Soyasapogenol C from Fermented Soybean (Glycine Max) Acting as a Novel AMPK/PPARα Dual Activator Ameliorates Hepatic Steatosis: A Novel SANDA Methodology
Abstract
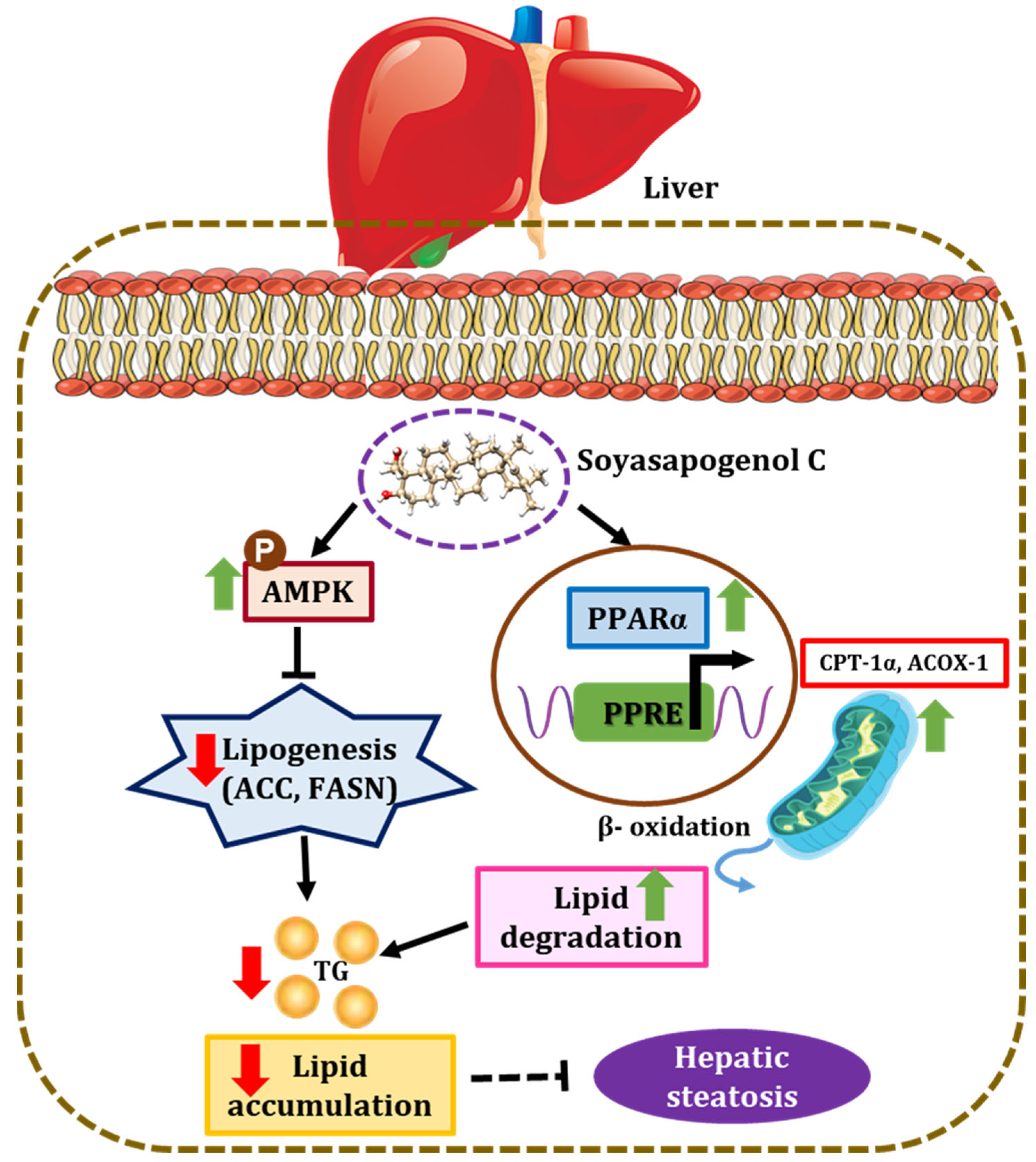
:1. Introduction
2. Results
2.1. Screening of Effective Soyasaponins from Fermented Soybean (Glycine Max) Using in Silico Evaluation
2.2. SSC Had Better Pharmacokinetic Properties Than SSB
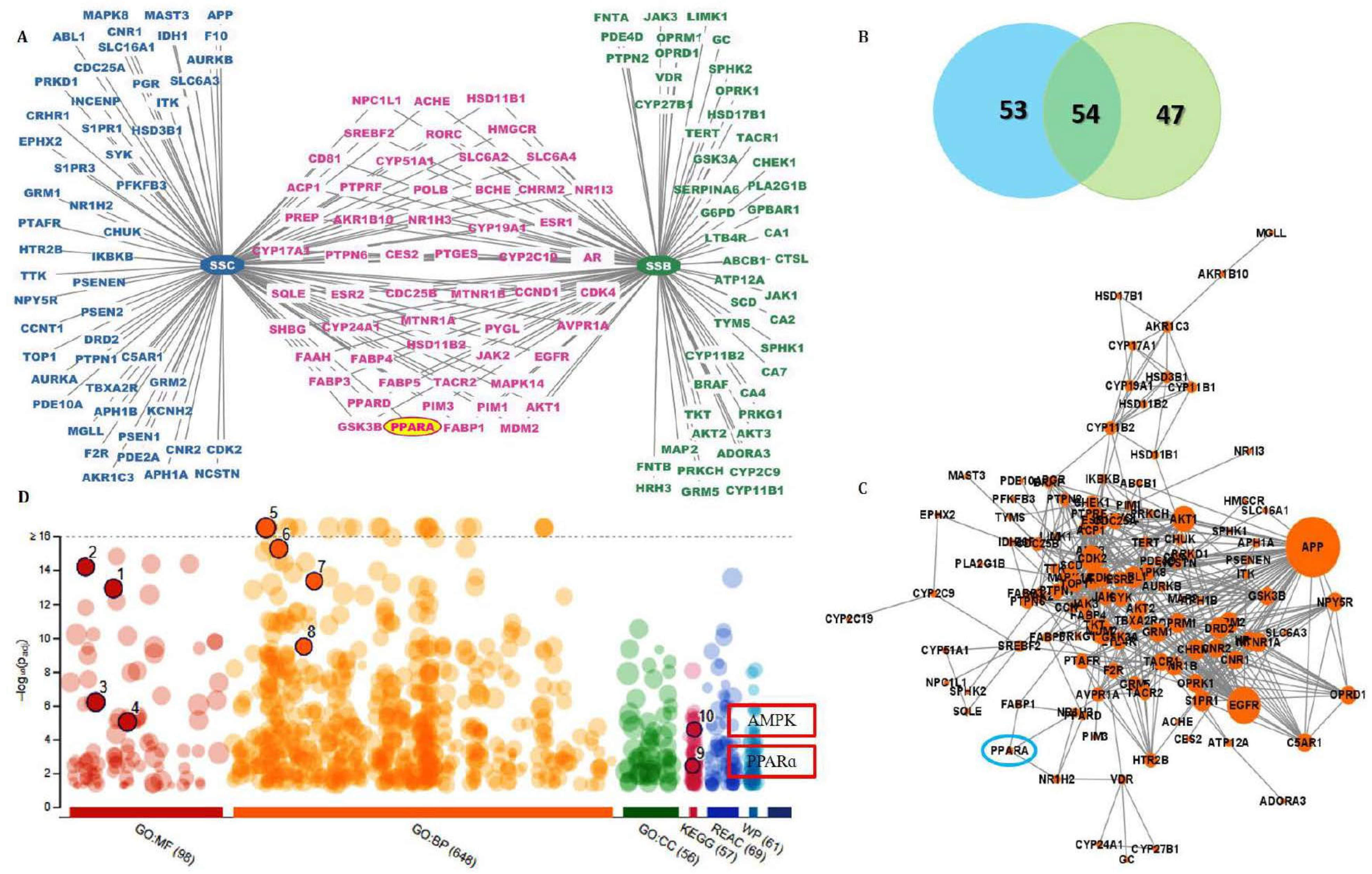
2.3. AMPK and PPARα Were Predicted as Targets of SSC Using Network Pharmacology
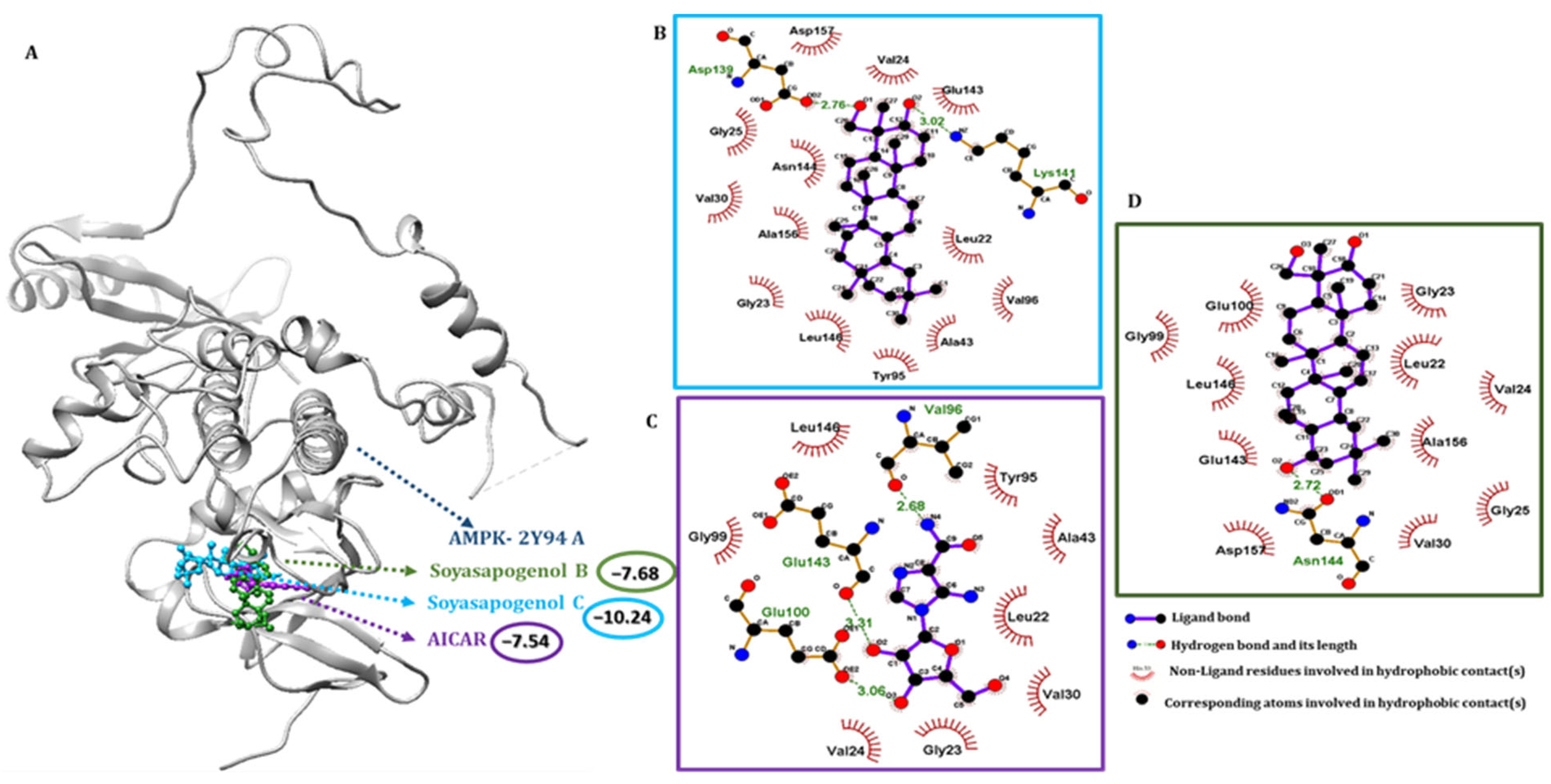
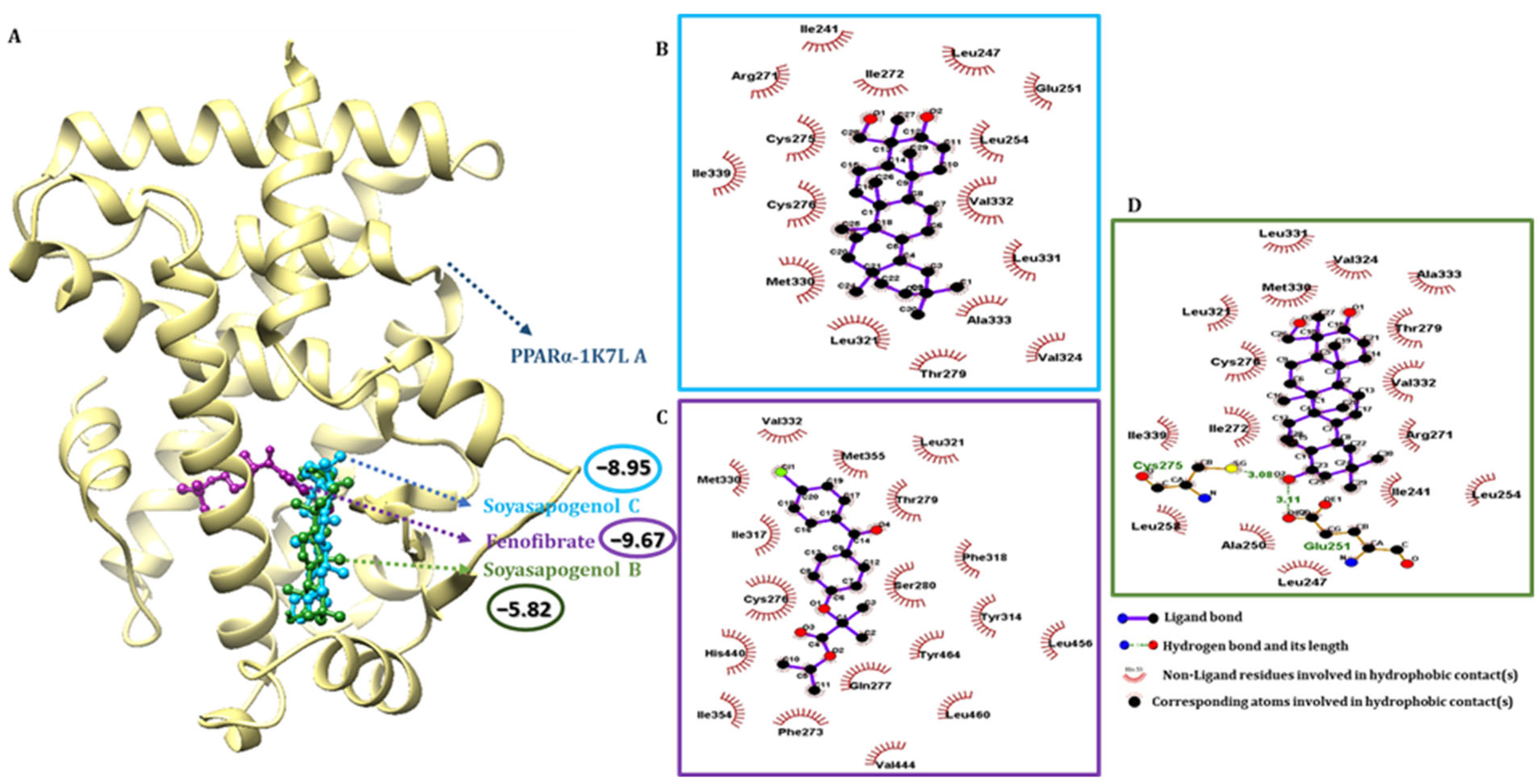
2.4. SSC Had a Higher Binding Affinity Than SSB with Target Receptors
2.5. Pharmacophore Validation of SSC and SSB Properties
2.6. Molecular Dynamics (MD) Simulation
2.7. SSC Inhibited Lipid Accumulation in Palmitate-Treated HepG2 Cells
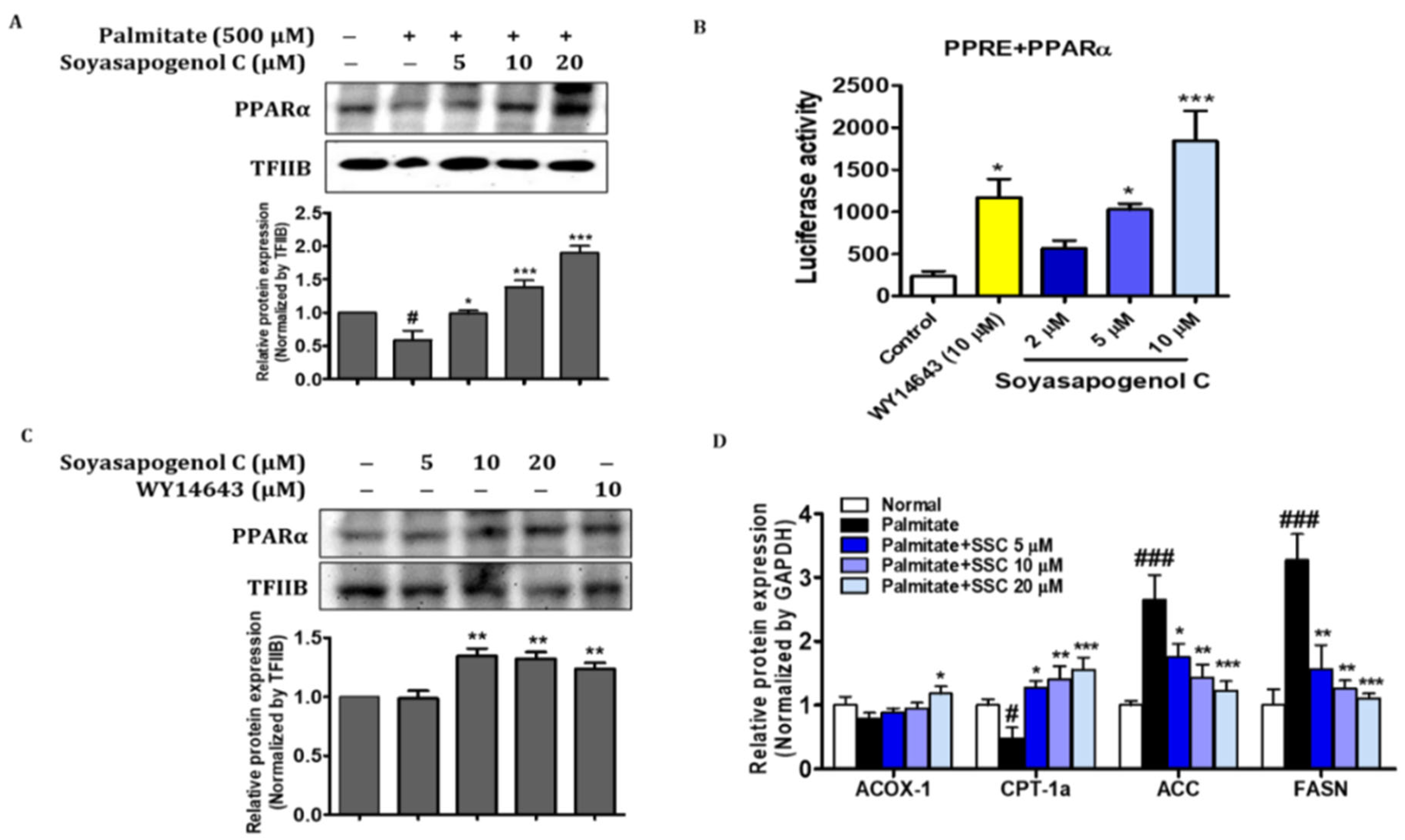
2.8. SSC Attenuated Hepatic Steatosis via Activation of AMPK in HepG2 Cells
2.9. SSC Is an Activator of PPARα in HepG2 Cells
3. Discussion
4. Materials and Methods
4.1. Compound Screening
4.2. Pharmacokinetic Properties Prediction
4.3. Target Prediction and Network Pharmacology
4.4. In Silico Experiment Materials
4.5. Molecular Docking
4.6. Molecular Dynamics Simulations
4.7. Reagents and Antibodies
4.8. Cell Culture and Treatment
4.9. Cell Viability
4.10. Preparation and Treatment with Sodium Palmitate in HepG2 Cells
4.11. Oil Red O Staining
4.12. Measurements of Intracellular Triglyceride Contents
4.13. Luciferase Assay
4.14. RNA Isolation and Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
4.15. Western Blotting
4.16. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Di Lorenzo, C.; Dell’Agli, M.; Badea, M.; Dima, L.; Colombo, E.; Sangiovanni, E.; Restani, P.; Bosisio, E. Plant Food Supplements with Anti-Inflammatory Properties: A Systematic Review (II). Crit. Rev. Food Sci. Nutr. 2013, 53, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Guang, C.; Chen, J.; Sang, S.; Cheng, S. Biological Functionality of Soyasaponins and Soyasapogenols. J. Agric. Food Chem. 2014, 62, 8247–8255. [Google Scholar] [CrossRef] [PubMed]
- Konoshima, T. Anti-Tumor-Promoting Activities of Triterpenoid Glycosides; Cancer Chemoprevention by Saponins. Adv. Exp. Med. Biol. 1996, 404, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Kinjo, J.; Imagire, M.; Udayama, M.; Arao, T.; Nohara, T. Studies on the hepatoprotective drugs part 3—Studies on the constituents of the leguminous plants part 56—Structure-Hepatoprotective Relationships Study of Soyasaponins I-IV Having Soyasapogenol B as Aglycone. Planta Med. 1998, 64, 233–236. [Google Scholar] [CrossRef]
