MicroPET Imaging Assessment of Brain Tau and Amyloid Deposition in 6 × Tg Alzheimer’s Disease Model Mice
Abstract
:1. Introduction
2. Results
2.1. In Vivo Amyloid, Inflammation and Tau PET Imaging of 6 × Tg Model Mice
2.2. In Vivo [18F]Flutemetamol PET Imaging Detected Aging-Related and AD-Associated Elevation in Brain Aβ
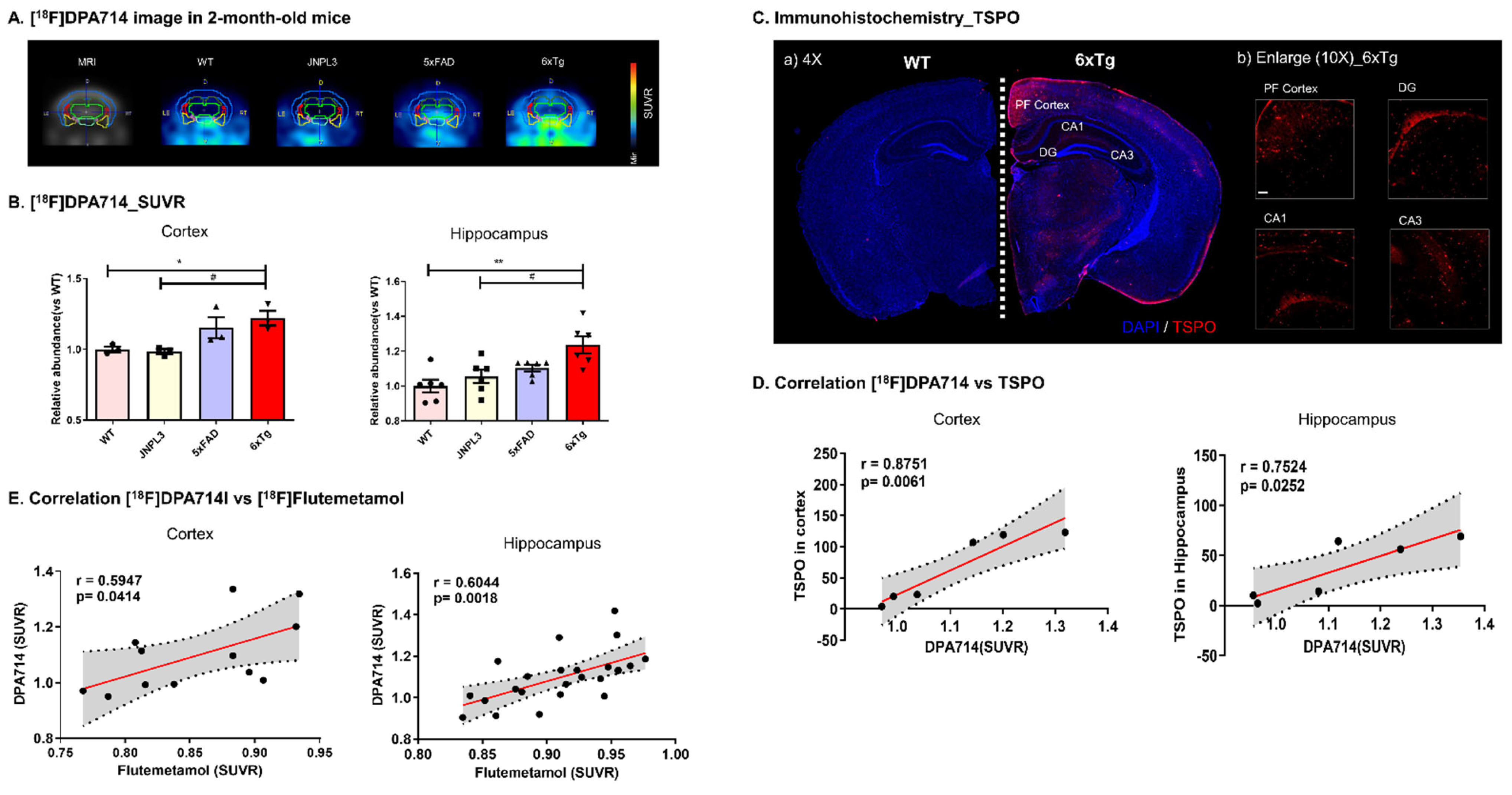
2.3. In Vivo [18F]DPA714 PET Imaging Detected AD-Associated Inflammation in the Brain
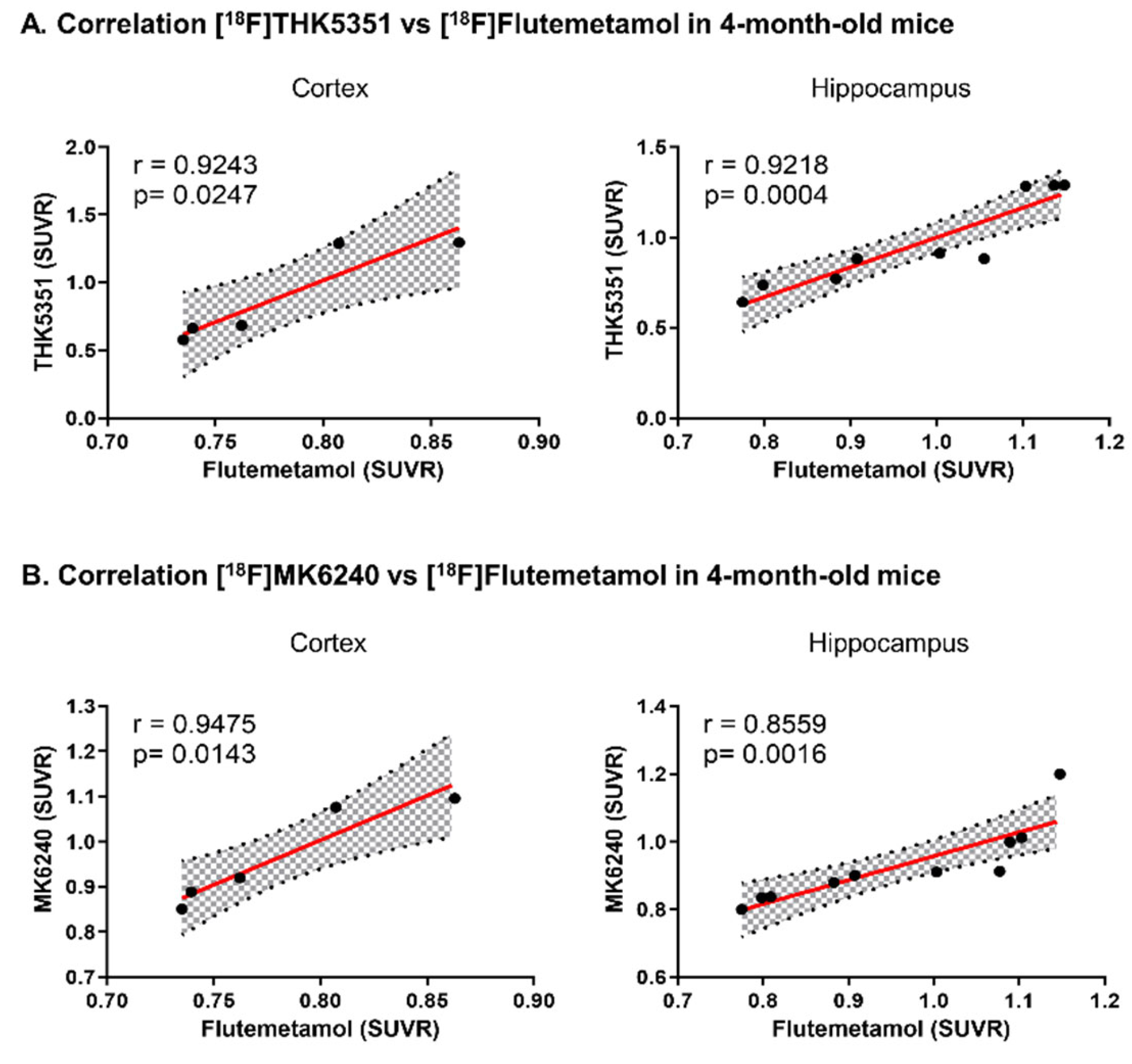
2.4. In Vivo [18F]THK5351 and [18F]MK6240 PET Imaging Detected AD-Associated Elevation in Brain Tau
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. MicroPET Studies
4.3. Immunohistochemistry
4.4. ELISA
4.5. Western Blot
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, J.; Gu, B.J.; Masters, C.L.; Wang, Y.J. A systemic view of Alzheimer disease—Insights from amyloid-beta metabolism beyond the brain. Nat. Rev. Neurol. 2017, 13, 703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henriksen, G.; Yousefi, B.H.; Drzezga, A.; Wester, H.J. Development and evaluation of compounds for imaging of beta-amyloid plaque by means of positron emission tomography. Eur. J. Nucl. Med. Mol. Imaging 2008, 35 (Suppl. 1), S75–S81. [Google Scholar] [CrossRef] [PubMed]
- Rinne, J.O.; Wong, D.F.; Wolk, D.A.; Leinonen, V.; Arnold, S.E.; Buckley, C.; Smith, A.; McLain, R.; Sherwin, P.F.; Farrar, G.; et al. [(18)F]Flutemetamol PET imaging and cortical biopsy histopathology for fibrillar amyloid beta detection in living subjects with normal pressure hydrocephalus: Pooled analysis of four studies. Acta Neuropathol. 2012, 124, 833–845. [Google Scholar] [CrossRef] [PubMed]
- Hatashita, S.; Wakebe, D.; Kikuchi, Y.; Ichijo, A. Longitudinal Assessment of Amyloid-beta Deposition by [18F]-Flutemetamol PET Imaging Compared With [11C]-PIB Across the Spectrum of Alzheimer’s Disease. Front. Aging Neurosci. 2019, 11, 251. [Google Scholar] [CrossRef]
- Harada, R.; Okamura, N.; Furumoto, S.; Furukawa, K.; Ishiki, A.; Tomita, N.; Tago, T.; Hiraoka, K.; Watanuki, S.; Shidahara, M.; et al. 18F-THK5351: A Novel PET Radiotracer for Imaging Neurofibrillary Pathology in Alzheimer Disease. J. Nucl. Med. 2016, 57, 208–214. [Google Scholar] [CrossRef] [Green Version]
