Lead Toxicity in Cereals: Mechanistic Insight into Toxicity, Mode of Action, and Management. Front. Plant Sci. 2021, 11, 587785. [Google Scholar] [CrossRef]
- Li, J.; Jia, Y.; Dong, R.; Huang, R.; Liu, P.; Li, X.; Wang, Z.; Liu, G.; Chen, Z. Advances in the Mechanisms of Plant Tolerance to Manganese Toxicity. Int. J. Mol. Sci. 2019, 20, 5096. [Google Scholar] [CrossRef] [Green Version]
- Bączek-Kwinta, R.; Antonkiewicz, J.; Łopata-Stasiak, A.; Kępka, W. Smoke Compounds Aggravate Stress Inflicted on Brassica Seedlings by Unfavourable Soil Conditions. Photosynthetica 2019, 57, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Manzoor, J.; Sharma, M.; Wani, K.A. Heavy Metals in Vegetables and Their Impact on the Nutrient Quality of Vegetables: A Review. J. Plant Nutr. 2018, 41, 1744–1763. [Google Scholar] [CrossRef]
- Kim, R.Y.; Yoon, J.K.; Kim, T.S.; Yang, J.E.; Owens, G.; Kim, K.R. Bioavailability of Heavy Metals in Soils: Definitions and Practical Implementation—A Critical Review. Environ. Geochem. Health 2015, 37, 1041–1061. [Google Scholar] [CrossRef]
- Thakur, M.; Praveen, S.; Divte, P.R.; Mitra, R.; Kumar, M.; Gupta, C.K.; Kalidindi, U.; Bansal, R.; Roy, S.; Anand, A.; et al. Metal Tolerance in Plants: Molecular and Physicochemical Interface Determines the “Not so Heavy Effect” of Heavy Metals. Chemosphere 2022, 287, 131957. [Google Scholar] [CrossRef] [PubMed]
- Haddad, A.; Mohiuddin, S.S. Biochemistry, Citric Acid Cycle; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Zaheer, I.E.; Ali, S.; Rizwan, M.; Farid, M.; Shakoor, M.B.; Gill, R.A.; Najeeb, U.; Iqbal, N.; Ahmad, R. Citric Acid Assisted Phytoremediation of Copper by Brassica napus L. Ecotoxicol. Environ. Saf. 2015, 120, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Ehsan, S.; Ali, S.; Noureen, S.; Mahmood, K.; Farid, M.; Ishaque, W.; Shakoor, M.B.; Rizwan, M. Citric Acid Assisted Phytoremediation of Cadmium by Brassica napus L. Ecotoxicol. Environ. Saf. 2014, 106, 164–172. [Google Scholar] [CrossRef]
- Afshan, S.; Ali, S.; Bharwana, S.A.; Rizwan, M.; Farid, M.; Abbas, F.; Ibrahim, M.; Mehmood, M.A.; Abbasi, G.H. Citric Acid Enhances the Phytoextraction of Chromium, Plant Growth, and Photosynthesis by Alleviating the Oxidative Damages in Brassica napus L. Environ. Sci. Pollut. Res. 2015, 22, 11679–11689. [Google Scholar] [CrossRef] [PubMed]
- Qiu, R.; Liu, W.; Zeng, X.; Tang, Y.; Brewer, E.; Fang, X. Effects of Exogenous Citric Acid and Malic Acid Addition on Nickel Uptake and Translocation in Leaf Mustard (Brassica juncea Var. Foliosa Bailey) and Alyssum corsicum. Int. J. Environ. Pollut. 2009, 38, 26639. [Google Scholar] [CrossRef]
- Kumar, A.; Pal, L.; Agrawal, V. Glutathione and Citric Acid Modulates Lead- and Arsenic-Induced Phytotoxicity and Genotoxicity Responses in Two Cultivars of Solanum lycopersicum L. Acta Physiol. Plant. 2017, 39, 151. [Google Scholar] [CrossRef]
- Amir, W.; Farid, M.; Ishaq, H.K.; Farid, S.; Zubair, M.; Alharby, H.F.; Bamagoos, A.A.; Rizwan, M.; Raza, N.; Hakeem, K.R.; et al. Accumulation Potential and Tolerance Response of Typha latifolia L. under Citric Acid Assisted Phytoextraction of Lead and Mercury. Chemosphere 2020, 257, 127247. [Google Scholar] [CrossRef]
- Mohammadi, S.; Pourakbar, L.; Siavash Moghaddam, S.; Popović-Djordjević, J. The Effect of EDTA and Citric Acid on Biochemical Processes and Changes in Phenolic Compounds Profile of Okra (Abelmoschus esculentus L.) under Mercury Stress. Ecotoxicol. Environ. Saf. 2021, 208, 111607. [Google Scholar] [CrossRef]
- Tahjib-Ul-Arif, M.; Zahan, M.I.; Karim, M.M.; Imran, S.; Hunter, C.T.; Islam, M.S.; Mia, M.A.; Hannan, M.A.; Rhaman, M.S.; Hossain, M.A.; et al. Citric Acid-Mediated Abiotic Stress Tolerance in Plants. Int. J. Mol. Sci. 2021, 22, 7235. [Google Scholar] [CrossRef]
- Chen, H.C.; Zhang, S.L.; Wu, K.J.; Li, R.; He, X.R.; He, D.N.; Huang, C.; Wei, H. The Effects of Exogenous Organic Acids on the Growth, Photosynthesis and Cellular Ultrastructure of Salix variegata Franch. Under Cd Stress. Ecotoxicol. Environ. Saf. 2020, 187, 109790. [Google Scholar] [CrossRef]
- Kaur, R.; Yadav, P.; Sharma, A.; Kumar Thukral, A.; Kumar, V.; Kaur Kohli, S.; Bhardwaj, R. Castasterone and Citric Acid Treatment Restores Photosynthetic Attributes in Brassica juncea L. under Cd(II) Toxicity. Ecotoxicol. Environ. Saf. 2017, 145, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Miao, C.; Mao, L.; Zhou, P.; Jin, Z.; Shi, W. Improvement of Phytoextraction and Antioxidative Defense in Solanum nigrum L. under Cadmium Stress by Application of Cadmium-Resistant Strain and Citric Acid. J. Hazard. Mater. 2010, 181, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Anwer, S.; Yasin Ashraf, M.; Hussain, M.; Ashraf, M.; Jamil, A. Citric Acid Mediated Phytoextraction of Cadmium By Maize (Zea mays L.). Pak. J. Bot. 2012, 44, 1831–1836. [Google Scholar]