- Potter, S.M. Overview of proposed mechanisms for the hypocholesterolemic effect of soy. J. Nutr. 1995, 125, 606S–611S. [Google Scholar] [CrossRef]
- Lee, H.-J.; Lim, S.-M.; Ko, D.-B.; Jeong, J.-J.; Hwang, Y.-H.; Kim, D.-H. Soyasapogenol B and Genistein Attenuate Lipopolysaccharide-Induced Memory Impairment in Mice by the Modulation of NF-κB-Mediated BDNF Expression. J. Agric. Food Chem. 2017, 65, 6877–6885. [Google Scholar] [CrossRef]
- Zhou, X.; Shen, P.; Wang, W.; Zhou, J.; Raj, R.; Du, Z.; Xu, S.; Wang, W.; Yu, B.; Zhang, J. Derivatization of Soyasapogenol A through Microbial Transformation for Potential Anti-inflammatory Food Supplements. J. Agric. Food Chem. 2021, 69, 6791–6798. [Google Scholar] [CrossRef]
- Omar, A.; Kalra, R.S.; Putri, J.; Elwakeel, A.; Kaul, S.C.; Wadhwa, R. Soyasapogenol-A targets CARF and results in suppression of tumor growth and metastasis in p53 compromised cancer cells. Sci. Rep. 2020, 10, 6323. [Google Scholar] [CrossRef]
- Hu, J.; Reddy, M.B.; Hendrich, S.; Murphy, P.A. Soyasaponin I and Sapongenol B Have Limited Absorption by Caco-2 Intestinal Cells and Limited Bioavailability in Women. J. Nutr. 2004, 134, 1867–1873. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.; Badger, T.M.; Ronis, M.J.J.; Wu, X. Non-isoflavone Phytochemicals in Soy and Their Health Effects. J. Agric. Food Chem. 2010, 58, 8119–8133. [Google Scholar] [CrossRef]
- Kwon, Y.S.; Lee, S.; Lee, S.H.; Kim, H.J.; Lee, C.H. Comparative Evaluation of Six Traditional Fermented Soybean Products in East Asia: A Metabolomics Approach. Metabolites 2019, 9, 183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, J.-G.; Saibara, T.; Chitturi, S.; Kim, B.I.; Sung, J.J.Y.; Chutaputti, A.; Party, A.P.W. What are the risk factors and settings for non-alcoholic fatty liver disease in Asia?Pacific? J. Gastroenterol. Hepatol. 2007, 22, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Postic, C.; Girard, J. The role of the lipogenic pathway in the development of hepatic steatosis. Diabetes Metab. 2008, 34, 643–648. [Google Scholar] [CrossRef]
- Cohen, J.C.; Horton, J.D.; Hobbs, H.H. Human Fatty Liver Disease: Old Questions and New Insights. Science 2011, 332, 1519–1523. [Google Scholar] [CrossRef] [Green Version]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262. [Google Scholar] [CrossRef] [Green Version]
