The Promises of Natural Killer Cell Therapy in Endometriosis
Abstract
:1. Introduction
2. Results
2.1. Blocking of Inhibitory Receptors
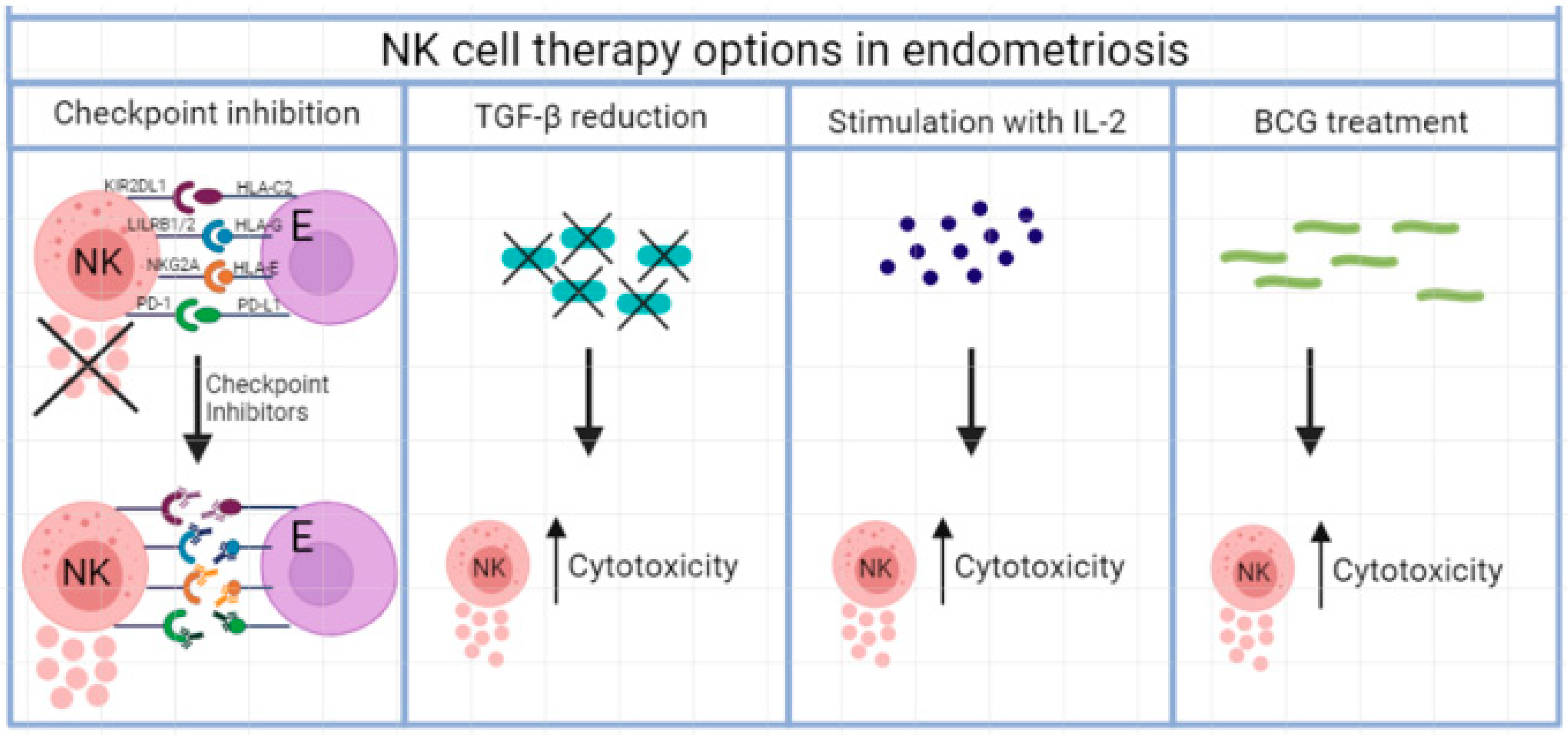
2.2. Checkpoint Inhibition
2.3. Inhibitory Cytokine Therapy
2.4. Stimulatory Cytokine Therapy
2.5. Mycobacterial Treatment
2.6. Adoptive NK Cell Therapy
3. Discussion
4. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thiruchelvam, U.; Wingfield, M.; O’Farrelly, C. Natural Killer Cells: Key Players in Endometriosis. Am. J. Reprod. Immunol. 2015, 74, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, T.; Yoshimura, Y. Molecular and cellular mechanisms for differentiation and regeneration of the uterine endometrium. Endocr. J. 2008, 55, 795–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burney, R.O.; Giudice, L.C. Pathogenesis and pathophysiology of endometriosis. Fertil. Steril. 2012, 98, 511–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stratton, P.; Berkley, K.J. Chronic pelvic pain and endometriosis: Translational evidence of the relationship and implications. Hum. Reprod. Update 2011, 17, 327–346. [Google Scholar] [CrossRef] [Green Version]
- Nnoaham, K.E.; Hummelshoj, L.; Webster, P.; d’Hooghe, T.; de Cicco Nardone, F.; de Cicco Nardone, C.; Jenkinson, C.; Kennedy, S.H.; Zondervan, K.T.; World Endometriosis Research Foundation Global Study of Women’s Health. Impact of endometriosis on quality of life and work productivity: A multicenter study across ten countries. Fertil. Steril. 2011, 96, 366–373.e8. [Google Scholar] [CrossRef] [Green Version]
- Alimi, Y.; Iwanaga, J.; Loukas, M.; Tubbs, R.S. The Clinical Anatomy of Endometriosis: A Review. Cureus 2018, 10, e3361. [Google Scholar] [CrossRef] [Green Version]
- Falcone, T.; Flyckt, R. Clinical Management of Endometriosis. Obstet. Gynecol. 2018, 131, 557–571. [Google Scholar] [CrossRef] [Green Version]
- Bulun, S.E.; Yilmaz, B.D.; Sison, C.; Miyazaki, K.; Bernardi, L.; Liu, S.; Kohlmeier, A.; Yin, P.; Milad, M.; Wei, J. Endometriosis. Endocr. Rev. 2019, 40, 1048–1079. [Google Scholar] [CrossRef]
- Mehedintu, C.; Plotogea, M.N.; Ionescu, S.; Antonovici, M. Endometriosis still a challenge. J. Med. Life 2014, 7, 349–357. [Google Scholar]
- Riccio, L.; Santulli, P.; Marcellin, L.; Abrao, M.S.; Batteux, F.; Chapron, C. Immunology of endometriosis. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 50, 39–49. [Google Scholar] [CrossRef]
- Izumi, G.; Koga, K.; Takamura, M.; Makabe, T.; Satake, E.; Takeuchi, A.; Taguchi, A.; Urata, Y.; Fujii, T.; Osuga, Y. Involvement of immune cells in the pathogenesis of endometriosis. J. Obstet. Gynaecol. Res. 2018, 44, 191–198. [Google Scholar] [CrossRef] [Green Version]