- Sebastian, A.; Prasad, M.N.V. Exogenous Citrate and Malate Alleviate Cadmium Stress in Oryza sativa L.: Probing Role of Cadmium Localization and Iron Nutrition. Ecotoxicol. Environ. Saf. 2018, 166, 215–222. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Zeng, G.; Zhou, L.; Wang, X.; Wang, Y.; Wang, C.; Hu, X.; Xu, W. Enhanced Efficiency of Cadmium Removal by Boehmeria nivea (L.) Gaud. in the Presence of Exogenous Citric and Oxalic Acids. J. Environ. Sci. 2014, 26, 2508–2516. [Google Scholar] [CrossRef]
- Bilal Shakoor, M.; Ali, S.; Hameed, A.; Farid, M.; Hussain, S.; Yasmeen, T.; Najeeb, U.; Aslam Bharwana, S.; Hasan Abbasi, G. Citric Acid Improves Lead (Pb) Phytoextraction in Brassica napus L. by Mitigating Pb-Induced Morphological and Biochemical Damages. Ecotoxicol. Environ. Saf. 2014, 109, 38–47. [Google Scholar] [CrossRef]
- Saffari, V.R.; Saffari, M. Effects of EDTA, Citric Acid, and Tartaric Acid Application on Growth, Phytoremediation Potential, and Antioxidant Response of Calendula officinalis L. in a Cadmium-Spiked Calcareous Soil. Int. J. Phytoremed. 2020, 22, 1204–1214. [Google Scholar] [CrossRef]
- Arsenov, D.; Župunski, M.; Borišev, M.; Nikolić, N.; Pilipovic, A.; Orlovic, S.; Kebert, M.; Pajevic, S. Citric Acid as Soil Amendment in Cadmium Removal by Salix viminalis L., Alterations on Biometric Attributes and Photosynthesis. Int. J. Phytoremed. 2020, 22, 29–39. [Google Scholar] [CrossRef]
- Song, J.F.; Markewitz, D.; Wu, S.; Sang, Y.; Duan, C.; Cui, X.Y. Exogenous Oxalic Acid and Citric Acid Improve Lead (Pb) Tolerance of Larix olgensis A. Henry Seedlings. Forests 2018, 9, 510. [Google Scholar] [CrossRef] [Green Version]
- Xue, W.; Wang, P.; Tang, L.; Zhang, C.; Wang, C.; Huang, Y.; Zhang, X.; Li, Y.; Zhao, B.; Liu, Z. Citric Acid Inhibits Cd Uptake by Improving the Preferential Transport of Mn and Triggering the Defense Response of Amino Acids in Grains. Ecotoxicol. Environ. Saf. 2021, 211, 111921. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, H.; He, D.; He, X.; Yan, Y.; Wu, K.; Wei, H. Effects of Exogenous Organic Acids on Cd Tolerance Mechanism of Salix variegata Franch. Under Cd Stress. Front. Plant Sci. 2020, 11, 594352. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Chen, H.; Hong, C.; Jiang, D.; Zheng, B. Exogenous Malic Acid Alleviates Cadmium Toxicity in Miscanthus sacchariflorus through Enhancing Photosynthetic Capacity and Restraining ROS Accumulation. Ecotoxicol. Environ. Saf. 2017, 141, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Iqbal, M.; Ashraf, M.Y.; Ashraf, M.A.; Ali, S. Organic Chelants-Mediated Enhanced Lead (Pb) Uptake and Accumulation Is Associated with Higher Activity of Enzymatic Antioxidants in Spinach (Spinacea oleracea L.). J. Hazard. Mater. 2016, 317, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi-Fasaei, R. Malic Acid and Phosphorus Influences on Nickel Phytoremediation Efficiency and Metal Nutrients Relationships in a Ni-Polluted Calcareous Soil. Int. Res. J. Appl. Basic Sci. 2012, 3, 2805–2808. [Google Scholar]
- Hawrylak-Nowak, B.; Dresler, S.; Matraszek, R. Exogenous Malic and Acetic Acids Reduce Cadmium Phytotoxicity and Enhance Cadmium Accumulation in Roots of Sunflower Plants. Plant Physiol. Biochem. 2015, 94, 225–234. [Google Scholar] [CrossRef]
- Guo, D.; Ali, A.; Ren, C.; Du, J.; Li, R.; Lahori, A.H.; Xiao, R.; Zhang, Z.; Zhang, Z. EDTA and Organic Acids Assisted Phytoextraction of Cd and Zn from a Smelter Contaminated Soil by Potherb Mustard (Brassica juncea, Coss) and Evaluation of Its Bioindicators. Ecotoxicol. Environ. Saf. 2019, 167, 396–403. [Google Scholar] [CrossRef]
- Liang, Y.; Xiao, X.; Guo, Z.; Peng, C.; Zeng, P.; Wang, X. Co-Application of Indole-3-Acetic Acid/Gibberellin and Oxalic Acid for Phytoextraction of Cadmium and Lead with Sedum alfredii Hance from Contaminated Soil. Chemosphere 2021, 285, 131420. [Google Scholar] [CrossRef]
- Sakouhi, L.; Kharbech, O.; Massoud, M.B.; Munemasa, S.; Murata, Y.; Chaoui, A. Oxalic Acid Mitigates Cadmium Toxicity in Cicer arietinum L. Germinating Seeds by Maintaining the Cellular Redox Homeostasis. J. Plant Growth Regul. 2022, 41, 697–709. [Google Scholar] [CrossRef]
- Turk, H.; Erdal, S.; Karayel, U.; Dumlupinar, R. Attenuation of Lead Toxicity by Promotion of Tolerance Mechanism in Wheat Roots by Lipoic Acid. Cereal Res. Commun. 2018, 46, 424–435. [Google Scholar] [CrossRef]
- Salavati, J.; Fallah, H.; Niknejad, Y.; Barari Tari, D. Methyl Jasmonate Ameliorates Lead Toxicity in Oryza sativa by Modulating Chlorophyll Metabolism, Antioxidative Capacity and Metal Translocation. Physiol. Mol. Biol. Plants 2021, 27, 1089–1104. [Google Scholar] [CrossRef]
- Mousavi, S.R.; Niknejad, Y.; Fallah, H.; Tari, D.B. Methyl Jasmonate Alleviates Arsenic Toxicity in Rice. Plant Cell Rep. 2020, 39, 1041–1060. [Google Scholar] [CrossRef] [PubMed]