- Smith, B.K.; Marcinko, K.; Desjardins, E.M.; Lally, J.S.; Ford, R.J.; Steinberg, G.R. Treatment of nonalcoholic fatty liver disease: Role of AMPK. Am. J. Physiol. Metab. 2016, 311, E730–E740. [Google Scholar] [CrossRef] [Green Version]
- Ouchi, N.; Shibata, R.; Walsh, K. AMP-Activated Protein Kinase Signaling Stimulates VEGF Expression and Angiogenesis in Skeletal Muscle. Circ. Res. 2005, 96, 838–846. [Google Scholar] [CrossRef] [Green Version]
- Yoon, Y.; Lee, H.; Kang, R.; Bae, S. AICAR, an activator of AMPK, inhibits adipogenesis via the WNT/β-catenin pathway in 3T3-L1 adipocytes. Int. J. Mol. Med. 2011, 28, 65–71. [Google Scholar] [CrossRef]
- Lian, Z.; Li, Y.; Gao, J.; Qu, K.; Li, J.; Hao, L.; Wu, S.; Zhu, H. A novel AMPK activator, WS070117, improves lipid metabolism discords in hamsters and HepG2 cells. Lipids Health Dis. 2011, 10, 67. [Google Scholar] [CrossRef] [Green Version]
- Peng, X.; Li, J.; Wang, M.; Qu, K.; Zhu, H. A novel AMPK activator improves hepatic lipid metabolism and leukocyte trafficking in experimental hepatic steatosis. J. Pharmacol. Sci. 2019, 140, 153–161. [Google Scholar] [CrossRef]
- Yun, Y.-R.; Kim, J.-H.; Jung, M.H. Protective effects of gomisin N against hepatic steatosis through AMPK activation. Biochem. Biophys. Res. Commun. 2016, 482, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-X. PPARs: Diverse regulators in energy metabolism and metabolic diseases. Cell Res. 2010, 20, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Gross, B.; Pawlak, M.; Lefebvre, P.; Staels, B. PPARs in obesity-induced T2DM, dyslipidaemia and NAFLD. Nat. Rev. Endocrinol. 2017, 13, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Zardi, E.M.; Navarini, L.; Sambataro, G.; Piccinni, P.; Sambataro, F.M.; Spina, C.; Dobrina, A. Hepatic PPARs: Their Role in Liver Physiology, Fibrosis and Treatment. Curr. Med. Chem. 2013, 20, 3370–3396. [Google Scholar] [CrossRef]
- Desvergne, B.; Wahli, W. Peroxisome Proliferator-Activated Receptors: Nuclear Control of Metabolism. Endocr. Rev. 1999, 20, 649–688. [Google Scholar] [CrossRef] [Green Version]
- Mandard, S.; Müller, M.; Kersten, S. Peroxisome proliferator-activated receptor a target genes. Cell. Mol. Life Sci. 2004, 61, 393–416. [Google Scholar] [CrossRef]
- Brandt, J.M.; Djouadi, F.; Kelly, D.P. Fatty Acids Activate Transcription of the Muscle Carnitine Palmitoyltransferase I Gene in Cardiac Myocytes via the Peroxisome Proliferator-activated Receptor α. J. Biol. Chem. 1998, 273, 23786–23792. [Google Scholar] [CrossRef] [Green Version]
