Oxygen Binding by Co(II) Complexes with Oxime-Containing Schiff Bases in Solution
Abstract
1. Introduction
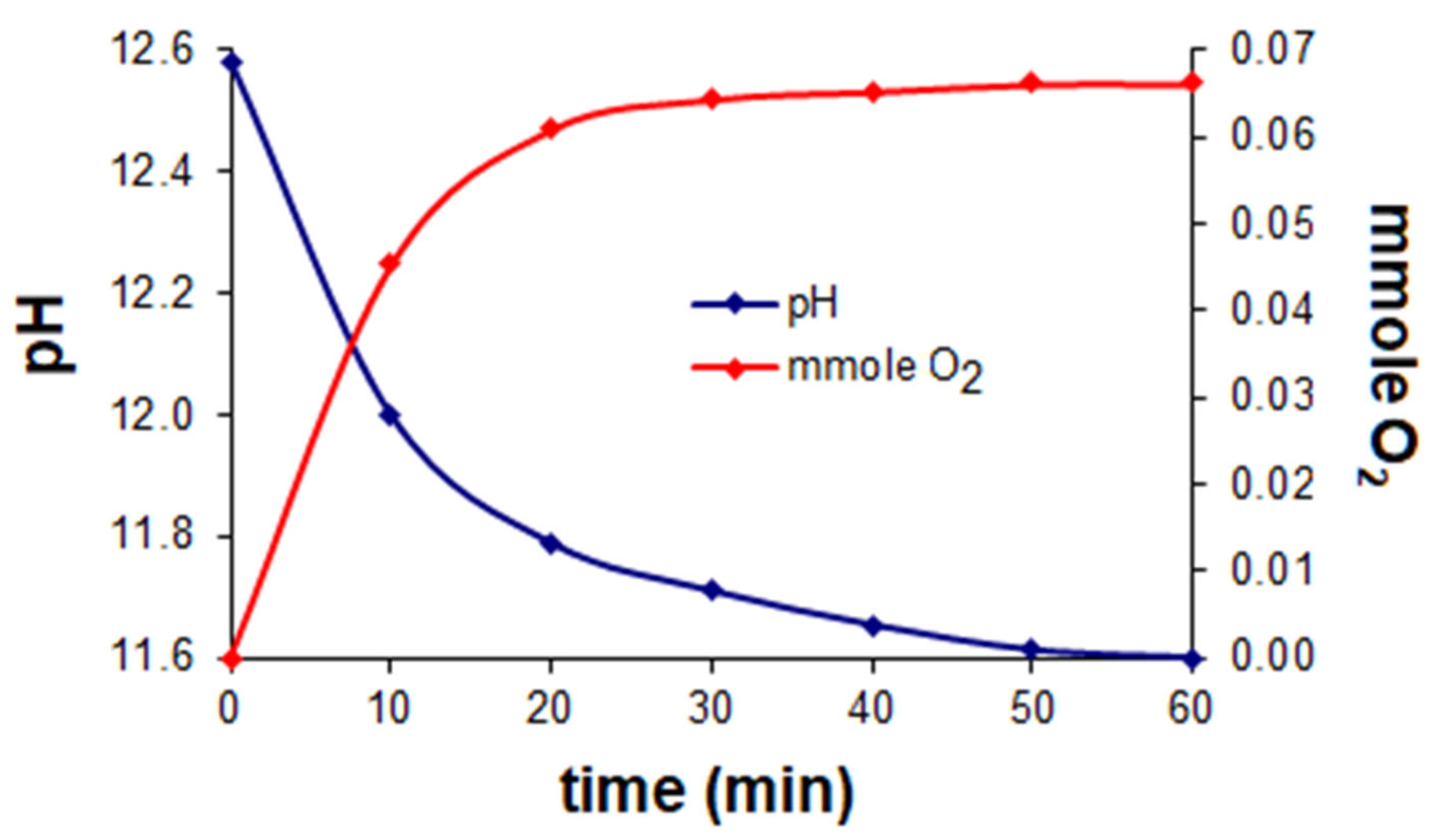
2. Results and Discussion
3. Materials and Methods
3.1. Reagents
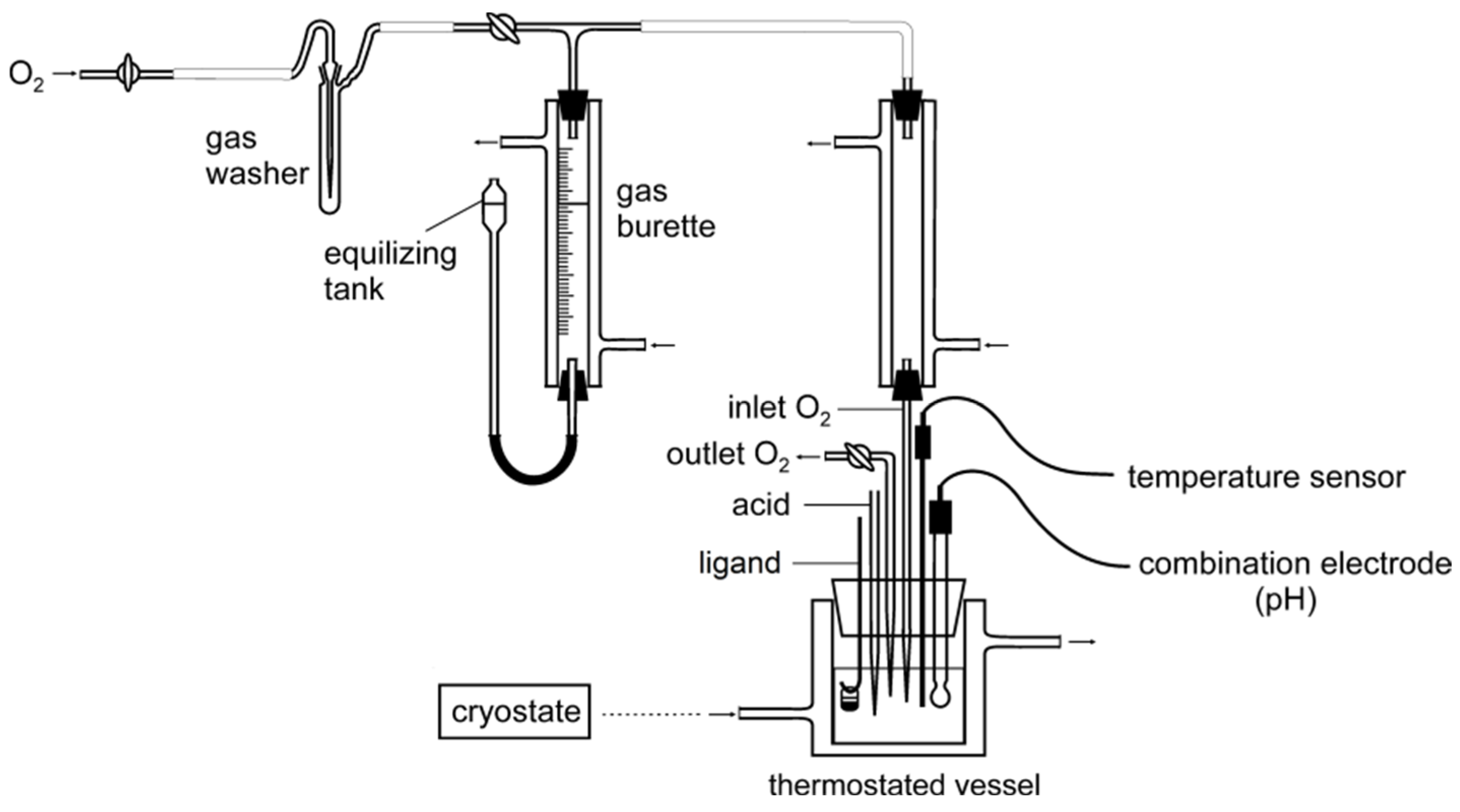
3.2. Apparatus
3.3. Oxygenation Reaction of the Co(II)–Hpoa and Co(II)–Hpop Systems
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abu-Dief, A.M.; Mohamed, I.M.A. A review on versatile applications of transition metal complexes incorporating Schiff bases. Beni-Suef Univ. J. Basic Appl. Sci. 2015, 4, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Moali, C.; Brollo, M.; Custot, J.; Sari, M.A.; Boucher, J.L.; Stuehr, D.J.; Mansuy, D. Recognition of alpha-amino acids bearing various C=NOH functions by nitric oxide synthase and arginase involves very different structural determinants. Biochemistry 2000, 39, 8208–8218. [Google Scholar] [CrossRef] [PubMed]
- Custot, J.; Boucher, J.L.; Vadon, S.; Guedes, C.; Dijols, S.; Delaforge, M.; Mansuy, D. N omega-hydroxyamino-alpha-amino acids as a new class of very strong inhibitors of arginases. J. Biol. Inorg. Chem. 1996, 1, 73–82. [Google Scholar] [CrossRef]
- Moroz, Y.S.; Kulon, K.; Haukka, M.; Gumienna-Kontecka, E.; Kozłowski, H.; Meyer, F.; Fritsky, I. Synthesis and structure of 2 × 2 molecular grid copper(II) and nickel(II) complexes with a new polydentate oxime-containing Schiff base ligand. Inorg. Chem. 2008, 47, 5656–5665. [Google Scholar] [CrossRef] [PubMed]