- Bali, S.; Jamwal, V.L.; Kohli, S.K.; Kaur, P.; Tejpal, R.; Bhalla, V.; Ohri, P.; Gandhi, S.G.; Bhardwaj, R.; Al-Huqail, A.A.; et al. Jasmonic Acid Application Triggers Detoxification of Lead (Pb) Toxicity in Tomato through the Modifications of Secondary Metabolites and Gene Expression. Chemosphere 2019, 235, 734–748. [Google Scholar] [CrossRef] [PubMed]
- Lei, G.J.; Sun, L.; Sun, Y.; Zhu, X.F.; Li, G.X.; Zheng, S.J. Jasmonic Acid Alleviates Cadmium Toxicity in Arabidopsis via Suppression of Cadmium Uptake and Translocation. J. Integr. Plant Biol. 2020, 62, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Kamran, M.; Wang, D.; Alhaithloul, H.A.S.; Alghanem, S.M.; Aftab, T.; Xie, K.; Lu, Y.; Shi, C.; Sun, J.; Gu, W.; et al. Jasmonic Acid-Mediated Enhanced Regulation of Oxidative, Glyoxalase Defense System and Reduced Chromium Uptake Contributes to Alleviation of Chromium (VI) Toxicity in Choysum (Brassica parachinensis L.). Ecotoxicol. Environ. Saf. 2021, 208, 111758. [Google Scholar] [CrossRef]
- Kaya, C.; Ugurlar, F.; Ashraf, M.; Noureldeen, A.; Darwish, H.; Ahmad, P. Methyl Jasmonate and Sodium Nitroprusside Jointly Alleviate Cadmium Toxicity in Wheat (Triticum aestivum L.) Plants by Modifying Nitrogen Metabolism, Cadmium Detoxification, and AsA–GSH Cycle. Front. Plant Sci. 2021, 12, 654780. [Google Scholar] [CrossRef]
- Hanaka, A.; Wójcik, M.; Dresler, S.; Mroczek-Zdyrska, M.; Maksymiec, W. Does Methyl Jasmonate Modify the Oxidative Stress Response in Phaseolus coccineus Treated with Cu? Ecotoxicol. Environ. Saf. 2016, 124, 480–488. [Google Scholar] [CrossRef]
- Dai, H.; Wei, S.; Pogrzeba, M.; Rusinowski, S.; Krzyżak, J.; Jia, G. Exogenous Jasmonic Acid Decreased Cu Accumulation by Alfalfa and Improved Its Photosynthetic Pigments and Antioxidant System. Ecotoxicol. Environ. Saf. 2020, 190, 110176. [Google Scholar] [CrossRef]
- Li, Q.; Wang, G.; Wang, Y.; Yang, D.; Guan, C.; Ji, J. Foliar Application of Salicylic Acid Alleviate the Cadmium Toxicity by Modulation the Reactive Oxygen Species in Potato. Ecotoxicol. Environ. Saf. 2019, 172, 317–325. [Google Scholar] [CrossRef]
- Safari, F.; Akramian, M.; Salehi-Arjmand, H.; Khadivi, A. Physiological and Molecular Mechanisms Underlying Salicylic Acid-Mitigated Mercury Toxicity in Lemon Balm (Melissa officinalis L.). Ecotoxicol. Environ. Saf. 2019, 183, 109542. [Google Scholar] [CrossRef]
- Metwally, A.; Finkemeier, I.; Georgi, M.; Dietz, K.J. Salicylic Acid Alleviates the Cadmium Toxicity in Barley Seedlings. Plant Physiol. 2003, 132, 272–281. [Google Scholar] [CrossRef] [Green Version]
- Kohli, S.K.; Handa, N.; Sharma, A.; Gautam, V.; Arora, S.; Bhardwaj, R.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P. Interaction of 24-Epibrassinolide and Salicylic Acid Regulates Pigment Contents, Antioxidative Defense Responses, and Gene Expression in Brassica juncea L. Seedlings under Pb Stress. Environ. Sci. Pollut. Res. 2018, 25, 15159–15173. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Pandey, N.; Pandey-Rai, S. Exogenous Salicylic Acid-Mediated Modulation of Arsenic Stress Tolerance with Enhanced Accumulation of Secondary Metabolites and Improved Size of Glandular Trichomes in Artemisia annua L. Protoplasma 2018, 255, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.L.; Wang, X.H.; Wei, T.; Wang, M.; Liu, X.; Hua, L.; Ren, X.H.; Guo, J.K.; Li, J. Exogenous Salicylic Acid Regulates Cell Wall Polysaccharides Synthesis and Pectin Methylation to Reduce Cd Accumulation of Tomato. Ecotoxicol. Environ. Saf. 2021, 207, 111550. [Google Scholar] [CrossRef]
- Lu, Q.; Zhang, T.; Zhang, W.; Su, C.; Yang, Y.; Hu, D.; Xu, Q. Alleviation of Cadmium Toxicity in Lemna minor by Exogenous Salicylic Acid. Ecotoxicol. Environ. Saf. 2018, 147, 500–508. [Google Scholar] [CrossRef]
- Soltani Maivan, E.; Radjabian, T.; Abrishamchi, P.; Talei, D. Physiological and Biochemical Responses of Melissa officinalis L. to Nickel Stress and the Protective Role of Salicylic Acid. Arch. Agron. Soil Sci. 2017, 63, 330–343. [Google Scholar] [CrossRef]
- Sihag, S.; Brar, B.; Joshi, U.N. Salicylic Acid Induces Amelioration of Chromium Toxicity and Affects Antioxidant Enzyme Activity in Sorghum bicolor L. Int. J. Phytoremed. 2019, 21, 293–304. [Google Scholar] [CrossRef]
- Saidi, I.; Guesmi, F.; Kharbech, O.; Hfaiedh, N.; Djebali, W. Gallic Acid Improves the Antioxidant Ability against Cadmium Toxicity: Impact on Leaf Lipid Composition of Sunflower (Helianthus annuus) Seedlings. Ecotoxicol. Environ. Saf. 2021, 210, 111906. [Google Scholar] [CrossRef] [PubMed]
- Yetişsin, F.; Kurt, F. Gallic Acid (GA) Alleviating Copper (Cu) Toxicity in Maize (Zea mays L.) Seedlings. Int. J. Phytoremed. 2020, 22, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Ozfidan-Konakci, C.; Kabakci, M. The Impacts of Gallic Acid on Redox State of Antioxidants Related to Ascorbate–Glutathione Cycle in Wheat (Triticum aestivum) Grown Under Cadmium Toxicity. Agric. Res. 2020, 9, 543–553. [Google Scholar] [CrossRef]