- Betthauser, T.J.; Lao, P.J.; Murali, D.; Barnhart, T.E.; Furumoto, S.; Okamura, N.; Stone, C.K.; Johnson, S.C.; Christian, B.T. In Vivo Comparison of Tau Radioligands (18)F-THK-5351 and (18)F-THK-5317. J. Nucl. Med. 2017, 58, 996–1002. [Google Scholar] [CrossRef] [Green Version]
- McGeer, P.L.; Itagaki, S.; Boyes, B.E.; McGeer, E.G. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology 1988, 38, 1285–1291. [Google Scholar] [CrossRef]
- Veiga, S.; Carrero, P.; Pernia, O.; Azcoitia, I.; Garcia-Segura, L.M. Translocator protein 18 kDa is involved in the regulation of reactive gliosis. Glia 2007, 55, 1426–1436. [Google Scholar] [CrossRef] [Green Version]
- Rupprecht, R.; Papadopoulos, V.; Rammes, G.; Baghai, T.C.; Fan, J.; Akula, N.; Groyer, G.; Adams, D.; Schumacher, M. Translocator protein (18 kDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders. Nat. Rev. Drug. Discov. 2010, 9, 971–988. [Google Scholar] [CrossRef]
- Dupont, A.C.; Largeau, B.; Santiago Ribeiro, M.J.; Guilloteau, D.; Tronel, C.; Arlicot, N. Translocator Protein-18 kDa (TSPO) Positron Emission Tomography (PET) Imaging and Its Clinical Impact in Neurodegenerative Diseases. Int. J. Mol. Sci. 2017, 18, 785. [Google Scholar] [CrossRef]
- Bonsack, F.; Foss, C.A.; Arbab, A.S.; Alleyne, C.H., Jr.; Pomper, M.G.; Sukumari-Ramesh, S. [(125) I]IodoDPA-713 Binding to 18 kDa Translocator Protein (TSPO) in a Mouse Model of Intracerebral Hemorrhage: Implications for Neuroimaging. Front. Neurosci. 2018, 12, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavisse, S.; Inoue, K.; Jan, C.; Peyronneau, M.A.; Petit, F.; Goutal, S.; Dauguet, J.; Guillermier, M.; Dolle, F.; Rbah-Vidal, L.; et al. [18F]DPA-714 PET imaging of translocator protein TSPO (18 kDa) in the normal and excitotoxically-lesioned nonhuman primate brain. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 478–494. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Kim, J.; Chang, K.A. Spatial memory deficiency early in 6 × Tg Alzheimer’s disease mouse model. Sci. Rep. 2021, 11, 1334. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.J.; Lee, H.J.; Kang, K.J.; Han, S.J.; Lee, Y.J.; Lee, K.C.; Lim, S.M.; Chi, D.Y.; Kim, K.M.; Park, J.A.; et al. Early Detection of Abeta Deposition in the 5 × FAD Mouse by Amyloid PET. Contrast Media Mol. Imaging 2018, 2018, 5272014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okamura, N.; Harada, R.; Ishiki, A.; Kikuchi, A.; Nakamura, T.; Kudo, Y. The development and validation of tau PET tracers: Current status and future directions. Clin. Transl. Imaging 2018, 6, 305–316. [Google Scholar] [CrossRef] [Green Version]
- Kinney, J.W.; Bemiller, S.M.; Murtishaw, A.S.; Leisgang, A.M.; Salazar, A.M.; Lamb, B.T. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimer’s Dementia Transl. Res. Clin. Interv. 2018, 4, 575–590. [Google Scholar] [CrossRef]
- Lemoine, L.; Saint-Aubert, L.; Nennesmo, I.; Gillberg, P.G.; Nordberg, A. Cortical laminar tau deposits and activated astrocytes in Alzheimer’s disease visualised by (3)H-THK5117 and (3)H-deprenyl autoradiography. Sci. Rep. 2017, 7, 45496. [Google Scholar] [CrossRef]
- Ng, K.P.; Pascoal, T.A.; Mathotaarachchi, S.; Therriault, J.; Kang, M.S.; Shin, M.; Guiot, M.C.; Guo, Q.; Harada, R.; Comley, R.A.; et al. Monoamine oxidase B inhibitor, selegiline, reduces (18)F-THK5351 uptake in the human brain. Alzheimers Res. Ther. 2017, 9, 25. [Google Scholar] [CrossRef] [Green Version]
- Collier, T.L.; Yokell, D.L.; Livni, E.; Rice, P.A.; Celen, S.; Serdons, K.; Neelamegam, R.; Bormans, G.; Harris, D.; Walji, A.; et al. cGMP production of the radiopharmaceutical [(18) F]MK-6240 for PET imaging of human neurofibrillary tangles. J. Label. Comp. Radiopharm. 2017, 60, 263–269. [Google Scholar] [CrossRef]
- Hostetler, E.D.; Walji, A.M.; Zeng, Z.; Miller, P.; Bennacef, I.; Salinas, C.; Connolly, B.; Gantert, L.; Haley, H.; Holahan, M.; et al. Preclinical Characterization of 18F-MK-6240, a Promising PET Tracer for In Vivo Quantification of Human Neurofibrillary Tangles. J. Nucl. Med. 2016, 57, 1599–1606. [Google Scholar] [CrossRef] [Green Version]