- Kersten, S.; Seydoux, J.; Peters, J.M.; Gonzalez, F.J.; Desvergne, B.; Wahli, W. Peroxisome proliferator–activated receptor α mediates the adaptive response to fasting. J. Clin. Investig. 1999, 103, 1489–1498. [Google Scholar] [CrossRef] [Green Version]
- Xiao, X.; Su, G.; Brown, S.N.; Chen, L.; Ren, J.; Zhao, P. Peroxisome proliferator-activated receptors γ and α agonists stimulate cardiac glucose uptake via activation of AMP-activated protein kinase. J. Nutr. Biochem. 2010, 21, 621–626. [Google Scholar] [CrossRef]
- Tu, Z.; Moss-Pierce, T.; Ford, P.; Jiang, T.A. Rosemary (Rosmarinus officinalis L.) Extract Regulates Glucose and Lipid Metabolism by Activating AMPK and PPAR Pathways in HepG2 Cells. J. Agric. Food Chem. 2013, 61, 2803–2810. [Google Scholar] [CrossRef]
- Tian, Y.; Feng, H.; Han, L.; Wu, L.; Lv, H.; Shen, B.; Li, Z.; Zhang, Q.; Liu, G. Magnolol Alleviates Inflammatory Responses and Lipid Accumulation by AMP-Activated Protein Kinase-Dependent Peroxisome Proliferator-Activated Receptor α Activation. Front. Immunol. 2018, 9, 147. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Lee, S.; Lee, S.-H.; Kim, H.-J.; Singh, D.; Lee, C.-H. Integrated Metabolomics and Volatolomics for Comparative Evaluation of Fermented Soy Products. Foods 2021, 10, 2516. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.H.; Ahn, E.-K.; Lee, J.A.; Shin, T.-S.; Tsukamoto, C.; Suh, J.-W.; Mei, I.; Chung, G. Soyasaponins Aa and Ab Exert an Anti-Obesity Effect in 3T3-L1 Adipocytes Through Downregulation of PPARγ. Phytother. Res. 2015, 29, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalid, S.; Hanif, R.; Jabeen, I.; Mansoor, Q.; Ismail, M. Pharmacophore modeling for identification of anti-IGF-1R drugs and in-vitro validation of fulvestrant as a potential inhibitor. PLoS ONE 2018, 13, e0196312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, Z.; Wang, H.; Song, P.; Zhang, M.; Geng, X.; Zou, M.-H. Nicotine-induced Activation of AMP-activated Protein Kinase Inhibits Fatty Acid Synthase in 3T3L1 Adipocytes—A role for oxidant stress. J. Biol. Chem. 2007, 282, 26793–26801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pyo, Y.-H.; Lee, T.-C. The Potential Antioxidant Capacity and Angiotensin I-Converting Enzyme Inhibitory Activity of Monascus-Fermented Soybean Extracts: Evaluation of Monascus-Fermented Soybean Extracts as Multifunctional Food Additives. J. Food Sci. 2007, 72, S218–S223. [Google Scholar] [CrossRef]
