Metabolic Syndrome and Male Fertility: Beyond Heart Consequences of a Complex Cardiometabolic Endocrinopathy
Abstract
1. Introduction
2. Definition of Metabolic Syndrome
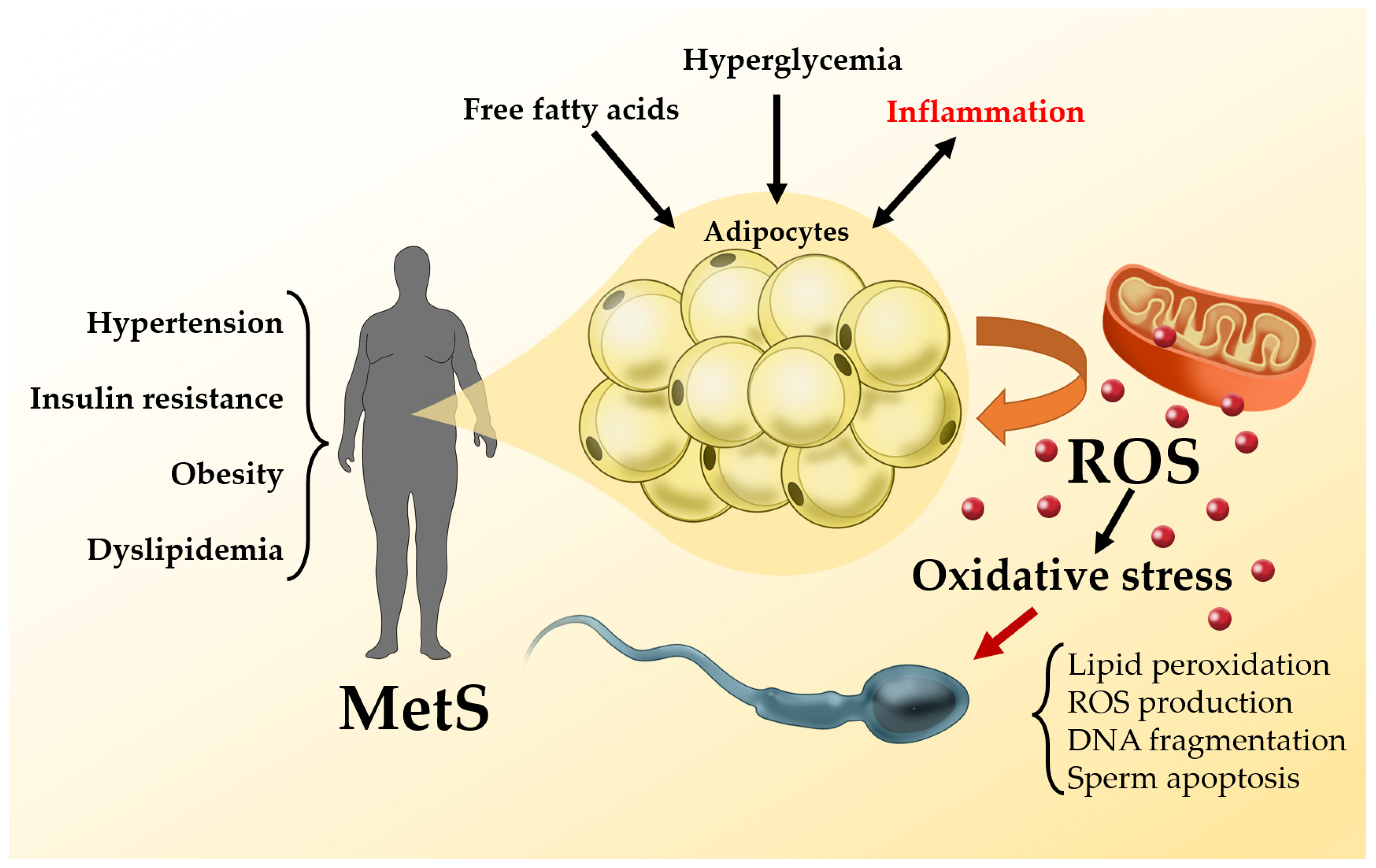
3. Metabolic Syndrome and Male Infertility: Pathophysiological Aspects
3.1. Metabolic Syndrome and Oxidative Stress
3.1.1. Seminal Oxidative Stress
3.1.2. Methods to Measure Oxidative Stress
3.1.3. Effects of Oxidative Stress on Sperm Quality and the Treatment with Antioxidant Molecules
3.2. Metabolic Syndrome and Semen Quality
3.2.1. Standard Semen Parameters
3.2.2. Sperm DNA Fragmentation
3.2.3. Mitochondrial Membrane Potential
3.3. Metabolic Syndrome and Reproductive Hormones
4. Metabolic Syndrome and Drugs: Fertility Issues
5. Metabolic Syndrome and Reproductive Outcomes
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metabolism 2019, 92, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-Johnson, C.; Mincey, K.D. Obesity Epidemiology Trends by Race/Ethnicity, Gender, and Education. Gastroenterol. Clin. N. Am. 2016, 45, 571–579. [Google Scholar] [CrossRef] [PubMed]
- McCracken, E.; Monaghan, M.; Sreenivasan, S. Pathophysiology of the metabolic syndrome. Clin. Dermatol. 2018, 36, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Vander Borght, M.; Wyns, C. Fertility and infertility: Definition and epidemiology. Clin. Biochem. 2018, 62, 2–10. [Google Scholar] [CrossRef]
- Mascarenhas, M.N.; Flaxman, S.R.; Boerma, T.; Vanderpoel, S.; Stevens, G.A. National, Regional, and Global Trends in Infertility Prevalence Since 1990: A Systematic Analysis of 277 Health Surveys. PLoS Med. 2012, 9, e1001356. [Google Scholar] [CrossRef]
- Agarwal, A.; Baskaran, S.; Parekh, N.; Cho, C.L.; Henkel, R.; Vij, S.; Arafa, M.; Panner Selvam, M.K.; Shah, R. Male infertility. Lancet 2021, 397, 319–333. [Google Scholar] [CrossRef]
- Agarwal, A.; Parekh, N.; Panner Selvam, M.K.; Henkel, R.; Shah, R.; Homa, S.T.; Ramasamy, R.; Ko, E.; Tremellen, K.; Esteves, S.; et al. Male Oxidative Stress Infertility (MOSI): Proposed Terminology and Clinical Practice Guidelines for Management of Idiopathic Male Infertility. World J. Mens. Health 2019, 37, 296. [Google Scholar] [CrossRef]
- Kylin, E. Studien ueber das hypertonie-hyperglykamie-hyperurikamiesyndrom. Zent. Inn. Med. 1923, 44, 105–127. [Google Scholar]
- Reaven, G.M. Role of insulin resistance in human disease. Diabetes 1988, 37, 1595–1607. [Google Scholar] [CrossRef]
- Haffner, S.M.; Valdez, R.A.; Hazuda, H.P.; Mitchell, B.D.; Morales, P.A.; Stern, M.P. Prospective Analysis of the Insulin-Resistance Syndrome (Syndrome X). Diabetes 1992, 41, 715–722. [Google Scholar] [CrossRef]
- World Health Organization. Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications: Report of a WHO Consultation. Part 1, Diagnosis and Classification of Diabetes Mellitus; WHO/NCD/NCS/99.2; World Health Organization: Geneva, Switzerland, 1999.
- Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA J. Am. Med. Assoc. 2001, 285, 2486–2497. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.M.M.; Zimmet, P.; Shaw, J. The metabolic syndrome—A new worldwide definition. Lancet 2005, 366, 1059–1062. [Google Scholar] [CrossRef]
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.T.; Loria, C.M.; Smith, S.C. Harmonizing the metabolic syndrome: A joint interim statement of the international diabetes federation task force on epidemiology and prevention; National heart, lung, and blood institute; American heart association; World heart federation; International. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, K.; Shoemaker, J.K.; Overend, T.J.; Petrella, R.J. Metabolic syndrome, endothelial function and lifestyle modification. Diabetes Vasc. Dis. Res. 2009, 6, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, V.O.; Grattagliano, I.; Portincasa, P.; Palasciano, G. Systemic oxidative alterations are associated with visceral adiposity and liver steatosis in patients with metabolic syndrome. J. Nutr. 2006, 136, 3022–3026. [Google Scholar] [CrossRef]
- Armutcu, F.; Ataymen, M.; Atmaca, H.; Gurel, A. Oxidative stress markers, C-reactive protein and heat shock protein 70 levels in subjects with metabolic syndrome. Clin. Chem. Lab. Med. 2008, 46, 785–790. [Google Scholar] [CrossRef]