- Tosti, C.; Pinzauti, S.; Santulli, P.; Chapron, C.; Petraglia, F. Pathogenetic Mechanisms of Deep Infiltrating Endometriosis. Reprod. Sci. 2015, 22, 1053–1059. [Google Scholar] [CrossRef]
- Selam, B.; Kayisli, U.A.; Garcia-Velasco, J.A.; Arici, A. Extracellular matrix-dependent regulation of Fas ligand expression in human endometrial stromal cells. Biol. Reprod. 2002, 66, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Fainaru, O.; Adini, A.; Benny, O.; Adini, I.; Short, S.; Bazinet, L.; Nakai, K.; Pravda, E.; Hornstein, M.D.; D’Amato, R.J.; et al. Dendritic cells support angiogenesis and promote lesion growth in a murine model of endometriosis. FASEB J. 2008, 22, 522–529. [Google Scholar] [CrossRef]
- Pencovich, N.; Luk, J.; Hantisteanu, S.; Hornstein, M.D.; Fainaru, O. The development of endometriosis in a murine model is dependent on the presence of dendritic cells. Reprod. Biomed. Online 2014, 28, 515–521. [Google Scholar] [CrossRef] [Green Version]
- Stanic, A.K.; Kim, M.; Styer, A.K.; Rueda, B.R. Dendritic cells attenuate the early establishment of endometriosis-like lesions in a murine model. Reprod. Sci. 2014, 21, 1228–1236. [Google Scholar] [CrossRef]
- de Barros, I.B.L.; Malvezzi, H.; Gueuvoghlanian-Silva, B.Y.; Piccinato, C.A.; Rizzo, L.V.; Podgaec, S. What do we know about regulatory T cells and endometriosis? A systematic review. J. Reprod. Immunol. 2017, 120, 48–55. [Google Scholar] [CrossRef]
- Delbandi, A.A.; Mahmoudi, M.; Shervin, A.; Moradi, Z.; Arablou, T.; Zarnani, A.H. Higher frequency of circulating, but not tissue regulatory T cells in patients with endometriosis. J. Reprod. Immunol. 2020, 139, 103119. [Google Scholar] [CrossRef]
- Jeung, I.; Cheon, K.; Kim, M.R. Decreased Cytotoxicity of Peripheral and Peritoneal Natural Killer Cell in Endometriosis. BioMed Res. Int. 2016, 2016, 2916070. [Google Scholar] [CrossRef] [Green Version]
- Gaynor, L.M.; Colucci, F. Uterine Natural Killer Cells: Functional Distinctions and Influence on Pregnancy in Humans and Mice. Front. Immunol. 2017, 8, 467. [Google Scholar] [CrossRef] [Green Version]
- Flynn, L.; Byrne, B.; Carton, J.; Kelehan, P.; O’Herlihy, C.; O’Farrelly, C. Menstrual cycle dependent fluctuations in NK and T-lymphocyte subsets from non-pregnant human endometrium. Am. J. Reprod. Immunol. 2000, 43, 209–217. [Google Scholar] [CrossRef]
- Wu, X.G.; Chen, J.J.; Zhou, H.L.; Wu, Y.; Lin, F.; Shi, J.; Wu, H.Z.; Xiao, H.Q.; Wang, W. Identification and Validation of the Signatures of Infiltrating Immune Cells in the Eutopic Endometrium Endometria of Women With Endometriosis. Front. Immunol. 2021, 12, 671201. [Google Scholar] [CrossRef]
- Vallve-Juanico, J.; Houshdaran, S.; Giudice, L.C. The endometrial immune environment of women with endometriosis. Hum. Reprod. Update 2019, 25, 564–591. [Google Scholar] [CrossRef]