- Lee, K.W.; Rahman, A.; Zada, M.; Lee, D.G.; Kim, K.Y.; Hwang, T.Y.; Ji, H.J.; Lee, S.H. Identification of Copper and Cadmium Induced Genes in Alfalfa Leaves through Annealing Control Primer Based Approach. J. Korean Soc. Grassl. Forage Sci. 2015, 35, 264–268. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, W.; Zhang, B.; Zhang, S.; Wang, W.; Ming, F. One Novel Mitochondrial Citrate Synthase from Oryza sativa L. Can Enhance Aluminum Tolerance in Transgenic Tobacco. Mol. Biotechnol. 2009, 42, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Luo, K.; Li, Z.; Yang, Y.; Hu, N.; Wu, Y. Overexpression of Citrus Junos Mitochondrial Citrate Synthase Gene in Nicotiana benthamiana Confers Aluminum Tolerance. Planta 2009, 230, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Anjum, N.A.; Hasanuzzaman, M.; Hossain, M.A.; Thangavel, P.; Roychoudhury, A.; Gill, S.S.; Merlos Rodrigo, M.A.; Adam, V.; Fujita, M.; Kizek, R.; et al. Jacks of Metal/Metalloid Chelation Trade in Plants—An Overview. Front. Plant Sci. 2015, 6, 192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.W.; Han, M.S.; Choi, S.; Yi, J.; Lee, T.W.; Lee, S.Y. Organic Acids: Succinic and Malic Acids. In Comprehensive Biotechnology, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2011; Volume 3, pp. 149–161. [Google Scholar]
- Gurtler, J.B.; Mai, T.L. Preservatives: Traditional Preservatives—Organic Acids. In Encyclopedia of Food Microbiology, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2014; pp. 119–130. [Google Scholar]
- Wang, Q.F.; Zhao, Y.; Yi, Q.; Li, K.Z.; Yu, Y.X.; Chen, L.M. Overexpression of Malate Dehydrogenase in Transgenic Tobacco Leaves: Enhanced Malate Synthesis and Augmented Al-Resistance. Acta Physiol. Plant. 2010, 32, 1209–1220. [Google Scholar] [CrossRef]
- Chen, Z.; Sun, L.; Liu, P.; Liu, G.; Tian, J.; Liao, H. Malate Synthesis and Secretion Mediated by a Manganese-Enhanced Malate Dehydrogenase Confers Superior Manganese Tolerance in Stylosanthes guianensis. Plant Physiol. 2015, 167, 176–188. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Fan, T.; Guan, L.; Ren, Y.; Han, Y.; Liu, Q.; Liu, Y.; Cao, S. CMDH4 Encodes a Protein That Is Required for Lead Tolerance in Arabidopsis. Plant Soil 2017, 412, 317–330. [Google Scholar] [CrossRef]
- Franceschi, V.R.; Nakata, P.A. Calcium Oxalate in Plants: Formation and Function. Annu. Rev. Plant Biol. 2005, 56, 41–71. [Google Scholar] [CrossRef]
- Nakata, P.A. Advances in Our Understanding of Calcium Oxalate Crystal Formation and Function in Plants. Plant Sci. 2003, 164, 901–909. [Google Scholar] [CrossRef]
- Osmolovskaya, N.; Dung, V.V.; Kuchaeva, L. The Role of Organic Acids in Heavy Metal Tolerance in Plants. Biol. Commun. 2018, 63, 9–16. [Google Scholar]
- Araya-Flores, J.; Miranda, S.; Covarrubias, M.P.; Stange, C.; Handford, M. Solanum lycopersicum (tomato) possesses mitochondrial and plastidial lipoyl synthases capable of increasing lipoylation levels when expressed in bacteria. Plant Physiol. Biochem. 2020, 151, 264–270. [Google Scholar] [CrossRef]
- Godoy, F.; Olivos-Hernández, K.; Stange, C.; Handford, M. Abiotic Stress in Crop Species: Improving Tolerance by Applying Plant Metabolites. Plants 2021, 10, 186. [Google Scholar] [CrossRef] [PubMed]
- Navari-Izzo, F.; Quartacci, M.F.; Sgherri, C. Lipoic Acid: A Unique Antioxidant in the Detoxification of Activated Oxygen Species. Plant Physiol. Biochem. 2002, 40, 463–470. [Google Scholar] [CrossRef]
- Sgherri, C.; Quartacci, M.F.; Izzo, R.; Navari-Izzo, F. Relation between Lipoic Acid and Cell Redox Status in Wheat Grown in Excess Copper. Plant Physiol. Biochem. 2002, 40, 591–597. [Google Scholar] [CrossRef]
- Raza, A.; Charagh, S.; Zahid, Z.; Mubarik, M.S.; Javed, R.; Siddiqui, M.H.; Hasanuzzaman, M. Jasmonic Acid: A Key Frontier in Conferring Abiotic Stress Tolerance in Plants. Plant Cell Rep. 2021, 40, 1513–1541. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, W.; Tong, T.; Chen, G.; Zeng, F.; Jang, S.; Gao, W.; Li, Z.; Mak, M.; Deng, F.; et al. Molecular Interaction and Evolution of Jasmonate Signaling with Transport and Detoxification of Heavy Metals and Metalloids in Plants. Front. Plant Sci. 2021, 12, 665842. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Trinh, N.N.; Fu, S.F.; Hsiung, Y.C.; Chia, L.C.; Lin, C.W.; Huang, H.J. Comparison of Early Transcriptome Responses to Copper and Cadmium in Rice Roots. Plant Mol. Biol. 2013, 81, 507–522. [Google Scholar] [CrossRef]
- Huang, T.L.; Nguyen, Q.T.T.; Fu, S.F.; Lin, C.Y.; Chen, Y.C.; Huang, H.J. Transcriptomic Changes and Signalling Pathways Induced by Arsenic Stress in Rice Roots. Plant Mol. Biol. 2012, 80, 587–608. [Google Scholar] [CrossRef]