- Champagne, C.P.; Tompkins, T.A.; Buckley, N.D.; Green-Johnson, J.M. Effect of fermentation by pure and mixed cultures of Streptococcus thermophilus and Lactobacillus helveticus on isoflavone and B-vitamin content of a fermented soy beverage. Food Microbiol. 2010, 27, 968–972. [Google Scholar] [CrossRef]
- Kaimal, V.; Sardana, D.; Bardes, E.E.; Gudivada, R.C.; Chen, J.; Jegga, A.G. Integrative Systems Biology Approaches to Identify and Prioritize Disease and Drug Candidate Genes. Methods Mol. Biol. 2011, 700, 241–259. [Google Scholar] [CrossRef]
- Sharma, A.; Anand, S.K.; Singh, N.; Dwarkanath, A.; Dwivedi, U.N.; Kakkar, P. Berbamine induced activation of the SIRT1/LKB1/AMPK signaling axis attenuates the development of hepatic steatosis in high-fat diet-induced NAFLD rats. Food Funct. 2021, 12, 892–909. [Google Scholar] [CrossRef]
- Seo, M.S.; Kim, J.H.; Kim, H.J.; Chang, K.C.; Park, S.W. Honokiol activates the LKB1–AMPK signaling pathway and attenuates the lipid accumulation in hepatocytes. Toxicol. Appl. Pharmacol. 2015, 284, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Rakhshandehroo, M.; Knoch, B.; Müller, M.; Kersten, S. Peroxisome Proliferator-Activated Receptor Alpha Target Genes. PPAR Res. 2010, 2010, 1095–1101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, L.-H.; Wang, A.; Luo, P.; Wang, X.; Jiang, D.-S.; Deng, W.; Zhang, X.; Wang, T.; Liu, Y.; Gao, L.; et al. Mindin/Spondin 2 inhibits hepatic steatosis, insulin resistance, and obesity via interaction with peroxisome proliferator-activated receptor α in mice. J. Hepatol. 2014, 60, 1046–1054. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, D.T.; Wu, J.; Back, S.-H.; Callaghan, M.U.; Ferris, S.P.; Iqbal, J.; Clark, R.; Miao, H.; Hassler, J.R.; Fornek, J.; et al. UPR Pathways Combine to Prevent Hepatic Steatosis Caused by ER Stress-Mediated Suppression of Transcriptional Master Regulators. Dev. Cell 2008, 15, 829–840. [Google Scholar] [CrossRef] [Green Version]
- Tian, X.; Ru, Q.; Xiong, Q.; Wen, R.; Chen, Y. Catalpol Attenuates Hepatic Steatosis by Regulating Lipid Metabolism via AMP-Activated Protein Kinase Activation. BioMed Res. Int. 2020, 2020, 6708061. [Google Scholar] [CrossRef]
- Guan, L.; Yang, H.; Cai, Y.; Sun, L.; Di, P.; Li, W.; Liu, G.; Tang, Y. ADMET-score—A comprehensive scoring function for evaluation of chemical drug-likeness. MedChemComm 2019, 10, 148–157. [Google Scholar] [CrossRef]