- Faas, M.M.; de Vos, P. Uterine NK cells and macrophages in pregnancy. Placenta 2017, 56, 44–52. [Google Scholar] [CrossRef]
- Fukaya, T.; Sugawara, J.; Yoshida, H.; Murakami, T.; Yajima, A. Intercellular adhesion molecule-1 and hepatocyte growth factor in human endometriosis: Original investigation and a review of literature. Gynecol. Obstet. Investig. 1999, 47 (Suppl. 1), 11–16. [Google Scholar] [CrossRef]
- Maeda, N.; Izumiya, C.; Yamamoto, Y.; Oguri, H.; Kusume, T.; Fukaya, T. Increased killer inhibitory receptor KIR2DL1 expression among natural killer cells in women with pelvic endometriosis. Fertil. Steril. 2002, 77, 297–302. [Google Scholar] [CrossRef] [Green Version]
- Valipour, B.; Velaei, K.; Abedelahi, A.; Karimipour, M.; Darabi, M.; Charoudeh, H.N. NK cells: An attractive candidate for cancer therapy. J. Cell. Physiol. 2019, 234, 19352–19365. [Google Scholar] [CrossRef]
- Hoogstad-van Evert, J.S.; Bekkers, R.; Ottevanger, N.; Jansen, J.H.; Massuger, L.; Dolstra, H. Harnessing natural killer cells for the treatment of ovarian cancer. Gynecol. Oncol. 2020, 157, 810–816. [Google Scholar] [CrossRef]
- Geller, M.A.; Miller, J.S. Use of allogeneic NK cells for cancer immunotherapy. Immunotherapy 2011, 3, 1445–1459. [Google Scholar] [CrossRef] [Green Version]
- Sharma, R.; Das, A. IL-2 mediates NK cell proliferation but not hyperactivity. Immunol. Res. 2018, 66, 151–157. [Google Scholar] [CrossRef]
- Mira, T.A.A.; Buen, M.M.; Borges, M.G.; Yela, D.A.; Benetti-Pinto, C.L. Systematic review and meta-analysis of complementary treatments for women with symptomatic endometriosis. Int. J. Gynaecol. Obstet. 2018, 143, 2–9. [Google Scholar] [CrossRef]
- Sciezynska, A.; Komorowski, M.; Soszynska, M.; Malejczyk, J. NK Cells as Potential Targets for Immunotherapy in Endometriosis. J. Clin. Med. 2019, 8, 1468. [Google Scholar] [CrossRef] [Green Version]
- Matsuoka, S.; Maeda, N.; Izumiya, C.; Yamashita, C.; Nishimori, Y.; Fukaya, T. Expression of inhibitory-motif killer immunoglobulin-like receptor, KIR2DL1, is increased in natural killer cells from women with pelvic endometriosis. Am. J. Reprod. Immunol. 2005, 53, 249–254. [Google Scholar] [CrossRef]
- Bylinska, A.; Wilczynska, K.; Malejczyk, J.; Milewski, L.; Wagner, M.; Jasek, M.; Niepieklo-Miniewska, W.; Wisniewski, A.; Ploski, R.; Barcz, E.; et al. The impact of HLA-G, LILRB1 and LILRB2 gene polymorphisms on susceptibility to and severity of endometriosis. Mol. Genet. Genom. 2018, 293, 601–613. [Google Scholar] [CrossRef] [Green Version]