- Devaraj, S.; Leonard, S.; Traber, M.G.; Jialal, I. Gamma-tocopherol supplementation alone and in combination with alpha-tocopherol alters biomarkers of oxidative stress and inflammation in subjects with metabolic syndrome. Free Radic. Biol. Med. 2008, 44, 1203–1208. [Google Scholar] [CrossRef]
- Prasun, P. Mitochondrial dysfunction in metabolic syndrome. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165838. [Google Scholar] [CrossRef]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders—A step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1066–1077. [Google Scholar] [CrossRef]
- Spahis, S.; Borys, J.M.; Levy, E. Metabolic Syndrome as a Multifaceted Risk Factor for Oxidative Stress. Antioxid. Redox Signal. 2017, 26, 445–461. [Google Scholar] [CrossRef]
- Ferder, L.; Inserra, F.; Martínez-Maldonado, M. Inflammation and the metabolic syndrome: Role of angiotensin II and oxidative stress. Curr. Hypertens. Rep. 2006, 8, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Šebeková, K.; Krivošíková, Z.; Gajdoš, M. Total plasma Nε-(carboxymethyl)lysine and sRAGE levels are inversely associated with a number of metabolic syndrome risk factors in non-diabetic young-to-middle-aged medication-free subjects. Clin. Chem. Lab. Med. 2014, 52, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.L.; Maddux, B.A.; Goldfine, I.D. The Molecular Basis for Oxidative Stress-Induced Insulin Resistance. Antioxid. Redox Signal. 2005, 7, 1040–1052. [Google Scholar] [CrossRef] [PubMed]
- Akoumianakis, I.; Antoniades, C. Impaired Vascular Redox Signaling in the Vascular Complications of Obesity and Diabetes Mellitus. Antioxid. Redox Signal. 2019, 30, 333–353. [Google Scholar] [CrossRef] [PubMed]
- Van Guilder, G.P.; Hoetzer, G.L.; Greiner, J.J.; Stauffer, B.L.; DeSouza, C.A. Influence of metabolic syndrome on biomarkers of oxidative stress and inflammation in obese adults. Obesity 2006, 14, 2127–2131. [Google Scholar] [CrossRef] [PubMed]
- Holvoet, P.; Lee, D.H.; Steffes, M.; Gross, M.; Jacobs, D.R. Association between circulating oxidized low-density lipoprotein and incidence of the metabolic syndrome. JAMA J. Am. Med. Assoc. 2008, 299, 2287–2293. [Google Scholar] [CrossRef]
- Huang, P.L. eNOS, metabolic syndrome and cardiovascular disease. Trends Endocrinol. Metab. 2009, 20, 295–302. [Google Scholar] [CrossRef]
- Aitken, R.J. Reactive oxygen species as mediators of sperm capacitation and pathological damage. Mol. Reprod. Dev. 2017, 84, 1039–1052. [Google Scholar] [CrossRef]
- Bisht, S.; Faiq, M.; Tolahunase, M.; Dada, R. Oxidative stress and male infertility. Nat. Rev. Urol. 2017, 14, 470–485. [Google Scholar] [CrossRef]
- Sinha, K.; Das, J.; Pal, P.B.; Sil, P.C. Oxidative stress: The mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch. Toxicol. 2013, 87, 1157–1180. [Google Scholar] [CrossRef]
- Shekarriz, M.; Sharma, R.K.; Thomas, A.J.; Agarwal, A. Positive myeloperoxidase staining (Endtz test) as an indicator of excessive reactive oxygen species formation in semen. J. Assist. Reprod. Genet. 1995, 12, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Saleh, R.A.; Bedaiwy, M.A. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil. Steril. 2003, 79, 829–843. [Google Scholar] [CrossRef]
- Dutta, S.; Sengupta, P.; Slama, P.; Roychoudhury, S. Oxidative stress, testicular inflammatory pathways, and male reproduction. Int. J. Mol. Sci. 2021, 22, 43. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Qiu, E.; Sharma, R. Laboratory assessment of oxidative stress in semen. Arab J. Urol. 2018, 16, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Douglas, C.; Parekh, N.; Kahn, L.G.; Henkel, R.; Agarwal, A. A novel approach to improving the reliability of manual semen analysis: A paradigm shift in the workup of infertile men. World J. Mens. Health 2019, 37, 172–185. [Google Scholar] [CrossRef] [PubMed]
- Politch, J.A.; Tucker, L.; Bowman, F.P.; Anderson, D.J. Concentrations and significance of cytokines and other immunologic factors in semen of healthy fertile men. Hum. Reprod. 2007, 22, 2928–2935. [Google Scholar] [CrossRef]
- Fraczek, M.; Sanocka, D.; Kamieniczna, M.; Kurpisz, M. Proinflammatory cytokines as an intermediate factor enhancing lipid sperm membrane peroxidation in in vitro conditions. J. Androl. 2008, 29, 85–92. [Google Scholar] [CrossRef]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 6th ed.; World Health Organization: Geneva, Switzerland, 2021; ISBN 978-92-4-154778-9.