- Lipinski, C.A. Lead- and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Hou, T.J.; Zhang, W.; Xia, K.; Qiao, X.B.; Xu, X.J. ADME Evaluation in Drug Discovery. 5. Correlation of Caco-2 Permeation with Simple Molecular Properties. J. Chem. Inf. Comput. Sci. 2004, 44, 1585–1600. [Google Scholar] [CrossRef] [Green Version]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef] [Green Version]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Sherman, B.T.; Tan, Q.; Kir, J.; Liu, D.; Bryant, D.; Guo, Y.; Stephens, R.; Baseler, M.W.; Lane, H.C.; et al. DAVID Bioinformatics Resources: Expanded annotation database and novel algorithms to better extract biology from large gene lists. Nucleic Acids Res. 2007, 35, W169–W175. [Google Scholar] [CrossRef] [PubMed]
- Raudvere, U.; Kolberg, L.; Kuzmin, I.; Arak, T.; Adler, P.; Peterson, H.; Vilo, J. g:Profiler: A web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic Acids Res. 2019, 47, W191–W198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, B.; Sanders, M.J.; Underwood, E.; Heath, R.; Mayer, F.V.; Carmena, D.; Jing, C.; Walker, P.A.; Eccleston, J.F.; Haire, L.F.; et al. Structure of mammalian AMPK and its regulation by ADP. Nature 2011, 472, 230–233. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.E.; Lambert, M.H.; Montana, V.G.; Plunket, K.D.; Moore, L.B.; Collins, J.L.; Oplinger, J.A.; Kliewer, S.A.; Gampe, R.T., Jr.; McKee, D.D.; et al. Structural determinants of ligand binding selectivity between the peroxisome proliferator-activated receptors. Proc. Natl. Acad. Sci. USA 2001, 98, 13919–13924. [Google Scholar] [CrossRef] [Green Version]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera-a visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem in 2021: New data content and improved web interfaces. Nucleic Acids Res. 2021, 49, D1388–D1395. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [Green Version]
- Fuhrmann, J.; Rurainski, A.; Lenhof, H.-P.; Neumann, D. A new Lamarckian genetic algorithm for flexible ligand-receptor docking. J. Comput. Chem. 2010, 31, 1911–1918. [Google Scholar] [CrossRef]
- Grosdidier, A.; Zoete, V.; Michielin, O. Fast docking using the CHARMM force field with EADock DSS. J. Comput. Chem. 2011, 32, 2149–2159. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Sun, H.; Yao, X.; Li, D.; Xu, L.; Li, Y.; Tian, S.; Hou, T. Comprehensive evaluation of ten docking programs on a diverse set of protein–ligand complexes: The prediction accuracy of sampling power and scoring power. Phys. Chem. Chem. Phys. 2016, 18, 12964–12975. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, R.A.; Swindells, M.B. LigPlot+: Multiple ligand–protein interaction diagrams for drug discovery. J. Chem. Inf. Model. 2011, 51, 2778–2786. [Google Scholar] [CrossRef] [PubMed]