- Galandrini, R.; Porpora, M.G.; Stoppacciaro, A.; Micucci, F.; Capuano, C.; Tassi, I.; Di Felice, A.; Benedetti-Panici, P.; Santoni, A. Increased frequency of human leukocyte antigen-E inhibitory receptor CD94/NKG2A-expressing peritoneal natural killer cells in patients with endometriosis. Fertil. Steril. 2008, 89 (Suppl. 5), 1490–1496. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Lv, C.; Su, Y.; Li, C.; Zhang, H.; Zhao, X.; Li, M. Expression of programmed death-1 (PD-1) and its ligand PD-L1 is upregulated in endometriosis and promoted by 17beta-estradiol. Gynecol. Endocrinol. 2019, 35, 251–256. [Google Scholar] [CrossRef]
- Sikora, J.; Smycz-Kubanska, M.; Mielczarek-Palacz, A.; Bednarek, I.; Kondera-Anasz, Z. The involvement of multifunctional TGF-beta and related cytokines in pathogenesis of endometriosis. Immunol. Lett. 2018, 201, 31–37. [Google Scholar] [CrossRef]
- Zhang, Z.; Yuan, Y.; He, L.; Yao, X.; Chen, J. Involvement of angiotensin II receptor type 1/NF-kappaB signaling in the development of endometriosis. Exp. Ther. Med. 2020, 20, 3269–3277. [Google Scholar] [CrossRef]
- Cakmak, B.; Cavusoglu, T.; Ates, U.; Meral, A.; Nacar, M.C.; Erbas, O. Regression of experimental endometriotic implants in a rat model with the angiotensin II receptor blocker losartan. J. Obstet. Gynaecol. Res. 2015, 41, 601–607. [Google Scholar] [CrossRef]
- Nataatmadja, M.; West, J.; Prabowo, S.; West, M. Angiotensin II Receptor Antagonism Reduces Transforming Growth Factor Beta and Smad Signaling in Thoracic Aortic Aneurysm. Ochsner. J. 2013, 13, 42–48. [Google Scholar] [PubMed]
- Velasco, I.; Quereda, F.; Bermejo, R.; Campos, A.; Acien, P. Intraperitoneal recombinant interleukin-2 activates leukocytes in rat endometriosis. J. Reprod. Immunol. 2007, 74, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Clayton, R.D.; Duffy, S.R.; Wilkinson, N.; Garry, R.; Jackson, A.M. Increase in peripheral blood mononuclear cell (PBMC)- and CD56+ cell-mediated killing of endometrial stromal cells by mycobacteria; a possible role in endometriosis immunotherapy? Hum. Reprod. 2004, 19, 1886–1893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Binyamin, L.; Alpaugh, R.K.; Hughes, T.L.; Lutz, C.T.; Campbell, K.S.; Weiner, L.M. Blocking NK cell inhibitory self-recognition promotes antibody-dependent cellular cytotoxicity in a model of anti-lymphoma therapy. J. Immunol. 2008, 180, 6392–6401. [Google Scholar] [CrossRef] [PubMed]
- Carosella, E.D.; Rouas-Freiss, N.; Tronik-Le Roux, D.; Moreau, P.; LeMaoult, J. HLA-G: An Immune Checkpoint Molecule. Adv. Immunol. 2015, 127, 33–144. [Google Scholar] [CrossRef]
- Amodio, G.; Sales de Albuquerque, R.; Gregori, S. New insights into HLA-G mediated tolerance. Tissue Antigens 2014, 84, 255–263. [Google Scholar] [CrossRef]