- Padnya, P.; Shibaeva, K.; Arsenyev, M.; Baryshnikova, S.; Terenteva, O.; Shiabiev, I.; Khannanov, A.; Boldyrev, A.; Gerasimov, A.; Grishaev, D.; et al. Catechol-Containing Schiff Bases on Thiacalixarene: Synthesis, Copper (II) Recognition, and Formation of Organic-Inorganic Copper-Based Materials. Molecules 2021, 26, 2334. [Google Scholar] [CrossRef]
- Pitucha, M.; Korga-Plewko, A.; Czylkowska, A.; Rogalewicz, B.; Drozd, M.; Iwan, M.; Kubik, J.; Humeniuk, E.; Adamczuk, G.; Karczmarzyk, Z.; et al. Influence of Complexation of Thiosemicarbazone Derivatives with Cu (II) Ions on Their Antitumor Activity against Melanoma Cells. Int. J. Mol. Sci. 2021, 22, 3104. [Google Scholar] [CrossRef]
- Szymańska, M.; Pospieszna-Markiewicz, I.; Mańka, M.; Insińska-Rak, M.; Dutkiewicz, G.; Patroniak, V.; Fik-Jaskółka, M.A. Synthesis and Spectroscopic Investigations of Schiff Base Ligand and Its Bimetallic Ag(I) Complex as DNA and BSA Binders. Biomolecules 2021, 11, 1449. [Google Scholar] [CrossRef]
- Moroz, Y.S.; Sliva, T.Y.; Kulon, K.; Kozlowski, H.; Fritsky, I.O. Dichlorido{2-hydroxyimino-N′-1-(2-pyridyl)ethylidene propanohydrazide-kappa N-3,N′,O}zinc(II) hemihydrate. Acta Crystallogr. 2008, E64, m353–m354. [Google Scholar] [CrossRef]
- Kufelnicki, A.; Tomyn, S.V.; Moroz, Y.S.; Haukka, M.; Jaciubek-Rosińska, J.; Fritsky, I.O. Synthesis of cobalt(III) complexes with new oxime-containing Schiff base ligands and metal-ligand coordination in solution. Polyhedron 2012, 33, 410–416. [Google Scholar] [CrossRef]