- Van Der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J.C. GROMACS: Fast, flexible, and free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef]
- Basith, S.; Manavalan, B.; Shin, T.H.; Lee, G. A Molecular Dynamics Approach to Explore the Intramolecular Signal Transduction of PPAR-α. Int. J. Mol. Sci. 2019, 20, 1666. [Google Scholar] [CrossRef] [Green Version]
- Tang, H.-C.; Chen, C.Y.-C. In Silico Design for Adenosine Monophosphate-Activated Protein Kinase Agonist from Traditional Chinese Medicine for Treatment of Metabolic Syndromes. Evid.-Based Complement. Altern. Med. 2014, 2014, 928589. [Google Scholar] [CrossRef] [Green Version]
- Klauda, J.B.; Venable, R.M.; Freites, J.A.; O’Connor, J.W.; Tobias, D.J.; Mondragon-Ramirez, C.; Vorobyov, I.; MacKerell, A.D., Jr.; Pastor, R.W. Update of the CHARMM All-Atom Additive Force Field for Lipids: Validation on Six Lipid Types. J. Phys. Chem. B 2010, 114, 7830–7843. [Google Scholar] [CrossRef] [Green Version]
- Stroet, M.; Caron, B.; Visscher, K.M.; Geerke, D.P.; Malde, A.K.; Mark, A.E. Automated Topology Builder Version 3.0: Prediction of Solvation Free Enthalpies in Water and Hexane. J. Chem. Theory Comput. 2018, 14, 5834–5845. [Google Scholar] [CrossRef]
- Racine, J. gnuplot 4.0: A portable interactive plotting utility. J. Appl. Econ. 2006, 21, 133–141. [Google Scholar] [CrossRef]
- Park, J.-Y.; Kim, Y.; Im, J.A.; Lee, H. Oligonol suppresses lipid accumulation and improves insulin resistance in a palmitate-induced in HepG2 hepatocytes as a cellular steatosis model. BMC Complement. Altern. Med. 2015, 15, 185. [Google Scholar] [CrossRef] [Green Version]
- Rakhshandehroo, M.; Hooiveld, G.; Müller, M.; Kersten, S. Comparative Analysis of Gene Regulation by the Transcription Factor PPARα between Mouse and Human. PLoS ONE 2009, 4, e6796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habib, A.; Créminon, C.; Frobert, Y.; Grassi, J.; Pradelles, P.; Maclouf, J. Demonstration of an inducible cyclooxygenase in human endothelial cells using antibodies raised against the carboxyl-terminal region of the cyclooxygenase-2. J. Biol. Chem. 1993, 268, 23448–23454. [Google Scholar] [CrossRef]



| Properties | AMPK (AICAR a) | PPARα (FF b) | SSB | SSC |
|---|---|---|---|---|
| Absorption | ||||