- Boitrelle, F.; Shah, R.; Saleh, R.; Henkel, R.; Kandil, H.; Chung, E.; Vogiatzi, P.; Zini, A.; Arafa, M.; Agarwal, A. The Sixth Edition of the WHO Manual for Human Semen Analysis: A Critical Review and SWOT Analysis. Life 2021, 11, 1368. [Google Scholar] [CrossRef]
- Aitken, R.J.; Clarkson, J.S.; Fishel, S. Generation of reactive oxygen species, lipid peroxidation, and human sperm function. Biol. Reprod. 1989, 41, 183–197. [Google Scholar] [CrossRef]
- Aitken, R.J.; Baker, M.A. Reactive oxygen species generation by human spermatozoa: A continuing enigma. Int. J. Androl. 2002, 25, 191–194. [Google Scholar] [CrossRef]
- Majzoub, A.; Agarwal, A. Systematic review of antioxidant types and doses in male infertility: Benefits on semen parameters, advanced sperm function, assisted reproduction and live-birth rate. Arab J. Urol. 2018, 16, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Chianese, R.; Pierantoni, R. Mitochondrial reactive oxygen species (ROS) production alters sperm quality. Antioxidants 2021, 10, 92. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.; Milne, S.; Leeson, H. Sperm DNA damage caused by oxidative stress: Modifiable clinical, lifestyle and nutritional factors in male infertility. Reprod. Biomed. Online 2014, 28, 684–703. [Google Scholar] [CrossRef] [PubMed]
- Smits, R.M.; Mackenzie-Proctor, R.; Yazdani, A.; Stankiewicz, M.T.; Jordan, V.; Showell, M.G. Antioxidants for male subfertility. Cochrane Database Syst. Rev. 2019, 2019. [Google Scholar] [CrossRef]
- Salas-Huetos, A.; Rosique-Esteban, N.; Becerra-Tomás, N.; Vizmanos, B.; Bulló, M.; Salas-Salvadó, J. The effect of nutrients and dietary supplements on sperm quality parameters: A systematic review andmeta-analysis of randomized clinical trials. Adv. Nutr. 2018, 9, 833–848. [Google Scholar] [CrossRef]
- Balercia, G.; Regoli, F.; Armeni, T.; Koverech, A.; Mantero, F.; Boscaro, M. Placebo-controlled double-blind randomized trial on the use of L-carnitine, L-acetylcarnitine, or combined L-carnitine and L-acetylcarnitine in men with idiopathic asthenozoospermia. Fertil. Steril. 2005, 84, 662–671. [Google Scholar] [CrossRef]
- Salvio, G.; Cutini, M.; Ciarloni, A.; Giovannini, L.; Perrone, M.; Balercia, G. Coenzyme Q10 and male infertility: A systematic review. Antioxidants 2021, 10, 874. [Google Scholar] [CrossRef]
- Moslemi, M.K.; Tavanbakhsh, S. Selenium-vitamin E supplementation in infertile men: Effects on semen parameters and pregnancy rate. Int. J. Gen. Med. 2011, 4, 99–104. [Google Scholar] [CrossRef]
- Cyrus, A.; Kabir, A.; Goodarzi, D.; Moghimi, M. The effect of adjuvant vitamin C after varicocele surgery on sperm quality and quantity in infertile men: A double blind placebo controlled clinical trial. Int. Braz J Urol 2015, 41, 230–238. [Google Scholar] [CrossRef]
- Kobori, Y.; Ota, S.; Sato, R.; Yagi, H.; Soh, S.; Arai, G.; Okada, H. Antioxidant cosupplementation therapy with vitamin C, vitamin E, and coenzyme Q10 in patients with oligoasthenozoospermia. Arch. Ital. Urol. Androl. 2014, 86, 1–4. [Google Scholar] [CrossRef]
- Zhao, J.; Dong, X.; Hu, X.; Long, Z.; Wang, L.; Liu, Q.; Sun, B.; Wang, Q.; Wu, Q.; Li, L. Zinc levels in seminal plasma and their correlation with male infertility: A systematic review and meta-analysis. Sci. Rep. 2016, 6, 22386. [Google Scholar] [CrossRef] [PubMed]
- Condorelli, R.A.; La Vignera, S.; Mongioì, L.M.; Vitale, S.G.; Laganà, A.S.; Cimino, L.; Calogero, A.E. Myo-inositol as a male fertility molecule: Speed them up! Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 30–35. [Google Scholar] [PubMed]
- Artini, P.G.; Casarosa, E.; Carletti, E.; Monteleone, P.; Di Noia, A.; Di Berardino, O.M. In vitro effect of myo-inositol on sperm motility in normal and oligoasthenospermia patients undergoing in vitro fertilization. Gynecol. Endocrinol. 2017, 33, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Minhas, S.; Bettocchi, C.; Boeri, L.; Capogrosso, P.; Carvalho, J.; Cilesiz, N.C.; Cocci, A.; Corona, G.; Dimitropoulos, K.; Gül, M.; et al. European Association of Urology Guidelines on Male Sexual and Reproductive Health: 2021 Update on Male Infertility. Eur. Urol. 2021, 80, 603–620. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, P.N.; Sigman, M.; Collura, B.; De Jonge, C.J.; Eisenberg, M.L.; Lamb, D.J.; Mulhall, J.P.; Niederberger, C.; Sandlow, J.I.; Sokol, R.Z.; et al. Diagnosis and treatment of infertility in men: AUA/ASRM guideline part II. Fertil. Steril. 2021, 115, 62–69. [Google Scholar] [CrossRef]