- Sharma, A.; Sidhu, G.P.S.; Araniti, F.; Bali, A.S.; Shahzad, B.; Tripathi, D.K.; Brestic, M.; Skalicky, M.; Landi, M. The Role of Salicylic Acid in Plants Exposed to Heavy Metals. Molecules 2020, 25, 540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Li, X. Salicylic Acid: Biosynthesis, Perception, and Contributions to Plant Immunity. Curr. Opin. Plant Biol. 2019, 50, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Liu, C.; Liang, Y.; Li, N.; Fu, Q. Salicylic Acid Signals Plant Defence against Cadmium Toxicity. Int. J. Mol. Sci. 2019, 20, 2960. [Google Scholar] [CrossRef] [Green Version]
- Szalai, G.; Krantev, A.; Yordanova, R.; Popova, L.P.; Janda, T. Influence of Salicylic Acid on Phytochelatin Synthesis in Zea mays during Cd Stress. Turk. J. Bot. 2013, 37, 708–714. [Google Scholar]
- Zawoznik, M.S.; Groppa, M.D.; Tomaro, M.L.; Benavides, M.P. Endogenous Salicylic Acid Potentiates Cadmium-Induced Oxidative Stress in Arabidopsis thaliana. Plant Sci. 2007, 173, 190–197. [Google Scholar] [CrossRef]
- Zanganeh, R.; Jamei, R.; Rahmani, F. Impacts of Seed Priming with Salicylic Acid and Sodium Hydrosulfide on Possible Metabolic Pathway of Two Amino Acids in Maize Plant under Lead Stress. Mol. Biol. Res. Commun. 2018, 7, 83–88. [Google Scholar] [PubMed]
- Daglia, M.; Lorenzo, A.; Nabavi, S.; Talas, Z.; Nabavi, S. Polyphenols: Well Beyond the Antioxidant Capacity: Gallic Acid and Related Compounds as Neuroprotective Agents: You Are What You Eat! Curr. Pharm. Biotechnol. 2014, 15, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Daglia, M. Polyphenols as Antimicrobial Agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef]
- Bontpart, T.; Marlin, T.; Vialet, S.; Guiraud, J.L.; Pinasseau, L.; Meudec, E.; Sommerer, N.; Cheynier, V.; Terrier, N. Two Shikimate Dehydrogenases, VvSDH3 and VvSDH4, Are Involved in Gallic Acid Biosynthesis in Grapevine. J. Exp. Bot. 2016, 67, 3537–3550. [Google Scholar] [CrossRef] [Green Version]
- Muir, R.M.; Ibáñez, A.M.; Uratsu, S.L.; Ingham, E.S.; Leslie, C.A.; McGranahan, G.H.; Batra, N.; Goyal, S.; Joseph, J.; Jemmis, E.D.; et al. Mechanism of Gallic Acid Biosynthesis in Bacteria (Escherichia coli) and Walnut (Juglans regia). Plant Mol. Biol. 2011, 75, 555–565. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Gupta, R.; Pandey, R. Exogenous Application of Rutin and Gallic Acid Regulate Antioxidants and Alleviate Reactive Oxygen Generation in Oryza sativa L. Physiol. Mol. Biol. Plants 2017, 23, 301–309. [Google Scholar] [CrossRef] [Green Version]
- Tavsan, Z.; Kayali, H. Phenylpropanoid Pathway Response to Cadmium and Lead Stress in Phaselous vulgaris Roots and Leaves. Asian J. Biotechnol. Bioresour. Technol. 2018, 3, 301–309. [Google Scholar] [CrossRef]
- Imperato, F. Phytochemistry: Advances in Research: 2006; Research Signpost: Trivandrum, Kerala, India, 2006. [Google Scholar]
- Riaz, U.; Kharal, M.A.; Murtaza, G.; Zaman, Q.U.; Javaid, S.; Malik, H.A.; Aziz, H.; Abbas, Z. Prospective Roles and Mechanisms of Caffeic Acid in Counter Plant Stress: A Mini Review. Pak. J. Agric. Res. 2018, 32, 8–19. [Google Scholar] [CrossRef]
- Díaz, J.; Bernal, A.; Pomar, F.; Merino, F. Induction of Shikimate Dehydrogenase and Peroxidase in Pepper ( Capsicum Annuum L.) Seedlings in Response to Copper Stress and Its Relation to Lignification. Plant Sci. 2001, 161, 179–188. [Google Scholar]
- Gülçin, I. Antioxidant Activity of Caffeic Acid (3,4-Dihydroxycinnamic Acid). Toxicology 2006, 217, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Badri, D.V.; Vivanco, J.M. Regulation and Function of Root Exudates. Plant Cell Environ. 2009, 32, 666–681. [Google Scholar] [PubMed]
- do Prado, N.B.; de Abreu, C.B.; Pinho, C.S.; de Junior, M.M.N.; Silva, M.D.; Espino, M.; Silva, M.F.; de Dias, F.S. Application of Multivariate Analysis to Assess Stress by Cd, Pb and Al in Basil (Ocimum basilicum L.) Using Caffeic Acid, Rosmarinic Acid, Total Phenolics, Total Flavonoids and Total Dry Mass in Response. Food Chem. 2022, 367, 130682. [Google Scholar] [PubMed]
- Lee, K.; Choi, G.H.; Back, K. Cadmium-Induced Melatonin Synthesis in Rice Requires Light, Hydrogen Peroxide, and Nitric Oxide: Key Regulatory Roles for Tryptophan Decarboxylase and Caffeic Acid O-Methyltransferase. J. Pineal Res. 2017, 63, e12441. [Google Scholar] [CrossRef] [PubMed]
- Narnoliya, L.K.; Sangwan, N.; Jadaun, J.S.; Bansal, S.; Sangwan, R.S. Defining the Role of a Caffeic Acid 3-O-Methyltransferase from Azadirachta Indica Fruits in the Biosynthesis of Ferulic Acid through Heterologous over-Expression in Ocimum Species and Withania somnifera. Planta 2021, 253, 20. [Google Scholar]
- Sami, A.; Shah, F.A.; Abdullah, M.; Zhou, X.; Yan, Y.; Zhu, Z.; Zhou, K. Melatonin Mitigates Cadmium and Aluminium Toxicity through Modulation of Antioxidant Potential in Brassica napus L. Plant Biol. 2020, 22, 679–690. [Google Scholar] [CrossRef]
- 10 Chemicals of Public Health Concern. Available online: https://www.who.int/news-room/photo-story/photo-story-detail/10-chemicals-of-public-health-concern (accessed on 29 April 2022).