| Human intestinal absorption (HIA %) | 18.27 | 97.39 | 92.18 | 94.56 |
| Caco-2 cell permeability (nm s−1) | 6.80 | 44.24 | 22.25 | 24.63 |
| MDCK cell permeability (nm s−1) | 0.58 | 15.527 | 0.044 | 0.048 |
| Skin permeability (logKp, cm h−1) | −5.17 | −1.55 | −3.60 | −2.41 |
| Distribution | ||||
| Plasma protein binding (%) | 5.12 | 100 | 100 | 100 |
| Blood–brain barrier penetration (Cbrain/Cblood) | 0.63 | 0.11 | 6.36 | 13.17 |
| Metabolism | ||||
| CYP2C19 inhibition | Non | Inhibitor | Non | Non |
| CYP2C19 Substrate | Non | Non | Non | Non |
| CYP2C9 inhibition | Non | Inhibitor | Inhibitor | Inhibitor |
| CYP2C9 Substrate | Non | Non | Non | Non |
| CYP2D6 inhibition | Non | Non | Non | Non |
| CYP2D6 Substrate | Non | Non | Non | Non |
| CYP3A4 inhibition | Inhibitor | Inhibitor | Inhibitor | Inhibitor |
| CYP3A4 Substrate | Weakly | Substrate | Substrate | Substrate |
| Excretion | ||||
| P-gp inhibition | Non | Inhibitor | Inhibitor | Inhibitor |
| Toxicity | ||||
| Ames test | Mutagen | Mutagen | Non | Non |
| Carcino_Mouse | Carcinogen | Carcinogen | Non-carcinogen | Non-carcinogen |
| Carcino_Rat | Non-carcinogen | Carcinogen | Non-carcinogen | Non-carcinogen |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arulkumar, R.; Jung, H.J.; Noh, S.G.; Chung, H.Y. Soyasapogenol C from Fermented Soybean (Glycine Max) Acting as a Novel AMPK/PPARα Dual Activator Ameliorates Hepatic Steatosis: A Novel SANDA Methodology. Int. J. Mol. Sci. 2022, 23, 5468. https://doi.org/10.3390/ijms23105468
Arulkumar R, Jung HJ, Noh SG, Chung HY. Soyasapogenol C from Fermented Soybean (Glycine Max) Acting as a Novel AMPK/PPARα Dual Activator Ameliorates Hepatic Steatosis: A Novel SANDA Methodology. International Journal of Molecular Sciences. 2022; 23(10):5468. https://doi.org/10.3390/ijms23105468
Chicago/Turabian StyleArulkumar, Radha, Hee Jin Jung, Sang Gyun Noh, and Hae Young Chung. 2022. "Soyasapogenol C from Fermented Soybean (Glycine Max) Acting as a Novel AMPK/PPARα Dual Activator Ameliorates Hepatic Steatosis: A Novel SANDA Methodology" International Journal of Molecular Sciences 23, no. 10: 5468. https://doi.org/10.3390/ijms23105468
APA StyleArulkumar, R., Jung, H. J., Noh, S. G., & Chung, H. Y. (2022). Soyasapogenol C from Fermented Soybean (Glycine Max) Acting as a Novel AMPK/PPARα Dual Activator Ameliorates Hepatic Steatosis: A Novel SANDA Methodology. International Journal of Molecular Sciences, 23(10), 5468. https://doi.org/10.3390/ijms23105468