- Moroz, Y.S.; Kalibabchuk, V.A.; Gumienna-Kontecka, E.; Skopenko, V.V.; Pavlova, S.V. (2E)-2-Hydroxyimino-N′-(E)-2-pyridylmethylene propanohydrazide. Acta Crystallogr. 2009, E65, o2413. [Google Scholar] [CrossRef]
- McLendon, G.; Martell, A.E. Inorganic oxygen carriers as models for biological-systems. Coord. Chem. Rev. 1976, 19, 1–39. [Google Scholar] [CrossRef]
- Fallab, S. Reactions with molecular oxygen. Angew. Chem. 1967, 79, 500–511. [Google Scholar] [CrossRef]
- Pająk, M.; Woźniczka, M.; Vogt, A.; Kufelnicki, A. Reversible uptake of molecular oxygen by heteroligand Co(II)-L-alpha-amino acid-imidazole systems: Equilibrium models at full mass balance. Chem. Cent. J. 2017, 11, 90. [Google Scholar] [CrossRef] [PubMed]
- Jeżowska-Trzebiatowska, B. Complex-compounds as model of biologically-active systems. Pure Appl. Chem. 1974, 38, 367–390. [Google Scholar] [CrossRef][Green Version]
- Shukla, S.N.; Gaur, P.; Raidas, M.L.; Chaurasia, B. Tailored synthesis of unsymmetrical tetradentate ONNO schiff base complexes of Fe(III), Co(II) and Ni(II): Spectroscopic characterization, DFT optimization, oxygen-binding study, antibacterial and anticorrosion activity. J. Mol. Struct. 2020, 1202, 127362. [Google Scholar] [CrossRef]
- Ochiai, E.-I. Oxygenation of cobalt(II) complexes. J. Inorg. Nucl. Chem. 1973, 35, 3375–3389. [Google Scholar] [CrossRef]
- Fritsky, I.O.; Kozłowski, H.; Prisyazhnaya, E.V.; Rzączyńska, Z.; Karaczyn, A.; Sliva, T.Y.; Głowiak, T. Co-ordination ability of novel tetradentate amide-and-oxime ligands: Differential binding to Cu-II and Ni-II. J. Chem. Soc. Dalton Trans. 1998, 21, 3629–3633. [Google Scholar] [CrossRef]