- Calogero, A.E.; Aversa, A.; La Vignera, S.; Corona, G.; Ferlin, A. The use of nutraceuticals in male sexual and reproductive disturbances: Position statement from the Italian Society of Andrology and Sexual Medicine (SIAMS). J. Endocrinol. Investig. 2017, 40, 1389–1397. [Google Scholar] [CrossRef]
- Panner Selvam, M.K.; Agarwal, A.; Henkel, R.; Finelli, R.; Robert, K.A.; Iovine, C.; Baskaran, S. The effect of oxidative and reductive stress on semen parameters and functions of physiologically normal human spermatozoa. Free Radic. Biol. Med. 2020, 152, 375–385. [Google Scholar] [CrossRef]
- Creta, M.; Arcaniolo, D.; Celentano, G.; Napolitano, L.; Rocca, R.L.; Capece, M.; Califano, G.; Mangiapia, F.; Spirito, L.; Crocetto, F.; et al. Toxicity of antioxidant supplements in patients with male factor infertility: A systematic review and meta-analysis of randomized controlled trials. Antioxidants 2022, 11, 89. [Google Scholar] [CrossRef]
- Zhao, L.; Pang, A. Effects of Metabolic Syndrome on Semen Quality and Circulating Sex Hormones: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2020, 11, 428. [Google Scholar] [CrossRef]
- Ventimiglia, E.; Capogrosso, P.; Serino, A.; Boeri, L.; Colicchia, M.; La Croce, G.; Scano, R.; Papaleo, E.; Damiano, R.; Montorsi, F.; et al. Metabolic syndrome in White-European men presenting for secondary couple’s infertility: An investigation of the clinical and reproductive burden. Asian J. Androl. 2016, 18, 368–373. [Google Scholar] [CrossRef]
- Leisegang, K.; Bouic, P.J.D.; Henkel, R.R. Metabolic syndrome is associated with increased seminal inflammatory cytokines and reproductive dysfunction in a case-controlled male cohort. Am. J. Reprod. Immunol. 2016, 76, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Saikia, U.K.; Saikia, K.; Sarma, D.; Appaiah, S. Sertoli cell function in young males with metabolic syndrome. Indian J. Endocrinol. Metab. 2019, 23, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Leisegang, K.; Udodong, A.; Bouic, P.J.D.; Henkel, R.R. Effect of the metabolic syndrome on male reproductive function: A case-controlled pilot study. Andrologia 2014, 46, 167–176. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ozturk, U.; Sener, N.C.; Nalbant, I.; Karabacak, O.R.; Ulusoy, M.G.; Imamoglu, M.A. The effect of metabolic syndrome upon the success of varicocelectomy. Sci. World J. 2012, 2012, 985201. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Kao, T.W.; Peng, T.C.; Yang, H.F.; Chen-Jung, W.U.; Chen, W.L. Metabolic syndrome and semen quality in adult population. J. Diabetes 2020, 12, 294–304. [Google Scholar] [CrossRef]
- Lotti, F.; Corona, G.; Degli Innocenti, S.; Filimberti, E.; Scognamiglio, V.; Vignozzi, L.; Forti, G.; Maggi, M. Seminal, ultrasound and psychobiological parameters correlate with metabolic syndrome in male members of infertile couples. Andrology 2013, 1, 229–239. [Google Scholar] [CrossRef]
- Elsamanoudy, A.Z.; Abdalla, H.A.; Hassanien, M.; Gaballah, M.A. Spermatozoal cell death-inducing DNA fragmentation factor-α-like effector A (CIDEA) gene expression and DNA fragmentation in infertile men with metabolic syndrome and normal seminogram. Diabetol. Metab. Syndr. 2016, 8, 76. [Google Scholar] [CrossRef]
- Du Plessis, S.S.; Cabler, S.; McAlister, D.A.; Sabanegh, E.; Agarwal, A. The effect of obesity on sperm disorders and male infertility. Nat. Rev. Urol. 2010, 7, 153–161. [Google Scholar] [CrossRef]
- Palmer, N.O.; Bakos, H.W.; Fullston, T.; Lane, M. Impact of obesity on male fertility, sperm function and molecular composition. Spermatogenesis 2012, 2, 253–263. [Google Scholar] [CrossRef]
- Craig, J.R.; Jenkins, T.G.; Carrell, D.T.; Hotaling, J.M. Obesity, male infertility, and the sperm epigenome. Fertil. Steril. 2017, 107, 848–859. [Google Scholar] [CrossRef]