- Kumar, S.; Dubey, R.S.; Tripathi, R.D.; Chakrabarty, D.; Trivedi, P.K. Omics and Biotechnology of Arsenic Stress and Detoxification in Plants: Current Updates and Prospective. Environ. Int. 2015, 74, 221–230. [Google Scholar]
- de la Fuente, J.M.; Ramirez-Rodriguez, V.; Cabrera-Ponce, J.L.; Herrera-Estrella, L. Aluminum Tolerance in Transgenic Plants by Alteration of Citrate Synthesis. Science 1997, 276, 1566–1568. [Google Scholar] [CrossRef]
- Tesfaye, M.; Dufault, N.S.; Dornbusch, M.R.; Allan, D.L.; Vance, C.P.; Samac, D.A. Influence of Enhanced Malate Dehydrogenase Expression by Alfalfa on Diversity of Rhizobacteria and Soil Nutrient Availability. Soil Biol. Biochem. 2003, 35, 1103–1113. [Google Scholar] [CrossRef]
- Rono, J.K.; le Wang, L.; Wu, X.C.; Cao, H.W.; Zhao, Y.N.; Khan, I.U.; Yang, Z.M. Identification of a New Function of Metallothionein-like Gene OsMT1e for Cadmium Detoxification and Potential Phytoremediation. Chemosphere 2021, 265, 129136. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Shi, W.; Jie, Y. Overexpression of BnPCS1, a Novel Phytochelatin Synthase Gene from Ramie (Boehmeria Nivea), Enhanced Cd Tolerance, Accumulation, and Translocation in Arabidopsis thaliana. Front. Plant Sci. 2021, 12, 639189. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, A.; Shukla, P.; Nangia, A.K.; Prasad, M.N.V. Transgenics in Phytoremediation of Metals and Metalloids: From Laboratory to Field. From Laboratory to Field. In Transgenic Plant Technology for Remediation of Toxic Metals and Metalloids; Elsevier: Amsterdam, The Netherlands, 2018; pp. 3–22. [Google Scholar]
- Geilfus, C.M. Controlled Environment Horticulture: Improving Quality of Vegetables and Medicinal Plants; Springer International Publishing: Cham, Switzerland, 2019. [Google Scholar]
- Kumar, A.; Singh, B.; Raigond, P.; Sahu, C.; Mishra, U.N.; Sharma, S.; Lal, M.K. Phytic Acid: Blessing in Disguise, a Prime Compound Required for Both Plant and Human Nutrition. Food Res. Int. 2021, 142, 110193. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Guo, S. Phytic Acid and Its Interactions: Contributions to Protein Functionality, Food Processing, and Safety. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2081–2105. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Liu, X.; Wu, X.; Tao, X.; Fei, W. Electrospun Polyurethane/Phytic Acid Nanofibrous Membrane for High Efficient Removal of Heavy Metal Ions. Environ. Technol. 2021, 42, 1053–1060. [Google Scholar] [CrossRef] [PubMed]

| Organic Acid | Structure | IUPAC Name |
|---|---|---|
| Citric acid | 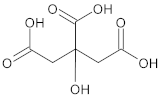 | 2-hydroxypropane-1,2,3-tricarboxylic acid |
| Malic acid |  | 2-hydroxybutanedioic acid |
| Oxalic acid |  | 1,2-ethanedioic acid |
| Lipoic acid | 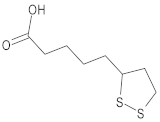 | 5-[(3R)-dithiolan-3-yl]pentanoic acid |
| Jasmonic acid |  | 2-[(1R,2R)-3-oxo-2-[(Z)-pent-2-enyl]cyclopentyl]acetic acid |
| Salicylic acid | 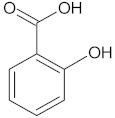 | 2-hydroxybenzoic acid |
| Gallic acid | 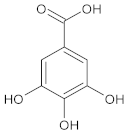 | 3,4,5-trihydroxybenzoic acid |
| Caffeic acid | 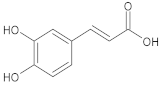 | (E)-3-(3,4-dihydroxyphenyl)prop-2-enoic acid |
| Organic Acid | Species | HM Stress | OA Application | Gene Expression Responses * | Physiological and Morphological Outcomes | Reference |
|---|---|---|---|---|---|---|
| Citric acid | Brassica juncea | Cd as CdCl2 (0.6 mM) | 0.6 mM in soil | Up-regulation: PSY, CHS. Down-regulation: CHLASE. | Increased: Growth, biomass, total chlorophyll, carotenoids, anthocyanins, flavonoids, gaseous exchange parameters, activities of antioxidant enzymes SOD, POD, CAT, GPOX. Decreased: MDA. | [23] |
| Oryza sativa | Cd as CdCl2 (25 μM) | 50 μM in nutritive medium | Up-regulation: OsNramp1, OsIRT1, OsHMA3, OsNAS1. Down-regulation: OsSOD, OsCAT. | Increased: Biomass, photosynthetic pigments, activities of antioxidant enzymes. Decreased: Cd content in leaves | [26] | |
| Oryza sativa | Cd as CdCl2 (0.1, 0.6, 0.9, 1.2, 2.4 mg kg−1) | 5 mM by spraying | Up-regulation of OsNramp1, 2, 3, 5. | Increased: Content of Glu, Phe, His, Ser and Thr, Arg; Mn mobilization, Mn/Cd ratio. Decreased: Cd mobilization. | [32] | |
| Salix variegata | Cd as CdCl2 (50 μM) | 100 μM in aqueous solution | Up-regulation: HMA1, PCS1, HMA3, Nramp5, MTP1, MTP4, HMA3, MT1A, MT2B. | Increased: Growth, biomass, activities of antioxidant enzymes SOD, POD, CAT, APX; Non-Protein Sulfhydryl compounds (NPT), GSH, and non-GSH NPT. Decreased: MDA. | [33] | |
| Solanum lycopersicum | Pb as Pb(NO3)2 or As as Na2HAsO4 (10 μM) | 250 μM in nutritive solution | - | Increased: Growth rate, photosynthetic pigments, activities of antioxidant enzymes CAT, APX, GR. Decreased: MDA, DNA damage. | [18] | |
| Brassica napus | Cu as CuSO4 (50, 100 μM) | 2.5, 5 mM in nutritive medium | - | Increased: Biomass, photosynthetic pigment, activities of antioxidant enzymes CAT, POX, SOD. Decreased: MDA, EL, H2O2. | [14] | |
| Brassica juncea | Cr (100, 500 μM) in solution | 2.5, 5 mM in nutritive medium | - | Increased: Biomass, photosynthetic pigments, activities of antioxidant enzymes SOD, POD, CAT, APX. Decreased: ROS, MDA. | [16] | |
| Solanum nigram | Cd (50 mg Cd2+) in dry soil | 20 mM in dry soil | - | Increased: Growth, biomass, plant weight, activities of antioxidant enzymes SOD, POD. Decreased: MDA. | [24] | |
| Zea mays | Cd as CdCl2 (300 mg kg −1) | 0.25, 0.5, 1, 2 g kg−1 by irrigation | - | Increased: Biomass, shoot and root length. Decreased: Cd uptake. | [25] | |
| Helianthus annuus | Cr (5, 10, 20 mg kg−1) in dry soil | 2.5, 5 mM | - | Increased: Growth, biomass, photosynthetic pigments, activities of antioxidant enzymes. Decreased: ROS, MDA. | [19] | |