- Duda, A.M.; Karaczyn, A.; Kozłowski, H.; Fritsky, I.O.; Głowiak, T.; Prisyazhnaya, E.V.; Sliva, T.Y.; Świątek-Kozłowska, J. Co-ordination of copper(II) and nickel(II) ions by a novel open chain oxime ligand, J. Chem. Soc. Dalton Trans. 1997, 20, 3853–3860. [Google Scholar] [CrossRef]
- Kufelnicki, A.; Tomyn, S.V.; Nedelkov, R.V.; Haukka, M.; Jaciubek-Rosińska, J.; Pająk, M.; Jaszczak, J.; Świątek, M.; Fritsky, I.O. Synthesis of cobalt(III) complexes with novel open chain oxime ligands and metal-ligand coordination in aqueous solution. Inorg. Chim. Acta 2010, 363, 2996–3003. [Google Scholar] [CrossRef]
- Busetto, C.; Cariati, F.; Fusi, A.; Gullotti, M.; Morazzon, F.; Pasini, A.; Ugo, R.; Valenti, V. Optically-active complexes of Schiff-bases. 2. Complexes of cobalt(II) with tetradentate Schiff-bases and their reactivity with oxygen. J. Chem. Soc. Dalton Trans. 1973, 7, 754–765. [Google Scholar] [CrossRef]
- Yang, J.; Hsiue, G. Epoxidized styrene-butadiene-styrene block copolymer membrane complexes with cobalt Schiff-bases for oxygen permeation. Macromolecules 1991, 24, 4010–4016. [Google Scholar] [CrossRef]
- Worayingyong, A.; Kangvansura, P.; Ausadasuk, S.; Praserthdam, P. The effect of preparation: Pechini and Schiff base methods, on adsorbed oxygen of LaCoO3 perovskite oxidation catalysts. Colloids Surf. A 2008, 315, 217–225. [Google Scholar] [CrossRef]
- Emara, A.A.A.; Ali, A.M.; El-Asmy, A.F.; Ragab, E.M. Investigation of the oxygen affinity of manganese(II), cobalt(II) and nickel(II) complexes with some tetradentate Schiff bases. J. Saudi Chem. Soc. 2014, 18, 762–773. [Google Scholar] [CrossRef]