- Oliveira, P.F.; Sousa, M.; Silva, B.M.; Monteiro, M.P.; Alves, M.G. Obesity, energy balance and spermatogenesis. Reproduction 2017, 153, R173–R185. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Ye, Z.X.; Wang, X.F.; Chang, J.; Yang, M.W.; Zhong, H.H.; Hong, F.F.; Yang, S. Nitric oxide bioavailability dysfunction involves in atherosclerosis. Biomed. Pharmacother. 2018, 97, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Elfassy, Y.; Bongrani, A.; Levy, P.; Foissac, F.; Fellahi, S.; Faure, C.; McAvoy, C.; Capeau, J.; Dupont, J.; Fève, B.; et al. Relationships between metabolic status, seminal adipokines, and reproductive functions in men from infertile couples. Eur. J. Endocrinol. 2020, 182, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Palmer, N.O.; Bakos, H.W.; Owens, J.A.; Setchell, B.P.; Lane, M. Diet and exercise in an obese mouse fed a high-fat diet improve metabolic health and reverse perturbed sperm function. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E768–E780. [Google Scholar] [CrossRef] [PubMed]
- La Vignera, S.; Condorelli, R.; Vicari, E.; D’Agata, R.; Calogero, A.E. Diabetes mellitus and minireview sperm parameters. J. Androl. 2012, 33, 145–153. [Google Scholar] [CrossRef]
- Marchetti, C.; Jouy, N.; Leroy-Martin, B.; Defossez, A.; Formstecher, P.; Marchetti, P. Comparison of four fluorochromes for the detection of the inner mitochondrial membrane potential in human spermatozoa and their correlation with sperm motility. Hum. Reprod. 2004, 19, 2267–2276. [Google Scholar] [CrossRef]
- Marchetti, C.; Obert, G.; Deffosez, A.; Formstecher, P.; Marchetti, P. Study of mitochondrial membrane potential, reactive oxygen species, DNA fragmentation and cell viability by flow cytometry in human sperm. Hum. Reprod. 2002, 17, 1257–1265. [Google Scholar] [CrossRef]
- Tamakoshi, K.; Yatsuya, H.; Kondo, T.; Hori, Y.; Ishikawa, M.; Zhang, H.; Murata, C.; Otsuka, R.; Zhu, S.; Toyoshima, H. The metabolic syndrome is associated with elevated circulating C-reactive protein in healthy reference range, a systemic low-grade inflammatory state. Int. J. Obes. 2003, 27, 443–449. [Google Scholar] [CrossRef]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef]
- Henkel, R. The impact of oxidants on sperm function. Andrologia 2005, 37, 205–206. [Google Scholar] [CrossRef]
- Martínez, P.; Proverbio, F.; Camejo, M.I. Sperm lipid peroxidation and pro-inflammatory cytokines. Asian J. Androl. 2007, 9, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Kershaw, E.E.; Flier, J.S. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 2004, 89, 2548–2556. [Google Scholar] [CrossRef]
- Roumaud, P.; Martin, L.J. Roles of leptin, adiponectin and resistin in the transcriptional regulation of steroidogenic genes contributing to decreased Leydig cells function in obesity. Horm. Mol. Biol. Clin. Investig. 2015, 24, 25–45. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Han, L.; Liu, M.; Lu, J.; Liu, M. Impact of Metabolic Syndrome on Sex Hormones and Reproductive Function: A Meta—Analysis of 2923 Cases and 14062 Controls. Aging 2020, 13, 1962–1971. [Google Scholar] [CrossRef]
- Cheung, A.S.; Hoermann, R.; Dupuis, P.; Joon, D.L.; Zajac, J.D.; Grossmann, M. Relationships between insulin resistance and frailty with body composition and testosterone in men undergoing androgen deprivation therapy for prostate cancer. Eur. J. Endocrinol. 2016, 175, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Berg, W.T.; Miner, M. Hypogonadism and metabolic syndrome: Review and update. Curr. Opin. Endocrinol. Diabetes. Obes. 2020, 27, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Miljić, D.; Popovic, V. Metabolic Syndrome in Hypopituitarism. Front. Horm. Res. 2018, 49, 1–19. [Google Scholar] [CrossRef]
- La Vignera, S.; Vita, R. Thyroid dysfunction and semen quality. Int. J. Immunopathol. Pharmacol. 2018, 32, 2058738418775241. [Google Scholar] [CrossRef]
- Lisovskaya, T.V.; Dubrovina, O.S.; Treshchilov, I.M.; Senturina, L.B.; Sevostyanova, O.Y.; Mayasina, E.N.; Buev, Y.E.; Salimov, D.F. Thyroid disorders and pathospermia in the ART clinic patients. Gynecol. Endocrinol. 2021, 37, 4–7. [Google Scholar] [CrossRef]
- Mehran, L.; Amouzegar, A.; Azizi, F. Thyroid disease and the metabolic syndrome. Curr. Opin. Endocrinol. Diabetes Obes. 2019, 26, 256–265. [Google Scholar] [CrossRef]
- Ejaz, M.; Kumar, P.; Thakur, M.; Bachani, P.; Naz, S.; Lal, K.; Shahid, W.; Shahid, S.; Jahangir, M.; Rizwan, A. Comparison of Lipid Profile in Patients With and Without Subclinical Hypothyroidism. Cureus 2021, 13, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Prasitlumkum, N.; Randhawa, S.; Banerjee, D. Association between subclinical hypothyroidism and incident hypertension in women: A systematic review and meta-analysis. J. Clin. Med. 2021, 10, 3318. [Google Scholar] [CrossRef] [PubMed]