| Calendula officinalis | Cd-spiked soils (50, 100 mg kg−1) | 0.05, 0.1 mM in soil | - | Increased: Root and shoot dry weight, photosynthetic pigments, activities of antioxidant enzymes SOD, CAT, APX. Decreased: MDA, H2O2. | [29] | |
| Salix viminalis | Cd as Cd(NO3)2 (3, 6 mg kg−1) in spray | 20 mM in aqueous solution | - | Increased: Biomass, Cd plant uptake, photosynthetic pigments, leaf gas exchange, photosynthetic rate. Decreased: Pro content. | [30] | |
| Larix olgensis | Pb as Pb (NO3)2 (100 mg kg−1) | 0.2, 1, 5, 10 mM by irrigation and leaf spray | - | Increased: Survival rate, biomass, photosynthetic pigments, activities of antioxidant enzymes SOD, POX, Pro content. Decreased: Pb content, MDA. | [31] | |
| Typha latifolia | Pb as Pb(NO3)2 and Hg as HgSO4 (0, 1, 2.5, 5 mM) in nutritive medium | 5 mM in nutritive medium | - | Increased: root, stem and leaf biomass, leaf number and areas, plant height, root length, photosynthetic pigments, activities of antioxidant enzymes SOD, POX, CAT, APX. Decreased: MDA, EL, ROS. | [19] | |
| Malic acid | Salix variegata | Cd as CdCl2 (50 μM) in aqueous solution | 100 μM in aqueous solution | Up-regulation: HMA1, PCS1, HMA3, Nramp5, MTP4. Down-regulation: MTP1, HMA3, MT1A. | Increased: Growth, biomass, activities of antioxidant enzymes SOD, POD, CAT, APX; non-protein sulfhydryl compounds (NPT), GSH, non-GSH NPT. Decreased: MDA. | [33] |
| Miscanthus Sacchariflorus | Cd as CdCl2 (100 μM) in nutritive solution | 100 μM in nutritive solution | Up-regulation: Cu/Zn-SOD, POD1, GPX1, GST1, MDHAR, DHAR. Down-regulation: CAT1. | Increased: Growth, root and shoot length, photosynthetic pigments, total antioxidant capacity, activities of antioxidant enzymes SOD, CAT, POD, APX, GR, GPX, and GST; concentration GSH and GSSG. Decreased: MDA, ROS | [34] | |
| Oryza sativa | Cd as CdCl2 (25 μM) | 50 μM in nutritive solution | Up-regulation: OsCDT1, OsNramp1, OsIRT1, HMA3. Down-regulation: OsNAS1, OsSOD. | Increased: Biomass, photosynthetic pigments, activities of antioxidant enzymes. Decreased: Cd content in leaves. | [26] | |
| Brassica juncea | Ni as NiSO4 (0.003 mM) in nutritive solution | 0.5, 1, 5 mM in nutritive solution | - | Increased: Ni leaf concentration. Decreased: Leaf biomass, Ni root uptake. | [17] | |
| Alyssum corsicum | Ni as NiSO4 (0.3 mM) in nutritive solution | 0.5, 1, 5 mM in nutritive solution | - | Increased: Shoot and root biomass. Decreased: Ni shoot concentration. | [17] | |
| Spinacea oleracea | Pb (2.42, 4.83 mM) in nutritive solution | 2.4 mM in nutritive solution | - | Increased: Biomass, shoot length, photosynthetic pigments, activities of antioxidant enzymes SOD, GPOX, CAT, APX, AsA contents, total phenolics. Decreased: MDA, ROS, flavonoid content. | [35] | |
| Zea mays | Soil polluted with 250 mg Ni kg−1 | 0.1 mM in nutritive solution | - | Increased: Shoot dry weight, Ni uptake efficiency (without soil P). Decreased: Ni uptake efficiency (with soil P). | [36] | |
| Helianthus annuus | Cd as CdCl2 (5 μM) in nutritive solution | 250, 500 μM in nutritive solution | - | Increased: Growth, biomass, shoot and root length, photosynthetic pigments, OA content, activities of root dehydrogenases. Decreased: ROS, H2O2. | [37] | |
| Oxalic acid | Brassica juncea | Cd and Zn resulted from smelting waste emissions | Drip irrigation system (5 mM) | - | Increased: Biomass, root and shoot dry weight, Zn and Cd mobilization, activities of antioxidant enzymes of PAL, PPO, and CAT. | [38] |
| Sedum alfredii | Cd (10.71 mg kg−1) and Pb (438.4 mg kg−1) in contaminated soil | 2.5 mM by leaf spray | - | Increased: Biomass, plant growth, Cd and Pb mobilization, photosynthetic pigments, K content. Decreased: MDA. | [39] | |
| Cicer arietinum | Cd as CdCl2 (200 µM) by seed imbibition | 100 μM in aqueous solution | - | Increased: root and shoot growth, activities of antioxidant enzymes GPX, GR, glutathione redox state, NADP+/NAD+ ratio, NADH+ NADPH ratio. Decreased: MDA, ROS, carbonyl group contents. | [40] | |
| Lipoic acid | Triticum aestivum | Pb as Pb(NO3)2 (1.5 mM) by seed imbibition | 2 μM by seed imbibition | - | Increased: Enzymatic activity amylase, SOD, GSH, GSH/GSSH ratio. Decreased: O2− y H2O2. | [41] |
| Jasmonic acid | Oryza sativa | Pb as Pb(NO3)2 (150, 300 μM) in hydroponic solution | 0.5, 1 μM in hydroponic solution | Up-regulation: HMA3, HMA4, PCS1, PCS2, ABCC1. Down-regulation: HMA2. | Increased: Growth, photosynthetic pigments, Pro. Decreased: MDA, ROS. | [42] |
| Oryza sativa | As (0, 25, 50 µM) in hydroponic solution | 0.5, 1 µM MJ in hydroponic solution | Up-regulation: IRO6, FRDL1, YSL2. Down-regulation: Lsi1, Lsi2, Lsi6, Nramp1, Nramp5. | Increased: Height, biomass, photosynthetic pigments, endogenous JA content, activities of antioxidant enzymes CAT, SOD, APX, POD. Decreased: MDA, ROS, As concentration in roots and leaves. | [43] | |
| Solanum lycopersicum | Pb (0, 0.25, 0.50, 0.75 mM) on filter paper | 100 nM by seed imbibition | Up-regulation: succinyl CoA ligase, succinate dehydrogenase, fumarate hydratase, CHS, PAL. Down-regulation: CHLASE, CS, malate synthase. | Increased: RWC, photosynthetic pigments, antioxidant molecules. Decreased: Pb concentration. | [44] | |
| Arabidopsis thaliana | Cd as CdCl2 (50 μM) in nutrient solution | 0.01, 0.025 μM MJ in nutrient solution | Down-regulation: AtIRT1, AtHMA2, AtHMA4. | Increased: Cd content in root cell wall. Decreased: chlorosis, Cd content in shoot and root cell sap. | [45] | |