- Andre, P.; Denis, C.; Soulas, C.; Bourbon-Caillet, C.; Lopez, J.; Arnoux, T.; Blery, M.; Bonnafous, C.; Gauthier, L.; Morel, A.; et al. Anti-NKG2A mAb Is a Checkpoint Inhibitor that Promotes Anti-tumor Immunity by Unleashing Both T and NK Cells. Cell 2018, 175, 1731–1743.e13. [Google Scholar] [CrossRef] [Green Version]
- Tinker, A.V.; Hirte, H.W.; Provencher, D.; Butler, M.; Ritter, H.; Tu, D.; Azim, H.A., Jr.; Paralejas, P.; Grenier, N.; Hahn, S.A.; et al. Dose-Ranging and Cohort-Expansion Study of Monalizumab (IPH2201) in Patients with Advanced Gynecologic Malignancies: A Trial of the Canadian Cancer Trials Group (CCTG): IND221. Clin. Cancer Res. 2019, 25, 6052–6060. [Google Scholar] [CrossRef] [Green Version]
- Marchetti, A.; Di Lorito, A.; Buttitta, F. Why anti-PD1/PDL1 therapy is so effective? Another piece in the puzzle. J. Thorac. Dis. 2017, 9, 4863–4866. [Google Scholar] [CrossRef] [Green Version]
- Spiers, L.; Coupe, N.; Payne, M. Toxicities associated with checkpoint inhibitors-an overview. Rheumatology 2019, 58 (Suppl. 7), vii7–vii16. [Google Scholar] [CrossRef]
- Hawinkels, L.J.; Ten Dijke, P. Exploring anti-TGF-beta therapies in cancer and fibrosis. Growth Factors 2011, 29, 140–152. [Google Scholar] [CrossRef]
- Fabregat, I.; Fernando, J.; Mainez, J.; Sancho, P. TGF-beta signaling in cancer treatment. Curr. Pharm. Des. 2014, 20, 2934–2947. [Google Scholar] [CrossRef] [PubMed]
- Varga, J.; Pasche, B. Transforming growth factor beta as a therapeutic target in systemic sclerosis. Nat. Rev. Rheumatol. 2009, 5, 200–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, T.; Zhou, C.; Ren, S. Role of IL-2 in cancer immunotherapy. Oncoimmunology 2016, 5, e1163462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oosterlynck, D.J.; Lacquet, F.A.; Waer, M.; Koninckx, P.R. Lymphokine-activated killer activity in women with endometriosis. Gynecol. Obstet. Investig. 1994, 37, 185–190. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoogstad-van Evert, J.; Paap, R.; Nap, A.; van der Molen, R. The Promises of Natural Killer Cell Therapy in Endometriosis. Int. J. Mol. Sci. 2022, 23, 5539. https://doi.org/10.3390/ijms23105539
Hoogstad-van Evert J, Paap R, Nap A, van der Molen R. The Promises of Natural Killer Cell Therapy in Endometriosis. International Journal of Molecular Sciences. 2022; 23(10):5539. https://doi.org/10.3390/ijms23105539
Chicago/Turabian StyleHoogstad-van Evert, Janneke, Romy Paap, Annemiek Nap, and Renate van der Molen. 2022. "The Promises of Natural Killer Cell Therapy in Endometriosis" International Journal of Molecular Sciences 23, no. 10: 5539. https://doi.org/10.3390/ijms23105539
APA StyleHoogstad-van Evert, J., Paap, R., Nap, A., & van der Molen, R. (2022). The Promises of Natural Killer Cell Therapy in Endometriosis. International Journal of Molecular Sciences, 23(10), 5539. https://doi.org/10.3390/ijms23105539