- Stoica, R.; Ancuceanu, R.; Costache, A.; Ștefan, S.; Stoian, A.; Guja, C.; Staden, R.; Popa-Tudor, I.; Serafinceanu, C.; Ionescu-Tîrgoviște, C. Subclinical hypothyroidism has no association with insulin resistance indices in adult females: A case-control study. Exp. Ther. Med. 2021, 22, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Sue, L.Y.; Leung, A.M. Levothyroxine for the Treatment of Subclinical Hypothyroidism and Cardiovascular Disease. Front. Endocrinol. 2020, 11, 824. [Google Scholar] [CrossRef] [PubMed]
- Zijlstra, L.E.; Jukema, J.W.; Westendorp, R.G.J.; Du Puy, R.S.; Poortvliet, R.K.E.; Kearney, P.M.; O’Keeffe, L.; Dekkers, O.M.; Blum, M.R.; Rodondi, N.; et al. Levothyroxine Treatment and Cardiovascular Outcomes in Older People With Subclinical Hypothyroidism: Pooled Individual Results of Two Randomised Controlled Trials. Front. Endocrinol. 2021, 12, 526. [Google Scholar] [CrossRef]
- Pearce, S.H.S.; Brabant, G.; Duntas, L.H.; Monzani, F.; Peeters, R.P.; Razvi, S.; Wemeau, J.-L. 2013 ETA Guideline: Management of Subclinical Hypothyroidism. Eur. Thyroid J. 2014, 2, 215–228. [Google Scholar] [CrossRef] [PubMed]
- ATA/AACE American Thyroid Association Clinical Practice Guidelines. Endocr. Pract. 2012, 18, 4–7.
- Arnaldi, G.; Martino, M. Androgens in Cushing’s Syndrome. Front. Horm. Res. 2019, 53, 77–91. [Google Scholar] [CrossRef]
- Barbot, M.; Zilio, M.; Scaroni, C. Cushing’s syndrome: Overview of clinical presentation, diagnostic tools and complications. Best Pract. Res. Clin. Endocrinol. Metab. 2020, 34, 101380. [Google Scholar] [CrossRef]
- Tirabassi, G.; Muscogiuri, G.; Colao, A.; Balercia, G. Dysregulation of the hypothalamic-pituitary-adrenal axis increases central body fat accumulation in males affected by diabetes mellitus and late-onset hypogonadism. Endocr. Pract. 2016, 22, 427–433. [Google Scholar] [CrossRef]
- Elhassan, Y.S.; Alahdab, F.; Prete, A.; Delivanis, D.A.; Khanna, A.; Prokop, L.; Murad, M.H.; O’Reilly, M.W.; Arlt, W.; Bancos, I. Natural History of Adrenal Incidentalomas with and without Mild Autonomous Cortisol Excess A Systematic Review and Meta-analysis. Ann. Intern. Med. 2019, 171, 107–116. [Google Scholar] [CrossRef]
- Cosarderelioglu, C.; Nidadavolu, L.S.; George, C.J.; Oh, E.S.; Bennett, D.A.; Walston, J.D.; Abadir, P.M. Brain Renin–Angiotensin System at the Intersect of Physical and Cognitive Frailty. Front. Neurosci. 2020, 14, 981. [Google Scholar] [CrossRef] [PubMed]
- Leal, M.C.; Pinheiro, S.V.B.; Ferreira, A.J.; Santos, R.A.S.; Bordoni, L.S.; Alenina, N.; Bader, M.; de França, L.R. The role of angiotensin-(1-7) receptor Mas in spermatogenesis in mice and rats. J. Anat. 2009, 214, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Moura, A.A.; Memili, E.; Portela, A.M.R.; Viana, A.G.; Velho, A.L.C.; Bezerra, M.J.B.; Vasconselos, F.R. Seminal plasma proteins and metabolites: Effects on sperm function and potential as fertility markers. Anim. Reprod. 2018, 15, 691–702. [Google Scholar] [CrossRef]
- Bechara, G.R.; De Souza, D.B.; Simoes, M.; Felix-Patrício, B.; Medeiros, J.L.; Costa, W.S.; Sampaio, F.J.B. Testicular Morphology and Spermatozoid Parameters in Spontaneously Hypertensive Rats Treated with Enalapril. J. Urol. 2015, 194, 1498–1503. [Google Scholar] [CrossRef]
- Altıntaş Aykan, D. The effects of sacubitril/valsartan and ramipril on the male fertility in hypertensive rats. North. Clin. Istanbul 2020, 7, 425–432. [Google Scholar] [CrossRef]
- Gianzo, M.; Subirán, N. Regulation of male fertility by the renin-angiotensin system. Int. J. Mol. Sci. 2020, 21, 7943. [Google Scholar] [CrossRef]
- Brouwers, S.; Sudano, I.; Kokubo, Y.; Sulaica, E.M. Arterial hypertension. Lancet 2021, 398, 249–261. [Google Scholar] [CrossRef]
- Brown, S.G.; Publicover, S.J.; Barratt, C.L.R.; Martins da Silva, S.J. Human sperm ion channel (dys)function: Implications for fertilization. Hum. Reprod. Update 2019, 25, 758–776. [Google Scholar] [CrossRef]
- Morakinyo, A.O.; Iranloye, B.O.; Adegoke, O.A. Antireproductive effect of calcium channel blockers on male rats. Reprod. Med. Biol. 2009, 8, 97–102. [Google Scholar] [CrossRef]