- Walji, A.M.; Hostetler, E.D.; Selnick, H.; Zeng, Z.; Miller, P.; Bennacef, I.; Salinas, C.; Connolly, B.; Gantert, L.; Holahan, M.; et al. Discovery of 6-(Fluoro-(18)F)-3-(1H-pyrrolo[2,3-c]pyridin-1-yl)isoquinolin-5-amine ([(18)F]-MK-6240): A Positron Emission Tomography (PET) Imaging Agent for Quantification of Neurofibrillary Tangles (NFTs). J. Med. Chem. 2016, 59, 4778–4789. [Google Scholar] [CrossRef] [PubMed]
- Malarte, M.L.; Nordberg, A.; Lemoine, L. Characterization of MK6240, a tau PET tracer, in autopsy brain tissue from Alzheimer’s disease cases. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1093–1102. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Ha, S.; Park, H.; Nam, E.; Suh, W.H.; Suh, Y.H.; Chang, K.A. Effects of a Dehydroevodiamine-Derivative on Synaptic Destabilization and Memory Impairment in the 5 × FAD, Alzheimer’s Disease Mouse Model. Front. Behav. Neurosci. 2018, 12, 273. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lim, D.K.; Suh, Y.H.; Chang, K.A. Long-Term Treatment of Cuban Policosanol Attenuates Abnormal Oxidative Stress and Inflammatory Response via Amyloid Plaques Reduction in 5 × FAD Mice. Antioxidants 2021, 10, 1321. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, S.; Kim, J.; Lee, S.-Y.; Okamura, N.; Chang, K.-A. MicroPET Imaging Assessment of Brain Tau and Amyloid Deposition in 6 × Tg Alzheimer’s Disease Model Mice. Int. J. Mol. Sci. 2022, 23, 5485. https://doi.org/10.3390/ijms23105485
Kang S, Kim J, Lee S-Y, Okamura N, Chang K-A. MicroPET Imaging Assessment of Brain Tau and Amyloid Deposition in 6 × Tg Alzheimer’s Disease Model Mice. International Journal of Molecular Sciences. 2022; 23(10):5485. https://doi.org/10.3390/ijms23105485
Chicago/Turabian StyleKang, ShinWoo, Jinho Kim, Sang-Yoon Lee, Nobuyuki Okamura, and Keun-A Chang. 2022. "MicroPET Imaging Assessment of Brain Tau and Amyloid Deposition in 6 × Tg Alzheimer’s Disease Model Mice" International Journal of Molecular Sciences 23, no. 10: 5485. https://doi.org/10.3390/ijms23105485
APA StyleKang, S., Kim, J., Lee, S.-Y., Okamura, N., & Chang, K.-A. (2022). MicroPET Imaging Assessment of Brain Tau and Amyloid Deposition in 6 × Tg Alzheimer’s Disease Model Mice. International Journal of Molecular Sciences, 23(10), 5485. https://doi.org/10.3390/ijms23105485