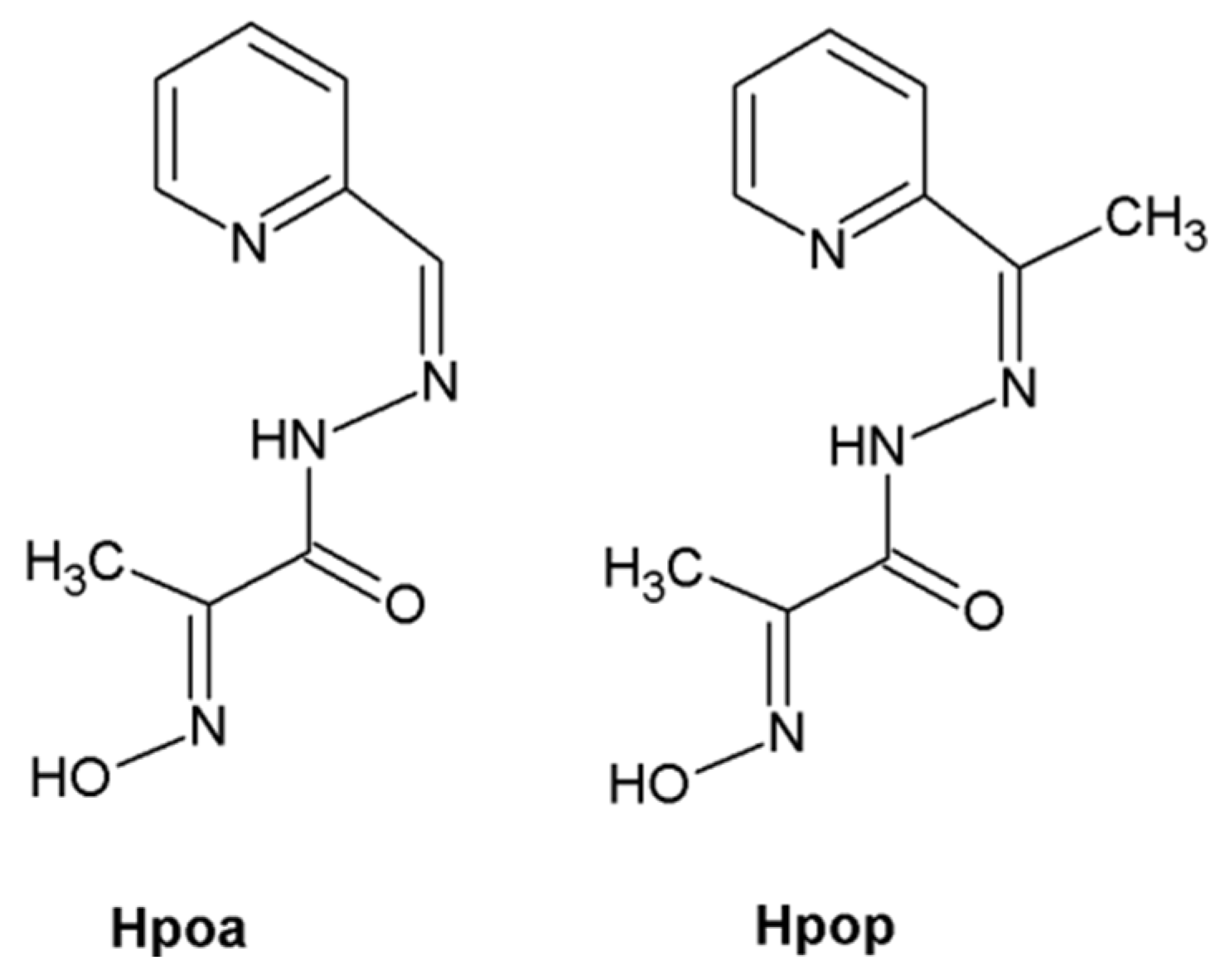

| Co(II) Systems | pH | Mmoles O2 | % Revers | Type of Bridge |
|---|---|---|---|---|
| Hpoa | 11.600 | 0.0661 | 3.44 | O22−OH |
| Hpop | 12.201 | 0.0472 | 6.30 | |
| H2pap | ~9.00 | ~0.45 | <10.0 | O22− |
| H2pen | ~9.00 | ~0.44 | <10.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pająk, M.; Woźniczka, M.; Lichawska, M.E.; Czerwiński, B.; Włodarczyk, J.; Fichna, J. Oxygen Binding by Co(II) Complexes with Oxime-Containing Schiff Bases in Solution. Int. J. Mol. Sci. 2022, 23, 5492. https://doi.org/10.3390/ijms23105492
Pająk M, Woźniczka M, Lichawska ME, Czerwiński B, Włodarczyk J, Fichna J. Oxygen Binding by Co(II) Complexes with Oxime-Containing Schiff Bases in Solution. International Journal of Molecular Sciences. 2022; 23(10):5492. https://doi.org/10.3390/ijms23105492
Chicago/Turabian StylePająk, Marek, Magdalena Woźniczka, Marta E. Lichawska, Bartłomiej Czerwiński, Jakub Włodarczyk, and Jakub Fichna. 2022. "Oxygen Binding by Co(II) Complexes with Oxime-Containing Schiff Bases in Solution" International Journal of Molecular Sciences 23, no. 10: 5492. https://doi.org/10.3390/ijms23105492
APA StylePająk, M., Woźniczka, M., Lichawska, M. E., Czerwiński, B., Włodarczyk, J., & Fichna, J. (2022). Oxygen Binding by Co(II) Complexes with Oxime-Containing Schiff Bases in Solution. International Journal of Molecular Sciences, 23(10), 5492. https://doi.org/10.3390/ijms23105492