- Morakinyo, A.; Iranloye, B.; Adegoke, O. Calcium antagonists modulate oxidative stress and acrosomal reaction in rat spermatozoa. Arch. Med. Sci. 2011, 7, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Aprioku, J.S. Early effects of concurrent administration of artesunate-amodiaquine and nifedipine on sperm parameters and sex hormones in Guinea pigs: An experimental study. Int. J. Reprod. Biomed. 2018, 16, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Wiysonge, C.S.; Bradley, H.A.; Volmink, J.; Mayosi, B.M.; Mbewu, A.; Opie, L.H. Beta-blockers for hypertension. Cochrane Database Syst. Rev. 2012, 11, CD002003. [Google Scholar] [CrossRef]
- Hong, C.; Turner, P. Influence of lipid solubility on the sperm immobilizing effect of beta- adrenoceptor blocking drugs. Br. J. Clin. Pharmacol. 1982, 14, 269–272. [Google Scholar] [CrossRef]
- Manolis, A.; Doumas, M.; Ferri, C.; Mancia, G. Erectile dysfunction and adherence to antihypertensive therapy: Focus on β-blockers. Eur. J. Intern. Med. 2020, 81, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Rangel, E.; Inzucchi, S.E. Metformin: Clinical use in type 2 diabetes. Diabetologia 2017, 60, 1586–1593. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.J.; Mu, Y.; Yu, N.; Yi, T.L.; Zhang, Y.; Pang, X.L.; Cheng, D.; Yang, J. Protective effects of metformin on reproductive function in obese male rats induced by high-fat diet. J. Assist. Reprod. Genet. 2015, 32, 1097–1104. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Y.; Chang, T.C.; Lin, S.H.; Wu, S.T.; Cha, T.L.; Tsao, C.W. Metformin ameliorates testicular function and spermatogenesis in male mice with high-fat and high-cholesterol diet-induced obesity. Nutrients 2020, 12, 1932. [Google Scholar] [CrossRef]
- Alves, M.G.; Martins, A.D.; Vaz, C.V.; Correia, S.; Moreira, P.I.; Oliveira, P.F.; Socorro, S. Metformin and male reproduction: Effects on Sertoli cell metabolism. Br. J. Pharmacol. 2014, 171, 1033–1042. [Google Scholar] [CrossRef]
- Cannarella, R.; Calogero, A.E.; Condorelli, R.A.; Greco, E.A.; Aversa, A.; La Vignera, S. Is there a role for glucagon-like peptide-1 receptor agonists in the treatment of male infertility? Andrology 2021, 9, 1499–1503. [Google Scholar] [CrossRef]
- Heeba, G.H.; Hamza, A.A. Rosuvastatin ameliorates diabetes-induced reproductive damage via suppression of oxidative stress, inflammatory and apoptotic pathways in male rats. Life Sci. 2015, 141, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Gabbar, M.; Esmail, M.; Kandeil, M.; El-Zanaty, A.M. The ameliorative effect of atorvastatin on serum testosterone and testicular oxidant/antioxidant system of HFD-fed male albino rats. F1000Research 2020, 9, 1300. [Google Scholar] [CrossRef]
- Pons-Rejraji, H.; Brugnon, F.; Sion, B.; Maqdasy, S.; Gouby, G.; Pereira, B.; Marceau, G.; Gremeau, A.S.; Drevet, J.; Grizard, G.; et al. Evaluation of atorvastatin efficacy and toxicity on spermatozoa, accessory glands and gonadal hormones of healthy men: A pilot prospective clinical trial. Reprod. Biol. Endocrinol. 2014, 12, 65. [Google Scholar] [CrossRef] [PubMed]
- Kasman, A.M.; Zhang, C.A.; Li, S.; Lu, Y.; Lathi, R.B.; Stevenson, D.K.; Shaw, G.M.; Eisenberg, M.L. Association between preconception paternal health and pregnancy loss in the USA: An analysis of US claims data. Hum. Reprod. 2021, 36, 785–793. [Google Scholar] [CrossRef]
- Murugappan, G.; Li, S.; Leonard, S.A.; Winn, V.D.; Druzin, M.L.; Eisenberg, M.L. Association of preconception paternal health and adverse maternal outcomes among healthy mothers. Am. J. Obstet. Gynecol. MFM 2021, 3, 100384. [Google Scholar] [CrossRef]
- Lan, L.; Harrison, C.L.; Misso, M.; Hill, B.; Teede, H.J.; Mol, B.W.; Moran, L.J. Systematic review and meta-analysis of the impact of preconception lifestyle interventions on fertility, obstetric, fetal, anthropometric and metabolic outcomes in men and women. Hum. Reprod. 2017, 32, 1925–1940. [Google Scholar] [CrossRef]
- Lotti, F.; Marchiani, S.; Corona, G.; Maggi, M. Metabolic Syndrome and Reproduction. Int. J. Mol. Sci. 2021, 22, 1988. [Google Scholar] [CrossRef]
- Cohen, D.J.; Giaccagli, M.M.; Herzfeld, J.D.; González, L.N.; Cuasnicú, P.S.; Da Ros, V.G. Metabolic Syndrome and Male Fertility Disorders: Is There a Causal Link? Rev. Endocr. Metab. Disord. 2021, 22, 1057–1071. [Google Scholar] [CrossRef]
| WHO | EGSIR | ATP III | AACE | IDF | AHA/NHLBI | AHA/NHLBI + IDF | |
|---|---|---|---|---|---|---|---|