| Brassica parachinensis | Cr as K2Cr2O7 (150, 300 μM) in solution | 5, 10, 20 µM by leaf spray | - | Increased: Growth, biomass, plant height, leaf area and number, photosynthetic pigments, activities of antioxidant enzymes SOD, APX, CAT, GPX, GST, GR, MDHAR, DHAR, AsA, and GSH contents. Decreased: MDA, ROS, Cr uptake. | [46] | |
| Triticum aestivum | Cd as CdCl2 (100 μM) in solution | 10 μM MJ by leaf spray | - | Increased: Growth, biomass, RWC, photosynthetic pigments, activities of antioxidant enzymes CAT, SOD. Decreased: MDA, ROS, chlorosis. | [47] | |
| Phaseolus coccineus | Cu as CuSO4 (50 μM) in hydroponic solution | 10 mM MJ preincubation in hydroponic solution | - | Increased: Activities of antioxidant enzymes CAT, APX, POX. Decreased: MDA, ROS. | [48] | |
| Medicago sativa | Cu as CuSO4 (100 µM) in nutritive medium | 1, 5, 10 nM MJ in nutritive medium | - | Increased: Biomass, photosynthetic pigments, activities of antioxidant enzymes CAT, SOD, POD, APX GR. Decreased: MDA, ROS, Cu concentration in roots and leaves. | [49] | |
| Salicylic acid | Solanum tuberosum | Cd as CdCl2 (200 μM) | 600 μM by leaf spray | Up-regulation: StSABP2, StSOD, StAPX. | Increased: RWC, photosynthetic pigments, Pro and SA content. Decreased: MDA, H2O2, O2. | [50] |
| Melissa officinalis | Hg as HgCl2 (50 μM) in nutritive solution | 50 μM in nutritive solution | Up-regulation: CHLG, PAL | Increased: Growth, biomass, RWC, photosynthetic pigments, total phenolics, antioxidant activities, Pro content. Decreased: MDA, ROS. | [51] | |
| Hordeum vulgare | Cd (25 μM) in hydroponic culture | 500 μM priming of dry caryopses | Up-regulation: GS | Increased: Growth, fresh and dry weight of roots and shoots, antioxidant activities CAT, APX, GPX Decreased: MDA. | [52] | |
| Zea mays | Pb as Pb(NO3)2 (2.5 mM) | 0.5 mM pretreated seed | Up-regulation: ZmACS6, ZmSAMD. | Increased: Glycine betaine and nitric oxide contents. Decreased: Met, Arg, Pro contents. | [51] | |
| Brassica juncea | Pb (0.25, 0.50, 0.75 mM) in solution | 1 mM by seed imbibition | Up-regulation: PSY, CAT, POD, DHAR, GST, GR. Down regulation: CHLASE. | Increased: Growth, root and shoot length, photosynthetic pigments, activities of antioxidant enzymes POD, APOX, GR, DHAR, MDHAR, GST, and GR, activities non-enzymatic antioxidants glutathione, ascorbic acid tocopherol. Decreased: ROS. | [53] | |
| Artemisia annua | As as Na2HAsO4 (100, 150 μM) | 100 μM in nutritive solution | Up-regulation: ADS, CYP71AV1, DBR2, ALDH1. | Increased: Growth, biomass, photosynthetic pigments, activities of antioxidant enzymes SOD, CAT, APX, GR artemisinin and dihydroartemisinin. Decreased: ROS. | [54] | |
| Solanum lycopersicum | Cd (10 μM) in pretreatment and hydroponic culture | 25, 50, 100, 200 μM in pretreatment and leaf spray | Up-regulation: TAP2, LAC, CesA1, CesA6. Down-regulation: PME1, PME2. | Increased: Pectin, cellulose, hemicellulose, lignin and callose synthesis in root and leaf cell wall. Decreased: Cd accumulation in cell wall, cytoplasm, organelles. | [55] | |
| Lemna minor | Cd as (10 μM Cd2+) in nutritive medium | 50 μM in nutritive medium | - | Increased: Fe, Mg, Ca, Mo, photosynthetic pigments, activities of antioxidant enzymes SOD, GPX, CAT, APX, GR, endogenous SA, and PAL activity. Decreased: Chlorosis, MDA, ROS, ascorbate, Pro. | [56] | |
| Melissa officinalis | Ni as NiCl2 (500 μM) in nutritive solution | 1 mM by leaf spray | - | Increased: Growth, shoot and root fresh and dry weights, photosynthetic pigments, root Pro content. Decreased: leaf Pro content, MDA, H2O2, EL. | [57] | |
| Sorghum bicolor | Cr as potassium dichromate (1.0, 2.0, 4.0 mg kg−1 soil) | 0.5 nM pretreatment and leaf spray | - | Increased: Growth, number of leaves, activities of antioxidant enzymes POX, APX. Decreased: MDA, ROS. | [58] | |
| Gallic acid | Helianthus annuus | Cd as CdCl2 (5, 10, 15, 20, 50, 100 μM) in nutritive solution | 75 μM by seed imbibition | - | Increased: Growth, biomass, photosynthetic pigments, activities of antioxidant enzymes CAT, APX, SOD, GR; leaf lipid and fatty acid composition. Decreased: MDA, ROS, EL, Cd concentration in roots and leaves. | [59] |
| Zea mays | Cu as CuSO4 (1 mM) by seed imbibition | 1.5 mM by seed imbibition | - | Increased: photosynthetic pigments, Cu content, Pro, activities of antioxidant enzymes GPX, CAT, SOD, APX. Decreased: MDA, ROS. | [60] | |
| Triticum aestivum | Cd (100, 200, 300 μM) in nutritive solution | 25, 75 μM, 1 mM in nutritive solution | - | Increased: Growth, Pro, activities of antioxidant enzymes SOD CAT, POX APX, Gr, NOX, MDHAR, DHAR¸ activities non-enzymatic antioxidants GSH, GSSG, AsA. Decreased: MDA. | [61] | |
| Caffeic acid | Medicago sativa | Cu as CuSO4 (250 µM) and Cd as CdCl2 (250 µM) in solution | - | Up-regulation: COMT in Cd stress | - | [62] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vega, A.; Delgado, N.; Handford, M. Increasing Heavy Metal Tolerance by the Exogenous Application of Organic Acids. Int. J. Mol. Sci. 2022, 23, 5438. https://doi.org/10.3390/ijms23105438
Vega A, Delgado N, Handford M. Increasing Heavy Metal Tolerance by the Exogenous Application of Organic Acids. International Journal of Molecular Sciences. 2022; 23(10):5438. https://doi.org/10.3390/ijms23105438
Chicago/Turabian StyleVega, Andrea, Ninoska Delgado, and Michael Handford. 2022. "Increasing Heavy Metal Tolerance by the Exogenous Application of Organic Acids" International Journal of Molecular Sciences 23, no. 10: 5438. https://doi.org/10.3390/ijms23105438
APA StyleVega, A., Delgado, N., & Handford, M. (2022). Increasing Heavy Metal Tolerance by the Exogenous Application of Organic Acids. International Journal of Molecular Sciences, 23(10), 5438. https://doi.org/10.3390/ijms23105438