| Definition | Insulin resistance + any other two components | Plasma insulin concentration > 75th percentile of nondiabetic patients + any of two components | Any of three out of five components | Insulin resistance + at least two other components | Central Obesity + at least two other components | Any of three out five components | Any of three out five components |
| Obesity | Waist/hip ratio > 0.9 in males and >0.85 in females or BMI > 30 kg/m2 | Waist circumference ≥ 94 cm in males and ≥80 cm in females | Waist circumference >102 cm in males and >80 cm in females | BMI > 25 kg/m2 | Obesity defined as waist circumference with ethnicity specific values or BMI >30 or kg/m2 | Waist circumference > 40 inches in males and >35 inches in females | Raised waist circumference (Population- and country-specific definitions) |
| HDL | <35 mg/dL: males <39 mg/dL: females | <39 mg/dL: males and females, or specific treatment | <40 mg/dL: males <50 mg/dL: females, or specific treatment | <40 mg/dL: males <50 mg/dL: females | <40 mg/dL: males <50 mg/dL: females, or specific treatment | <40 mg/dL: males <50 mg/dL: females, or specific treatment | <40 mg/dL: males <50 mg/dL: females, or specific treatment |
| TG | ≥150 mg/dL | ≥150 mg/dL or specific treatment | ≥150 mg/dL or specific treatment | ≥150 mg/dL | ≥150 mg/dL or specific treatment | ≥150 mg/dL or specific treatment | ≥150 mg/dL or specific treatment |
| Hyperglycemia | Impaired glucose tolerance, impaired fasting glucose, or lowered insulin sensitivity | Fasting plasma glucose > 110 mg/dL | Fasting plasma glucose > 110 mg/dL or specific treatment | Impaired glucose tolerance or impaired fasting glucose (but not diabetes) | Fasting plasma glucose > 100 mg/dL or previously diagnosed type 2 diabetes | Fasting plasma glucose > 100 mg/dL or specific treatment | Fasting plasma glucose > 100 mg/dL or specific treatment |
| Blood Pressure | ≥140/90 mm Hg | ≥140/90 mm Hg or specific treatment | SBP ≥ 130 or DBP ≥ 85 mm Hg or specific treatment | ≥130/85 mm Hg | SBP ≥ 130 or DBP ≥ 85 mm Hg or specific treatment | ≥130/85 mm Hg or specific treatment | ≥130/85 mm Hg or specific treatment |
| Urine albumin ≥ 20 μg/min or albumin: creatinine ratio ≥ 30 mg/g |
| Reference | Semen Volume | Sperm Concentration | Sperm Motility | Sperm Morphology | Sperm Vitality | SDF | MMP |
|---|---|---|---|---|---|---|---|
| Ventimiglia et al. [62] | ↓ | ↓ | = | ↓ | = | NE | NE |
| Leisegang et al. [63] | ↓ | ↓ | ↓ Total and progressive motility | NE | NE | ↑ | ↓ |
| Saikia et al. [64] | ↓ | Lower total count | ↓ Total and progressive motility | = | NE | NE | NE |
| Leisegang et al. [65] | = | ↓ | ↓ Only progressive motility | NE | ↓ | ↑ | ↓ |
| Ozturk et al. [66] | NE | Lower total count | ↓ | = | NE | NE | NE |
| Chen et al. [67] | = | NE | ↓ | ↓ | ↓ | NE | NE |
| Lotti et al. [68] | = | = | = | ↓ | NE | NE | NE |
| Elsamanoudy et al. [69] | = | NE | ↓ | ↓ | ↓ | NE | NE |
| Elfassy et al. [75] | = | ↑ | ↓ | = | ↓ | = | NE |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvio, G.; Ciarloni, A.; Cutini, M.; delli Muti, N.; Finocchi, F.; Perrone, M.; Rossi, S.; Balercia, G. Metabolic Syndrome and Male Fertility: Beyond Heart Consequences of a Complex Cardiometabolic Endocrinopathy. Int. J. Mol. Sci. 2022, 23, 5497. https://doi.org/10.3390/ijms23105497
Salvio G, Ciarloni A, Cutini M, delli Muti N, Finocchi F, Perrone M, Rossi S, Balercia G. Metabolic Syndrome and Male Fertility: Beyond Heart Consequences of a Complex Cardiometabolic Endocrinopathy. International Journal of Molecular Sciences. 2022; 23(10):5497. https://doi.org/10.3390/ijms23105497
Chicago/Turabian StyleSalvio, Gianmaria, Alessandro Ciarloni, Melissa Cutini, Nicola delli Muti, Federica Finocchi, Michele Perrone, Silvia Rossi, and Giancarlo Balercia. 2022. "Metabolic Syndrome and Male Fertility: Beyond Heart Consequences of a Complex Cardiometabolic Endocrinopathy" International Journal of Molecular Sciences 23, no. 10: 5497. https://doi.org/10.3390/ijms23105497
APA StyleSalvio, G., Ciarloni, A., Cutini, M., delli Muti, N., Finocchi, F., Perrone, M., Rossi, S., & Balercia, G. (2022). Metabolic Syndrome and Male Fertility: Beyond Heart Consequences of a Complex Cardiometabolic Endocrinopathy. International Journal of Molecular Sciences, 23(10), 5497. https://doi.org/10.3390/ijms23105497







